Low Expression of Selenoprotein S Modulates Osteogenic Differentiation Through Bidirectional Regulation of the SP7–HSP47/COL1A1/SPARC Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Transfection
2.3. Osteogenic Induction
2.4. Quantitative Real-Time PCR
2.5. Animals
2.6. Western Blotting
2.7. Immunohistochemical Staining
2.8. Immunofluorescence
2.9. Transcription Factor Target Gene Screening and Binding Sequence Prediction
2.10. The Dual-Luciferase Reporter Assay
2.11. Chromatin Immunoprecipitation
2.12. Transcriptome Analyses
2.13. Statistical Analysis
3. Results
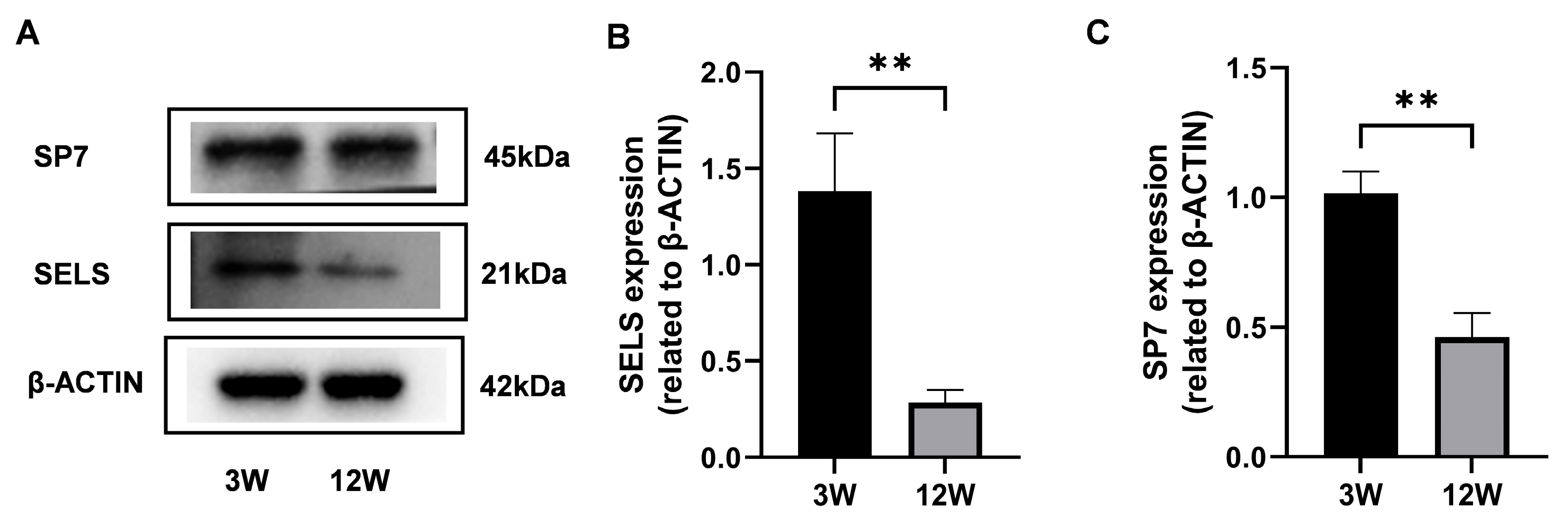
3.1. Expression of SELS During the Active Phase of Osteogenesis
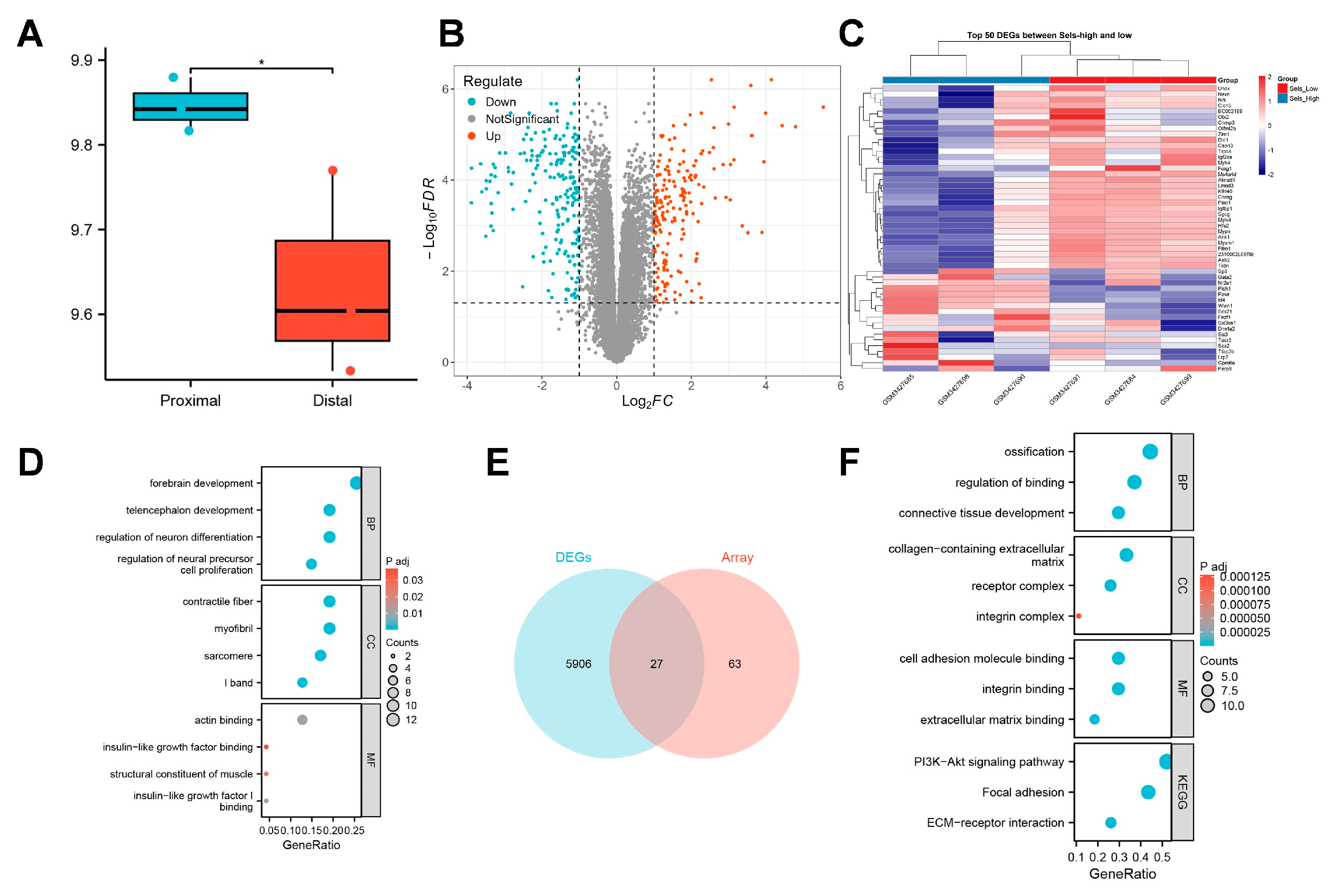
3.2. SELS Expression Patterns and Related Transcriptomic Changes During Mandibular Osteogenesis
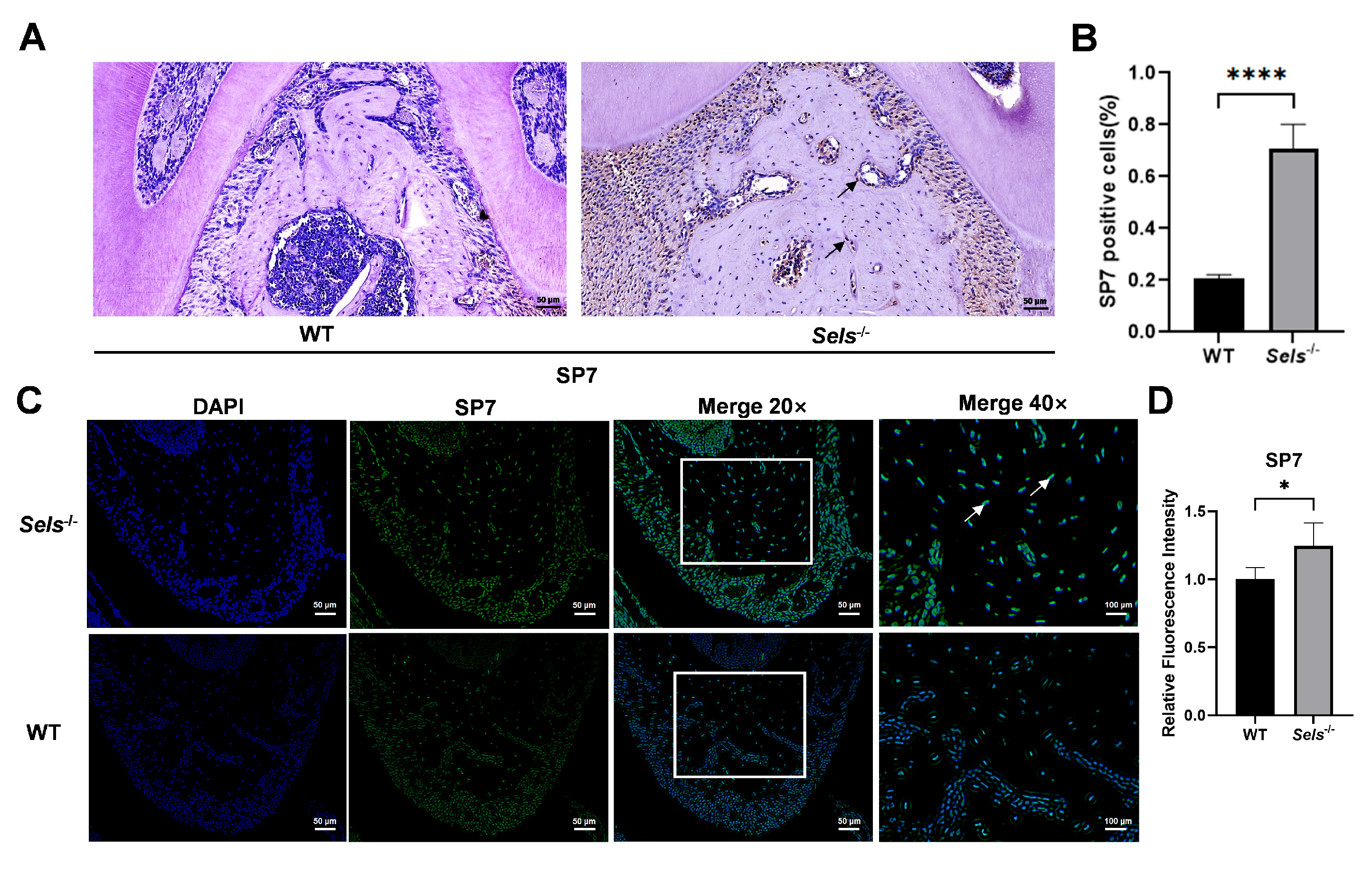
3.3. Histological Analysis of the Impact of Low SELS Expression on Mandibular Bone
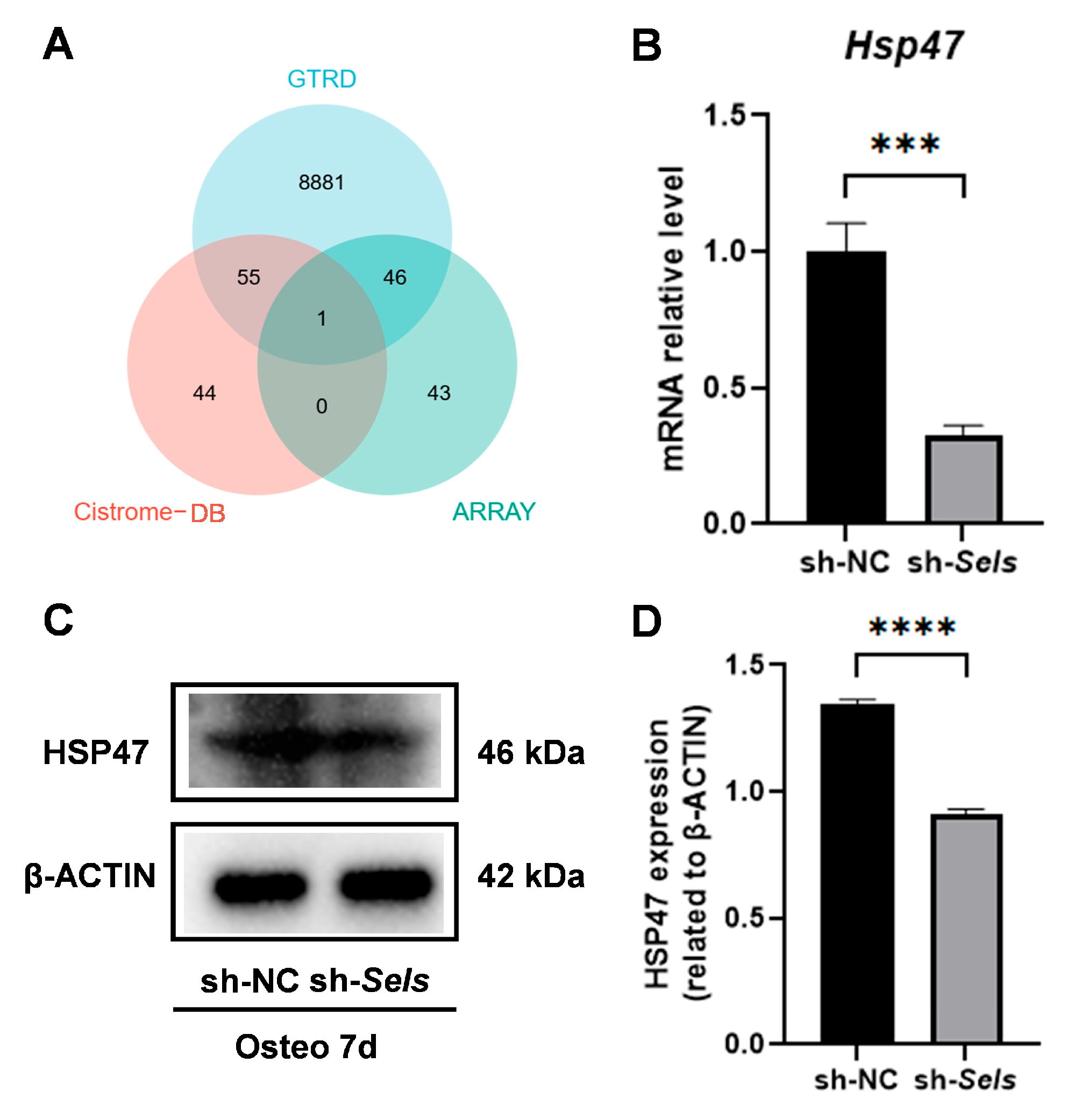
3.4. Identification of HSP47 as a Downstream Target of SP7 and Its Expression During Osteogenic Differentiation in BMSCs with Low SELS Expression
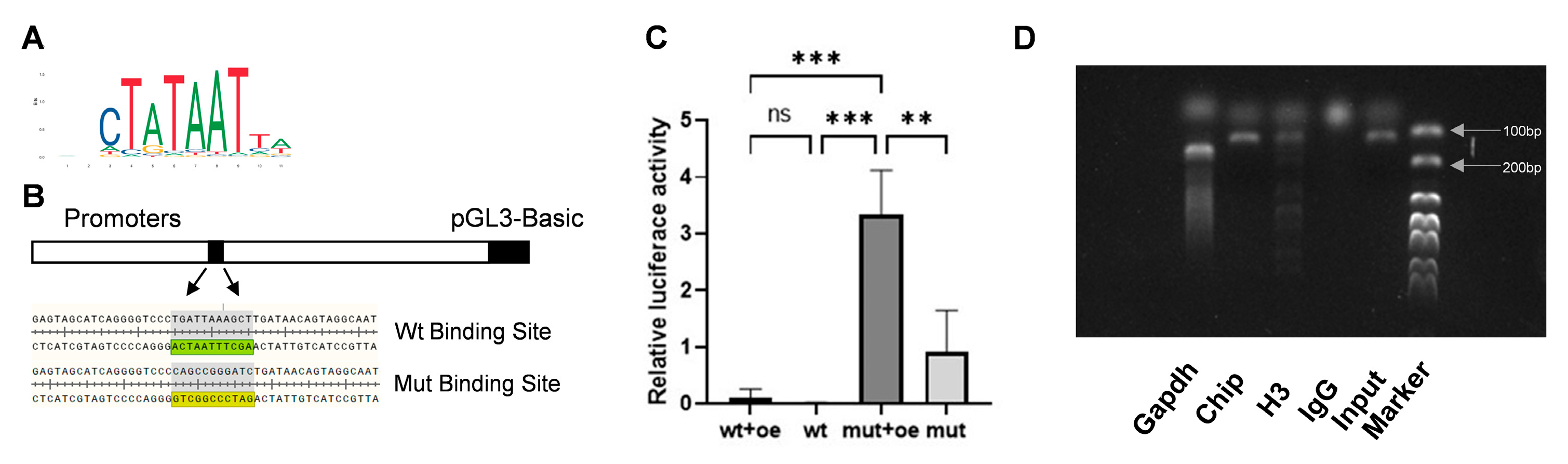
3.5. HSP47 Expression Is Regulated by SP7 Binding at Its Promoter Region
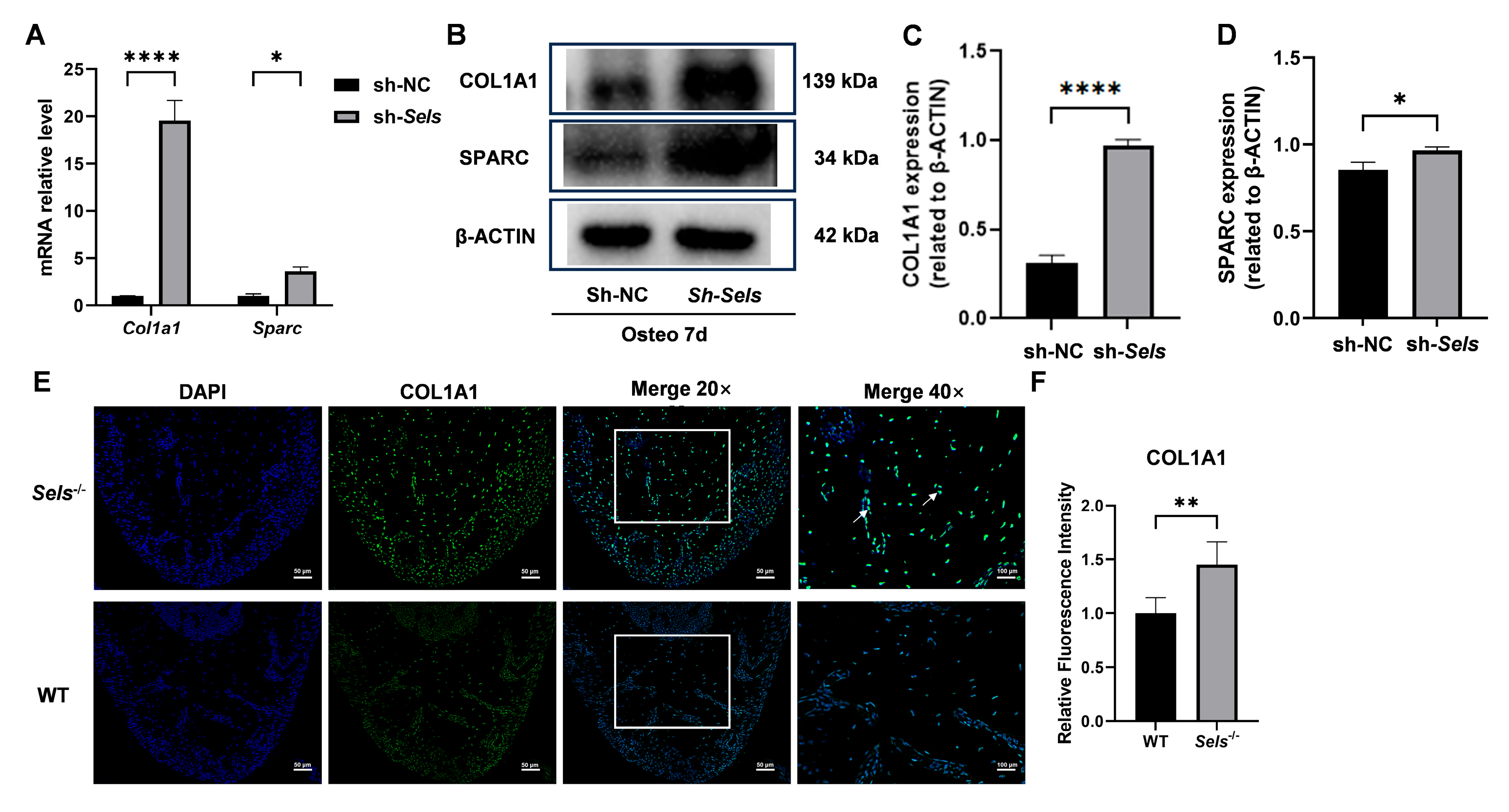
3.6. Upregulation of SP7 Downstream Targets COL1A1 and SPARC Under Conditions of Low SELS Expression
3.7. Mechanism Diagram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bubenik, J.L.; Miniard, A.C.; Driscoll, D.M. Alternative transcripts and 3′UTR elements govern the incorporation of selenocysteine into selenoprotein S. PLoS ONE 2013, 8, e62102. [Google Scholar] [CrossRef]
- Ghelichkhani, F.; Gonzalez, F.A.; Kapitonova, M.A.; Schaefer-Ramadan, S.; Liu, J.; Cheng, R.; Rozovsky, S. Selenoprotein S: A versatile disordered protein. Arch. Biochem. Biophys. 2022, 731, 109427. [Google Scholar] [CrossRef]
- Blackwood, E.A.; MacDonnell, L.F.; Thuerauf, D.J.; Bilal, A.S.; Murray, V.B.; Bedi, K.C., Jr.; Margulies, K.B.; Glembotski, C.C. Noncanonical Form of ERAD Regulates Cardiac Hypertrophy. Circulation 2023, 147, 66–82. [Google Scholar] [CrossRef]
- Gao, Y.; Hannan, N.R.; Wanyonyi, S.; Konstantopolous, N.; Pagnon, J.; Feng, H.C.; Jowett, J.B.; Kim, K.H.; Walder, K.; Collier, G.R. Activation of the selenoprotein SEPS1 gene expression by pro-inflammatory cytokines in HepG2 cells. Cytokine 2006, 33, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Bian, W.; Fu, F.; Hu, J.; Liu, H. Selenoprotein S inhibits inflammation-induced vascular smooth muscle cell calcification. J. Biol. Inorg. Chem. 2018, 23, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Huang, R.; Chang, Y.; Yi, X. Roles and mechanisms of optineurin in bone metabolism. Biomed. Pharmacother. 2024, 172, 116258. [Google Scholar] [CrossRef]
- Cichocka, E.; Górczyńska-Kosiorz, S.B.; Niemiec, P.; Trautsolt, W.; Gumprecht, J. The Impact of Selected COL1A1 and COL1A2 Gene Polymorphisms on Bone Mineral Density and the Risk of Metabolic Diseases in Postmenopausal Women. Int. J. Mol. Sci. 2025, 26, 4981. [Google Scholar] [CrossRef]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation. Front. Cell Dev. Biol. 2020, 8, 601224. [Google Scholar] [CrossRef]
- Qu, S.; Wu, J.; Bao, Q.; Yao, B.; Duan, R.; Chen, X.; Li, L.; Yuan, H.; Jin, Y.; Ma, C. Osterix promotes the migration and angiogenesis of breast cancer by upregulation of S100A4 expression. J. Cell Mol. Med. 2019, 23, 1116–1127. [Google Scholar] [CrossRef]
- Ortuño, M.J.; Susperregui, A.R.; Artigas, N.; Rosa, J.L.; Ventura, F. Osterix induces COL1A1 gene expression through binding to Sp1 sites in the bone enhancer and proximal promoter regions. Bone 2013, 52, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Niger, C.; Lima, F.; Yoo, D.J.; Gupta, R.R.; Buo, A.M.; Hebert, C.; Stains, J.P. The transcriptional activity of osterix requires the recruitment of Sp1 to the osteocalcin proximal promoter. Bone 2011, 49, 683–692. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Y.; Zhang, L.; Zhang, C. Transcriptional regulation of bone sialoprotein gene expression by Osx. Biochem. Biophys. Res. Commun. 2016, 476, 574–579. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, E.H.; Park, S.Y.; Kim, J.E. Induction of fibrillin-2 and periostin expression in Osterix-knockdown MC3T3-E1 cells. Gene 2017, 596, 123–129. [Google Scholar] [CrossRef]
- Wu, H.; Shi, J.Y.; Zhao, Y.S.; Shi, Y.W.; Zhong, L.Q.; Feng, H.N.; Liu, Y.; Chen, J.H.; Chen, X. Low Expression of Selenoprotein S Promotes Osteogenic Differentiation in Bone Marrow Mesenchymal Stromal Cells. Biol. Trace Elem. Res. 2025, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kolmykov, S.; Yevshin, I.; Kulyashov, M.; Sharipov, R.; Kondrakhin, Y.; Makeev, V.J.; Kulakovskiy, I.V.; Kel, A.; Kolpakov, F. GTRD: An integrated view of transcription regulation. Nucleic Acids Res. 2021, 49, D104–D111. [Google Scholar] [CrossRef] [PubMed]
- Taing, L.; Dandawate, A.; L’Yi, S.; Gehlenborg, N.; Brown, M.; Meyer, C.A. Cistrome Data Browser: Integrated search, analysis and visualization of chromatin data. Nucleic Acids Res. 2024, 52, D61–D66. [Google Scholar] [CrossRef]
- Rauluseviciute, I.; Riudavets-Puig, R.; Blanc-Mathieu, R.; Castro-Mondragon, J.A.; Ferenc, K.; Kumar, V.; Lemma, R.B.; Lucas, J.; Chèneby, J.; Baranasic, D.; et al. JASPAR 2024: 20th anniversary of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2024, 52, D174–D182. [Google Scholar] [CrossRef]
- Kim, C.Y.; Kim, K.H. Dexamethasone-induced selenoprotein S degradation is required for adipogenesis. J. Lipid Res. 2013, 54, 2069–2082. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Chai, H.; Wang, S.; Sun, C.; Huang, Q.; Xu, W. SOST gene suppression stimulates osteocyte Wnt/β-catenin signaling to prevent bone resorption and attenuates particle-induced osteolysis. J. Mol. Med. 2023, 101, 607–620. [Google Scholar] [CrossRef]
- Selvaraj, V.; Sekaran, S.; Dhanasekaran, A.; Warrier, S. Type 1 collagen: Synthesis, structure and key functions in bone mineralization. Differentiation 2024, 136, 100757. [Google Scholar] [CrossRef]
- Su, H.; Liao, Y.; Yuan, X.; Huang, J.; Chen, Y.; Zhao, B. G/ β- TCP composite scaffold material promotes osteogenic differentiation of bone marrow mesenchymal stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 2025–2031. [Google Scholar] [CrossRef]
- Xu, S.; Chen, Y.; Miao, J.; Li, Y.; Liu, J.; Zhang, J.; Liang, J.; Chen, S.; Hou, S. Esculin inhibits hepatic stellate cell activation and CCl4-induced liver fibrosis by activating the Nrf2/GPX4 signaling pathway. Phytomedicine 2024, 128, 155465. [Google Scholar] [CrossRef]
- Sofyanti Putri, R.; Putra, A.; Chodidjah, D.; Masyitah Darlan, D.; Trisnadi, S.; Thomas, S.; Amalina, N.D.; Candra Irawan, R. Clitorea ternatea flower extract induces platelet-derived growth factor(PDGF) and GPx gene overexpression in ultraviolet (UV) B irradiationinduced collagen loss. Med. Glas. 2023, 20, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Robin, M.; Mouloungui, E.; Castillo Dali, G.; Wang, Y.; Saffar, J.L.; Pavon-Djavid, G.; Divoux, T.; Manneville, S.; Behr, L.; Cardi, D.; et al. Mineralized collagen plywood contributes to bone autograft performance. Nature 2024, 636, 100–107. [Google Scholar] [CrossRef]
- Mojadadi, A.; Au, A.; Salah, W.; Witting, P.; Ahmad, G. Role for Selenium in Metabolic Homeostasis and Human Reproduction. Nutrients 2021, 13, 3256. [Google Scholar] [CrossRef]
- Qi, Z.; Duan, A.; Ng, K. Selenoproteins in Health. Molecules 2023, 29, 136. [Google Scholar] [CrossRef]
- Hu, X.; Li, B.; Wu, F.; Liu, X.; Liu, M.; Wang, C.; Shi, Y.; Ye, L. GPX7 Facilitates BMSCs Osteoblastogenesis via ER Stress and mTOR Pathway. J. Cell Mol. Med. 2021, 25, 10454–10465. [Google Scholar] [CrossRef]
- Qin, Q.; Yang, H.; Guo, R.; Zheng, Y.; Huang, Y.; Jin, L.; Fan, Z.; Li, W. FAM96B negatively regulates FOSL1 to modulate the osteogenic differentiation and regeneration of periodontal ligament stem cells via ferroptosis. Stem Cell Res. Ther. 2024, 15, 471. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Liu, Y.; Zhang, M.; Shi, Y.; Zhang, Y.; Mi, G.; Wang, M.; He, Y.; Chen, Y.; et al. Effects of Selenoprotein S Knockdown on Endoplasmic Reticulum Stress in ATDC5 Cells and Gene Expression Profiles in Hypertrophic Chondrocytes. Biol. Trace Elem. Res. 2023, 201, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, G.; Luan, X.; Diekwisch, T.G.H. Epigenetic Repression of RUNX2 and OSX Promoters Controls the Nonmineralized State of the Periodontal Ligament. Genes. 2023, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Cui, C.; Rosen, C.J.; Sato, T.; Xu, R.; Li, P.; Wei, X.; Bi, R.; Yuan, Q.; Zhou, C. Klotho in Osx+-mesenchymal progenitors exerts pro-osteogenic and anti-inflammatory effects during mandibular alveolar bone formation and repair. Signal Transduct. Target. Ther. 2022, 7, 155. [Google Scholar] [CrossRef]
- Bellaye, P.S.; Burgy, O.; Bonniaud, P.; Kolb, M. HSP47: A potential target for fibrotic diseases and implications for therapy. Expert. Opin. Ther. Targets 2021, 25, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.C.; Blissett, A.R. New genes in bone development: What’s new in osteogenesis imperfecta. J. Clin. Endocrinol. Metab. 2013, 98, 3095–3103. [Google Scholar] [CrossRef] [PubMed]
- Herbert, A. Osteogenesis imperfecta type 10 and the cellular scaffolds underlying common immunological diseases. Genes. Immun. 2024, 25, 265–276. [Google Scholar] [CrossRef]
- Khan, E.S.; Däinghaus, T. HSP47 in human diseases: Navigating pathophysiology, diagnosis and therapy. Clin. Transl. Med. 2024, 14, e1755. [Google Scholar] [CrossRef]
- Ito, S.; Nagata, K. Biology of HSP47 (Serpin H1), a collagen-specific molecular chaperone. Semin. Cell Dev. Biol. 2017, 62, 142–151. [Google Scholar] [CrossRef]
- Abraham, E.T.; Oecal, S.; Mörgelin, M.; Schmid, P.W.N.; Buchner, J.; Baumann, U.; Gebauer, J.M. Collagen’s primary structure determines collagen:HSP47 complex stoichiometry. J. Biol. Chem. 2021, 297, 101169. [Google Scholar] [CrossRef]
- Devos, H.; Zoidakis, J.; Roubelakis, M.G.; Latosinska, A.; Vlahou, A. Reviewing the Regulators of COL1A1. Int. J. Mol. Sci. 2023, 24, 10004. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Therapeutic Potential of Exercise-Induced SPARC in Bone Health? Biomedicines 2025, 13, 945. [Google Scholar] [CrossRef] [PubMed]

| Genes | Forward | Reverse |
|---|---|---|
| Gapdh | 5′-TTGATGGCAACAATCTCCAC-3′ | 5′-CGTCCCGTAGACAAAATGGT-3′ |
| Selenos | 5′-GCТGGСТАGТТGGТАGGТТGА-3′ | 5′-CGCATCCCGTCACAGAGA-3′ |
| HSP47 | 5′-GCAGCAGCAAGCAACACTACAACT-3′ | 5′-AGAACATGGCGTTCACAAGCAGTG-3′ |
| COL1A1 | 5′-GCTCCTCTTAGGGGCCACT-3′ | 5′-CCACGTCTCACCATTGGGG-3′ |
| SPARC | 5′-ACCCCCGGCAATTTCATGG-3′ | 5′-TGTCTTCCCAGCTCTTGATGTAA-3′ |
| Matrix ID | Name | Score | Sequence ID | Start | End | Strand | Predicted Sequence |
|---|---|---|---|---|---|---|---|
| UN0264.1 | UN0264.1.SP7 | 8.447574 | NC_000073.7:c99004321-99002222 | 1515 | 1525 | − | AGCTTTAATCA |
| UN0264.1 | UN0264.1.SP7 | 8.249999 | NC_000073.7:c99004321-99002222 | 22 | 32 | − | TTCTATTATCA |
| UN0264.1 | UN0264.1.SP7 | 7.20972 | NC_000073.7:c99004321-99002222 | 1379 | 1389 | + | AGCTAGAATTC |
| UN0264.1 | UN0264.1.SP7 | 7.020698 | NC_000073.7:c99004321-99002222 | 1264 | 1274 | + | TGCTGTATTTA |
| UN0264.1 | UN0264.1.SP7 | 6.8002434 | NC_000073.7:c99004321-99002222 | 615 | 625 | + | CTCTATAACTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Zhao, Y.-S.; Li, C.-S.; Shi, J.-Y.; Li, Y.; Zhong, L.-Q.-Y.; Liu, Y.; Chen, X. Low Expression of Selenoprotein S Modulates Osteogenic Differentiation Through Bidirectional Regulation of the SP7–HSP47/COL1A1/SPARC Axis. Curr. Issues Mol. Biol. 2025, 47, 677. https://doi.org/10.3390/cimb47090677
Wu H, Zhao Y-S, Li C-S, Shi J-Y, Li Y, Zhong L-Q-Y, Liu Y, Chen X. Low Expression of Selenoprotein S Modulates Osteogenic Differentiation Through Bidirectional Regulation of the SP7–HSP47/COL1A1/SPARC Axis. Current Issues in Molecular Biology. 2025; 47(9):677. https://doi.org/10.3390/cimb47090677
Chicago/Turabian StyleWu, Hao, Yun-Shan Zhao, Chun-Shen Li, Jing-Yi Shi, Yi Li, Liang-Qiu-Yue Zhong, Yan Liu, and Xi Chen. 2025. "Low Expression of Selenoprotein S Modulates Osteogenic Differentiation Through Bidirectional Regulation of the SP7–HSP47/COL1A1/SPARC Axis" Current Issues in Molecular Biology 47, no. 9: 677. https://doi.org/10.3390/cimb47090677
APA StyleWu, H., Zhao, Y.-S., Li, C.-S., Shi, J.-Y., Li, Y., Zhong, L.-Q.-Y., Liu, Y., & Chen, X. (2025). Low Expression of Selenoprotein S Modulates Osteogenic Differentiation Through Bidirectional Regulation of the SP7–HSP47/COL1A1/SPARC Axis. Current Issues in Molecular Biology, 47(9), 677. https://doi.org/10.3390/cimb47090677






