Extracellular Vesicles in Osteogenesis: A Comprehensive Review of Mechanisms and Therapeutic Potential for Bone Regeneration
Abstract
1. Introduction
2. Biology of EVs
2.1. Classification of EVs
2.2. Classification by Functional Roles
2.3. EV Size Categories
2.4. Biogenesis and Composition of EVs
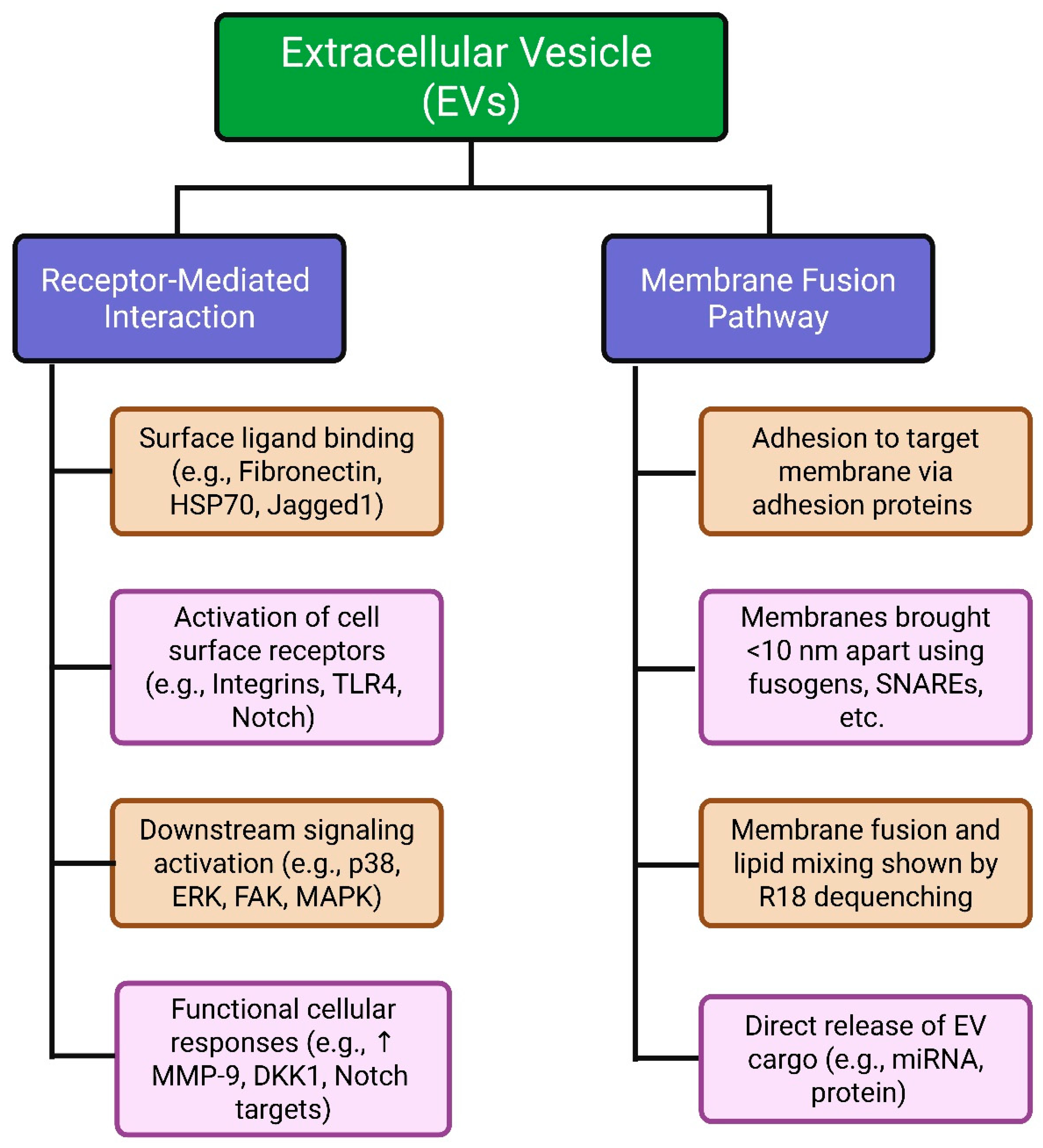
2.5. Mechanisms of EV Uptake and Cell Interaction
2.6. Role of Extracellular Vesicles in Articular Cartilage Homeostasis and Repair
3. Role of EVs in Osteogenesis
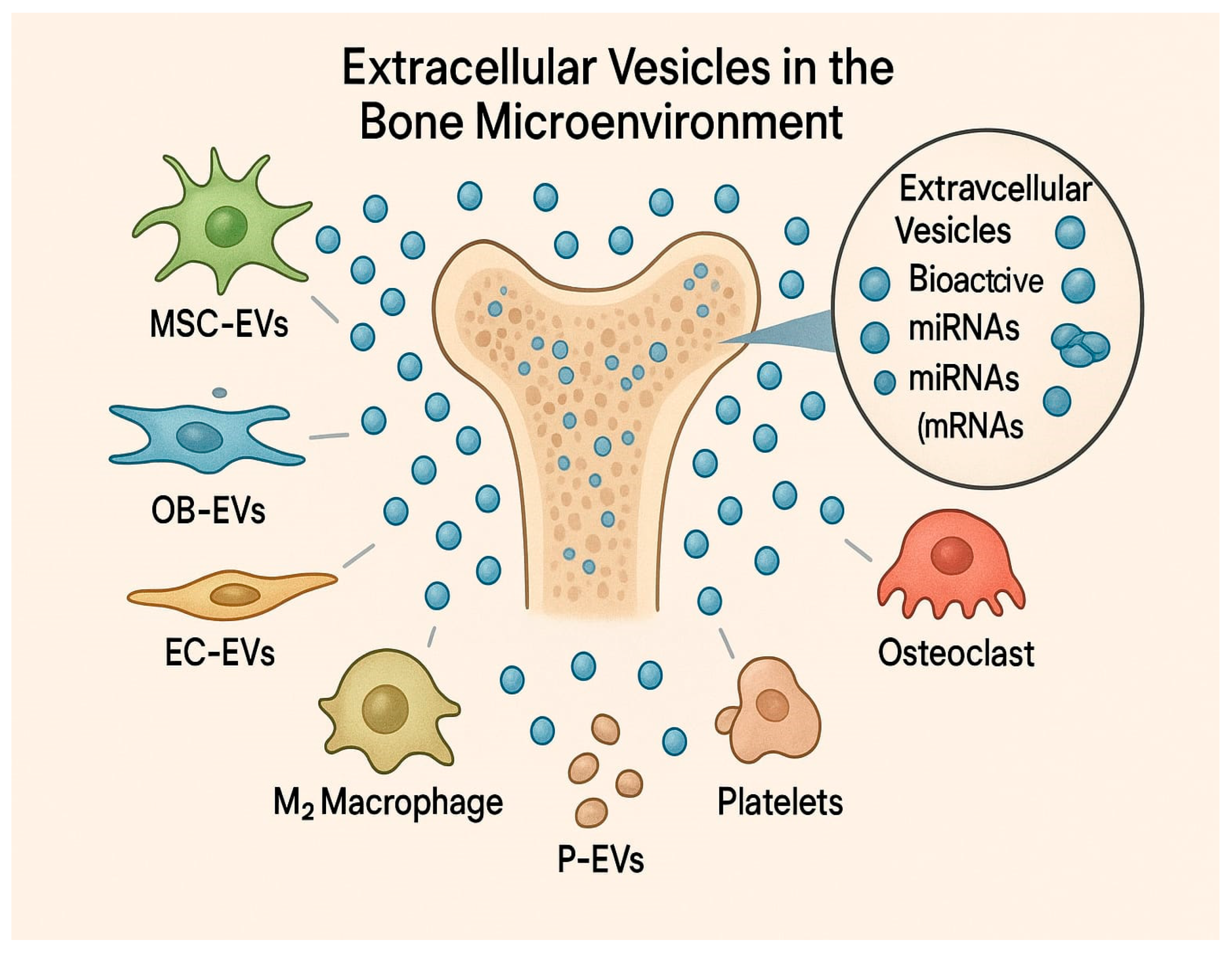
3.1. EVs Derived from Different Cell Types and Their Effects on Bone Formation
3.2. Molecular Mechanisms Mediated by EVs in Bone Regeneration
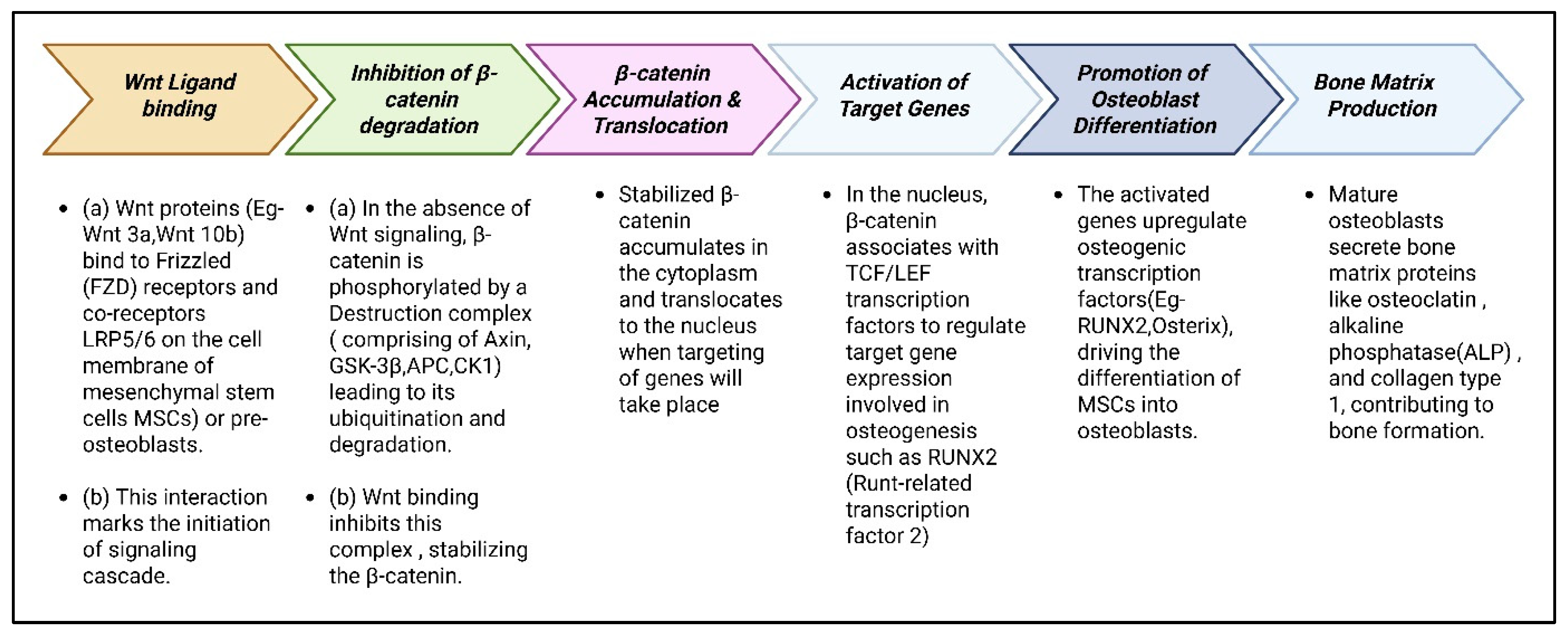
3.2.1. Wnt/β-Catenin Pathway: Activating Osteogenesis
Overview of Wnt/β-Catenin Signaling
Wnt/β-Catenin Pathway in Osteogenesis
Transcription Factors in Wnt/β-Catenin-Mediated Osteogenesis
3.2.2. BMP/TGF-β Pathway
Induction of Bone Formation
Ligand Synthesis and Activation
Ligand–Receptor Binding and Receptor Complex Formation
SMAD Protein Activation
Nuclear Translocation and Gene Transcription
MSC Differentiation into Osteoblasts
Osteoblast Function and Bone Matrix Formation
Osteoblast Fate and Bone Maturation
Negative Regulation and Feedback Loops
3.2.3. PI3K/Akt and MAPK Signaling: Enhancing Cell Survival and Proliferation
The Phosphoinositide 3-Kinase (PI3K/Akt) Signaling Pathway
MAPK Signaling Pathway
Three-Tiered Kinase Cascade
Nuclear Translocation and Transcriptional Regulation
Gene Expression and Proliferation Outcomes
3.3. Endothelial Cell-Derived EVs: Angiogenesis and Osteogenesis Coupling
3.4. Macrophage-Derived EVs: Modulating Inflammatory Responses for Bone Healing
4. Therapeutic Applications of EVs in Bone Regeneration
4.1. EVs for Fracture Healing
4.1.1. Facilitating Bone Regeneration
4.1.2. Regulation of the Immune Response
4.1.3. Enhancement of Vascularization (Angiogenesis)
4.1.4. Comparative Effects of EVs, Demineralized Bone Matrix, and Decellularized Bone Matrix on Bone Regeneration
4.1.5. Advantages over Conventional Therapies
4.1.6. Mechanisms of Action
- ➢
- Cargo delivery: EVs deliver proteins, lipids, mRNAs, and miRNAs that modulate recipient cell behavior, promoting osteogenic differentiation, angiogenesis, and immunomodulation.
- ➢
- Signaling pathway modulation: EVs can activate or suppress key signaling pathways in target cells involved in bone repair, such as Wnt/β-catenin, TGF-β/SMAD, and PI3K/Akt.
- ➢
- Extracellular matrix interaction: Certain EV components interact directly with the extracellular matrix, modifying its structure and facilitating mineralization.
4.1.7. Sources of EVs for Fracture Healing
- ➢
- Bone marrow mesenchymal stem cells (BMSCs): BMSC-derived EVs are extensively studied for their osteogenic and immunomodulatory properties.
- ➢
- Adipose-derived stem cells (ASCs): ASC-derived EVs can enhance the osteogenic potential of BMSCs and support bone regeneration.
- ➢
- Umbilical cord mesenchymal stem cells (uMSCs): EVs from uMSCs exhibit pro-angiogenic and fracture healing activities.
- ➢
- ➢
- Exosomes derived from endothelial progenitor cells (EPCs) accelerate bone repair by promoting differentiation and recruitment of osteoclast precursors via LncRNA-MALAT1.
4.1.8. Delivery Strategies
- ➢
- Local injection: Direct injection of EVs into the fracture site enables targeted delivery and higher local concentrations.
- ➢
- Scaffold integration: Incorporating EVs into biomaterial scaffolds provides sustained release and augments bone regeneration at the defect site.
- ➢
- Systemic administration: Although less targeted, systemic delivery may be beneficial in cases involving systemic inflammation.
4.2. EVs in Osteoporosis Therapy
4.2.1. Promoting Bone Formation (Osteogenesis)
- ○
- EVs derived from osteoblasts, endothelial cells, myocytes, and especially mesenchymal stem cells (MSCs) deliver proteins (including BMPs, OPG, CLEC11A, CTHRC1), microRNAs (miRNAs), and other factors that stimulate osteoblast proliferation, differentiation, and matrix mineralization. For example, MSC-derived EVs can upregulate β-catenin, a key signaling molecule in osteogenesis.
- ○
- EVs from multiple sources, such as osteoblasts, osteoclasts, and MSCs, regulate the balance between bone formation and resorption, thereby affecting the development and progression of osteoporosis. Additionally, EVs can serve as drug carriers to enhance drug targeting and bioavailability in bone tissue, offering a promising strategy for osteoporosis diagnosis and therapy.
- ○
- Specific miRNAs transported by EVs, such as miR-186 and miR-150-3p, have been shown to promote osteogenic differentiation. Conversely, miRNAs like miR-206 and miR-31 suppress osteogenesis, highlighting the context-dependent effects of EVs. Similarly, certain long non-coding RNAs (lncRNAs), including lncRNA-MALAT1, may inhibit osteogenic differentiation.
- ○
- Engineered EVs loaded with osteogenic factors or miRNAs can further augment bone formation.
4.2.2. Inhibiting Bone Resorption (Osteoclastogenesis)
- ○
- EVs can inhibit both the activity and formation of osteoclasts, which are responsible for bone resorption. For example, EVs derived from adipose-derived stem cells (ADSCs) containing miR-21-5p suppress osteoclast differentiation.
- ○
- EVs from mesenchymal stem cells (MSCs) can disrupt the RANKL/RANK pathway—a key axis in osteoclastogenesis—thereby reducing bone resorption. Some EVs also deliver osteoprotegerin (OPG), a decoy receptor for RANKL that blocks osteoclast activation [107].
- ○
- EVs loaded with specific osteoclastogenesis inhibitors, such as antagomiR-31a-5p, have been shown to reduce bone loss.
4.2.3. Modulating the Bone Microenvironment and Inflammation
- ○
- Osteoporosis is typically associated with a pro-inflammatory milieu that promotes bone resorption. EVs, particularly those derived from MSCs, possess immunomodulatory properties. They can modulate the balance between pro- and anti-inflammatory signaling, indirectly supporting bone health.
- ○
- MSC-derived EVs also induce angiogenesis—the formation of new blood vessels—which is essential for nutrient and oxygen supply to bone tissue and supports bone regeneration.
4.2.4. Sources of EVs for the Treatment of Osteoporosis
4.2.5. Cell Sources for Therapeutic EVs in Osteoporosis
- ✓
- Mesenchymal stem cells (MSCs): EVs from bone marrow (BMSCs), adipose tissue (ADSCs), and umbilical cord (UCMSCs). MSCs are the most extensively studied due to their multipotent differentiation capacity and paracrine signaling, which enhance bone regeneration and suppress bone loss.
- ✓
- Osteoblasts: Osteoblast-derived EVs may directly deliver factors that promote bone matrix deposition and regulate osteoclast activity.
- ✓
- Endothelial cells: EVs from endothelial cells can stimulate angiogenesis, crucial for maintaining bone integrity.
- ✓
- Immune cells (e.g., macrophages): The effects of immune cell–derived EVs in osteoporosis are complex and context-dependent; EVs from M2-polarized macrophages are generally associated with pro-regenerative activities.
- ✓
- Urine-derived stem cells (USCs): EVs from USCs have demonstrated the capacity to promote bone formation and inhibit osteoclasts [96].
4.2.6. Advantages of EV Therapy for Osteoporosis
- ➢
- Cell-free approach: Lower risk of complications related to cell transplantation, such as immune rejection and ectopic tissue formation.
- ➢
- Improved safety profile: Typically less immunogenic and safer than cell-based therapies.
- ➢
- Potential for targeted delivery: EVs can be engineered to target specific cell types or tissues within the bone microenvironment.
- ➢
- Stability and storage: EVs exhibit greater stability and are easier to store than live cells.
- ➢
- Customizable cargo: The therapeutic properties of EVs can be optimized by preconditioning donor cells or directly loading EVs with drugs or genetic material.
4.3. EV-Based Approaches in Bone Tissue Engineering
4.4. Comparison of EV Therapy with Traditional Bone Grafting
5. Engineering and Enhancement of EVs for Osteogenic Therapy
5.1. Genetic and Chemical Modifications to Enhance EV Cargo
5.2. Loading of Osteoinductive Molecules into EVs
5.3. Surface Modification for Targeted EV Delivery
5.4. Integration of EVs with 3D Bioprinting and Scaffold Technologies
6. Challenges and Future Perspectives
6.1. Standardization and Large-Scale Production of EVs
6.1.1. Sample Collection, Matching, Sample Size, and Data Collection
6.1.2. Sample Handling and Processing
6.1.3. Sample Stability and Storage
Upstream Manufacturing Process of EVs
Downstream Manufacturing Process of EVs
6.2. Dosing, Stability, and Storage Concerns
6.3. Safety and Immunogenicity of EV-Based Therapies
6.3.1. Immune Responses to EVs
6.3.2. Immune Responses to Impurities Present in EVs
6.3.3. EV-Induced Immune Responses
6.4. Future Directions in EV-Based Bone Regeneration
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, L.; Ke, W.; Liao, Z.; Feng, X.; Lei, J.; Wang, K.; Wang, B.; Li, G.; Luo, R.; Shi, Y.; et al. Small Extracellular Vesicles with Nanomorphology Memory Promote Osteogenesis. Bioact. Mater. 2022, 17, 425–438. [Google Scholar] [CrossRef]
- Ravi Mythili, V.M.; Rajendran, R.L.; Arun, R.; Thasma Loganathbabu, V.K.; Reyaz, D.; Nagarajan, A.K.; Ahn, B.-C.; Gangadaran, P. Emerging Strategies for Revascularization: Use of Cell-Derived Extracellular Vesicles and Artificial Nanovesicles in Critical Limb Ischemia. Bioengineering 2025, 12, 92. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Lin, X.; Cao, J.; Liu, Y.-W.; Luo, Z.-W.; Rao, S.-S.; Wang, Q.; Wang, Y.-Y.; Chen, C.-Y.; Zhu, G.-Q.; et al. Young Osteocyte-Derived Extracellular Vesicles Facilitate Osteogenesis by Transferring Tropomyosin-1. J. Nanobiotechnol. 2024, 22, 208. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhao, I.S.; Pan, H.; Yang, J.; Wang, H.; Deng, Y.; Zhang, Y. Extracellular Vesicle-Functionalized Bioactive Scaffolds for Bone Regeneration. Asian J. Pharm. Sci. 2024, 19, 100945. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Dey, A.; Ghosh, S.; Bhuniya, T.; Koley, M.; Bera, A.; Guha, S.; Chakraborty, K.; Muthu, S.; Gorai, S.; Vorn, R.; et al. Clinical Theragnostic Signature of Extracellular Vesicles in Traumatic Brain Injury (TBI). ACS Chem. Neurosci. 2023, 14, 2981–2994. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Sogawa, C.; Ono, K.; Matsumoto, M.; Tran, M.T.; Okusha, Y.; Lang, B.J.; Okamoto, K.; Calderwood, S.K. Cell Stress Induced Stressome Release Including Damaged Membrane Vesicles and Extracellular HSP90 by Prostate Cancer Cells. Cells 2020, 9, 755. [Google Scholar] [CrossRef]
- Okusha, Y.; Eguchi, T.; Tran, M.T.; Sogawa, C.; Yoshida, K.; Itagaki, M.; Taha, E.A.; Ono, K.; Aoyama, E.; Okamura, H.; et al. Extracellular Vesicles Enriched with Moonlighting Metalloproteinase Are Highly Transmissive, Pro-Tumorigenic, and Trans-Activates Cellular Communication Network Factor (CCN2/CTGF): CRISPR against Cancer. Cancers 2020, 12, 881. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Spinelli, C.; Reis-Sobreiro, M.; Cavallini, L.; You, S.; Zandian, M.; Li, X.; Mishra, R.; Chiarugi, P.; Adam, R.M.; et al. MYC Mediates Large Oncosome-Induced Fibroblast Reprogramming in Prostate Cancer. Cancer Res. 2017, 77, 2306–2317, Erratum in Cancer Res. 2017, 77, 3961. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zucker, B.; Zhang, S.; Elias, S.; Zhu, Y.; Chen, H.; Ding, T.; Li, Y.; Sun, Y.; Lou, J.; et al. Migrasome Formation Is Mediated by Assembly of Micron-Scale Tetraspanin Macrodomains. Nat. Cell Biol. 2019, 21, 991–1002, Erratum in Nat. Cell Biol. 2019, 21, 1301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Higginbotham, J.N.; Jeppesen, D.K.; Yang, Y.-P.; Li, W.; McKinley, E.T.; Graves-Deal, R.; Ping, J.; Britain, C.M.; Dorsett, K.A.; et al. Transfer of Functional Cargo in Exomeres. Cell Rep. 2019, 27, 940–954.e6. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, F.; Chiricolo, M.; Mariani, E.; Facchini, A. Biosynthesis of the Cancer-Related Sialyl-Alpha 2,6-Lactosaminyl Epitope in Colon Cancer Cell Lines Expressing Beta-Galactoside Alpha 2,6-Sialyltransferase under a Constitutive Promoter. Eur. J. Biochem. 2001, 268, 5876–5884. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Gangadaran, P.; Khan, F.; Rajendran, R.L.; Onkar, A.; Goenka, A.; Ahn, B.-C. Unveiling Invisible Extracellular Vesicles: Cutting-Edge Technologies for Their in Vivo Visualization. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e2009. [Google Scholar] [CrossRef]
- Onkar, A.; Khan, F.; Goenka, A.; Rajendran, R.L.; Dmello, C.; Hong, C.M.; Mubin, N.; Gangadaran, P.; Ahn, B.-C. Smart Nanoscale Extracellular Vesicles in the Brain: Unveiling Their Biology, Diagnostic Potential, and Therapeutic Applications. ACS Appl. Mater. Interfaces 2024, 16, 6709–6742. [Google Scholar] [CrossRef]
- Gopal, A.; Gangadaran, P.; Rajendran, R.L.; Oh, J.M.; Lee, H.W.; Hong, C.M.; Kalimuthu, S.; Han, M.-H.; Lee, J.; Ahn, B.-C. Extracellular Vesicle Mimetics Engineered from Mesenchymal Stem Cells and Curcumin Promote Fibrosis Regression in a Mouse Model of Thioacetamide-Induced Liver Fibrosis. Regen. Ther. 2024, 26, 911–921. [Google Scholar] [CrossRef]
- Lee, M.-J.; Park, D.-H.; Kang, J.-H. Exosomes as the Source of Biomarkers of Metabolic Diseases. Ann. Pediatr. Endocrinol. Metab. 2016, 21, 119–125. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Tan, S.S.; Yin, Y.; Lee, T.; Lai, R.C.; Yeo, R.W.Y.; Zhang, B.; Choo, A.; Lim, S.K. Therapeutic MSC Exosomes Are Derived from Lipid Raft Microdomains in the Plasma Membrane. J. Extracell. Vesicles 2013, 2, 22614. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of Extracellular Vesicles (EV): Exosomes, Microvesicles, Retrovirus-like Vesicles, and Apoptotic Bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mears, R.; Craven, R.A.; Hanrahan, S.; Totty, N.; Upton, C.; Young, S.L.; Patel, P.; Selby, P.J.; Banks, R.E. Proteomic Analysis of Melanoma-Derived Exosomes by Two-Dimensional Polyacrylamide Gel Electrophoresis and Mass Spectrometry. Proteomics 2004, 4, 4019–4031. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.B.; Audhya, A. ESCRT-Dependent Cargo Sorting at Multivesicular Endosomes. Semin. Cell Dev. Biol. 2018, 74, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gao, H.; Lv, P.; Liu, J.; Liu, G. Extracellular Vesicles as an Efficient Nanoplatform for the Delivery of Therapeutics. Hum. Vaccines Immunother. 2017, 13, 2678–2687. [Google Scholar] [CrossRef]
- Campanella, C.; Bucchieri, F.; Merendino, A.M.; Fucarino, A.; Burgio, G.; Corona, D.F.V.; Barbieri, G.; David, S.; Farina, F.; Zummo, G.; et al. The Odyssey of Hsp60 from Tumor Cells to Other Destinations Includes Plasma Membrane-Associated Stages and Golgi and Exosomal Protein-Trafficking Modalities. PLoS ONE 2012, 7, e42008. [Google Scholar] [CrossRef]
- Kranendonk, M.E.G.; de Kleijn, D.P.V.; Kalkhoven, E.; Kanhai, D.A.; Uiterwaal, C.S.P.M.; van der Graaf, Y.; Pasterkamp, G.; Visseren, F.L.J.; SMART Study Group. Extracellular Vesicle Markers in Relation to Obesity and Metabolic Complications in Patients with Manifest Cardiovascular Disease. Cardiovasc. Diabetol. 2014, 13, 37. [Google Scholar] [CrossRef]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular Endosome Biogenesis in the Absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef]
- Savina, A.; Vidal, M.; Colombo, M.I. The Exosome Pathway in K562 Cells Is Regulated by Rab11. J. Cell Sci. 2002, 115, 2505–2515. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of Ultracentrifugation, Density Gradient Separation, and Immunoaffinity Capture Methods for Isolating Human Colon Cancer Cell Line LIM1863-Derived Exosomes. Methods 2012, 56, 293–304. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-‘t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of Sample Collection, Isolation and Analysis Methods in Extracellular Vesicle Research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef] [PubMed]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Mathivanan, S.; Ji, H.; Simpson, R.J. Two Distinct Populations of Exosomes Are Released from LIM1863 Colon Carcinoma Cell-Derived Organoids. Mol. Cell. Proteom. MCP 2013, 12, 587–598. [Google Scholar] [CrossRef]
- Palmisano, G.; Jensen, S.S.; Le Bihan, M.-C.; Lainé, J.; McGuire, J.N.; Pociot, F.; Larsen, M.R. Characterization of Membrane-Shed Microvesicles from Cytokine-Stimulated β-Cells Using Proteomics Strategies. Mol. Cell. Proteom. MCP 2012, 11, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Ignatchenko, V.; Ignatchenko, A.; Mejia-Guerrero, S.; Kislinger, T. In-Depth Proteomic Analyses of Ovarian Cancer Cell Line Exosomes Reveals Differential Enrichment of Functional Categories Compared to the NCI 60 Proteome. Biochem. Biophys. Res. Commun. 2014, 445, 694–701. [Google Scholar] [CrossRef]
- Buschow, S.I.; van Balkom, B.W.M.; Aalberts, M.; Heck, A.J.R.; Wauben, M.; Stoorvogel, W. MHC Class II-Associated Proteins in B-Cell Exosomes and Potential Functional Implications for Exosome Biogenesis. Immunol. Cell Biol. 2010, 88, 851–856. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of mRNAs and microRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Stolz, D.B.; Sullivan, M.L.G.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z.; et al. Mechanism of Transfer of Functional microRNAs between Mouse Dendritic Cells via Exosomes. Blood 2012, 119, 756–766. [Google Scholar] [CrossRef]
- Rajendran, R.L.; Ahn, B.-C.; Gangadaran, P. Potential of Mesenchymal Stem Cell-Derived Exosomes in the Treatment of Androgenetic Alopecia. World J. Stem Cells 2025, 17, 107078. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, A.; Bandari, S.K.; Liu, J.; Mobley, J.A.; Brown, E.E.; Sanderson, R.D. Fibronectin on the Surface of Myeloma Cell-Derived Exosomes Mediates Exosome-Cell Interactions. J. Biol. Chem. 2016, 291, 1652–1663. [Google Scholar] [CrossRef]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma Exosomes Protect the Myocardium from Ischemia-Reperfusion Injury. J. Am. Coll. Cardiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef]
- Gonzalez-King, H.; García, N.A.; Ontoria-Oviedo, I.; Ciria, M.; Montero, J.A.; Sepúlveda, P. Hypoxia Inducible Factor-1α Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells 2017, 35, 1747–1759. [Google Scholar] [CrossRef]
- Prada, I.; Meldolesi, J. Binding and Fusion of Extracellular Vesicles to the Plasma Membrane of Their Cell Targets. Int. J. Mol. Sci. 2016, 17, 1296. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, J.M.; Chernomordik, L.V. Flagging Fusion: Phosphatidylserine Signaling in Cell-Cell Fusion. J. Biol. Chem. 2021, 296, 100411. [Google Scholar] [CrossRef] [PubMed]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH Is a Key Factor for Exosome Traffic in Tumor Cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef]
- Han, K.-Y.; Tran, J.A.; Chang, J.-H.; Azar, D.T.; Zieske, J.D. Potential Role of Corneal Epithelial Cell-Derived Exosomes in Corneal Wound Healing and Neovascularization. Sci. Rep. 2017, 7, 40548. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Y.; Wei, R.; Zhang, X.; Wang, C.; Feng, M. Proinflammatory Macrophage-Derived Microvesicles Exhibit Tumor Tropism Dependent on CCL2/CCR2 Signaling Axis and Promote Drug Delivery via SNARE-Mediated Membrane Fusion. Theranostics 2020, 10, 6581–6598. [Google Scholar] [CrossRef]
- Ganesh, B.H.; Padinjarathil, H.; Rajendran, R.L.; Ramani, P.; Gangadaran, P.; Ahn, B.-C. The Role of Extracellular Vesicles in Aging and Age-Related Disorders. Antioxidants 2025, 14, 177. [Google Scholar] [CrossRef]
- Jin, P.; Liu, H.; Chen, X.; Liu, W.; Jiang, T. From Bench to Bedside: The Role of Extracellular Vesicles in Cartilage Injury Treatment. Biomater. Res. 2024, 28, 0110. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Jiang, S.; Yuan, C.; Lin, K. The Potential Therapeutic Role of Extracellular Vesicles in Osteoarthritis. Front. Bioeng. Biotechnol. 2022, 10, 1022368. [Google Scholar] [CrossRef]
- Yokoi, A.; Ochiya, T. Exosomes and Extracellular Vesicles: Rethinking the Essential Values in Cancer Biology. Semin. Cancer Biol. 2021, 74, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Weng, L.; Sun, J.; Jin, Y.; Xiao, C. Activation of Cancer Immunotherapy by Nanomedicine. Front. Pharmacol. 2022, 13, 1041073. [Google Scholar] [CrossRef]
- Murali, V.P.; Holmes, C.A. Mesenchymal Stromal Cell-Derived Extracellular Vesicles for Bone Regeneration Therapy. Bone Rep. 2021, 14, 101093. [Google Scholar] [CrossRef]
- Cheng, C.; Tang, S.; Cui, S.; Yang, T.; Li, L.; Zhai, M.; Wei, F.; Ding, G. Nerve Growth Factor Promote Osteogenic Differentiation of Dental Pulp Stem Cells through MEK/ERK Signalling Pathways. J. Cell. Mol. Med. 2024, 28, e18143. [Google Scholar] [CrossRef]
- Ansari, S.; de Wildt, B.W.M.; Vis, M.A.M.; de Korte, C.E.; Ito, K.; Hofmann, S.; Yuana, Y. Matrix Vesicles: Role in Bone Mineralization and Potential Use as Therapeutics. Pharmaceuticals 2021, 14, 289. [Google Scholar] [CrossRef]
- Yuan, F.-L.; Wu, Q.-Y.; Miao, Z.-N.; Xu, M.-H.; Xu, R.-S.; Jiang, D.-L.; Ye, J.-X.; Chen, F.-H.; Zhao, M.-D.; Wang, H.-J.; et al. Osteoclast-Derived Extracellular Vesicles: Novel Regulators of Osteoclastogenesis and Osteoclast-Osteoblasts Communication in Bone Remodeling. Front. Physiol. 2018, 9, 628. [Google Scholar] [CrossRef]
- Ateeq, M.; Broadwin, M.; Sellke, F.W.; Abid, M.R. Extracellular Vesicles’ Role in Angiogenesis and Altering Angiogenic Signaling. Med. Sci. 2024, 12, 4. [Google Scholar] [CrossRef]
- Liu, G.-S.; Chen, H.-A.; Chang, C.-Y.; Chen, Y.-J.; Wu, Y.-Y.; Widhibrata, A.; Yang, Y.-H.; Hsieh, E.-H.; Delila, L.; Lin, I.-C.; et al. Platelet-Derived Extracellular Vesicle Drug Delivery System Loaded with Kaempferol for Treating Corneal Neovascularization. Biomaterials 2025, 319, 123205. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone Marrow Stromal/Stem Cell-Derived Extracellular Vesicles Regulate Osteoblast Activity and Differentiation in Vitro and Promote Bone Regeneration in Vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/Tricalcium Phosphate Combination Scaffolds Can Enhance Bone Regeneration by Activating the PI3K/Akt Signaling Pathway. Stem Cell Res. Ther. 2016, 7, 136. [Google Scholar] [CrossRef]
- Yang, F.; Shi, K.; Jia, Y.-P.; Hao, Y.; Peng, J.-R.; Qian, Z.-Y. Advanced Biomaterials for Cancer Immunotherapy. Acta Pharmacol. Sin. 2020, 41, 911–927, Correction in Acta Pharmacol. Sin. 2021, 42, 844. [Google Scholar] [CrossRef]
- Zou, M.-L.; Chen, Z.-H.; Teng, Y.-Y.; Liu, S.-Y.; Jia, Y.; Zhang, K.-W.; Sun, Z.-L.; Wu, J.-J.; Yuan, Z.-D.; Feng, Y.; et al. The Smad Dependent TGF-β and BMP Signaling Pathway in Bone Remodeling and Therapies. Front. Mol. Biosci. 2021, 8, 593310. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Li, P.; Yuan, K.; Zhao, F.; Zhu, X.; Zhang, P.; Huang, Z. Extracellular Vesicles Derived from Human Dental Pulp Stem Cells Promote Osteogenesis of Adipose-Derived Stem Cells via the MAPK Pathway. J. Tissue Eng. 2020, 11, 2041731420975569. [Google Scholar] [CrossRef]
- Vermeulen, S.; Tahmasebi Birgani, Z.; Habibovic, P. Biomaterial-Induced Pathway Modulation for Bone Regeneration. Biomaterials 2022, 283, 121431. [Google Scholar] [CrossRef]
- Hashemi, A.; Ezati, M.; Nasr, M.P.; Zumberg, I.; Provaznik, V. Extracellular Vesicles and Hydrogels: An Innovative Approach to Tissue Regeneration. ACS Omega 2024, 9, 6184–6218. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Z.; Pan, D.; Xin, Z.; Bu, F.; Zhang, Y.; Tian, Q.; Feng, X. Circulating lncRNA UCA1 and lncRNA PGM5-AS1 Act as Potential Diagnostic Biomarkers for Early-Stage Colorectal Cancer. Biosci. Rep. 2021, 41, BSR20211115. [Google Scholar] [CrossRef]
- Morhayim, J.; van de Peppel, J.; Braakman, E.; Rombouts, E.W.J.C.; Ter Borg, M.N.D.; Dudakovic, A.; Chiba, H.; van der Eerden, B.C.J.; Raaijmakers, M.H.; van Wijnen, A.J.; et al. Osteoblasts Secrete miRNA-Containing Extracellular Vesicles That Enhance Expansion of Human Umbilical Cord Blood Cells. Sci. Rep. 2016, 6, 32034. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xu, M.; Lu, F.; He, Y. Development of Matrix Metalloproteinases-Mediated Extracellular Matrix Remodeling in Regenerative Medicine: A Mini Review. Tissue Eng. Regen. Med. 2023, 20, 661–670. [Google Scholar] [CrossRef]
- Gillessen, S.; Attard, G.; Beer, T.M.; Beltran, H.; Bjartell, A.; Bossi, A.; Briganti, A.; Bristow, R.G.; Chi, K.N.; Clarke, N.; et al. Management of Patients with Advanced Prostate Cancer: Report of the Advanced Prostate Cancer Consensus Conference 2019. Eur. Urol. 2020, 77, 508–547. [Google Scholar] [CrossRef]
- Nusse, R.; Varmus, H.E. Many Tumors Induced by the Mouse Mammary Tumor Virus Contain a Provirus Integrated in the Same Region of the Host Genome. Cell 1982, 31, 99–109. [Google Scholar] [CrossRef]
- Niehrs, C. The Complex World of WNT Receptor Signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/Beta-Catenin Signaling in Development and Disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, J.; Wang, F.; Fang, Y.; Yang, Y.; Zhou, Q.; Yuan, W.; Gu, X.; Hu, J.; Yang, S. Pre-Metastatic Niche: Formation, Characteristics and Therapeutic Implication. Signal Transduct. Target. Ther. 2024, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Xu, P.; Lin, X.; Feng, X.-H. Posttranslational Regulation of Smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022087. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef]
- Šromová, V.; Sobola, D.; Kaspar, P. A Brief Review of Bone Cell Function and Importance. Cells 2023, 12, 2576. [Google Scholar] [CrossRef]
- Dong, J.; Xu, X.; Zhang, Q.; Yuan, Z.; Tan, B. The PI3K/AKT Pathway Promotes Fracture Healing through Its Crosstalk with Wnt/β-Catenin. Exp. Cell Res. 2020, 394, 112137. [Google Scholar] [CrossRef]
- Hassan, D.; Menges, C.W.; Testa, J.R.; Bellacosa, A. AKT Kinases as Therapeutic Targets. J. Exp. Clin. Cancer Res. 2024, 43, 313. [Google Scholar] [CrossRef] [PubMed]
- Simanshu, D.K.; Nissley, D.V.; McCormick, F. RAS Proteins and Their Regulators in Human Disease. Cell 2017, 170, 17–33. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. MMBR 2011, 75, 50–83, Correction in Mol. Biol. Rev. MMBR 2012, 76, 496. [Google Scholar] [CrossRef] [PubMed]
- Wortzel, I.; Seger, R. The ERK Cascade: Distinct Functions within Various Subcellular Organelles. Genes Cancer 2011, 2, 195–209. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK Pathway for Cancer Therapy: From Mechanism to Clinical Studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone Physiological Microenvironment and Healing Mechanism: Basis for Future Bone-Tissue Engineering Scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef]
- Lange, M.; Babczyk, P.; Tobiasch, E. Exosomes: A New Hope for Angiogenesis-Mediated Bone Regeneration. Int. J. Mol. Sci. 2024, 25, 5204. [Google Scholar] [CrossRef]
- Zhai, Y.; Schilling, K.; Wang, T.; El Khatib, M.; Vinogradov, S.; Brown, E.B.; Zhang, X. Spatiotemporal Blood Vessel Specification at the Osteogenesis and Angiogenesis Interface of Biomimetic Nanofiber-Enabled Bone Tissue Engineering. Biomaterials 2021, 276, 121041. [Google Scholar] [CrossRef]
- De Sousa, K.P.; Rossi, I.; Abdullahi, M.; Ramirez, M.I.; Stratton, D.; Inal, J.M. Isolation and Characterization of Extracellular Vesicles and Future Directions in Diagnosis and Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1835. [Google Scholar] [CrossRef]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 Macrophages and Their Overlaps—Myth or Reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The Secretion Profile of Mesenchymal Stem Cells and Potential Applications in Treating Human Diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Ou, X.; Wang, Q.; Zhang, L. Macrophage-Derived Extracellular Vesicles: A Novel Therapeutic Alternative for Diabetic Wound. Int. J. Nanomed. 2025, 20, 5763–5777. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, X.; Ren, X.; Lin, Y.; Zeng, F.; Wang, Q. Synthetic Evolution of Saccharomyces Cerevisiae for Biomanufacturing: Approaches and Applications. mLife 2025, 4, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Zhou, C.; Bai, M.; Wan, Y.; Zheng, Q.; Fan, Z.; Wang, X.; Yang, C. Engineered Extracellular Vesicles for Tissue Repair and Regeneration. Burn. Trauma 2024, 12, tkae062. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Sun, X.; Dou, Y.; Wang, M.; Zhao, Y.; Yang, Q.; Zhao, Y. The Immuno-Modulation Effect of Macrophage-Derived Extracellular Vesicles in Chronic Inflammatory Diseases. Front. Immunol. 2021, 12, 785728. [Google Scholar] [CrossRef]
- Shi, H.; Yang, Y.; Xing, H.; Jia, J.; Xiong, W.; Guo, S.; Yang, S. Exosomal Non-Coding RNAs: Emerging Insights into Therapeutic Potential and Mechanisms in Bone Healing. J. Tissue Eng. 2024, 15, 20417314241286606. [Google Scholar] [CrossRef]
- Tacconi, S.; Vari, F.; Sbarigia, C.; Vardanyan, D.; Longo, S.; Mura, F.; Angilè, F.; Jalabert, A.; Blangero, F.; Eljaafari, A.; et al. M1-Derived Extracellular Vesicles Polarize Recipient Macrophages into M2-like Macrophages and Alter Skeletal Muscle Homeostasis in a Hyper-Glucose Environment. Cell Commun. Signal. CCS 2024, 22, 193. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, J.; Jiang, M.; Tan, W.; Zeng, Y.; Liu, X.; Wang, P.; Jiang, H.; Zhou, J.; Liu, X.; et al. 3D-Printed Multifunctional Bilayer Scaffold with Sustained Release of Apoptotic Extracellular Vesicles and Antibacterial Coacervates for Enhanced Wound Healing. Biomaterials 2025, 318, 123196. [Google Scholar] [CrossRef]
- Qiao, Z.; Greven, J.; Horst, K.; Pfeifer, R.; Kobbe, P.; Pape, H.-C.; Hildebrand, F. Fracture Healing and the Underexposed Role of Extracellular Vesicle-Based Cross Talk. Shock 2018, 49, 486–496. [Google Scholar] [CrossRef]
- Rozier, P.; Maumus, M.; Bony, C.; Maria, A.T.J.; Sabatier, F.; Jorgensen, C.; Guilpain, P.; Noël, D. Extracellular Vesicles Are More Potent Than Adipose Mesenchymal Stromal Cells to Exert an Anti-Fibrotic Effect in an In Vitro Model of Systemic Sclerosis. Int. J. Mol. Sci. 2021, 22, 6837. [Google Scholar] [CrossRef] [PubMed]
- Buzás, E.I.; Tóth, E.Á.; Sódar, B.W.; Szabó-Taylor, K.É. Molecular Interactions at the Surface of Extracellular Vesicles. Semin. Immunopathol. 2018, 40, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Wang, G.; Dai, L.; Wang, T.; Wang, F. Cellular and Molecular Connections Between Bone Fracture Healing and Exosomes. Physiol. Res. 2023, 72, 565–574. [Google Scholar] [CrossRef]
- Mao, B.; Zhang, Z.; Lai, S.; Zhang, K.; Li, J.; Fu, W. Demineralized Cortical Bone Matrix Augmented With Peripheral Blood-Derived Mesenchymal Stem Cells for Rabbit Medial Meniscal Reconstruction. Front. Bioeng. Biotechnol. 2022, 10, 855103. [Google Scholar] [CrossRef]
- Percival, K.M.; Paul, V.; Husseini, G.A. Recent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration. Int. J. Mol. Sci. 2024, 25, 6012. [Google Scholar] [CrossRef]
- Gangadaran, P.; Onkar, A.; Rajendran, R.L.; Goenka, A.; Oh, J.M.; Khan, F.; Nagarajan, A.K.; Muthu, S.; Krishnan, A.; Hong, C.M.; et al. Noninvasive in Vivo Imaging of Macrophages: Understanding Tumor Microenvironments and Delivery of Therapeutics. Biomark. Res. 2025, 13, 20. [Google Scholar] [CrossRef]
- Fang, F.; Yang, J.; Wang, J.; Li, T.; Wang, E.; Zhang, D.; Liu, X.; Zhou, C. The Role and Applications of Extracellular Vesicles in Osteoporosis. Bone Res. 2024, 12, 4. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Rajendran, R.L.; Chatterjee, G.; Reyaz, D.; Prakash, K.; Hong, C.M.; Ahn, B.-C.; ArulJothi, K.N.; Gangadaran, P. Mesenchymal Stem Cell-Derived Exosomes: A Paradigm Shift in Clinical Therapeutics. Exp. Cell Res. 2025, 450, 114616. [Google Scholar] [CrossRef]
- Muraca, M.; Cappariello, A. The Role of Extracellular Vesicles (EVs) in the Epigenetic Regulation of Bone Metabolism and Osteoporosis. Int. J. Mol. Sci. 2020, 21, 8682. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xie, H. Extracellular Vesicle-Integrated Biomaterials in Bone Tissue Engineering Applications: Current Progress and Future Perspectives. Int. J. Nanomed. 2025, 20, 7653–7683. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; He, W.; Huang, Q.; Zhang, R.; Feng, Q. Hydroxyapatite/Collagen Coating on PLGA Electrospun Fibers for Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. J. Biomed. Mater. Res. A 2018, 106, 2863–2870. [Google Scholar] [CrossRef]
- Jang, H.-J.; Yoon, J.-K. The Role of Vasculature and Angiogenic Strategies in Bone Regeneration. Biomimetics 2024, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone Grafts and Biomaterials Substitutes for Bone Defect Repair: A Review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Soufizadeh, P.; Nikbakht Brujeni, G.; Dehghan, M.M.; Jabbari Fakhr, M.; Houshmand, P.; Mohebbi, M.; Aminianfar, H.; Sadeghian Chaleshtori, S. Preventing the Rejection of Skin Allografts by Immunomodulatory and Regenerative Effects of Exosomes Derived from Bone Marrow Mesenchymal Stem Cells in Mice. Iran. J. Basic Med. Sci. 2025, 28, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Stöckl, S.; Li, S.; Herrmann, M.; Lukas, C.; Reinders, Y.; Sickmann, A.; Grässel, S. Effects of Extracellular Vesicles from Osteogenic Differentiated Human BMSCs on Osteogenic and Adipogenic Differentiation Capacity of Naïve Human BMSCs. Cells 2022, 11, 2491. [Google Scholar] [CrossRef]
- Huang, G.; Pan, S.-T. ROS-Mediated Therapeutic Strategy in Chemo-/Radiotherapy of Head and Neck Cancer. Oxid. Med. Cell. Longev. 2020, 2020, 5047987. [Google Scholar] [CrossRef]
- Cheng, G.; Karoui, H.; Hardy, M.; Kalyanaraman, B. Redox-Crippled MitoQ Potently Inhibits Breast Cancer and Glioma Cell Proliferation: A Negative Control for Verifying the Antioxidant Mechanism of MitoQ in Cancer and Other Oxidative Pathologies. Free Radic. Biol. Med. 2023, 205, 175–187. [Google Scholar] [CrossRef]
- Jin, P.; Jiang, J.; Zhou, L.; Huang, Z.; Nice, E.C.; Huang, C.; Fu, L. Mitochondrial Adaptation in Cancer Drug Resistance: Prevalence, Mechanisms, and Management. J. Hematol. Oncol. 2022, 15, 97. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Wang, X.; Sun, Y.; Zhang, J.; Chen, J.; Shi, Y. An Epigenetic Role of Mitochondria in Cancer. Cells 2022, 11, 2518. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, L.; Deng, B.; Zhao, K.; Chen, C.; Wang, W. Mitochondrial Uncoupling Protein 2: A Central Player in Pancreatic Disease Pathophysiology. Mol. Med. 2024, 30, 259. [Google Scholar] [CrossRef]
- Danilushkina, A.A.; Emene, C.C.; Barlev, N.A.; Gomzikova, M.O. Strategies for Engineering of Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 13247. [Google Scholar] [CrossRef]
- Coffman, A.A.; Basta-Pljakic, J.; Guerra, R.M.; Ebetino, F.H.; Lundy, M.W.; Majeska, R.J.; Schaffler, M.B. A Bisphosphonate With a Low Hydroxyapatite Binding Affinity Prevents Bone Loss in Mice After Ovariectomy and Reverses Rapidly With Treatment Cessation. JBMR Plus 2021, 5, e10476. [Google Scholar] [CrossRef] [PubMed]
- Jamee, R.; Araf, Y.; Naser, I.B.; Promon, S.K. The Promising Rise of Bioprinting in Revolutionalizing Medical Science: Advances and Possibilities. Regen. Ther. 2021, 18, 133–145. [Google Scholar] [CrossRef]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular Vesicles: A Rising Star for Therapeutics and Drug Delivery. J. Nanobiotechnol. 2023, 21, 231. [Google Scholar] [CrossRef]
- Uddin, M.J.; Mohite, P.; Munde, S.; Ade, N.; Oladosu, T.A.; Chidrawar, V.R.; Patel, R.; Bhattacharya, S.; Paliwal, H.; Singh, S. Extracellular Vesicles: The Future of Therapeutics and Drug Delivery Systems. Intell. Pharm. 2024, 2, 312–328. [Google Scholar] [CrossRef]
- Gandham, S.; Su, X.; Wood, J.; Nocera, A.L.; Alli, S.C.; Milane, L.; Zimmerman, A.; Amiji, M.; Ivanov, A.R. Technologies and Standardization in Research on Extracellular Vesicles. Trends Biotechnol. 2020, 38, 1066–1098. [Google Scholar] [CrossRef]
- Thakur, A.; Rai, D. Global Requirements for Manufacturing and Validation of Clinical Grade Extracellular Vesicles. J. Liq. Biopsy 2024, 6, 100278. [Google Scholar] [CrossRef]
- Syromiatnikova, V.; Prokopeva, A.; Gomzikova, M. Methods of the Large-Scale Production of Extracellular Vesicles. Int. J. Mol. Sci. 2022, 23, 10522. [Google Scholar] [CrossRef]
- Estes, S.; Konstantinov, K.; Young, J.D. Manufactured Extracellular Vesicles as Human Therapeutics: Challenges, Advances, and Opportunities. Curr. Opin. Biotechnol. 2022, 77, 102776. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Zickler, A.M.; El Andaloussi, S. Dosing Extracellular Vesicles. Adv. Drug Deliv. Rev. 2021, 178, 113961. [Google Scholar] [CrossRef]
- Yuan, F.; Li, Y.-M.; Wang, Z. Preserving Extracellular Vesicles for Biomedical Applications: Consideration of Storage Stability before and after Isolation. Drug Deliv. 2021, 28, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, S.; Jafari, N.; Tamadon, A.; Ghaffarzadeh, A.; Rahbarghazi, R.; Mahdipour, M. Different Storage and Freezing Protocols for Extracellular Vesicles: A Systematic Review. Stem Cell Res. Ther. 2024, 15, 453. [Google Scholar] [CrossRef]
- Görgens, A.; Corso, G.; Hagey, D.W.; Jawad Wiklander, R.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of Storage Conditions Stabilizing Extracellular Vesicles Preparations. J. Extracell. Vesicles 2022, 11, e12238. [Google Scholar] [CrossRef]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The Impact of Storage on Extracellular Vesicles: A Systematic Study. J. Extracell. Vesicles 2022, 11, e12162. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, J.; Liu, G.; Wolfram, J. Immunogenicity of Extracellular Vesicles. Adv. Mater. 2024, 36, e2403199. [Google Scholar] [CrossRef]
- Condrat, C.E.; Varlas, V.N.; Duică, F.; Antoniadis, P.; Danila, C.A.; Cretoiu, D.; Suciu, N.; Crețoiu, S.M.; Voinea, S.C. Pregnancy-Related Extracellular Vesicles Revisited. Int. J. Mol. Sci. 2021, 22, 3904. [Google Scholar] [CrossRef]
- Takakura, Y.; Hanayama, R.; Akiyoshi, K.; Futaki, S.; Hida, K.; Ichiki, T.; Ishii-Watabe, A.; Kuroda, M.; Maki, K.; Miura, Y.; et al. Quality and Safety Considerations for Therapeutic Products Based on Extracellular Vesicles. Pharm. Res. 2024, 41, 1573–1594. [Google Scholar] [CrossRef]
- Holkar, K.; Vaidya, A.; Pethe, P.; Kale, V.; Ingavle, G. Biomaterials and Extracellular Vesicles in Cell-Free Therapy for Bone Repair and Regeneration: Future Line of Treatment in Regenerative Medicine. Materialia 2020, 12, 100736. [Google Scholar] [CrossRef]
- Infante, A.; Alcorta-Sevillano, N.; Macías, I.; Rodríguez, C.I. Educating EVs to Improve Bone Regeneration: Getting Closer to the Clinic. Int. J. Mol. Sci. 2022, 23, 1865. [Google Scholar] [CrossRef]
- Gholami, L.; Nooshabadi, V.T.; Shahabi, S.; Jazayeri, M.; Tarzemany, R.; Afsartala, Z.; Khorsandi, K. Extracellular Vesicles in Bone and Periodontal Regeneration: Current and Potential Therapeutic Applications. Cell Biosci. 2021, 11, 16. [Google Scholar] [CrossRef]
- Youssef, E.; Palmer, D.; Fletcher, B.; Vaughn, R. Exosomes in Precision Oncology and Beyond: From Bench to Bedside in Diagnostics and Therapeutics. Cancers 2025, 17, 940. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ntege, E.H.; Inoue, Y.; Matsuura, N.; Sunami, H.; Sowa, Y. Optimizing Mesenchymal Stem Cell Extracellular Vesicles for Chronic Wound Healing: Bioengineering, Standardization, and Safety. Regen. Ther. 2024, 26, 260–274. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, S.; Li, W. Harnessing Stem Cell-Derived Extracellular Vesicles for the Regeneration of Degenerative Bone Conditions. Int. J. Nanomed. 2023, 18, 5561–5578. [Google Scholar] [CrossRef]
| Category | Mechanism | Details | Significance | References |
|---|---|---|---|---|
| Biogenesis markers | ESCRT (endosomal sorting complex required for transport) machinery ESCRT-independent pathway | Includes proteins such as TSG101, Alix, HSC70, and HSP90β Utilizes sphingomyelinase rather than ESCRT | These proteins shape and load exosomes, are present in exosomes from all cell types, and are termed “exosomal markers.” Exosome release persists despite ESCRT knockout, particularly for CD63-positive vesicles. | [29,31,32] |
| Membrane proteins | Tetraspanins (CD9, CD63, CD81) | Transmembrane proteins highly enriched in exosomes | Previously considered exosome-specific, now also identified in microvesicles and apoptotic bodies | [33,34] |
| Other surface proteins | Plasma membrane-associated proteins | Widely present in exosomes | Facilitate cargo selection, targeting, and intercellular communication | [35] |
| Protein content (internal) | Glycoproteins Post-translationally modified proteins | More abundant in exosomes compared to parent cells Includes phosphorylated and extensively glycosylated proteins | Glycosylation enhances cellular interaction and targeting More enriched in microvesicles than in exosomes, aiding vesicle classification | [35,36] |
| Unexpected proteins | Mitochondrial and nuclear proteins Golgi and ER proteins | Detected due to inter-organelle trafficking Present at low abundance, likely from contact with early endosomes | Challenges prior assumptions that these proteins are absent from exosomes Still classified as non-exosomal markers due to their low abundance relative to the total cell lysate | [29,37] |
| Cargo sorting proteins | CD63 (also a tetraspanin) Alix and TSG101 | Present in both ESCRT-dependent and ESCRT-independent exosomes Directly participate in cargo sorting during vesicle formation | Strong, though not exclusive, exosome marker Commonly used indicators of classical exosome biogenesis | [27,29,32,37] |
| Transcription Factor | Function | Mechanism |
|---|---|---|
| Co-activator | Associates with TCF/LEF in the nucleus to activate osteogenic genes; becomes stabilized following Wnt signaling |
| DNA-binding proteins | Forms complexes with β-catenin to promote transcription of genes driving osteoblast lineage specification |
| Master osteogenic regulator | Activated by Wnt/β-catenin signaling; induces osteoblast differentiation and bone matrix gene expression |
| Osteoblast-specific factor | Acts downstream of RUNX2; required for the maturation of pre-osteoblasts into functional osteoblasts |
| Transcriptional regulator | Upregulates RUNX2 expression and facilitates osteogenic differentiation |
| Regulator of early osteogenesis | Induced by Wnt signaling; stimulates osteoprogenitor cell proliferation |
| Bone matrix regulator | Cooperates with RUNX2 to increase osteocalcin and other matrix protein expression |
| Negative regulators | Suppress RUNX2; inhibition by Wnt signaling promotes osteogenesis |
| Ligand | Type II Receptor | Type I Receptor (ALKs) |
|---|---|---|
| BMP-2/4/7 | BMPR-II, ActR-IIA, ActR-IIB | ALK3 (BMPR-IA), ALK6 (BMPR-IB) |
| TGF-β1/β2/β3 | TGF-βRII | ALK5 (TGF-βRI), ALK1 (endothelial cells) |
| Gene | Role in Osteogenesis |
|---|---|
| Runx2 | Drives osteoblast lineage commitment |
| Osterix | Essential for osteoblast maturation |
| ALP | Promotes matrix mineralization |
| Osteocalcin | Mediates calcium binding in bone |
| Type I Collagen | Primary structural protein in bone matrix |
| Fate | Description |
|---|---|
| Osteocytes | Embedded in mineralized matrix; maintain bone homeostasis |
| Bone lining cells | Quiescent, flattened osteoblasts on bone surfaces |
| Apoptosis | Undergo programmed cell death when further activity is unnecessary |
| Mechanism | Regulators | Key Effects |
|---|---|---|
| Extracellular inhibitors | Noggin, Chordin, Gremlin | Prevent ligand–receptor interaction |
| Inhibitory SMADs | SMAD6, SMAD7 | Inhibit R-SMADs; promote receptor degradation |
| Ubiquitin ligases | Smurf1, Smurf2, NEDD4L | Target receptors or SMADs for degradation |
| Receptor endocytosis | Caveolin-mediated pathways | Internalize receptors from cell surface |
| MicroRNAs | miR-26a, miR-145, miR-20a | Suppress pathway gene expression post-transcriptionally |
| Feedback loops | SMAD6/7, Noggin (signaling-induced) | Self-regulate signaling intensity |
| Biological Process | Transcription Factor/Target | Akt-Mediated Effect |
|---|---|---|
| BAD | Phosphorylation at Ser136 promotes 14-3-3 binding, sequestering BAD in the cytoplasm and preventing apoptosis induction |
| Caspase | Phosphorylation suppresses protease activity, thereby blocking apoptosis initiation | |
| FOXO1/3a/4 | Phosphorylation retains FOXO in the cytoplasm, suppressing pro-apoptotic gene transcription | |
| NF-Κb | Activation induces transcription of genes promoting survival and proliferation | |
| mTORC1 | Activation stimulates protein synthesis and cellular growth |
| GSK-3β | Inhibition stabilizes cyclin D1, advancing cell cycle progression | |
| E2F1 | Akt-dependent phosphorylation modulates E2F1, affecting cell cycle advancement | |
| FOXO1 | Inhibition decreases gluconeogenic gene expression, reducing glucose output |
| SREBP-1c | Activation upregulates genes involved in lipid biosynthesis | |
| HIF-1α | Stabilization and activation induce angiogenic gene expression under hypoxic conditions |
| TEFB | Phosphorylation causes cytoplasmic retention, diminishing lysosomal biogenesis and autophagy |
| p21 | Phosphorylation at Thr145 restricts cell cycle progression |
| CREB | Phosphorylation promotes transcription of genes involved in survival and metabolism |
| STAT3 | Phosphorylation increases expression of genes governing cell growth and survival | |
| Runx2 | Phosphorylation enhances invasive gene expression associated with cell growth and survival | |
| YAP | Phosphorylation at Ser127 results in cytoplasmic retention and reduced transcriptional activity |
| Kinase Tier | Components | Functions | Ref. |
|---|---|---|---|
| MAPKKK | RAF (A-RAF, B-RAF, C-RAF) | Activated by RAS-GTP; phosphorylates and activates MAPKK (MEK) | [83] |
| MAPKK | MEK1/2 | Dual-specificity kinase; phosphorylates ERK1/2 on Thr and Tyr residues | [84] |
| MAPK | ERK1/2 | Activated ERK translocates to the nucleus and regulates transcription | [83] |
| Transcription Factors | Target Genes | Mechanism |
|---|---|---|
| Cyclin D1, Bcl-2 | AP-1 facilitates cell cycle progression and upregulates anti-apoptotic genes. |
| c-Fos | ERK-mediated phosphorylation activates Elk-1, which binds the serum response element (SRE) to induce c-Fos transcription. |
| Survivin, Mcl-1 | Phosphorylated CREB promotes cell survival by inducing anti-apoptotic gene expression. |
| E2F, Cyclin E | MYC promotes G1/S transition by activating cyclins and repressing cyclin-dependent kinase inhibitors. |
| EV Type | Parent Cell Origin | Bioactive Cargo | Osteogenic Effects |
|---|---|---|---|
| Exosomes | MSCs, osteoblasts | Proteins (RUNX2, BMPs), miRNAs (miR-21, miR-26a, miR-29b), lipids | Promote osteoblast differentiation; stimulate bone matrix synthesis; enhance angiogenesis; modulate immune cell phenotype |
| Microvesicles | Osteoblasts, ECs | Protein kinases, mRNAs, miRNAs (miR-210) | Support mineralization; enhance osteogenic gene expression; facilitate matrix remodeling |
| EC-derived EVs (EC-EVs) | Endothelial cells | VEGF, FGF2, Ang1, miR-126, MMPs | Couple angiogenesis and osteogenesis; promote vascularization; stimulate MSC differentiation |
| Macrophage-derived EVs (Mφ-EVs) | M2 macrophages | Anti-inflammatory cytokines, miRNAs | Modulate inflammation; induce osteogenesis and bone repair in inflammatory/aged tissue |
| Stress EVs | Bone/progenitor cells | Stress-adaptive proteins, regulatory RNAs | Adaptation to pathological stress: potential modulation of repair processes |
| Matrix vesicles | Osteoblasts | Mineralization proteins, enzymes (ALP) | Initiate and regulate mineral deposition in bone |
| Oncosomes | Cancer cells | Tumor-promoting proteins, oncogenic miRNAs | Not osteogenic; may disrupt bone homeostasis |
| Sl. No. | EV Source and Type | Storage Temperature | Storage Duration | Particle Changes Observed | Content Changes Observed |
|---|---|---|---|---|---|
| 1. | Blood (sEVs) | RT, 4 °C, 20 °C, 40 °C, 80 °C, 160 °C | Days or months | NA | Long-term storage at RT and 4 °C increased signal intensity, while short-term storage reduced signal intensity |
| 2. | Plasma (EVs) | –80 °C; single freeze–thaw cycle | 12–20 m | EV levels decreased over time; single freeze–thaw cycle increased EV count | NA |
| 3. | Platelet (nanovesicles) | –80 °C + DMSO | 1 h | Nanovesicle number increased | NA |
| 4. | Milk (EVs) | 4 °C to 80 °C | 2–8 weeks | NA | CD63 and CD9 expression remained unchanged |
| 5. | Urine (exosomes) | RT, 4 °C to 80 °C | 2 h, 1 day, 1 week | EV yield declined over time | NA |
| 6. | Plasma | 4 °C, 20 °C to 80 °C | 2 weeks to 2 years | NA | RNA levels decreased after weeks at 4 °C; freezing did not affect RNA or protein levels |
| 7. | Serum | RT, 4 °C | 6–168 h | NA | RT for 24 h and 4 °C for 1 week did not alter CD63, TSG101, or DNA concentration |
| Freeze–thaw cycles | 1, 3, and 5 cycles | NA | CD63 and TSG101 unchanged; DNA concentration markedly decreased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, S.; Gangadaran, P.; Dhara, C.; Ghosh, S.; Phadikar, S.D.; Chakraborty, A.; Mahajan, A.A.; Mondal, R.; Chattopadhyay, D.; Banerjee, T.; et al. Extracellular Vesicles in Osteogenesis: A Comprehensive Review of Mechanisms and Therapeutic Potential for Bone Regeneration. Curr. Issues Mol. Biol. 2025, 47, 675. https://doi.org/10.3390/cimb47080675
Biswas S, Gangadaran P, Dhara C, Ghosh S, Phadikar SD, Chakraborty A, Mahajan AA, Mondal R, Chattopadhyay D, Banerjee T, et al. Extracellular Vesicles in Osteogenesis: A Comprehensive Review of Mechanisms and Therapeutic Potential for Bone Regeneration. Current Issues in Molecular Biology. 2025; 47(8):675. https://doi.org/10.3390/cimb47080675
Chicago/Turabian StyleBiswas, Sreyee, Prakash Gangadaran, Chandrajeet Dhara, Shreya Ghosh, Soumya Deep Phadikar, Akash Chakraborty, Atharva Anand Mahajan, Ranit Mondal, Debdeep Chattopadhyay, Trisha Banerjee, and et al. 2025. "Extracellular Vesicles in Osteogenesis: A Comprehensive Review of Mechanisms and Therapeutic Potential for Bone Regeneration" Current Issues in Molecular Biology 47, no. 8: 675. https://doi.org/10.3390/cimb47080675
APA StyleBiswas, S., Gangadaran, P., Dhara, C., Ghosh, S., Phadikar, S. D., Chakraborty, A., Mahajan, A. A., Mondal, R., Chattopadhyay, D., Banerjee, T., Dey, A., Ghosh, S., Krishnan, A., Ahn, B.-C., & Rajendran, R. L. (2025). Extracellular Vesicles in Osteogenesis: A Comprehensive Review of Mechanisms and Therapeutic Potential for Bone Regeneration. Current Issues in Molecular Biology, 47(8), 675. https://doi.org/10.3390/cimb47080675













