Genome Wide Identification of Sesame Dof Transcription Factors and Functional Analysis of SiDof8, SiDof10 and SiDof34 in Fatty Acid Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification and Analysis of Physicochemical Properties of Dof Proteins
2.2. Phylogenetic and Gene Structure Analysis
2.3. Conservative Motif Analysis
2.4. Tissue Expression Analysis of Dof Genes
2.5. Functional Analysis of SiDof8, SiDof10 and SiDof34 for Regulating Fatty Acid Synthesis
2.6. Subcellular Localization Analysis
3. Results
3.1. Analysis of Physicochemical Properties of Sesame Dof Proteins
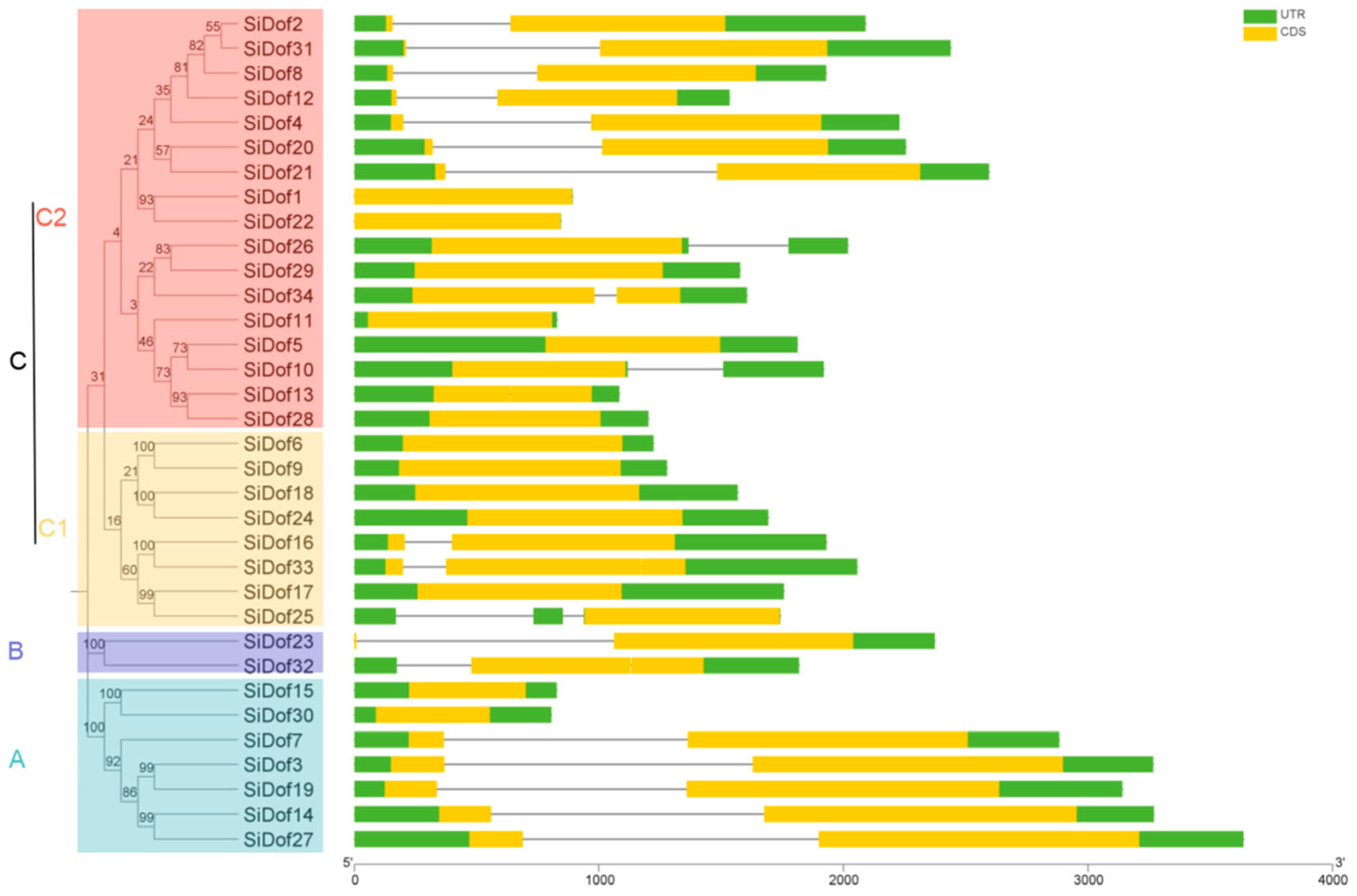
3.2. Genetic Structure and Phylogenetic Analysis of Sesame Dof Members
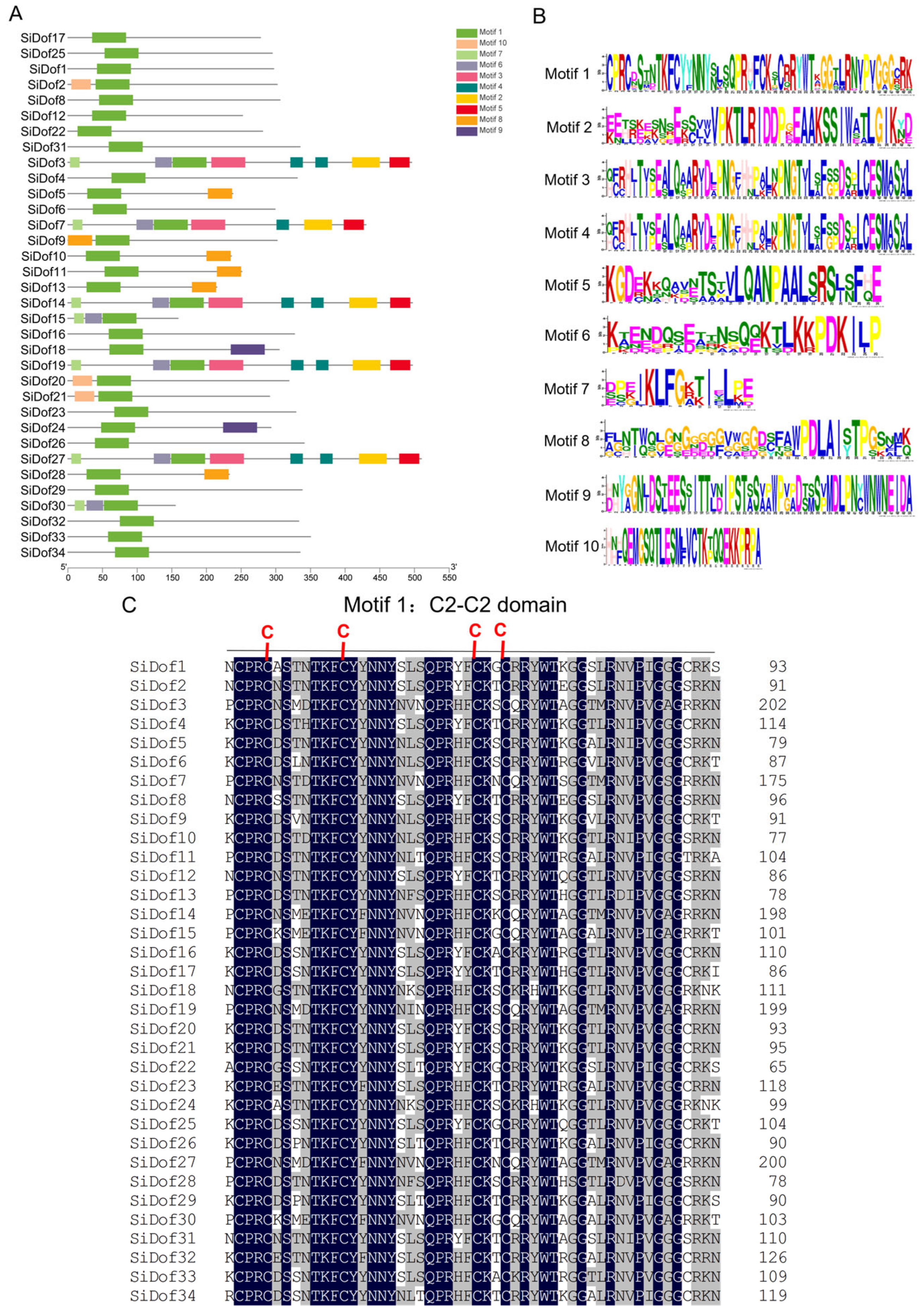
3.3. Analysis of Conserved Motifs of Sesame Dof Members
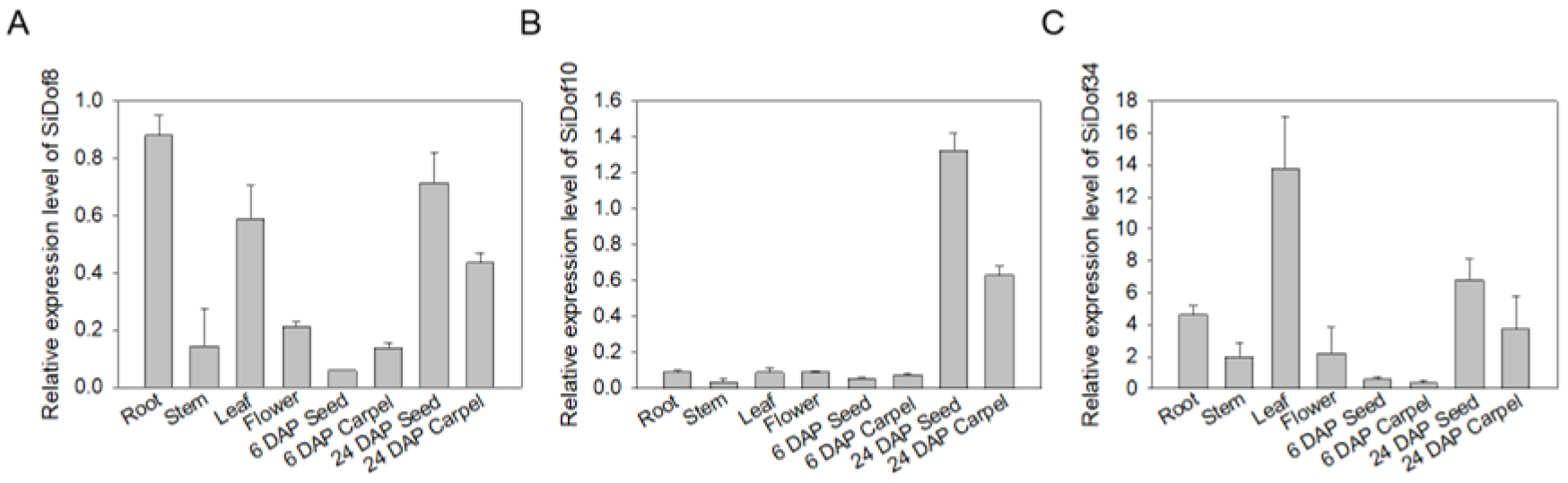
3.4. Tissue-Specific Expression Analysis of Sesame Dof Genes
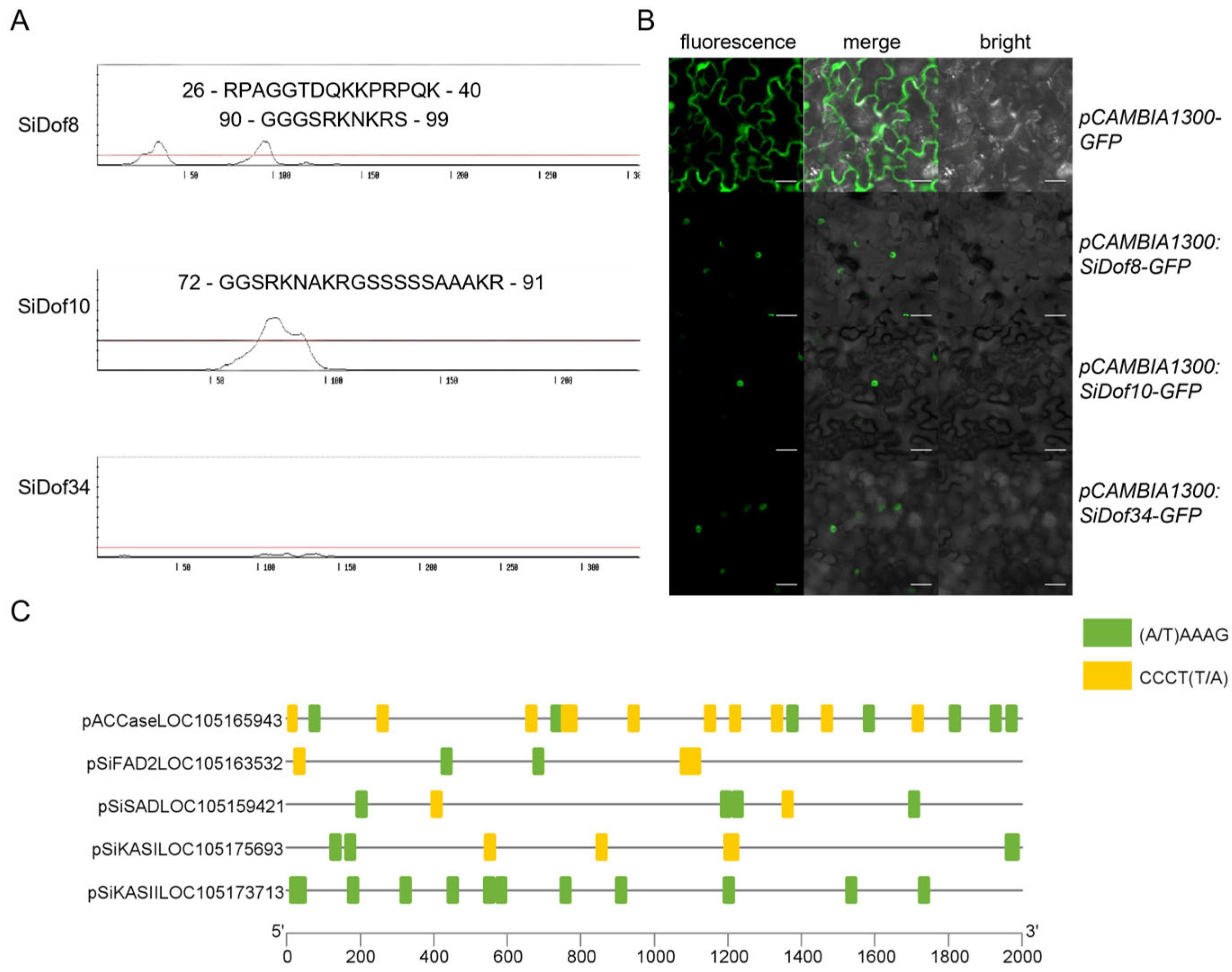
3.5. SiDof8, SiDof10 and SiDof34 Were All Located in the Nucleus and Their DNA Binding Sites Were Included in the Promoter Region of Fatty Acid Synthesis-Related Genes
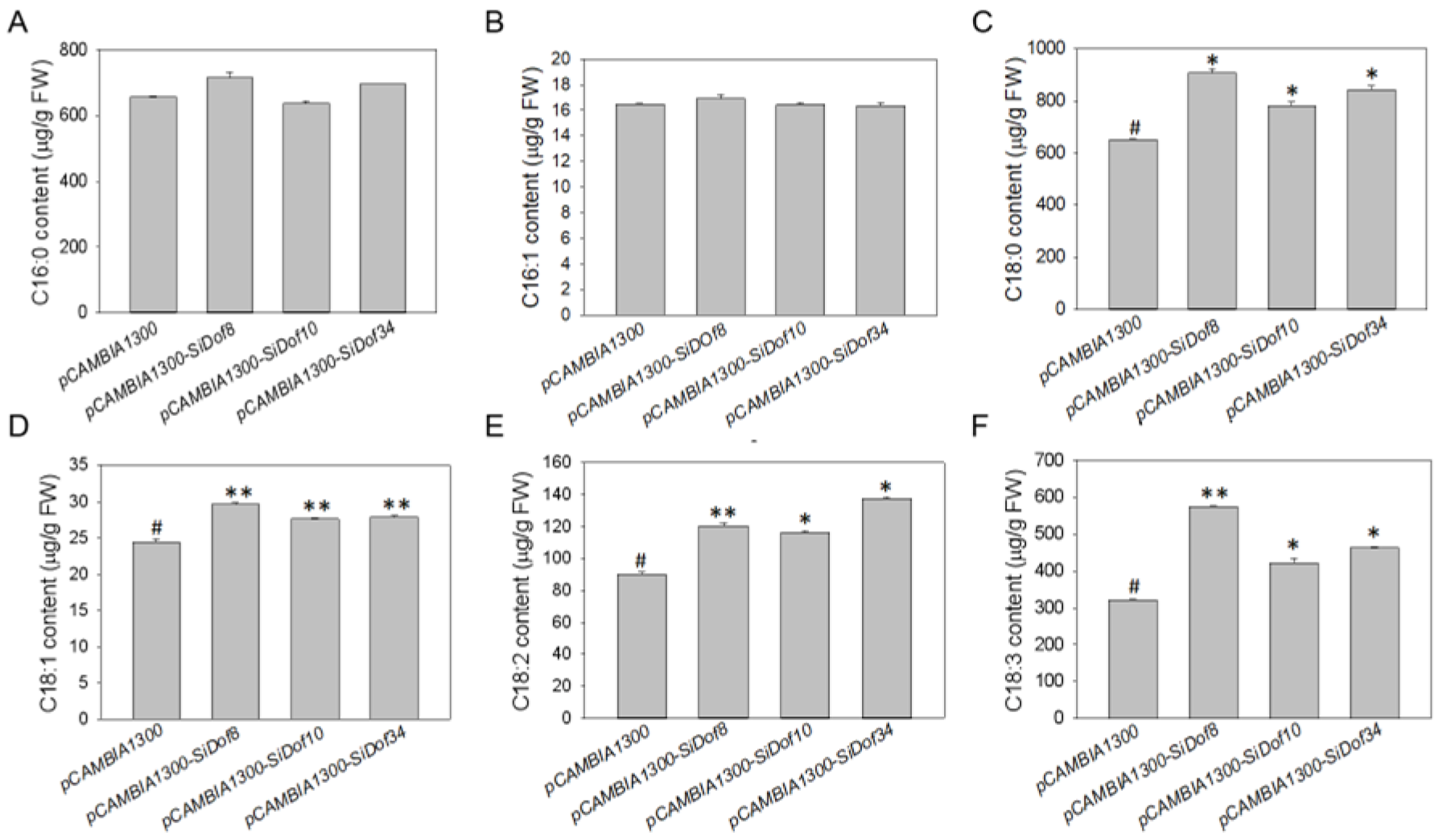
3.6. Overexpression of SiDof8, SiDof10 and SiDof34 Significantly Increased the Contents of C18:0, C18:1, C18:2 and C18:3
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yanagisawa, S. Dof DNA binding domains contain a novel zinc finger motif. Trends Plant Sci. 1996, 1, 213–214. [Google Scholar] [CrossRef]
- Gupta, S.; Malviya, N.; Kushwaha, H.; Nasim, J.; Bisht, N.C.; Singh, V.K.; Yadav, D. Insights into structural and functional diversity of Dof (DNA binding with one finger) transcription factor. Planta 2015, 241, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Carbajosa, J.; Moose, S.P.; Parsons, R.L.; Schmidt, R.J. A maize zinc-finger protein binds the prolamin box in zein gene promoters and interacts with the basic leucine zipper transcriptional activator. Proc. Natl. Acad. Sci. USA 1997, 94, 7685–7690. [Google Scholar] [CrossRef] [PubMed]
- Mena, M.; Vicente-Carbajosa, J.; Schmidt, R.J.; Carbonero, P. An endosperm-specific Dof protein from barley, highly conserved in wheat, binds to and activates transcription from the prolamin-box of a native b-hordein promoter in barley endosperm. Plant J. 1998, 16, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Ni, Z.; Yao, Y.; Nie, X.; Sun, Q. Wheat Dof transcription factor WPBF interacts with TaQM and activates transcription of an alpha-gliadin gene during wheat seed development. Plant Mol. Biol. 2007, 63, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Gualberti, G.; Papi, M.; Bellucci, L.; Ricci, I.; Bouchez, D.; Camilleri, C.; Costantino, P.; Vittorioso, P. Mutations in the Dof zinc finger genes DAG2 and DAG1 influence with opposite effects the germination of Arabidopsis seeds. Plant Cell 2002, 14, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Papi, M.; Sabatini, S.; Bouchez, D.; Camilleri, C.; Costantino, P.; Vittorioso, P. Identification and disruption of an Arabidopsis zinc finger gene controlling seed germination. Genes Dev. 2000, 14, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Skirycz, A.; Radziejwoski, A.; Busch, W.; Hannah, M.A.; Czeszejko, J.; Kwaśniewski, M.; Zanor, M.I.; Lohmann, J.U.; Veylder, L.D.; Witt, I.; et al. The Dof transcription factor OBP1 is involved in cell cycle regulation in Arab. Thaliana. Plant J. 2008, 56, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yan, M.; Li, L.; He, J.; Zhou, S.; Li, Z.; Niu, C.; Bao, C.; Zhi, F.; Ma, F.; et al. The apple DNA-binding one zinc-finger protein MdDof54 promotes drought resistance. Hortic. Res. 2020, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C.K. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Di, D.; Ma, M.; Zhang, X.; Lu, Y.; Kronzucker, H.J.; Shi, W. Potential Secretory Transporters and Biosynthetic Precursors of Biological Nitrification Inhibitor 1,9-Decanediol in Rice as Revealed by Transcriptome and Metabolome Analyses. Rice Sci. 2024, 31, 87–102. [Google Scholar]
- Buyachuihan, L.; Stegemann, F.; Grininger, M. How Acyl Carrier Proteins (ACPs) Direct Fatty Acid and Polyketide Biosynthesis. Scand J Immunol. 2018, 88, e12713. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Nagano, Y. Plant acetyl-CoA carboxylase: Structure, biosynthesis, regulation, and gene manipulation for plant breeding. Biosci. Biotechnol. Biochem. 2004, 68, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Yasuno, R.; Von, W.K.; Wada, H. Identification and molecular characterization of the beta-ketoacyl-[acyl carrier protein] synthase component of the Arabidopsis mitochondrial fatty acid synthase. J. Biol. Chem. 2004, 279, 8242–8251. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.S.; Labrie, S.T.; Kinney, A.J.; Von, W.K.; Browse, J. A KAS2 cDNA complements the phenotypes of the Arabidopsis fab1 mutant that differs in a single residue bordering the substrate binding pocket. Plant J. 2002, 29, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Okuley, J.; Lightner, J.; Feldmann, K.; Yadav, N.; Lark, E.; Browse, J. Arabidopsis FAD2 gene encodes the enzyme that is essential for polyunsaturated lipid synthesis. Plant Cell 1994, 6, 147–158. [Google Scholar] [PubMed]
- Reed, D.W.; Schafer, U.A.; Covello, P.S. Characterization of the Brassica napus extraplastidial linoleate desaturase by expression in Saccharomyces cerevisiae. Plant Physiol. 2000, 122, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Hitz, W.D.; Carlson, T.J.; Booth, J.R.; Kinney, A.J.; Stecca, K.L.; Yadav, N.S. Cloning of a higher-plant plastid omega-6 fatty acid desaturase cDNA and its expression in a cyanobacterium. Plant Physiol. 1994, 105, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.P. Biosynthesis of complex lipids. Fed Proc. 1961, 20, 934–940. [Google Scholar] [PubMed]
- Li, Q.; Shao, J.H.; Tang, S.H.; Shen, Q.W.; Wang, T.H.; Chen, W.L.; Hong, Y.Y. Corrigendum: WRINKLEDl accelerates flowering and regulates lipid homeostasis between oil accumulation and membrane lipid anabolism in Brassica napus. Front. Plant Sci. 2015, 6, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Pouvreau, B.; Baud, S.; Vernoud, V. Duplicate maize Wrinkled1 transcription factors activate target genes involved in seed oil biosynthesis. Plant Physiol. 2011, 156, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Zhang, B.; Hao, Y.J.; Huang, J.; Tian, A.G.; Liao, Y.; Zhang, J.S.; Chen, S.Y. The soybean Dof-type transcription factor genes, GmDof4 and GmDof11, enhance lipid content in the seeds of transgenic Arabidopsis plants. Plant J. 2007, 52, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liang, W.; Liu, Z.; Wang, Y.M.; Zhao, Y.P.; Ijaz, B.; Hua, J.P. Overexpression of GhDof1 improved salt and cold tolerance and seed oil content in Gossypium hirsutum. J. Plant Physiol. 2017, 218, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Chen, L.; Zhang, L.; Liao, X.; Wang, J. Identification and expression profiling of the CoDof genes involved in fatty acid/lipid biosynthesis of tetraploid Camellia oleifera. Front Plant Sci. 2025, 16, 1599849. [Google Scholar] [CrossRef] [PubMed]
- Oboulbiga, E.B.; Douamba, Z.; Compaoré-Sérémé, D.; Semporé, J.N.; Dabo, R.; Semde, Z.; Tapsoba, F.W.; Hama-Ba, F.; Songré-Ouattara, L.T.; Parkouda, C.; et al. Physicochemical, potential nutritional, antioxidant and health properties of sesame seed oil: A review. Front. Nutr. 2023, 1, 1127926. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhao, F.; Wang, Z.; Wang, Q.; Chai, X.; Hou, G.; Meng, Q. Sesame (Sesamum indicum L.): A Comprehensive Review of Nutritional Value, Phytochemical Composition, Health Benefits, Development of Food, and Industrial Applications. Nutrients 2022, 14, 4079. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S. The Dof family of plant transcription factors. Trends Plant Sci. 2002, 7, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Liu, T.; Ling, M.; Ma, Q.; Cao, W.; Xing, F.; Huang, T.; Zhang, Y.; Duan, H.; Liu, Z.Z. Genome-Wide Identification of DOF Gene Family and the Mechanism Dissection of SbDof21 Regulating Starch Biosynthesis in Sorghum. Int. J. Mol. Sci. 2022, 23, 12152. [Google Scholar] [CrossRef] [PubMed]
- Lohani, N.; Babaei, S.; Singh, M.B.; Bhalla, P.L. Genome-wide in silico identification and comparative analysis of Dof gene family in Brassica Napus. Plants (Basel) 2021, 10, 709. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Ji, X.; Wang, M.; Li, J.; Wang, X.; Meng, L.; Wei, P.; Xu, H.; Niu, T.; Liu, A. Genome-wide identification and expression analysis of the Dof gene family reveals their involvement in hormone response and abiotic stresses in sunflower (Helianthus annuus L.). Gene 2024, 910, 148336. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, K.; Zhang, Y.; Feng, Q.; Wang, L.H.; Zhao, Y.; Li, D.H.; Zhao, Q.; Zhu, X.D.; Zhu, X.F.; et al. Genetic discovery for oil production and quality in sesame. Nat. Commun. 2015, 6, 8609. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.P. Transcript Profilling, Cloning, and Comparative Function Analysis of Genes Involved in Lipid Biosynthesis from Oil Crops; Chinese Academy of Agricultural Sciences: Wuhan, China, 2009; pp. 109–111. [Google Scholar]
- Song, S.; You, J.; Shi, L.; Sheng, C.; Zhou, W.; Dossou, S.S.K.; Dossa, K.; Wang, L.; Zhang, X. Genome-Wide Analysis of nsLTP Gene Family and Identification of SiLTPs Contributing to High Oil Accumulation in Sesame (Sesamum indicum L.). Int. J. Mol. Sci. 2021, 22, 5291. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Chauhan, R.S. Regulation of FA and TAG biosynthesis pathway genes in endosperms and embryos of high and low oil content genotypes of Jatropha curcas L. Plant Physiol. Bioch. 2015, 94, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Song, Y.; Gao, H.; Wang, M.; Cui, H.; Ji, C.; Wang, J.; Yuan, L.; Li, R. Genome-wide identification and functional analysis of Dof transcription factor family in Camelina sativa. BMC Genom. 2022, 23, 812. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Xiao, L.; Wu, G.; Wu, Y.H.; Zhang, X.R.; Lu, C.M. Accumulation pattern of fatty acids during the seed development of sesame (Sesamum indicum L.). Chin. J. Oil Crop Sci. 2008, 30, 84–89. [Google Scholar]
- Yin, M.; Wang, Y.; Wang, Z.; Hu, T.; Chen, S.W.; Zhou, Y.L.; Xing, Z.; Wang, J.P.; Li, R.Z. Gene cloning and functional analysis of PfSAD3 and PfSAD4 in Perilla frutescens. J. Agric. Biotechnol. 2024, 32, 2526–2539. [Google Scholar]
- Chen, S.W.; Hu, T.; Lei, T.; Yang, H.L.; Wen, J.; Chai, X.D.; Wang, J.P.; Li, R.Z. Identification of the PfDof transcription factor family in Perilla frutescens and functional analysis of PfDof29 in lipid synthesis. Chin. J. Biotechnol. 2025, 41, 1–28. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Chen, S.; Song, F.; Shi, L.; Lv, X.; Zhu, Z.; Lu, H. Genome Wide Identification of Sesame Dof Transcription Factors and Functional Analysis of SiDof8, SiDof10 and SiDof34 in Fatty Acid Synthesis. Curr. Issues Mol. Biol. 2025, 47, 700. https://doi.org/10.3390/cimb47090700
Zhang F, Chen S, Song F, Shi L, Lv X, Zhu Z, Lu H. Genome Wide Identification of Sesame Dof Transcription Factors and Functional Analysis of SiDof8, SiDof10 and SiDof34 in Fatty Acid Synthesis. Current Issues in Molecular Biology. 2025; 47(9):700. https://doi.org/10.3390/cimb47090700
Chicago/Turabian StyleZhang, Feicui, Shanyu Chen, Feiling Song, Limin Shi, Xuegao Lv, Zhengmei Zhu, and Huabing Lu. 2025. "Genome Wide Identification of Sesame Dof Transcription Factors and Functional Analysis of SiDof8, SiDof10 and SiDof34 in Fatty Acid Synthesis" Current Issues in Molecular Biology 47, no. 9: 700. https://doi.org/10.3390/cimb47090700
APA StyleZhang, F., Chen, S., Song, F., Shi, L., Lv, X., Zhu, Z., & Lu, H. (2025). Genome Wide Identification of Sesame Dof Transcription Factors and Functional Analysis of SiDof8, SiDof10 and SiDof34 in Fatty Acid Synthesis. Current Issues in Molecular Biology, 47(9), 700. https://doi.org/10.3390/cimb47090700





