Effect of Dietary Exposure to Low-Density Polyethylene Microplastics and Their Potential Role as Estrogen Vectors In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Microplastic Pellets and Fish Selection
2.2. LDPE Microplastic Exposure in Wastewater Treatment Plant
2.3. Chemicals Extraction of LDPE Microplastics (Pristine and Exposed)
2.4. Dietary Exposure of LPDE Microplastics to Fish
2.5. Terminal Sampling
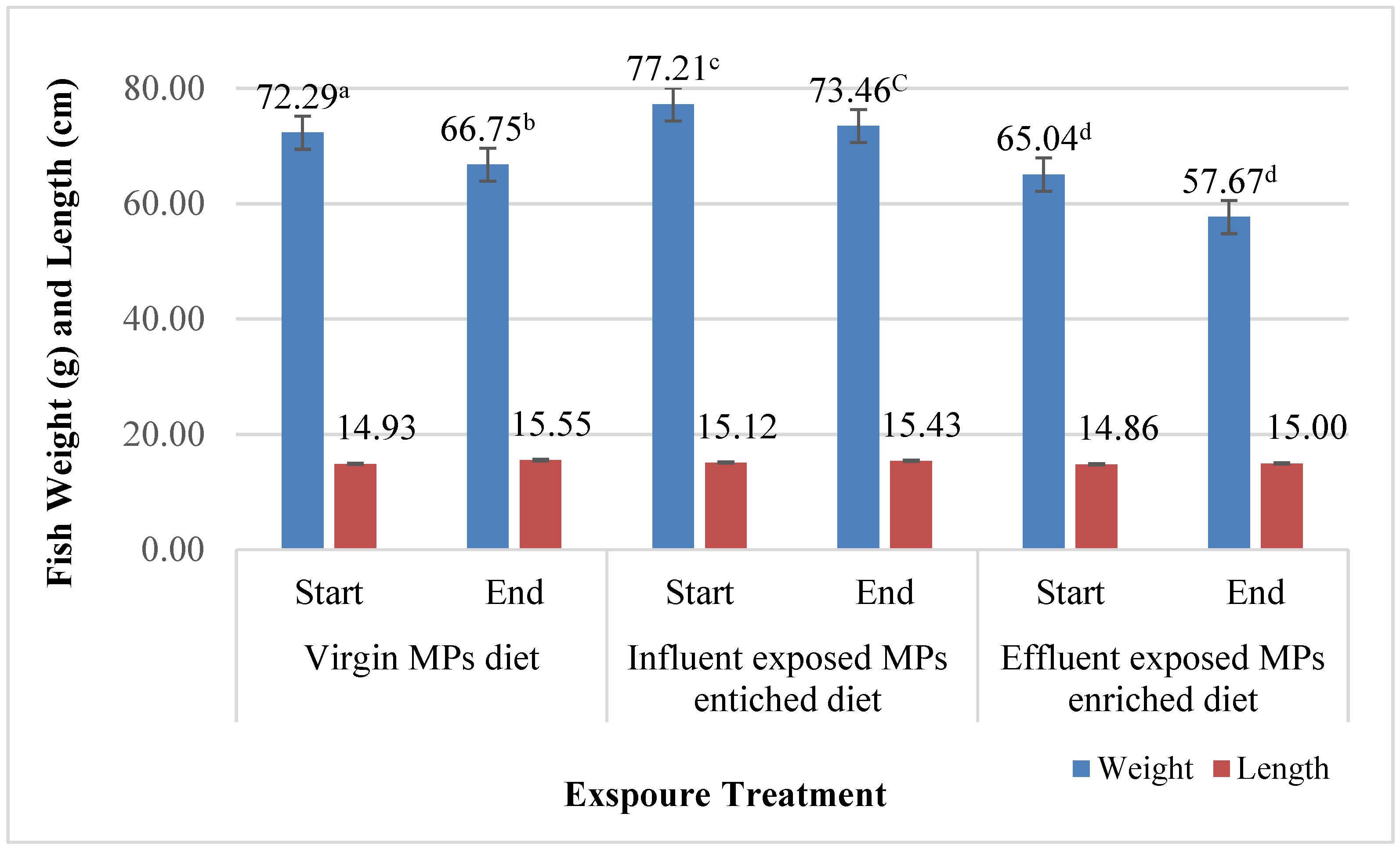
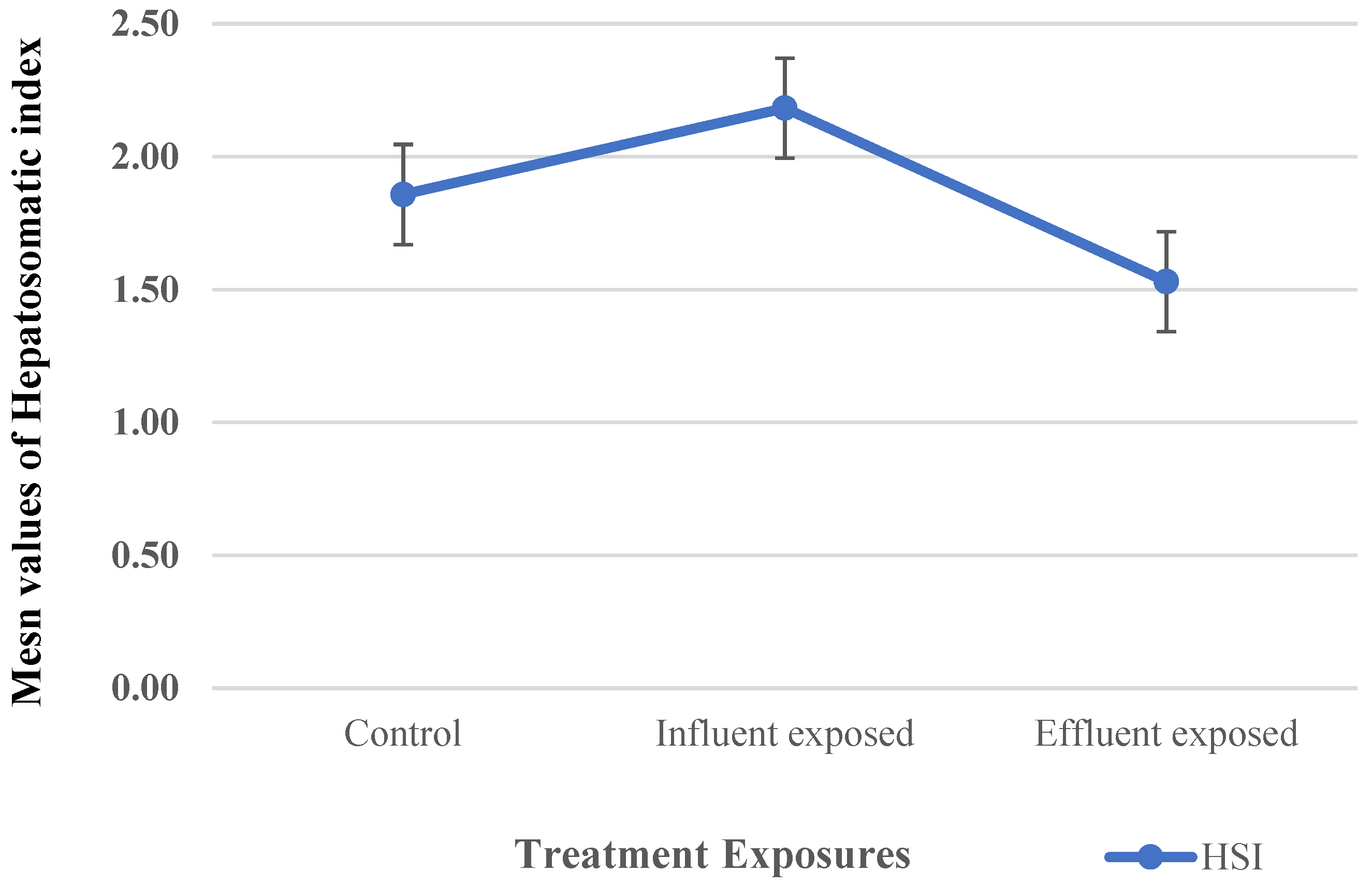
2.6. Biological Parameters
2.7. Serum Protein and Vitellogenin
2.7.1. Serum Protein
2.7.2. Serum Vitellogenin
2.7.3. qPCR Assay for CYP 1A1 and Vtg Genes in Liver Tissue
2.8. Statistical Analysis
3. Results
3.1. Biochemical Parameters
3.1.1. Serum Protein and Vitellogenin
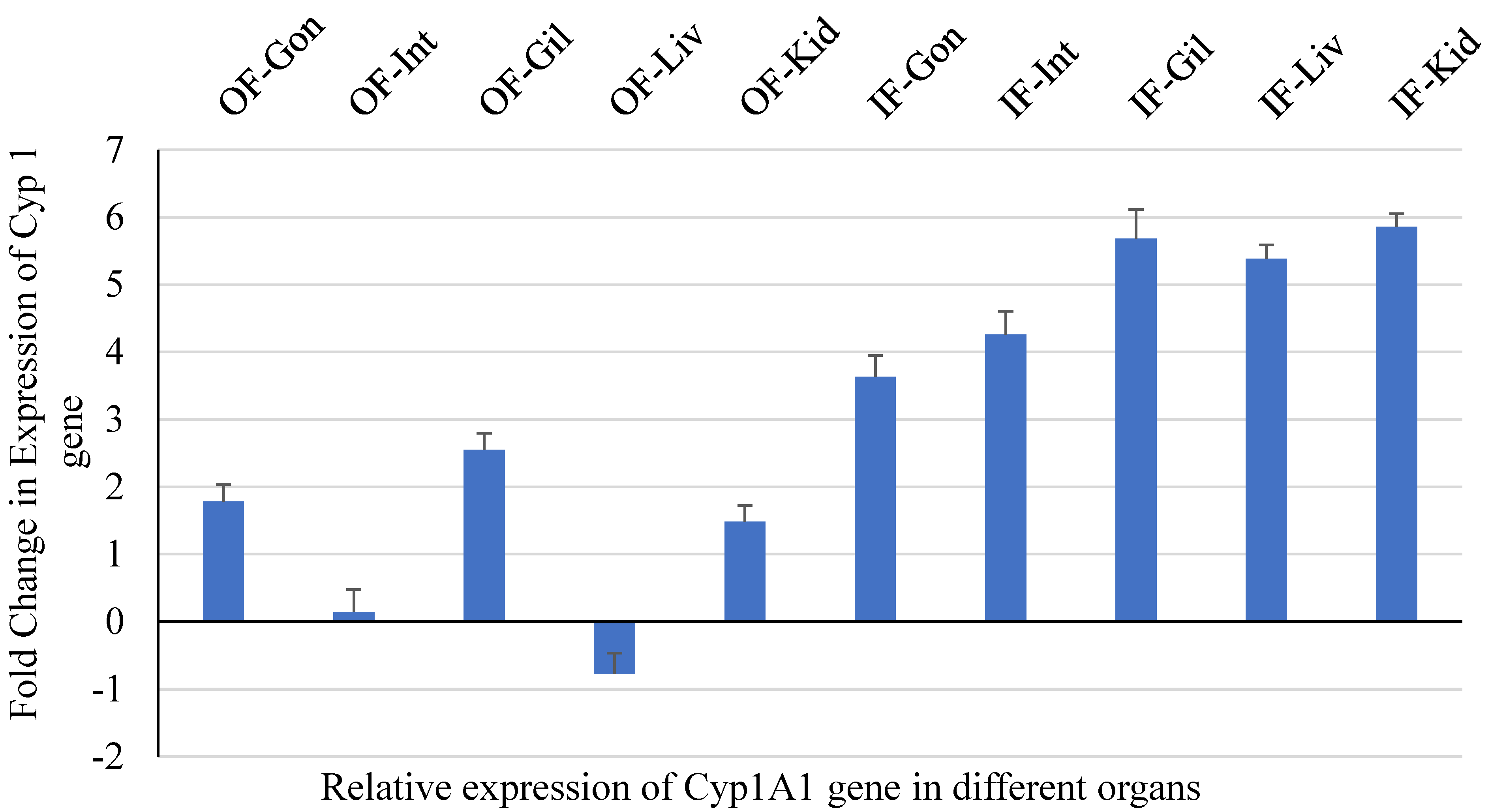
3.1.2. qPCR for CYP1A1 and Vtg Genes in Liver Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, F.R.; Patsiou, D.; Catarino, A.I. Pollutants Bioavailability and Toxicological Risk from Microplastics. In Handbook of Microplastics in the Environment; Rocha-Santos, T., Costa, M., Mouneyrac, C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–40. [Google Scholar]
- Prajapati, A.; Narayan Vaidya, A.; Kumar, A.R. Microplastic properties and their interaction with hydrophobic organic contaminants: A review. Environ. Sci. Pollut. Res. 2022, 29, 49490–49512. [Google Scholar] [CrossRef]
- Shahnawaz, M.; Sangale, M.K.; Ade, A.B. Microplastics. In Bioremediation Technology for Plastic Waste; Springer: Berlin/Heidelberg, Germany, 2019; pp. 11–19. [Google Scholar]
- Gao, N.; Yang, L.; Lu, X.; Duan, Z.; Zhu, L.; Feng, J. A review of interactions of microplastics and typical pollutants from toxicokinetics and toxicodynamics perspective. J. Hazard. Mater. 2022, 432, 128736. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Alagha, D.I.; Hahladakis, J.N.; Sayadi, S.; Al-Ghouti, M.A. Material flow analysis of plastic waste in the gulf co-operation countries (GCC) and the Arabian gulf: Focusing on Qatar. Sci. Total Environ. 2022, 830, 154745. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Kim, J.-W.; Pham, T.D.; Tarafdar, A.; Hong, S.; Chun, S.-H.; Lee, S.-H.; Kang, D.-Y.; Kim, J.-Y.; Kim, S.-B. Microplastics in food: A review on analytical methods and challenges. Int. J. Environ. Res. Public Health 2020, 17, 6710. [Google Scholar] [CrossRef]
- Kannan, K.; Vimalkumar, K. A review of human exposure to microplastics and insights into microplastics as obesogens. Front. Endocrinol. 2021, 12, 724989. [Google Scholar] [CrossRef]
- Atugoda, T.; Wijesekara, H.; Werellagama, D.R.I.B.; Jinadasa, K.B.S.N.; Bolan, N.S.; Vithanage, M. Adsorptive interaction of antibiotic ciprofloxacin on polyethylene microplastics: Implications for vector transport in water. Environ. Technol. Innov. 2020, 19, 100971. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, A.; Subbiah, S.; Anderson, T.A.; Green, M.J.; Zhao, X.; Cañas-Carrell, J.E. Sorption of three common nonsteroidal anti-inflammatory drugs (NSAIDs) to microplastics. Sci. Total Environ. 2020, 715, 136974. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, Q.; Hu, Z.; Yan, L.; Ieong, U.-I.; Xu, Y. Adsorption of chlorophenols on polyethylene terephthalate microplastics from aqueous environments: Kinetics, mechanisms and influencing factors. Environ. Pollut. 2020, 265, 114926. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Bakir, A.; O’Connor, I.A.; Rowland, S.J.; Hendriks, A.J.; Thompson, R.C. Relative importance of microplastics as a pathway for the transfer of hydrophobic organic chemicals to marine life. Environ. Pollut. 2016, 219, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M. The complex mixture, fate and toxicity of chemicals associated with plastic debris in the marine environment. In Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015; pp. 117–140. [Google Scholar]
- Wardrop, D.; Bott, C.; Criddle, C.; Hale, R.; McDevitt, J.; Morse, M.; Rochman, C. Technical Review of Microbeads/Microplastics in the Chesapeake Bay; STAC: Edgewater, MD, USA, 2016. [Google Scholar]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Al-Jandal, N.; AlKhubaizi, A.; Saeed, T.; Hajeyah, M. Potential Adsorption Affinity of Estrogens on LDPE and PET Microplastics Exposed to Wastewater Treatment Plant Effluents. Int. J. Environ. Res. Public Health 2022, 19, 16027. [Google Scholar] [CrossRef] [PubMed]
- Verla, A.W.; Enyoh, C.E.; Verla, E.N.; Nwarnorh, K.O. Microplastic–toxic chemical interaction: A review study on quantified levels, mechanism and implication. SN Appl. Sci. 2019, 1, 1400. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: The True’s beaked whale Mesoplodon mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Courtene-Jones, W.; Clark, N.J.; Fischer, A.C.; Smith, N.S.; Thompson, R.C. Ingestion of microplastics by Marine Animals. Plast. Ocean Orig. Charact. Fate Impacts 2022, 349–366. [Google Scholar]
- Du, S.; Zhu, R.; Cai, Y.; Xu, N.; Yap, P.-S.; Zhang, Y.; He, Y.; Zhang, Y. Environmental fate and impacts of microplastics in aquatic ecosystems: A review. RSC Adv. 2021, 11, 15762–15784. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: Implications for plastic marine debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; O’Connor, I.A.; Rowland, S.J.; Hendricks, A.J.; Thompson, R.C. Relative importance of microplastics as a pathway for the transfer of persistent organic pollutants to marine life. Model. Oral Uptake Chem. 2014, 219, 56–65. [Google Scholar]
- Chua, E.M.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Clarke, B.O. Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes compressa. Environ. Sci. Technol. 2014, 48, 8127–8134. [Google Scholar] [CrossRef] [PubMed]
- Gouin, T.; Roche, N.; Lohmann, R.; Hodges, G. A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplastic. Environ. Sci. Technol. 2011, 45, 1466–1472. [Google Scholar] [CrossRef]
- Zarfl, C.; Matthies, M. Are marine plastic particles transport vectors for organic pollutants to the Arctic? Mar. Pollut. Bull. 2010, 60, 1810–1814. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, H.; Allgeier, A.; Zhou, Q.; Ouellet, J.D.; Crawford, S.E.; Luo, Y.; Yang, Y.; Shi, H.; Hollert, H. Marine microplastics bound dioxin-like chemicals: Model explanation and risk assessment. J. Hazard. Mater. 2019, 364, 82–90. [Google Scholar] [CrossRef]
- Grinsted, R.A.; Clark, L.; Koenig, J.L. Study of cyclic sorption-desorption into poly (methyl methacrylate) rods using NMR imaging. Macromolecules 1992, 25, 1235–1241. [Google Scholar] [CrossRef]
- Pestana, C.J.; Moura, D.S.; Capelo-Neto, J.; Edwards, C.; Dreisbach, D.; Spengler, B.; Lawton, L.A. Potentially poisonous plastic particles: Microplastics as a vector for cyanobacterial toxins microcystin-LR and microcystin-LF. Environ. Sci. Technol. 2021, 55, 15940–15949. [Google Scholar] [CrossRef]
- Borges Ramirez, M.M.; Dzul Caamal, R.; Rendón von Osten, J. Occurrence and seasonal distribution of microplastics and phthalates in sediments from the urban channel of the Ria and coast of Campeche, Mexico. Sci. Total Environ. 2019, 672, 97–105. [Google Scholar] [CrossRef]
- Jeong, C.-B.; Won, E.-J.; Kang, H.-M.; Lee, M.-C.; Hwang, D.-S.; Hwang, U.-K.; Zhou, B.; Souissi, S.; Lee, S.-J.; Lee, J.-S. Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and p-JNK and p-p38 Activation in the Monogonont Rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef]
- Jin, Y.; Xia, J.; Pan, Z.; Yang, J.; Wang, W.; Fu, Z. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ. Pollut. 2018, 235, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, X.; Liu, H.; Wang, H.; Lu, K.; Gao, S. Desorption of pharmaceuticals from pristine and aged polystyrene microplastics under simulated gastrointestinal conditions. J. Hazard. Mater. 2020, 392, 122346. [Google Scholar] [CrossRef] [PubMed]
- Foekema, E.M.; De Gruijter, C.; Mergia, M.T.; van Franeker, J.A.; Murk, A.J.; Koelmans, A.A. Plastic in North Sea Fish. Environ. Sci. Technol. 2013, 47, 8818–8824. [Google Scholar] [CrossRef] [PubMed]
- Solomando, A.; Capó, X.; Alomar, C.; Álvarez, E.; Compa, M.; Valencia, J.M.; Pinya, S.; Deudero, S.; Sureda, A. Long-term exposure to microplastics induces oxidative stress and a pro-inflammatory response in the gut of S. aurata Linnaeus, 1758. Environ. Pollut. 2020, 266, 115295. [Google Scholar] [CrossRef]
- Compa, M.; Ventero, A.; Iglesias, M.; Deudero, S. Ingestion of microplastics and natural fibres in Sardina pilchardus (Walbaum, 1792) and Engraulis encrasicolus (Linnaeus, 1758) along the Spanish Mediterranean coast. Mar. Pollut. Bull. 2018, 128, 89–96. [Google Scholar] [CrossRef]
- Capó, X.; Company, J.J.; Alomar, C.; Compa, M.; Sureda, A.; Grau, A.; Hansjosten, B.; López-Vázquez, J.; Quintana, J.B.; Rodil, R.; et al. Long-term exposure to virgin and seawater exposed microplastic enriched-diet causes liver oxidative stress and inflammation in gilthead seabream Sparus aurata, Linnaeus 1758. Sci. Total Environ. 2021, 767, 144976. [Google Scholar] [CrossRef]
- Rios-Fuster, B.; Arechavala-Lopez, P.; García-Marcos, K.; Alomar, C.; Compa, M.; Álvarez, E.; Julià, M.M.; Martí, A.S.; Sureda, A.; Deudero, S. Experimental evidence of physiological and behavioral effects of microplastic ingestion in S. aurata. Aquat. Toxicol. 2021, 231, 105737. [Google Scholar] [CrossRef]
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO: Roma, Italy, 2017. [Google Scholar]
- Ory, N.C.; Gallardo, C.; Lenz, M.; Thiel, M. Capture, swallowing, and egestion of microplastics by a planktivorous juvenile fish. Environ. Pollut. 2018, 240, 566–573. [Google Scholar] [CrossRef]
- Jaafar, N.; Azfaralariff, A.; Musa, S.M.; Mohamed, M.; Yusoff, A.H.; Lazim, A.M. Occurrence, distribution and characteristics of microplastics in gastrointestinal tract and gills of commercial marine fish from Malaysia. Sci. Total Environ. 2021, 799, 149457. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, B. Ingestion of microplastics by fish and its potential consequences from a physical perspective. Integr. Environ. Assess. Manag. 2017, 13, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ge, J.; Yu, X. Bioavailability and toxicity of microplastics to fish species: A review. Ecotoxicol. Environ. Saf. 2020, 189, 109913. [Google Scholar] [CrossRef] [PubMed]
- Varó, I.; Osorio, K.; Estensoro, I.; Naya-Catala, F.; Sitja-Bobadilla, A.; Navarro, J.C.; Pérez-Sánchez, J.; Torreblanca, A.; Piazzon, M.C. Effect of virgin low density polyethylene microplastic ingestion on intestinal histopathology and microbiota of gilthead sea bream. Aquaculture 2021, 545, 737245. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef]
- Demirel, S.; Gençalioğlu, O.; Aslan, M.; Kirci, H. Investigation of some mechanical properties of composite materials made of LDPE and bamboo at two different rates. Orman. Araştırma Derg. 2022, 9, 291–298. [Google Scholar] [CrossRef]
- Antelava, A.; Jablonska, N.; Constantinou, A.; Manos, G.; Salaudeen, S.A.; Dutta, A.; Al-Salem, S.M. Energy potential of plastic waste valorization: A short comparative assessment of pyrolysis versus gasification. Energy Fuels 2021, 35, 3558–3571. [Google Scholar] [CrossRef]
- Al-Zaidan, A.S.; Akbar, A.; Bahbahani, H.; Al-Mohanna, S.Y.; Kolattukudy, B.; Balakrishna, V. Landing, consumption, and DNA barcoding of commercial seabream (Perciformes: Sparidae) in Kuwait. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 802–817. [Google Scholar] [CrossRef]
- Abusam, A. Fate of Estrogens in Kuwaiti Municipal Wastewater Treatment Plants. In Proceedings of the 14th Gulf Water Conference: Water in the GCC… Towards Economic Efficiency and Financial Sustainability, Riyadh, Saudi Arabia, 13–15 February 2022; p. 15. [Google Scholar]
- Abusam, A.; Saeed, T.; Al-Jandal, N. Removal of Estrogens in Kuwaiti Municipal Wastewater Treatment Plants. J. Environ. Treat. Tech. 2021, 9, 642–646. [Google Scholar]
- Al-Jandal, N.; Saeed, T.; Azad, I.; Al-Subiai, S.; Al-Zekri, W.; Hussain, S.; Al-Hasan, E. Impact of endocrine disrupting compounds in sewage impacted coastal area on seabream. Ecotoxicol. Environ. Saf. 2018, 150, 280–288. [Google Scholar] [CrossRef]
- Saeed, T.; Al-Jandal, N.; Abusam, A.; Taqi, H.; Al-Khabbaz, A.; Zafar, J. Sources and levels of endocrine disrupting compounds (EDCs) in Kuwait’s coastal areas. Mar. Pollut. Bull. 2017, 118, 407–412. [Google Scholar] [CrossRef]
- Jovanović, B.; Gökdağ, K.; Güven, O.; Emre, Y.; Whitley, E.M.; Kideys, A.E. Virgin microplastics are not causing imminent harm to fish after dietary exposure. Mar. Pollut. Bull. 2018, 130, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Facey, D.E.; Leclerc, C.; Dunbar, D.; Arruda, D.; Pyzocha, L.; Blazer, V. Physiological Indicators of Stress Among Fishes from Contaminated Areas of Lake Champlain. In Lake Champlain in Transition: From Research Toward Restoration; American Geophysical Union: Washington, DC, USA, 1999; pp. 349–359. [Google Scholar]
- Liebel, S.; Tomotake, M.E.M.; Ribeiro, C. Fish histopathology as biomarker to evaluate water quality. Ecotoxicol. Environ. Contam. 2013, 8, 9–15. [Google Scholar] [CrossRef]
- Cardosa, M.J.; Tio, P.H. Dot enzyme immunoassay: An alternative diagnostic aid for dengue fever and dengue haemorrhagic fever. Bull. World Health Organ. 1991, 69, 741–745. [Google Scholar]
- Espinosa, C.; Beltrán, J.M.G.; Esteban, M.A.; Cuesta, A. In vitro effects of virgin microplastics on fish head-kidney leucocyte activities. Environ. Pollut. 2018, 235, 30–38. [Google Scholar] [CrossRef]
- Azad, I.; Al-Jandal, N. Induction of vitellogenin in cultured fish: Vitellogenin gene expression response of blue fin and yellow fin breams to injected 17-estradiol. J. Environ. Chem. Ecotoxicol. 2020, 12, 8–16. [Google Scholar] [CrossRef]
- Legendre, M.; Albaret, J.J. Maximum observed length as an indicator of growth rate in tropical fishes. Aquaculture 1991, 94, 327–341. [Google Scholar] [CrossRef]
- Deng, D.-F.; Ding, Y.-Z.; Wen, H.; Jiang, M. Comprehensive understanding the impacts of dietary exposure to polyethylene microplastics on genetically improved farmed tilapia (Oreochromis niloticus): Tracking from growth, microbiota, metabolism to gene expressions. Sci. Total Environ. 2022, 841, 156571. [Google Scholar]
- Stagg, R.M.; McIntosh, A.; Mackie, P. Elevation of hepatic monooxygenase activity in the dab (Limanda limanda L.) in relation to environmental contamination with petroleum hydrocarbons in the northern North Sea. Aquat. Toxicol. 1995, 33, 245–264. [Google Scholar] [CrossRef]
- Klomp, F.; Wenzel, C.; Drozdzik, M.; Oswald, S. Drug–drug interactions involving intestinal and hepatic CYP1A enzymes. Pharmaceutics 2020, 12, 1201. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Kanjo, Y.; Mizutani, S. Urinary excretion rates of natural estrogens and androgens from humans, and their occurrence and fate in the environment: A review. Sci. Total Environ. 2009, 407, 4975–4985. [Google Scholar] [CrossRef]
- Kibambe, M.G.; Momba, M.N.; Daso, A.; Van Zijl, M.; Coetzee, M.A. Efficiency of selected wastewater treatment processes in removing estrogen compounds and reducing estrogenic activity using the T47D-KBLUC reporter gene assay. J. Environ. Manag. 2020, 260, 110135. [Google Scholar] [CrossRef]
- Sun, P.; Liu, X.; Zhang, M.; Li, Z.; Cao, C.; Shi, H.; Yang, Y.; Zhao, Y. Sorption and leaching behaviors between aged MPs and BPA in water: The role of BPA binding modes within plastic matrix. Water Res. 2021, 195, 116956. [Google Scholar] [CrossRef] [PubMed]
- Mommsen, T.P. Paradigms of growth in fish. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 129, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zuo, J.; Li, J.; Zhang, Y.; Ai, X.; Zhang, J.; Gong, D.; Sun, D. Effects of secondary polyethylene microplastic exposure on crucian (Carassius carassius) growth, liver damage, and gut microbiome composition. Sci. Total Environ. 2022, 802, 149736. [Google Scholar] [CrossRef]
- Espinosa, C.; Esteban, M.Á.; Cuesta, A. Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2019, 95, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, K.; Ding, D.; Liu, J.; Lei, Z.; Chen, X.; Ye, G.; Zhang, J.; Shen, H.; Yan, C. Size-dependent adverse effects of microplastics on intestinal microbiota and metabolic homeostasis in the marine medaka (Oryzias melastigma). Environ. Int. 2021, 151, 106452. [Google Scholar] [CrossRef]
- Nelson, E.R.; Habibi, H.R. Estrogen receptor function and regulation in fish and other vertebrates. Gen. Comp. Endocrinol. 2013, 192, 15–24. [Google Scholar] [CrossRef]
- Cleveland, B.; Weber, G. Effects of steroid treatment on growth, nutrient partitioning, and expression of genes related to growth and nutrient metabolism in adult triploid rainbow trout (Oncorhynchus mykiss). Domest. Anim. Endocrinol. 2016, 56, 1–12. [Google Scholar] [CrossRef]
- Sun, S.-X.; Wu, J.-L.; Lv, H.-B.; Zhang, H.-Y.; Zhang, J.; Limbu, S.M.; Qiao, F.; Chen, L.-Q.; Yang, Y.; Zhang, M.-L.; et al. Environmental estrogen exposure converts lipid metabolism in male fish to a female pattern mediated by AMPK and mTOR signaling pathways. J. Hazard. Mater. 2020, 394, 122537. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, H.; Han, Z.; Tang, Z.; Xiao, J.; Guo, Z.; Wang, F.; Luo, Y.; Zhou, Y. Effects of waterborne exposure to 17β-estradiol on hepatic lipid metabolism genes in tilapia (Oreochromis niloticus). Aquac. Rep. 2020, 17, 100382. [Google Scholar] [CrossRef]
- Jobling, S.; Casey, D.; Rodgers-Gray, T.; Oehlmann, J.; Schulte-Oehlmann, U.; Pawlowski, S.; Baunbeck, T.; Turner, A.; Tyler, C. Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent. Aquat. Toxicol. 2003, 65, 205–220. [Google Scholar] [CrossRef]
- Jabeen, K.; Li, B.; Chen, Q.; Su, L.; Wu, C.; Hollert, H.; Shi, H. Effects of virgin microplastics on goldfish (Carassius auratus). Chemosphere 2018, 213, 323–332. [Google Scholar] [CrossRef]
- Naidoo, T.; Glassom, D. Decreased growth and survival in small juvenile fish, after chronic exposure to environmentally relevant concentrations of microplastic. Mar. Pollut. Bull. 2019, 145, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Nehemia, A.; Maganira, J.D.; Rumisha, C. Length-Weight relationship and condition factor of tilapia species grown in marine and fresh water ponds. Agric. Biol. J. N. Am. 2012, 3, 117–124. [Google Scholar] [CrossRef]
- Adams, S.; McLean, R. Estimation of largemouth bass, Micropterus salmoides Lacepede, growth using the liver somatic index and physiological variables. J. Fish Biol. 1985, 26, 111–126. [Google Scholar] [CrossRef]
- Naidoo, S.; Vosloo, D.; Schoeman, M.C. Pollutant exposure at wastewater treatment works affects the detoxification organs of an urban adapter, the Banana Bat. Environ. Pollut. 2016, 208, 830–839. [Google Scholar] [CrossRef]
- Hamed, M.; Soliman, H.A.; Badrey, A.E.; Osman, A.G. Microplastics induced histopathological lesions in some tissues of tilapia (Oreochromis niloticus) early juveniles. Tissue Cell 2021, 71, 101512. [Google Scholar] [CrossRef]
- Hanachi, P.; Kazemi, S.; Zivary, S.; Karbalaei, S.; Ghadami, S.A. The effect of polyethylene terephthalate and abamectin on oxidative damages and expression of vtg and cyp1a genes in juvenile zebrafish. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100565. [Google Scholar] [CrossRef]


| Estrogens | Influent Streams | Effluent Streams |
|---|---|---|
| DES | 1.13 | 0.00 |
| E1 | 8.24 | 0.96 |
| E2 | 0.54 | 0.00 |
| EE2 | 2.24 | 3.83 |
| E3 | 3.54 | 0.00 |
| Σ Estrogens | 15.68 | 4.79 |
| Exposure Time | CF (100 × g cm−3) | ||
|---|---|---|---|
| Control | Influent-Exposed | Effluent-Exposed | |
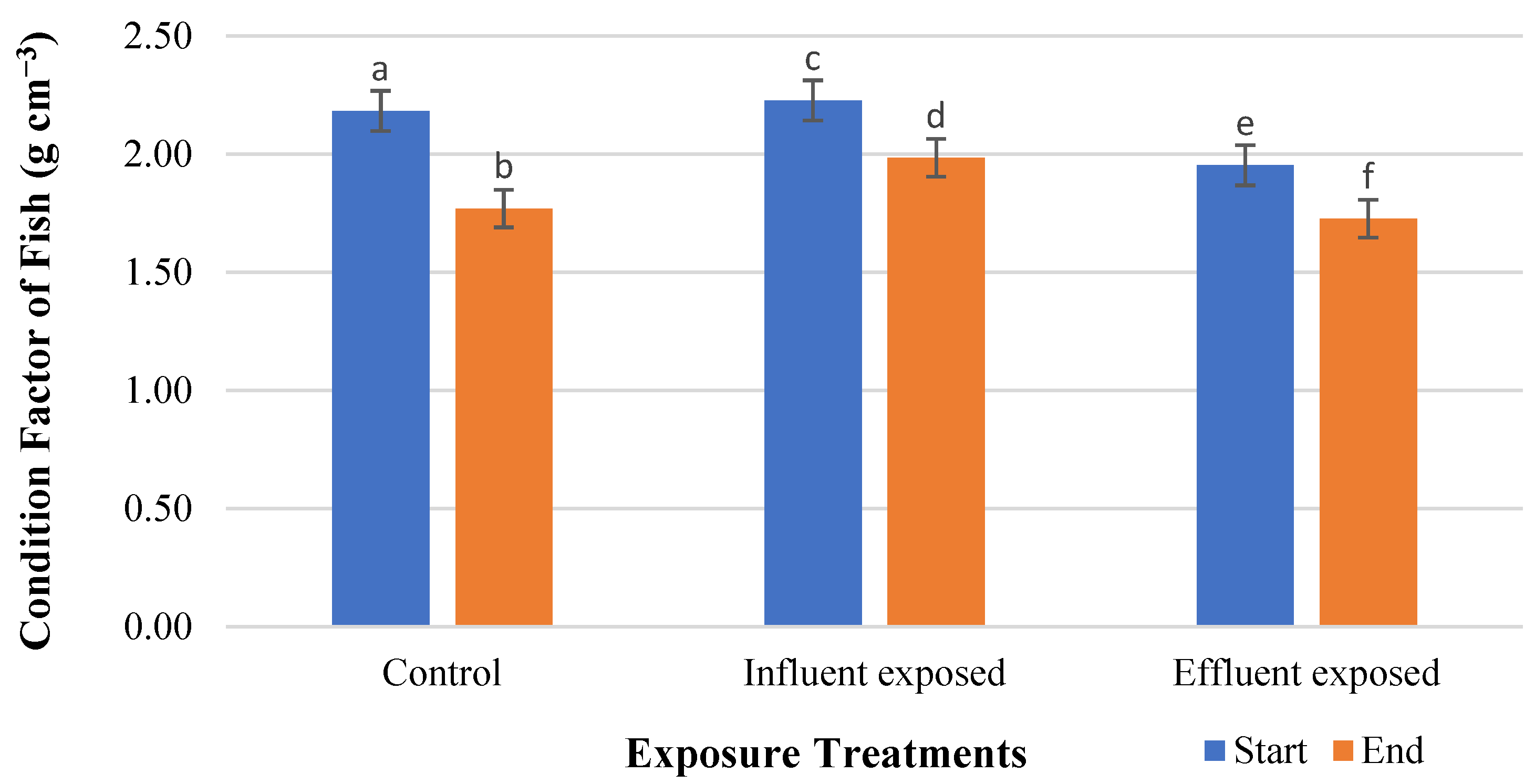
| Start | 2.18 ± 0.06 * | 2.23 ± 0.05 * | 1.95 ± 0.03 * |
| End | 1.77 ± 0.03 | 1.98 ± 0.03 | 1.73 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Jandal, N.; Saheb, A.I.; Alkhubaizi, A.; Akbar, A.; Al-Hasan, E.; Hussain, S.; Al-Mansour, H. Effect of Dietary Exposure to Low-Density Polyethylene Microplastics and Their Potential Role as Estrogen Vectors In Vivo. Curr. Issues Mol. Biol. 2025, 47, 701. https://doi.org/10.3390/cimb47090701
Al-Jandal N, Saheb AI, Alkhubaizi A, Akbar A, Al-Hasan E, Hussain S, Al-Mansour H. Effect of Dietary Exposure to Low-Density Polyethylene Microplastics and Their Potential Role as Estrogen Vectors In Vivo. Current Issues in Molecular Biology. 2025; 47(9):701. https://doi.org/10.3390/cimb47090701
Chicago/Turabian StyleAl-Jandal, Noura, Azad Ismail Saheb, Abdulaziz Alkhubaizi, Abrar Akbar, Enas Al-Hasan, Sumaiah Hussain, and Hamad Al-Mansour. 2025. "Effect of Dietary Exposure to Low-Density Polyethylene Microplastics and Their Potential Role as Estrogen Vectors In Vivo" Current Issues in Molecular Biology 47, no. 9: 701. https://doi.org/10.3390/cimb47090701
APA StyleAl-Jandal, N., Saheb, A. I., Alkhubaizi, A., Akbar, A., Al-Hasan, E., Hussain, S., & Al-Mansour, H. (2025). Effect of Dietary Exposure to Low-Density Polyethylene Microplastics and Their Potential Role as Estrogen Vectors In Vivo. Current Issues in Molecular Biology, 47(9), 701. https://doi.org/10.3390/cimb47090701







