Homologous Recombination Proficiency in High-Grade Serous Epithelial Ovarian Cancer Tumors: The Dark Side of the Moon
Abstract
1. Introduction
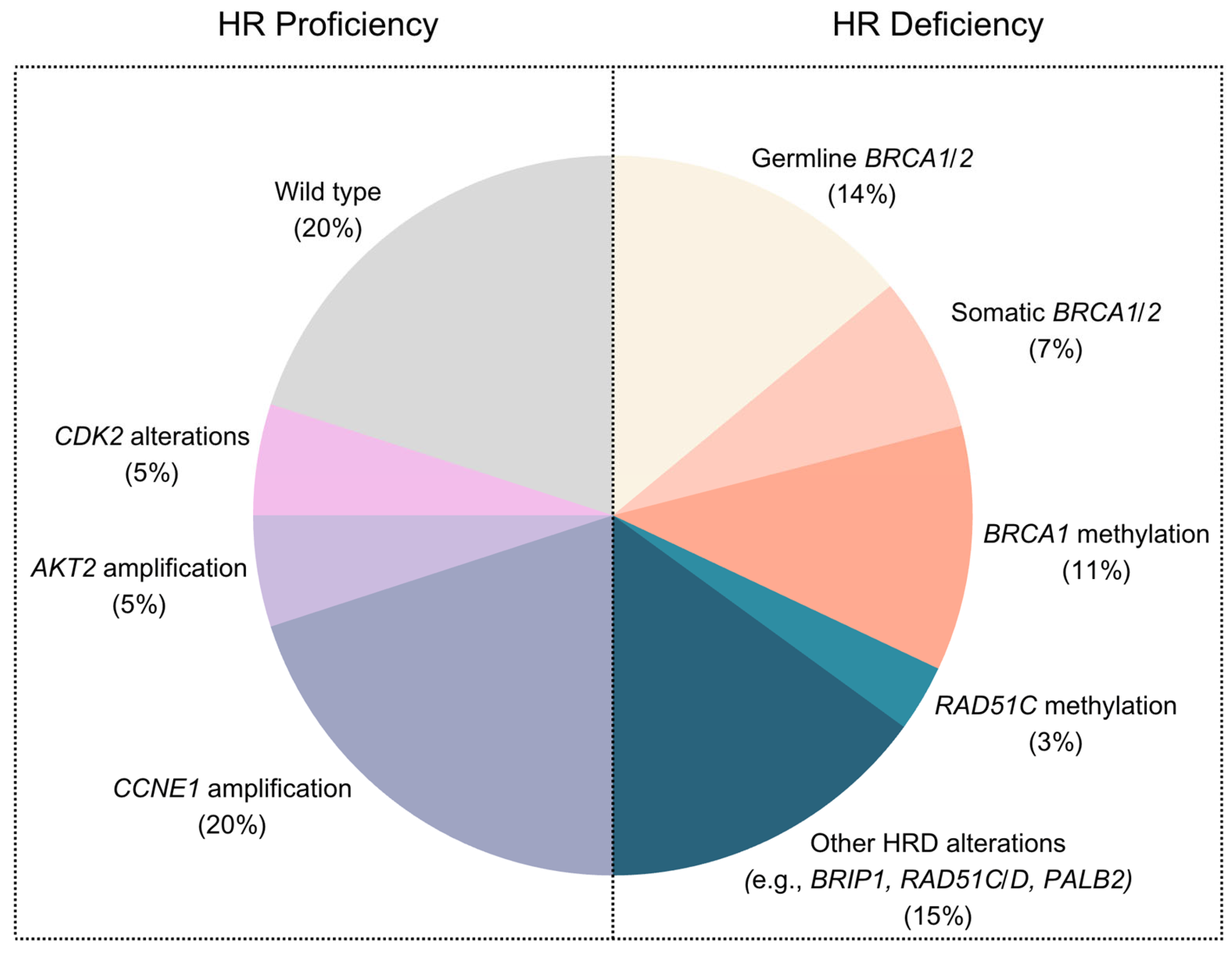
2. Homologous Recombination Deficient Tumors
3. Homologous-Recombination-Proficient Tumors
4. CCNE1 Amplification
5. Replication Stress
6. High Fold-Back Inversions
7. Antibody Drug Conjugates (ADCs) Targeting Folate Receptor-Alpha
8. ADCs Targeting NaPi2B
9. ADCs Targeting HER-2
10. Mismatch Repair Deficiency
11. Other Molecular Alterations and New Emerging Therapies
12. Additional Considerations Regarding Homologous Recombination Status in HGSOC
13. Discussion
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| ADC | Antibody drug conjugate |
| BET | Bromodomain and extra-terminal |
| CCOC | Clear cell ovarian cancer |
| CI | Confidence interval |
| EGFR | Epidermal growth factor receptor |
| ENOC | Endometrioid ovarian cancer |
| EOC | Epithelial ovarian cancer |
| FBI | Fold-back inversions |
| FDA | Food and Drug Administration |
| HDACi | Histone deacetylase inhibitor |
| HER2 | Human epidermal growth factor receptor 2 |
| HR | Homologous recombination |
| HRD | Homologous recombination deficient |
| HRP | Homologous recombination proficient |
| HRR | Homologous recombination repair |
| HGSOC | High-grade serous ovarian cancer |
| LGSOC | Low-grade serous ovarian cancer |
| LOH | Loss of heterozygosity |
| MIRV | Mirvetuximab soravtansine |
| MMR | Mismatch repair |
| MOC | Mucinous ovarian cancer |
| MSI | Microsatellite instability |
| NaPi2B | Sodium-dependent phosphate transport protein 2B |
| NCCN | The National Comprehensive Cancer Network |
| NCI | National Cancer Institute |
| NHEJ | Non-homologous end-joining |
| ORR | Overall response rate |
| OS | Overall survival |
| PARPi | Poly ADP-ribose polymerase inhibitors |
| PFS | Progression-free survival |
| PI3K | Phosphoinositide 3-kinase |
| T-DXd | Trastuzumab deruxtecan |
| TME | Tumor microenvironment |
| UpRi | Upifitamab rilsodotin |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Gaitskell, K.; Hermon, C.; Barnes, I.; Pirie, K.; Floud, S.; Green, J.; Beral, V.; Reeves, G.K. Ovarian cancer survival by stage, histotype, and pre-diagnostic lifestyle factors, in the prospective UK Million Women Study. Cancer Epidemiol. 2022, 76, 102074. [Google Scholar] [CrossRef] [PubMed]
- Bolton, K.L.; Chenevix-Trench, G.; Goh, C.; Sadetzki, S.; Ramus, S.J.; Karlan, B.Y.; Lambrechts, D.; Despierre, E.; Barrowdale, D.; McGuffog, L.; et al. Association Between BRCA1 and BRCA2 Mutations and Survival in Women with Invasive Epithelial Ovarian Cancer. JAMA 2012, 307, 382–390. [Google Scholar] [CrossRef]
- Pavanello, M.; Chan, I.H.; Ariff, A.; Pharoah, P.D.; Gayther, S.A.; Ramus, S.J. Rare Germline Genetic Variants and the Risks of Epithelial Ovarian Cancer. Cancers 2020, 12, 3046. [Google Scholar] [CrossRef]
- Gauci, M.; Calleja-Agius, J. Spotlight on Carcinosarcoma of the Ovary: A Scoping Review. Acta Medica 2024, 67, 1–11. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Etemadmoghadam, D.; Temple, J.; Lynch, A.G.; Riad, M.; Sharma, R.; Stewart, C.; Fereday, S.; Caldas, C.; Defazio, A.; et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J. Pathol. 2010, 221, 49–56. [Google Scholar] [CrossRef]
- Alsop, K.; Fereday, S.; Meldrum, C.; de Fazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef]
- Patch, A.-M.; Christie, E.L.; Etemadmoghadam, D.; Garsed, D.W.; George, J.; Fereday, S.; Nones, K.; Cowin, P.; Alsop, K.; Bailey, P.J.; et al. Whole–genome characterization of chemoresistant ovarian cancer. Nature 2015, 521, 489–494. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef]
- Bell, D.; Berchuck, A.; Birrer, M.; Chien, J.; Cramer, D.W.; Dao, F.; Dhir, R.; DiSaia, P.; Gabra, H.; Glenn, P.; et al. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Lord, C.J.; Tutt, A.N.; Ashworth, A. Synthetic lethality and cancer therapy: Lessons learned from the development of PARP inhibitors. Annu. Rev. Med. 2015, 66, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Garsed, D.W.; Pandey, A.; Fereday, S.; Kennedy, C.J.; Takahashi, K.; Alsop, K.; Hamilton, P.T.; Hendley, J.; Chiew, Y.E.; Traficante, N.; et al. The genomic and immune landscape of long-term survivors of high-grade serous ovarian cancer. Nat. Genet. 2022, 54, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Hollis, R.L.; Meynert, A.M.; Michie, C.O.; Rye, T.; Churchman, M.; Hallas-Potts, A.; Croy, I.; McCluggage, W.G.; Williams, A.R.W.; Bartos, C.; et al. Multiomic Characterization of High-Grade Serous Ovarian Carcinoma Enables High-Resolution Patient Stratification. Clin. Cancer Res. 2022, 28, 3546–3556. [Google Scholar] [CrossRef] [PubMed]
- Bowtell, D.D.; Bohm, S.; Ahmed, A.A.; Aspuria, P.J.; Bast, R.C., Jr.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A.; et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Ceccaldi, R.; Shapiro, G.I.; D’Andrea, A.D. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. 2015, 5, 1137–1154. [Google Scholar] [CrossRef]
- Etemadmoghadam, D.; Au-Yeung, G.; Wall, M.; Mitchell, C.; Kansara, M.; Loehrer, E.; Batzios, C.; George, J.; Ftouni, S.; Weir, B.A.; et al. Resistance to CDK2 Inhibitors Is Associated with Selection of Polyploid Cells in CCNE1-Amplified Ovarian Cancer. Clin. Cancer Res. 2013, 19, 5960–5971. [Google Scholar] [CrossRef]
- Au-Yeung, G.; Lang, F.; Azar, W.J.; Mitchell, C.; Jarman, K.E.; Lackovic, K.; Aziz, D.; Cullinane, C.; Pearson, R.B.; Mileshkin, L.; et al. Selective Targeting of Cyclin E1-Amplified High-Grade Serous Ovarian Cancer by Cyclin-Dependent Kinase 2 and AKT Inhibition. Clin. Cancer Res. 2017, 23, 1862–1874. [Google Scholar] [CrossRef]
- Foster, K.I.; Shaw, K.R.M.; Jin, J.; Westin, S.N.; Yap, T.A.; Glassman, D.M.; Jazaeri, A.A.; Rauh-Hain, J.A.; Lee, S.; Fellman, B.M.; et al. Clinical implications of tumor-based next-generation sequencing in high-grade epithelial ovarian cancer. Cancer 2023, 129, 1672–1680. [Google Scholar] [CrossRef]
- Landen, C.N.; Molinero, L.; Hamidi, H.; Sehouli, J.; Miller, A.; Moore, K.N.; Taskiran, C.; Bookman, M.; Lindemann, K.; Anderson, C.; et al. Influence of Genomic Landscape on Cancer Immunotherapy for Newly Diagnosed Ovarian Cancer: Biomarker Analyses from the IMagyn050 Randomized Clinical Trial. Clin. Cancer Res. 2023, 29, 1698–1707. [Google Scholar] [CrossRef]
- Wang, Y.K.; Bashashati, A.; Anglesio, M.S.; Cochrane, D.R.; Grewal, D.S.; Ha, G.; McPherson, A.; Horlings, H.M.; Senz, J.; Prentice, L.M.; et al. Genomic consequences of aberrant DNA repair mechanisms stratify ovarian cancer histotypes. Nat. Genet. 2017, 49, 856–865. [Google Scholar] [CrossRef]
- Wu, C.H.; Hsieh, C.S.; Chang, Y.C.; Huang, C.C.; Yeh, H.T.; Hou, M.F.; Chung, Y.C.; Tu, S.H.; Chang, K.J.; Chattopadhyay, A.; et al. Differential whole-genome doubling and homologous recombination deficiencies across breast cancer subtypes from the Taiwanese population. Commun. Biol. 2021, 4, 1052. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Barretina-Ginesta, M.P.; Pothuri, B.; Vergote, I.; Graybill, W.; Mirza, M.R.; McCormick, C.C.; Lorusso, D.; Moore, R.G.; Freyer, G.; et al. Niraparib first-line maintenance therapy in patients with newly diagnosed advanced ovarian cancer: Final overall survival results from the PRIMA/ENGOT-OV26/GOG-3012 trial. Ann. Oncol. 2024, 35, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Caldon, C.E.; Musgrove, E.A. Distinct and redundant functions of cyclin E1 and cyclin E2 in development and cancer. Cell Div. 2010, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.W.; Ueland, F.R.; Kolesar, J.M. CCNE1 Amplification as a Predictive Biomarker of Chemotherapy Resistance in Epithelial Ovarian Cancer. Diagnostics 2020, 10, 279. [Google Scholar] [CrossRef]
- Creeden, J.F.; Nanavaty, N.S.; Einloth, K.R.; Gillman, C.E.; Stanbery, L.; Hamouda, D.M.; Dworkin, L.; Nemunaitis, J. Homologous recombination proficiency in ovarian and breast cancer patients. BMC Cancer 2021, 21, 1154. [Google Scholar] [CrossRef]
- Etemadmoghadam, D.; Weir, B.A.; Au-Yeung, G.; Alsop, K.; Mitchell, G.; George, J.; Davis, S.; D’Andrea, A.D.; Simpson, K.; Hahn, W.C.; et al. Synthetic lethality between CCNE1 amplification and loss of BRCA1. Proc. Natl. Acad. Sci. USA 2013, 110, 19489–19494. [Google Scholar] [CrossRef]
- Etemadmoghadam, D.; deFazio, A.; Beroukhim, R.; Mermel, C.; George, J.; Getz, G.; Tothill, R.; Okamoto, A.; Raeder, M.B.; Harnett, P.; et al. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary chemoresistance in ovarian carcinomas. Clin. Cancer Res. 2009, 15, 1417–1427. [Google Scholar] [CrossRef]
- Westin, S.N.; Labrie, M.; Litton, J.K.; Blucher, A.; Fang, Y.; Vellano, C.P.; Marszalek, J.R.; Feng, N.; Ma, X.; Creason, A.; et al. Phase Ib Dose Expansion and Translational Analyses of Olaparib in Combination with Capivasertib in Recurrent Endometrial, Triple-Negative Breast, and Ovarian Cancer. Clin. Cancer Res. 2021, 27, 6354–6365. [Google Scholar] [CrossRef]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Gomez Moreno, H.L.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar] [CrossRef]
- Lheureux, S.; Cristea, M.C.; Bruce, J.P.; Garg, S.; Cabanero, M.; Mantia-Smaldone, G.; Olawaiye, A.B.; Ellard, S.L.; Weberpals, J.I.; Wahner Hendrickson, A.E.; et al. Adavosertib plus gemcitabine for platinum-resistant or platinum-refractory recurrent ovarian cancer: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2021, 397, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, G.; Bressel, M.; Prall, O.; Surace, D.; Andrews, J.; Mongta, S.; Lee, Y.C.; Gao, B.; Meniawy, T.; Baron-Hay, S.E.; et al. IGNITE: A phase II signal-seeking trial of adavosertib targeting recurrent high-grade, serous ovarian cancer with cyclin E1 overexpression with and without gene amplification. J. Clin. Oncol. 2022, 40, 5515. [Google Scholar] [CrossRef]
- Westin, S.N.; Coleman, R.L.; Fellman, B.M.; Yuan, Y.; Sood, A.K.; Soliman, P.T.; Wright, A.A.; Horowitz, N.S.; Campos, S.M.; Konstantinopoulos, P.A.; et al. EFFORT: EFFicacy of adavosertib in parp ResisTance: A randomized two-arm non-comparative phase II study of adavosertib with or without olaparib in women with PARP-resistant ovarian cancer. J. Clin. Oncol. 2021, 39, 5505. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Cheng, S.C.; Wahner Hendrickson, A.E.; Penson, R.T.; Schumer, S.T.; Doyle, L.A.; Lee, E.K.; Kohn, E.C.; Duska, L.R.; Crispens, M.A.; et al. Berzosertib plus gemcitabine versus gemcitabine alone in platinum-resistant high-grade serous ovarian cancer: A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 957–968. [Google Scholar] [CrossRef]
- Yap, T.A.; O’Carrigan, B.; Penney, M.S.; Lim, J.S.; Brown, J.S.; de Miguel Luken, M.J.; Tunariu, N.; Perez-Lopez, R.; Rodrigues, D.N.; Riisnaes, R.; et al. Phase I Trial of First-in-Class ATR Inhibitor M6620 (VX-970) as Monotherapy or in Combination with Carboplatin in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2020, 38, 3195–3204. [Google Scholar] [CrossRef]
- Campbell, P.J.; Yachida, S.; Mudie, L.J.; Stephens, P.J.; Pleasance, E.D.; Stebbings, L.A.; Morsberger, L.A.; Latimer, C.; McLaren, S.; Lin, M.L.; et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 2010, 467, 1109–1113. [Google Scholar] [CrossRef]
- Nesic, K.; Wakefield, M.; Kondrashova, O.; Scott, C.L.; McNeish, I.A. Targeting DNA repair: The genome as a potential biomarker. J. Pathol. 2018, 244, 586–597. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Kalli, K.R.; Oberg, A.L.; Keeney, G.L.; Christianson, T.J.H.; Low, P.S.; Knutson, K.L.; Hartmann, L.C. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol. Oncol. 2008, 108, 619–626. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Lorusso, D.; Oaknin, A.; Pignata, S.; Dean, A.; Denys, H.; Colombo, N.; Van Gorp, T.; Konner, J.A.; Marin, M.R.; et al. Efficacy and Safety of Mirvetuximab Soravtansine in Patients with Platinum-Resistant Ovarian Cancer with High Folate Receptor Alpha Expression: Results from the SORAYA Study. J. Clin. Oncol. 2023, 41, Jco2201900. [Google Scholar] [CrossRef] [PubMed]
- Naumann, R.W.; Coleman, R.L.; Burger, R.A.; Sausville, E.A.; Kutarska, E.; Ghamande, S.A.; Gabrail, N.Y.; DePasquale, S.E.; Nowara, E.; Gilbert, L.; et al. PRECEDENT: A Randomized Phase II Trial Comparing Vintafolide (EC145) and Pegylated Liposomal Doxorubicin (PLD) in Combination Versus PLD Alone in Patients with Platinum-Resistant Ovarian Cancer. J. Clin. Oncol. 2013, 31, 4400–4406. [Google Scholar] [CrossRef]
- Vergote, I.B.; Gilbert, L.G.; Lucy; Ghatage, P.; Lisyankaya, A.; Ghamande, S.; Chambers, S.K.; Arranz, J.A.; Provencher, D.M.; Bessette, P.; et al. A randomized double-blind phase III trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD/Doxil®/Caelyx®) in combination versus PLD in participants with platinum-resistant ovarian cancer (PROCEED) (NCT01170650). Gynecol. Oncol. 2015, 137, 5–6. [Google Scholar] [CrossRef]
- Moore, K.N.; Angelergues, A.; Konecny, G.E.; García, Y.; Banerjee, S.; Lorusso, D.; Lee, J.Y.; Moroney, J.W.; Colombo, N.; Roszak, A.; et al. Mirvetuximab Soravtansine in FRα-Positive, Platinum-Resistant Ovarian Cancer. N. Engl. J. Med. 2023, 389, 2162–2174. [Google Scholar] [CrossRef] [PubMed]
- Karpel, H.C.; Powell, S.S.; Pothuri, B. Antibody-Drug Conjugates in Gynecologic Cancer. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology: Alexandria, VA, USA, 2023; Volume 43, p. e390772. [Google Scholar] [CrossRef]
- O’Malley, D.M.; Myers, T.K.N.; Zamagni, C.; Diver, E.; Lorusso, D. GLORIOSA: A randomized, open-label, phase 3 study of mirvetuximab soravtansine with bevacizumab vs. bevacizumab as maintenance in platinum-sensitive ovarian, fallopian tube, or primary peritoneal cancer. J. Clin. Oncol. 2023, 41, TPS5622. [Google Scholar] [CrossRef]
- Nurgalieva, A.K.; Popov, V.E.; Skripova, V.S.; Bulatova, L.F.; Savenkova, D.V.; Vlasenkova, R.A.; Safina, S.Z.; Shakirova, E.Z.; Filonenko, V.V.; Bogdanov, M.V.; et al. Sodium-dependent phosphate transporter NaPi2b as a potential predictive marker for targeted therapy of ovarian cancer. Biochem. Biophys. Rep. 2021, 28, 101104. [Google Scholar] [CrossRef]
- Banerjee, S.; Oza, A.M.; Birrer, M.J.; Hamilton, E.P.; Hasan, J.; Leary, A.; Moore, K.N.; Mackowiak-Matejczyk, B.; Pikiel, J.; Ray-Coquard, I.; et al. Anti-NaPi2b antibody-drug conjugate lifastuzumab vedotin (DNIB0600A) compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer in a randomized, open-label, phase II study. Ann. Oncol. 2018, 29, 917–923. [Google Scholar] [CrossRef]
- Gorp, T.V.; Martin, A.G.; Sen, S.; Sehouli, J.; Spira, A.; Boni, V.; Kristeleit, R.; Richardson, D.L.; Spicer, J.F.; Wolf, J.; et al. NAPISTAR 1-01: An international phase I/II trial of the novel ADC TUB-040 in platinum-resistant ovarian cancer (PROC) and relapsed/refractory adenocarcinoma non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2025, 43, TPS8660. [Google Scholar] [CrossRef]
- Richardson, D.L.; Harter, P.; O’Malley, D.M.; Martin, A.G.; Herzog, T.J.; Rogalski, C.; Lemming, R.; Keeton, E.; Burger, R.A.; Mirza, M.R. UP-NEXT (GOG-3049/ENGOT-Ov71-NSGO-CTU): A study of upitifamab rilsodotin (UpRi), a NaPi2b-directed antibody drug conjugate (ADC), in platinum-sensitive recurrent ovarian cancer. J. Clin. Oncol. 2023, 41, TPS5614. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients with HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- Atwal, A.; Snowsill, T.; Dandy, M.C.; Krum, T.; Newton, C.; Evans, D.G.; Crosbie, E.J.; Ryan, N.A.J. The prevalence of mismatch repair deficiency in ovarian cancer: A systematic review and meta-analysis. Int. J. Cancer 2022, 151, 1626–1639. [Google Scholar] [CrossRef]
- Fan, C.A.; Reader, J.; Roque, D.M. Review of Immune Therapies Targeting Ovarian Cancer. Curr. Treat. Options Oncol. 2018, 19, 74. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients with Platinum-Resistant Ovarian Cancer. J. Clin. Oncol. 2015, 33, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.; Piha-Paul, S.; Ott, P.A.; Mehnert, J.M.; Berton-Rigaud, D.; Morosky, A.; Yang, P.; Ruman, J.; Matei, D. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: Analysis of KEYNOTE-028. Gynecol. Oncol. 2019, 152, 243–250. [Google Scholar] [CrossRef]
- Disis, M.L.; Taylor, M.H.; Kelly, K.; Beck, J.T.; Gordon, M.; Moore, K.M.; Patel, M.R.; Chaves, J.; Park, H.; Mita, A.C.; et al. Efficacy and Safety of Avelumab for Patients with Recurrent or Refractory Ovarian Cancer: Phase 1b Results From the JAVELIN Solid Tumor Trial. JAMA Oncol. 2019, 5, 393–401. [Google Scholar] [CrossRef]
- Matulonis, U.; Shapira-Frommer, R.; Santin, A.; Lisyanskaya, A.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.; Birrer, M.; Provencher, D. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Results from the phase II KEYNOTE-100 study. Ann. Oncol. 2019, 30, 1080–1087. [Google Scholar] [CrossRef]
- Monk, B.J.; Colombo, N.; Oza, A.M.; Fujiwara, K.; Birrer, M.J.; Randall, L.; Poddubskaya, E.V.; Scambia, G.; Shparyk, Y.V.; Lim, M.C.; et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1275–1289. [Google Scholar] [CrossRef]
- Bound, N.T.; Vandenberg, C.J.; Kartikasari, A.E.R.; Plebanski, M.; Scott, C.L. Improving PARP inhibitor efficacy in high-grade serous ovarian carcinoma: A focus on the immune system. Front. Genet. 2022, 13, 886170. [Google Scholar] [CrossRef]
- Kandalaft, L.E.; Dangaj Laniti, D.; Coukos, G. Immunobiology of high-grade serous ovarian cancer: Lessons for clinical translation. Nat. Rev. Cancer 2022, 22, 640–656. [Google Scholar] [CrossRef]
- Veneziani, A.C.; Gonzalez-Ochoa, E.; Alqaisi, H.; Madariaga, A.; Bhat, G.; Rouzbahman, M.; Sneha, S.; Oza, A.M. Heterogeneity and treatment landscape of ovarian carcinoma. Nat. Rev. Clin. Oncol. 2023, 20, 820–842. [Google Scholar] [CrossRef]
- Glasspool, R.M.; Brown, R.; Gore, M.E.; Rustin, G.J.; McNeish, I.A.; Wilson, R.H.; Pledge, S.; Paul, J.; Mackean, M.; Hall, G.D.; et al. A randomised, phase II trial of the DNA-hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in combination with carboplatin vs carboplatin alone in patients with recurrent, partially platinum-sensitive ovarian cancer. Br. J. Cancer 2014, 110, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, N.; Fang, F.; Ozes, A.R.; Tang, J.; Adewuyi, A.; Keer, H.; Lyons, J.; Baylin, S.B.; Matei, D.; Nakshatri, H.; et al. An Effective Epigenetic-PARP Inhibitor Combination Therapy for Breast and Ovarian Cancers Independent of BRCA Mutations. Clin. Cancer Res. 2018, 24, 3163–3175. [Google Scholar] [CrossRef] [PubMed]
- Karakashev, S.; Zhu, H.; Wu, S.; Yokoyama, Y.; Bitler, B.G.; Park, P.-H.; Lee, J.-H.; Kossenkov, A.V.; Gaonkar, K.S.; Yan, H.; et al. CARM1-expressing ovarian cancer depends on the histone methyltransferase EZH2 activity. Nat. Commun. 2018, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Karakashev, S.; Fukumoto, T.; Zhao, B.; Lin, J.; Wu, S.; Fatkhutdinov, N.; Park, P.-H.; Semenova, G.; Jean, S.; Cadungog, M.G.; et al. EZH2 Inhibition Sensitizes CARM1-High, Homologous Recombination Proficient Ovarian Cancers to PARP Inhibition. Cancer Cell 2020, 37, 157–167.e156. [Google Scholar] [CrossRef]
- El Messaoudi, S.; Fabbrizio, E.; Rodriguez, C.; Chuchana, P.; Fauquier, L.; Cheng, D.; Theillet, C.; Vandel, L.; Bedford, M.T.; Sardet, C. Coactivator-associated arginine methyltransferase 1 (CARM1) is a positive regulator of the Cyclin E1 gene. Proc. Natl. Acad. Sci. USA 2006, 103, 13351–13356. [Google Scholar] [CrossRef]
- Huang, T.T.; Lampert, E.J.; Coots, C.; Lee, J.M. Targeting the PI3K pathway and DNA damage response as a therapeutic strategy in ovarian cancer. Cancer Treat. Rev. 2020, 86, 102021. [Google Scholar] [CrossRef]
- Andrikopoulou, A.; Liontos, M.; Koutsoukos, K.; Dimopoulos, M.A.; Zagouri, F. Clinical perspectives of BET inhibition in ovarian cancer. Cell. Oncol. 2021, 44, 237–249. [Google Scholar] [CrossRef]
- Groeneweg, J.W.; Foster, R.; Growdon, W.B.; Verheijen, R.H.; Rueda, B.R. Notch signaling in serous ovarian cancer. J. Ovarian Res. 2014, 7, 95. [Google Scholar] [CrossRef]
- Gupta, V.G.; Hirst, J.; Petersen, S.; Roby, K.F.; Kusch, M.; Zhou, H.; Clive, M.L.; Jewell, A.; Pathak, H.B.; Godwin, A.K.; et al. Entinostat, a selective HDAC1/2 inhibitor, potentiates the effects of olaparib in homologous recombination proficient ovarian cancer. Gynecol. Oncol. 2021, 162, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Cadoo, K.A.; Meyers, M.L.; Burger, R.A.; Armstrong, D.K.; Penson, R.T.; Gordon, M.S.; Fleming, G.F.; Moroney, J.W.; Hamilton, E.P.; Duska, L.R.; et al. A phase II randomized study of avelumab plus entinostat versus avelumab plus placebo in patients (pts) with advanced epithelial ovarian cancer (EOC). J. Clin. Oncol. 2019, 37, 5511. [Google Scholar] [CrossRef]
- Koole, S.N.; Schouten, P.C.; Hauke, J.; Kluin, R.J.C.; Nederlof, P.; Richters, L.K.; Krebsbach, G.; Sikorska, K.; Alkemade, M.; Opdam, M.; et al. Effect of HIPEC according to HRD/BRCAwt genomic profile in stage III ovarian cancer: Results from the phase III OVHIPEC trial. Int. J. Cancer 2022, 151, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Ngoi, N.Y.L.; Tan, D.S.P. The role of homologous recombination deficiency testing in ovarian cancer and its clinical implications: Do we need it? ESMO Open 2021, 6, 100144. [Google Scholar] [CrossRef]
- Altman, D.G.; Royston, P. The cost of dichotomising continuous variables. BMJ 2006, 332, 1080. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Kim, J.; Haradhvala, N.J.; Huang, M.N.; Tian Ng, A.W.; Wu, Y.; Boot, A.; Covington, K.R.; Gordenin, D.A.; Bergstrom, E.N.; et al. The repertoire of mutational signatures in human cancer. Nature 2020, 578, 94–101. [Google Scholar] [CrossRef]
- Davies, H.; Glodzik, D.; Morganella, S.; Yates, L.R.; Staaf, J.; Zou, X.; Ramakrishna, M.; Martin, S.; Boyault, S.; Sieuwerts, A.M.; et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat. Med. 2017, 23, 517–525. [Google Scholar] [CrossRef]
- Drews, R.M.; Hernando, B.; Tarabichi, M.; Haase, K.; Lesluyes, T.; Smith, P.S.; Morrill Gavarró, L.; Couturier, D.-L.; Liu, L.; Schneider, M.; et al. A pan-cancer compendium of chromosomal instability. Nature 2022, 606, 976–983. [Google Scholar] [CrossRef]
- Liu, J.F.; Gordon, M.; Veneris, J.; Braiteh, F.; Balmanoukian, A.; Eder, J.P.; Oaknin, A.; Hamilton, E.; Wang, Y.; Sarkar, I.; et al. Safety, clinical activity and biomarker assessments of atezolizumab from a Phase I study in advanced/recurrent ovarian and uterine cancers. Gynecol. Oncol. 2019, 154, 314–322. [Google Scholar] [CrossRef]
- Nguyen, L.; Martens, J.W.M.; Van Hoeck, A.; Cuppen, E. Pan-cancer landscape of homologous recombination deficiency. Nat. Commun. 2020, 11, 5584. [Google Scholar] [CrossRef]
| Molecular Alteration | Main Features | Therapies/ Potential Therapies | Clinical Trials | Trial Status |
|---|---|---|---|---|
| CCNE1 amplification/overexpression | Occurs in 20% of HGSOC cases Largely mutually exclusive with BRCA1/2 mutations | CDK inhibitors with CDK2-specific activity | AT7519 (AT7519M, Astex Therapeutics Ltd., Cambridge, UK) AG-024322 (Pfizer) Dinaciclib (MK7965, SCH727965, Merck & Co.) CYC065 (Cyclacel Pharmaceuticals) Ronaciclib (BAY 1000394, Bayer) TG02 (Tragara Pharmaceuticals) Milciclib (PHA 848125, Tiziana Life Sciences) | None have passed phase II |
| Wee1 inhibitors | NCT02151292 (NCI): adavosertib plus gemcitabine | Active, not recruiting: reported benefit in PFS | ||
| IGNITE (AstraZeneca, Cambridge, UK): adavosertib | Completed, ORR 53% | |||
| NCT02272790 EFFORT (AstraZeneca): adavosertib with or without olaparib | Completed | |||
| High prevalence of replication stress | Most of the HGSOC cases, both HRD and HRP tumors In HRP, tumors are caused by premature entry into the S phase due to CCNE1 amplification. | ATR inhibitors | NCT02157792: Phase I trial of M6620 (VX-970) as monotherapy or in combination with carboplatin (Vertex Pharmaceuticals, Boston, MA, USA) | M6620 was well tolerated, with target engagement and preliminary antitumor responses observed |
| NCT02595892: Phase II trial testing gemcitabine hydrochloride alone or with M6620—National Cancer Institute (NCI) | Active, not recruiting | |||
| NCT03682289: Phase II trial testing AZD6738 alone and in combination with olaparib—AstraZeneca. | Recruiting | |||
| Phase I trial testing BAY 1895344 in combination with chemotherapy—NCI | Recruiting | |||
| NCT02595892: Phase II trial testing berzosertib plus gemcitabine versus gemcitabine alone in platinum-resistant HGSOC | Completed; benefit in PFS for the interventional arm | |||
| Fold-back inversions (FBI) | Most tumors with high FBI have BRCA1/2 wild-type status Associated with poor prognostic features such as CCNE1 amplification | NA | NA | NA |
| High folate receptor alpha expression | Associated with platinum and PARPi resistance | Mirvetuximab soravtansine | NCT04296890: SORAYA (ImmunoGen, Inc., Waltham, MA, USA): MIRV | Completed: meaningful antitumor activity |
| NCT04209855: MIRASOL (ImmunoGen, Inc.): MIRV | Completed | |||
| NCT05445778: GLORIOSA (Immunogen, Inc.) | Recruiting | |||
| Sodium-dependent phosphate transport protein 2B (NaPi2B) | Associated with platinum resistance | ADC Upifitamab rilsodotin (UpRi) | NCT03319628: UPLIFT | Active, not recruiting |
| NCT05329545: UP-NEXT | Terminated by sponsor; waiting results | |||
| ADC TUB-40 | NCT06303505 | Recruiting | ||
| MMR deficiency | High degree of microsatellite instability Large number of neoantigens | Checkpoint inhibitors | No trials have been conducted in the context of ovarian cancer HRP tumors | NA |
| Demethylation of BRCA1 and RAD51C | Mutually exclusive with BRCA1/2 mutations Demethylation of a single copy of initially BRCA1 and RAD51C methylated genes will restore the protein due to treatment pressure | Demethylating agents in combination with platinum-based therapies or PARPis | Phase II trial: Decitabine in combination with carboplatin vs. carboplatin alone (Cancer Research UK. CRUKD/07/065 and Eisai, Tokyo, Japan) | Terminated (no efficacy observed) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavanello, M.; Vieira, C.M.; Arenhardt, M.P.; Nogueira-Rodrigues, A. Homologous Recombination Proficiency in High-Grade Serous Epithelial Ovarian Cancer Tumors: The Dark Side of the Moon. Curr. Issues Mol. Biol. 2025, 47, 702. https://doi.org/10.3390/cimb47090702
Pavanello M, Vieira CM, Arenhardt MP, Nogueira-Rodrigues A. Homologous Recombination Proficiency in High-Grade Serous Epithelial Ovarian Cancer Tumors: The Dark Side of the Moon. Current Issues in Molecular Biology. 2025; 47(9):702. https://doi.org/10.3390/cimb47090702
Chicago/Turabian StylePavanello, Marina, Carolina Martins Vieira, Martina Parenza Arenhardt, and Angelica Nogueira-Rodrigues. 2025. "Homologous Recombination Proficiency in High-Grade Serous Epithelial Ovarian Cancer Tumors: The Dark Side of the Moon" Current Issues in Molecular Biology 47, no. 9: 702. https://doi.org/10.3390/cimb47090702
APA StylePavanello, M., Vieira, C. M., Arenhardt, M. P., & Nogueira-Rodrigues, A. (2025). Homologous Recombination Proficiency in High-Grade Serous Epithelial Ovarian Cancer Tumors: The Dark Side of the Moon. Current Issues in Molecular Biology, 47(9), 702. https://doi.org/10.3390/cimb47090702






