Mistletoe in Cancer Cell Biology: Recent Advances
Abstract
1. Introduction
2. Cell Death Mechanisms: Recent Discoveries
2.1. Molecular Mechanisms of Action
2.2. Cell-Type-Specific Responses and Therapeutic Windows
2.3. Synergistic Effects with Conventional Therapies
3. Immunomodulatory Properties: New Insights
3.1. Macrophage Reprogramming and TAM Modulation
3.2. Modulation T Cell and NK Cell Activation
3.3. Dendritic Cell (DC) Maturation and Antigen Presentation
3.4. Cytokine Network Modulation
4. Clinical Applications in the Modern Era
4.1. Breakthrough in Immunotherapy Combination
4.2. Integration with Conventional Therapies
4.3. Precision Medicine Applications
5. Clinical Translation: From Europe to Global Implementation
5.1. The Johns Hopkins Breakthrough
5.2. Regulatory Evolution and Future Directions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, E.H.; Jung, K.W.; Park, N.J.; Kang, M.J.; Yun, E.H.; Kim, H.J.; Kim, J.E.; Kong, H.J.; Im, J.S.; Seo, H.G. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2021. Cancer Res. Treat. 2024, 56, 357–371. [Google Scholar] [CrossRef]
- Kang, M.J.; Jung, K.W.; Bang, S.H.; Choi, S.H.; Park, E.H.; Yun, E.H.; Kim, H.J.; Kong, H.J.; Im, J.S.; Seo, H.G. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2020. Cancer Res. Treat. 2023, 55, 385–399. [Google Scholar] [CrossRef]
- Market, M.; Tennakoon, G.; Auer, R.C. Postoperative Natural Killer Cell Dysfunction: The Prime Suspect in the Case of Metastasis Following Curative Cancer Surgery. Int. J. Mol. Sci. 2021, 22, 11378. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Silva, A.S.; Gillies, R.J.; Frieden, B.R. Adaptive therapy. Cancer Res. 2009, 69, 4894–4903. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Daoud, S.; Mahmod, A.I.; Hamed, R.A.; Awajan, D.; Abuarab, S.F.; Odeh, L.H.; Khater, S.; Al Kury, L.T. Plants as a Source of Anticancer Agents: From Bench to Bedside. Molecules 2022, 27, 4818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xie, H.; Zhang, Z.; Wen, B.; Cao, H.; Bai, Y.; Che, Q.; Guo, J.; Su, Z. Applications and Biocompatibility of Mesoporous Silica Nanocarriers in the Field of Medicine. Front. Pharmacol. 2022, 13, 829796. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, D.C.; Rogerio, C.B.; de Oliveira, J.L.; Campos, E.V.R.; de Araújo, D.R.; Pampana, L.C.; Duarte, M.J.; Valadares, G.F.; Fraceto, L.F. Development of a Mosquito Repellent Formulation Based on Nanostructured Lipid Carriers. Front. Pharmacol. 2021, 12, 760682. [Google Scholar] [CrossRef]
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef]
- Corroon, J.; Kight, R. Regulatory Status of Cannabidiol in the United States: A Perspective. Cannabis Cannabinoid Res. 2018, 3, 190–194. [Google Scholar] [CrossRef]
- Izzo, A.A.; Stefanska, B. Natural products and cancer: From drug discovery to prevention and therapy. Br. J. Pharmacol. 2025, 182, 2069–2074. [Google Scholar] [CrossRef]
- Karaman Özlü, Z.; Klinç, T.; Özlü, İ.; Ünal, H.; Toraman, R.L. The relationship between individuals’ use of complementary and alternative medicine during the pandemic in Turkey and their attitudes towards perceived COVID-19 risk. Eur. J. Integr. Med. 2022, 56, 102194. [Google Scholar] [CrossRef]
- Hijazi, M.A.; Shatila, H.; Abu Qiyas, S.; Aboul-Ela, M.; El-Lakany, A.; Naja, F. Complementary and alternative medicine use during the COVID-19 pandemic: Community pharmacists’ knowledge, attitudes, and practices. Res. Social. Adm. Pharm. 2023, 19, 502–509. [Google Scholar] [CrossRef]
- Graetz, D.E.; Sniderman, E.; Villegas, C.A.; Kaye, E.C.; Ragab, I.; Laptsevich, A.; Maliti, B.; Naidu, G.; Huang, H.; Gassant, P.Y.; et al. Resilient health care in global pediatric oncology during the COVID-19 pandemic. Cancer 2022, 128, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Eliyas, S.; Gressel, O.; Ben-Arye, E.; Vagedes, J.; Samuels, N.; Kassem, S. Coming out of the Integrative Oncology Comfort Zone: Addressing Healthcare Providers’ Wartime-Related Concerns. Psychooncology 2024, 33, e70042. [Google Scholar] [CrossRef]
- Lim, K.H.J.; Murali, K.; Thorne, E.; Punie, K.; Kamposioras, K.; Oing, C.; O’Connor, M.; Élez, E.; Amaral, T.; Garrido, P.; et al. The impact of COVID-19 on oncology professionals-one year on: Lessons learned from the ESMO Resilience Task Force survey series. ESMO Open 2022, 7, 100374. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M. The Anti-Inflammatory Activity of Viscum album. Plants 2023, 12, 1460. [Google Scholar] [CrossRef]
- Weissenstein, U.; Kunz, M.; Urech, K.; Regueiro, U.; Baumgartner, S. Interaction of a standardized mistletoe (Viscum album) preparation with antitumor effects of Trastuzumab in vitro. BMC Complement. Altern. Med. 2016, 16, 271. [Google Scholar] [CrossRef]
- Melzer, J.; Iten, F.; Hostanska, K.; Saller, R. Efficacy and safety of mistletoe preparations (Viscum album) for patients with cancer diseases. A systematic review. Forsch. Komplementmed. 2009, 16, 217–226. [Google Scholar] [CrossRef]
- Legnani, W. Mistletoe in conventional oncological practice: Exemplary cases. Integr. Cancer Ther. 2008, 7, 162–171. [Google Scholar] [CrossRef]
- Duong Van Huyen, J.P.; Delignat, S.; Kazatchkine, M.D.; Kaveri, S.V. Comparative study of the sensitivity of lymphoblastoid and transformed monocytic cell lines to the cytotoxic effects of Viscum album extracts of different origin. Chemotherapy 2003, 49, 298–302. [Google Scholar] [CrossRef]
- Huguet Soler, M.; Stoeva, S.; Schwamborn, C.; Wilhelm, S.; Stiefel, T.; Voelter, W. Complete amino acid sequence of the A chain of mistletoe lectin I. FEBS Lett. 1996, 399, 153–157. [Google Scholar] [CrossRef]
- Frantz, M.; Jung, M.L.; Ribereau-Gayon, G.; Anton, R. Modulation of mistletoe (Viscum album L.) lectins cytotoxicity by carbohydrates and serum glycoproteins. Arzneimittelforschung 2000, 50, 471–478. [Google Scholar] [CrossRef]
- Loef, M.; Walach, H. Quality of life in cancer patients treated with mistletoe: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 227. [Google Scholar] [CrossRef]
- Loef, M.; Paepke, D.; Walach, H. Quality of Life in Breast Cancer Patients Treated With Mistletoe Extracts: A Systematic Review and Meta-Analysis. Integr. Cancer Ther. 2023, 22, 15347354231198074. [Google Scholar] [CrossRef]
- Ostermann, T.; Appelbaum, S.; Poier, D.; Boehm, K.; Raak, C.; Büssing, A. A Systematic Review and Meta-Analysis on the Survival of Cancer Patients Treated with a Fermented Viscum album L. Extract (Iscador): An Update of Findings. Complement. Med. Res. 2020, 27, 260–271. [Google Scholar] [CrossRef]
- Loef, M.; Walach, H. Survival of Cancer Patients Treated with Non-Fermented Mistletoe Extract: A Systematic Review and Meta-Analysis. Integr. Cancer Ther. 2022, 21, 15347354221133561. [Google Scholar] [CrossRef]
- Vanhaverbeke, C.; Touboul, D.; Elie, N.; Prévost, M.; Meunier, C.; Michelland, S.; Cunin, V.; Ma, L.; Vermijlen, D.; Delporte, C.; et al. Untargeted metabolomics approach to discriminate mistletoe commercial products. Sci. Rep. 2021, 11, 14205. [Google Scholar] [CrossRef]
- Lederer, A.K.; Rieger, S.; Schink, M.; Huber, R. Pharmakokinetics of Mistletoe Lectins after Intravenous Application of a Mistletoe Product in Healthy Subjects. Pharmaceuticals 2024, 17, 278. [Google Scholar] [CrossRef]
- Schad, F.; Thronicke, A.; Hofheinz, R.D.; Matthes, H.; Grah, C. Patients with Advanced or Metastasised Non-Small-Cell Lung Cancer with Viscum album L. Therapy in Addition to PD-1/PD-L1 Blockade: A Real-World Data Study. Cancers 2024, 16, 1609. [Google Scholar] [CrossRef]
- Bryant, S.; Duncan, L.; Feder, G.; Huntley, A.L. A pilot study of the mistletoe and breast cancer (MAB) trial: A protocol for a randomised double-blind controlled trial. Pilot. Feasibility Stud. 2022, 8, 78. [Google Scholar] [CrossRef]
- Vella, R.; Giardino, A.; Pizzocaro, E.; Frigerio, I.; Bannone, E.; Vieni, S.; Butturini, G. Unconventional Treatments for Pancreatic Cancer: A Systematic Review. Cancers 2025, 17, 1437. [Google Scholar] [CrossRef]
- Staupe, H.; Buentzel, J.; Keinki, C.; Buentzel, J.; Huebner, J. Systematic analysis of mistletoe prescriptions in clinical studies. J. Cancer Res. Clin. Oncol. 2023, 149, 5559–5571. [Google Scholar] [CrossRef] [PubMed]
- Matthes, H.; Thronicke, A.; Hofheinz, R.D.; Baars, E.; Martin, D.; Huber, R.; Breitkreuz, T.; Bar-Sela, G.; Galun, D.; Schad, F. Statement to an Insufficient Systematic Review on Viscum album L. Therapy. Evid. Based Complement. Alternat Med. 2020, 2020, 7091039. [Google Scholar] [CrossRef] [PubMed]
- Weissenstein, U.; Tschumi, S.; Leonhard, B.; Baumgartner, S. A fermented Mistletoe (Viscum album L.) extract elicits markers characteristic for immunogenic cell death driven by endoplasmic reticulum stress in vitro. BMC Complement. Med. Ther. 2025, 25, 175. [Google Scholar] [CrossRef] [PubMed]
- Beztsinna, N.; de Matos, M.B.C.; Walther, J.; Heyder, C.; Hildebrandt, E.; Leneweit, G.; Mastrobattista, E.; Kok, R.J. Quantitative analysis of receptor-mediated uptake and pro-apoptotic activity of mistletoe lectin-1 by high content imaging. Sci. Rep. 2018, 8, 2768. [Google Scholar] [CrossRef]
- Hong, C.E.; Lyu, S.Y. Modulation of Breast Cancer Cell Apoptosis and Macrophage Polarization by Mistletoe Lectin in 2D and 3D Models. Int. J. Mol. Sci. 2024, 25, 8459. [Google Scholar] [CrossRef]
- Lyu, S.Y.; Park, S.M.; Choung, B.Y.; Park, W.B. Comparative study of Korean (Viscum album var. coloratum) and European mistletoes (Viscum album). Arch. Pharm. Res. 2000, 23, 592–598. [Google Scholar] [CrossRef]
- Lyu, S.Y.; Meshesha, S.M.; Hong, C.E. Synergistic Effects of Mistletoe Lectin and Cisplatin on Triple-Negative Breast Cancer Cells: Insights from 2D and 3D In Vitro Models. Int. J. Mol. Sci. 2025, 26, 366. [Google Scholar] [CrossRef]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Reis, R.L.; Pirraco, R.P. Modelling the complex nature of the tumor microenvironment: 3D tumor spheroids as an evolving tool. J. Biomed. Sci. 2024, 31, 13. [Google Scholar] [CrossRef]
- Melo, M.N.O.; Ochioni, A.C.; Zancan, P.; Oliveira, A.P.; Grazi, M.; Garrett, R.; Holandino, C.; Baumgartner, S. Viscum album mother tinctures: Harvest conditions and host trees influence the plant metabolome and the glycolytic pathway of breast cancer cells. Front. Pharmacol. 2022, 13, 1027931. [Google Scholar] [CrossRef]
- Schröder, L.; Hohnjec, N.; Senkler, M.; Senkler, J.; Küster, H.; Braun, H.P. The gene space of European mistletoe (Viscum album). Plant J. 2022, 109, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Wang, L.; Fu, W.; Kumar, R.; Durham, J.N.; Azad, N.S.; Laheru, D.A.; Browner, I.; Kachhap, S.K.; Boyapati, K.; et al. Phase I Trial of Intravenous Mistletoe Extract in Advanced Cancer. Cancer Res. Commun. 2023, 3, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Thronicke, A.; Schad, F.; Debus, M.; Grabowski, J.; Soldner, G. Viscum album L. Therapy in Oncology: An Update on Current Evidence. Complement. Med. Res. 2022, 29, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Schad, F.; Thronicke, A.; Steele, M.L.; Merkle, A.; Matthes, B.; Grah, C.; Matthes, H. Overall survival of stage IV non-small cell lung cancer patients treated with Viscum album L. in addition to chemotherapy, a real-world observational multicenter analysis. PLoS ONE 2018, 13, e0203058. [Google Scholar] [CrossRef]
- Thronicke, A.; Steele, M.L.; Grah, C.; Matthes, B.; Schad, F. Clinical safety of combined therapy of immune checkpoint inhibitors and Viscum album L. therapy in patients with advanced or metastatic cancer. BMC Complement. Altern. Med. 2017, 17, 534. [Google Scholar] [CrossRef]
- Valle, A.C.V.; de Carvalho, A.C.; Rahme, S.W.; Araujo, A.d.R.B.; Malard, P.F.; Sena Brunel, H.S. Comparison of cytotoxicity caused by Viscum album in human mesenchymal stem cells and hepatocellular carcinoma cells. Med. Res. Arch. 2024, 12. [Google Scholar] [CrossRef]
- Xie, W.; Delebinski, C.; Gürgen, D.; Schröder, M.; Seifert, G.; Melzig, M.F. Inhibition of osteosarcoma by European Mistletoe derived val-miR218. Extracell. Vesicles Circ. Nucl. Acids 2023, 4, 306–322. [Google Scholar] [CrossRef]
- Hohneck, A.L.; Sadikaj, L.; Heinemann, L.; Schroeder, M.; Riess, H.; Gerhards, A.; Burkholder, I.; Heckel-Reusser, S.; Gottfried, J.; Hofheinz, R.D. Patients with Advanced Pancreatic Cancer Treated with Mistletoe and Hyperthermia in Addition to Palliative Chemotherapy: A Retrospective Single-Center Analysis. Cancers 2023, 15, 4929. [Google Scholar] [CrossRef]
- Shatat, M.A.; Gauthier, B.; Yoon, S.; Yuan, E.; Yang, P.; Narla, G.; Dowlati, A.; Lee, R.T. Mistletoe lectin inhibits growth of Myc-amplified small-cell lung cancer. Cancer Med. 2023, 12, 8378–8387. [Google Scholar] [CrossRef]
- Baek, J.H.; Jeon, Y.; Han, K.W.; Jung, D.H.; Kim, K.O. Effect of mistletoe extract on tumor response in neoadjuvant chemoradiotherapy for rectal cancer: A cohort study. World J. Surg. Oncol. 2021, 19, 178. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Phalke, S.; Stévigny, C.; Souard, F.; Vermijlen, D. Mistletoe-Extract Drugs Stimulate Anti-Cancer Vγ9Vδ2 T Cells. Cells 2020, 9, 1560. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J. Reducing Malignant Ascites and Long-Term Survival in a Patient with Recurrent Gastric Cancer Treated with a Combination of Docetaxel and Mistletoe Extract. Case Rep. Oncol. 2020, 13, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Ateş, Ş.; Ulger, H.; Yılmaz, S.; Şeker Karatoprak, G.; al, Ö.; Uçar, S.; Taştan, M.; Tokpinar, A.; Alpa, Ş.; Farooqi, A. Evaluation of antitumoral effect of mistletoe fruit extract on Ehrlich ascites tumor cells with muse cell analyzer and argyrophilic nucleolar organizer region staining method. Postępy Hig. Med. Dośw. 2022, 76, 209–219. [Google Scholar] [CrossRef]
- Pozdnyakov, D.; Adzhiahmetova, S.; Chervonnaya, N.; Voronkov, A.; Oganesyan, E. Some aspects of the adaptogenic potential of European mistletoe (Viscum album L.) extracts under variable physical performance. J. Med. Plants 2021, 20, 60–78. [Google Scholar] [CrossRef]
- Orange, M.; Poidimani, N.; Crosignani, A.; Werthmann, P.G.; Bertotto, C. Complete, Durable Remission of Advanced Hepatocellular Carcinoma under Treatment with Viscum album Extracts: A Case Report. Complement. Med. Res. 2022, 29, 483–491. [Google Scholar] [CrossRef]
- Elmelegy, M.; Abdella, H.; El-Bakly, W.; Tolba, M.; El-Demerdash, E. Effect of Viscum album Extract on Angiogenesis Mediators and Cytokines in Egyptian Patients with Intermediate Hepatocellular Carcinoma. Arch. Pharm. Sci. Ain Shams Univ. 2021, 5, 33–45. [Google Scholar] [CrossRef]
- Robev, B.; Iliev, I.; Tsoneva, I.; Momchilova, A.; Nesheva, A.; Kostadinova, A.; Staneva, G.; Nikolova, B. Antitumor Effect of Iscador on Breast Cancer Cell Lines with Different Metastatic Potential. Int. J. Mol. Sci. 2023, 24, 5247. [Google Scholar] [CrossRef]
- Mazalovska, M.; Kouokam, J.C. Transiently Expressed Mistletoe Lectin II in Nicotiana benthamiana Demonstrates Anticancer Activity In Vitro. Molecules 2020, 25, 2562. [Google Scholar] [CrossRef]
- Juengel, E.; Rutz, J.; Meiborg, M.; Markowitsch, S.D.; Maxeiner, S.; Grein, T.; Thomas, A.; Chun, F.K.; Haferkamp, A.; Tsaur, I.; et al. Mistletoe Extracts from Different Host Trees Disparately Inhibit Bladder Cancer Cell Growth and Proliferation. Cancers 2023, 15, 4849. [Google Scholar] [CrossRef]
- Menke, K.; Schwermer, M.; Eisenbraun, J.; Schramm, A.; Zuzak, T.J. Anticancer Effects of Viscum album Fraxini Extract on Medulloblastoma Cells in vitro. Complement. Med. Res. 2021, 28, 15–22. [Google Scholar] [CrossRef]
- Schad, F.; Steinmann, D.; Oei, S.L.; Thronicke, A.; Grah, C. Evaluation of quality of life in lung cancer patients receiving radiation and Viscum album L.: A real-world data study. Radiat. Oncol. 2023, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Schad, F.; Thronicke, A. Safety of Combined Targeted and Helixor® Viscum album L. Therapy in Breast and Gynecological Cancer Patients, a Real-World Data Study. Int. J. Environ. Res. Public Health 2023, 20, 2565. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hur, J.; Hong, S.C.; Jung, J.; Park, C.H.; Park, J.B.; Yoon, T.J.; Kim, J.B.; Yang, S.H. Modulated electro-hyperthermia therapy combined with Korean mistletoe extract treatment exerts a strong anti-tumor activity by enhancing cellular and humoral immune responses in mice. Anim. Cells Syst. 2025, 29, 163–172. [Google Scholar] [CrossRef]
- Oei, S.L.; Thronicke, A.; Kröz, M.; von Trott, P.; Schad, F.; Matthes, H. Impact of Oncological Therapy and Viscum album L. Treatment on Cancer-Related Fatigue and Internal Coherence in Nonmetastasized Breast Cancer Patients. Integr. Cancer Ther. 2020, 19, 1534735420917211. [Google Scholar] [CrossRef]
- Abe, C.; Bhaswant, M.; Miyazawa, T.; Miyazawa, T. The Potential Use of Exosomes in Anti-Cancer Effect Induced by Polarized Macrophages. Pharmaceutics 2023, 15, 1024. [Google Scholar] [CrossRef]
- Xu, C.; Chen, J.; Tan, M.; Tan, Q. The role of macrophage polarization in ovarian cancer: From molecular mechanism to therapeutic potentials. Front. Immunol. 2025, 16, 1543096. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Zhang, D.; Sun, X.; Wu, Y.; Wang, J.; Li, Q.; Jiang, G. The macrophage polarization by miRNAs and its potential role in the treatment of tumor and inflammation (Review). Oncol. Rep. 2023, 50, 190. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Y.; Mao, Y.; Li, Y.; Shen, Y.; Sheng, J.; Gu, N. Modulation of macrophage polarization by iron-based nanoparticles. Med. Rev. 2023, 3, 105–122. [Google Scholar] [CrossRef]
- Kozłowski, H.M.; Sobocińska, J.; Jędrzejewski, T.; Maciejewski, B.; Dzialuk, A.; Wrotek, S. Fever-Range Hyperthermia Promotes Macrophage Polarization towards Regulatory Phenotype M2b. Int. J. Mol. Sci. 2023, 24, 17574. [Google Scholar] [CrossRef]
- Lim, W.-T.; Hong, C.-E.; Lyu, S.-Y. Immuno-Modulatory Effects of Korean Mistletoe in MDA-MB-231 Breast Cancer Cells and THP-1 Macrophages. Sci. Pharm. 2023, 91, 48. [Google Scholar] [CrossRef]
- Magalhães, I.F.B.; Figueirêdo, A.L.M.; da Silva, E.M.; de Miranda, A.A.B.; da Rocha, C.Q.; da Silva Calabrese, K.; Almeida-Souza, F.; Abreu-Silva, A.L. Effects of Passovia ovata Mistletoe on Pro-Inflammatory Markers In Vitro and In Vivo. Plants 2023, 12, 1814. [Google Scholar] [CrossRef] [PubMed]
- Konozy, E.H.E.; Osman, M.E.M. From inflammation to immune regulation: The dual nature of dietary lectins in health and disease. Heliyon 2024, 10, e39471. [Google Scholar] [CrossRef]
- Bian, Z.; Wu, X.; Chen, Q.; Gao, Q.; Xue, X.; Wang, Y. Oct4 activates IL-17A to orchestrate M2 macrophage polarization and cervical cancer metastasis. Cancer Immunol. Immunother. 2024, 73, 73. [Google Scholar] [CrossRef] [PubMed]
- Kalim, H.; Pratama, M.Z.; Sermoati, I.; Yuniati, M.; Haryati, N.; Norahmawati, E.; Endharti, A.; Irwanto, Y.; Solikhin, M.; Hidayat, S. The Effect of Mango Mistletoes (Dendrophthoe pentandra) Leaves Extract on Percentage of CD4+CD28+, CD8+CD28+, and interleukin-2 Levels of Aged Balb/c Mice. Open Access Maced. J. Med. Sci. 2021, 9, 414–421. [Google Scholar] [CrossRef]
- Cogo, E.; Elsayed, M.; Bhardwaj, S.; Cooley, K.; Aycho, C.; Liang, V.; Papadogianis, P.; Psihogios, A.; Seely, D. Mistletoe Extracts during the Oncological Perioperative Period: A Systematic Review and Meta-Analysis of Human Randomized Controlled Trials. Curr. Oncol. 2023, 30, 8196–8219. [Google Scholar] [CrossRef]
- Kaesbach, S.; Hintze, A.; Engelbrecht, S.; Wartenberg, M.; Templeton, A.J. ER+ HER2- Invasive Breast Cancer: Tumor Remission following Viscum Album Extract/Influenza Vaccine Treatment—A Report of 2 Cases. Complement. Med. Res. 2025, 32, 176–181. [Google Scholar] [CrossRef]
- Kim, J.J.; Hwang, Y.H.; Kang, K.Y.; Kim, I.; Kim, J.B.; Park, J.H.; Yoo, Y.C.; Yee, S.T. Enhanced dendritic cell maturation by the B-chain of Korean mistletoe lectin (KML-B), a novel TLR4 agonist. Int. Immunopharmacol. 2014, 21, 309–319. [Google Scholar] [CrossRef]
- Jin, P.; Han, T.H.; Ren, J.; Saunders, S.; Wang, E.; Marincola, F.M.; Stroncek, D.F. Molecular signatures of maturing dendritic cells: Implications for testing the quality of dendritic cell therapies. J. Transl. Med. 2010, 8, 4. [Google Scholar] [CrossRef]
- Cruz, F.M.; Colbert, J.D.; Merino, E.; Kriegsman, B.A.; Rock, K.L. The Biology and Underlying Mechanisms of Cross-Presentation of Exogenous Antigens on MHC-I Molecules. Annu. Rev. Immunol. 2017, 35, 149–176. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Uddin, M.J.; Hossen, M.J.; Rahman, M.A.; Mohibbullah, M.; Hannan, M.A.; Choi, J.-S. Dendritic Cells (DCs)-Based Cancer Immunotherapy: A Review on the Prospects of Medicinal Plants and Their Phytochemicals as Potential Pharmacological Modulators. Appl. Sci. 2022, 12, 9452. [Google Scholar] [CrossRef]
- Hostanska, K.; Hajto, T.; Spagnoli, G.C.; Fischer, J.; Lentzen, H.; Herrmann, R. A plant lectin derived from Viscum album induces cytokine gene expression and protein production in cultures of human peripheral blood mononuclear cells. Nat. Immun. 1995, 14, 295–304. [Google Scholar] [PubMed]
- Pelzer, F.; Loef, M.; Martin, D.D.; Baumgartner, S. Cancer-related fatigue in patients treated with mistletoe extracts: A systematic review and meta-analysis. Support. Care Cancer 2022, 30, 6405–6418. [Google Scholar] [CrossRef] [PubMed]
- Schad, F.; Thronicke, A.; Hofheinz, R.D.; Klein, R.; Grabowski, P.; Oei, S.L.; Wüstefeld, H.; Grah, C. Immune Checkpoint Blockade Combined with AbnobaViscum® Therapy Is Linked to Improved Survival in Advanced or Metastatic Non-Small-Cell Lung Cancer Patients: A Registry Study in Accordance with the ESMO Guidance for Reporting Real-World Evidence. Pharmaceuticals 2024, 17, 1713. [Google Scholar] [CrossRef]
- Devi, S.; Gründemann, C.; Huber, R.; Kowarschik, S. Characterization of Viscum album L. Effect on Immune Escape Proteins PD-L1, PD-L2, and MHC-I in the Prostate, Colon, Lung, and Breast Cancer Cells. Complement. Med. Res. 2023, 30, 386–392. [Google Scholar] [CrossRef]
- Thronicke, A.; Grabowski, P.; Roos, J.; Wüstefeld, H.; Grah, C.; Johnson, S.; Schad, F. Combined Immune Checkpoint Blockade and Helixor® Therapy in Oncology: Real-World Tolerability and Subgroup Survival (ESMO GROW). Int. J. Mol. Sci. 2025, 26, 3669. [Google Scholar] [CrossRef]
- Thronicke, A.; Reinhold, T.; von Trott, P.; Grah, C.; Matthes, B.; Matthes, H.; Schad, F. Cost-effectiveness of real-world administration of chemotherapy and add-on Viscum album L. therapy compared to chemotherapy in the treatment of stage IV NSCLC patients. PLoS ONE 2020, 15, e0236426. [Google Scholar] [CrossRef]
- Wode, K.; Hök Nordberg, J.; Kienle, G.S.; Elander, N.O.; Bernhardson, B.M.; Sunde, B.; Sharp, L.; Henriksson, R.; Fransson, P. Efficacy of mistletoe extract as a complement to standard treatment in advanced pancreatic cancer: Study protocol for a multicentre, parallel group, double-blind, randomised, placebo-controlled clinical trial (MISTRAL). Trials 2020, 21, 783. [Google Scholar] [CrossRef]
- Oei, S.L.; Schad, F. Are Aspects of Integrative Concepts Helpful to Improve Pancreatic Cancer Therapy? Cancers 2023, 15, 1116. [Google Scholar] [CrossRef]
- Zimmermann-Klemd, A.M.; Reinhardt, J.K.; Winker, M.; Gründemann, C. Phytotherapy in Integrative Oncology-An Update of Promising Treatment Options. Molecules 2022, 27, 3209. [Google Scholar] [CrossRef]
- Peñaloza, E.; Holandino, C.; Scherr, C.; Araujo, P.I.P.; Borges, R.M.; Urech, K.; Baumgartner, S.; Garrett, R. Comprehensive Metabolome Analysis of Fermented Aqueous Extracts of Viscum album L. by Liquid Chromatography-High Resolution Tandem Mass Spectrometry. Molecules 2020, 25, 4006. [Google Scholar] [CrossRef] [PubMed]
- Korcan, S.E.; Çankaya, N.; Azarkan, S.Y.; Bulduk, İ.; Karaaslan, E.C.; Kargıoğlu, M.; Konuk, M.; Güvercin, G. Determination of Antioxidant Activities of Viscum album L.: First Report on Interaction of Phenolics with Survivin Protein using in silico Analysis. ChemistrySelect 2023, 8, e202300130. [Google Scholar] [CrossRef]
- Mascher, A.; Pelzer, F.; Duncan, L.J.; Martin, D.D.; Baumgartner, S.; Berger, B. The Introspective Patient Experience of Mistletoe Therapy in Cancer: A Qualitative Study. Integr. Cancer Ther. 2023, 22, 15347354231198474. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, G.; Baumgartner, S.; Scherr, C.; Martin, D.; Tournier, A.L. Chronobiology of Viscum album L.: A time series of daily metabolomic fingerprints spanning 27 years. Front. Physiol. 2024, 15, 1396212. [Google Scholar] [CrossRef]
- Acuña, C.; Kokornaczyk, M.O.; Baumgartner, S.; Castelán, M. Unsupervised Deep Learning Approach for Characterizing Fractality in Dried Drop Patterns of Differently Mixed Viscum album Preparations. Fractal Fract. 2023, 7, 733. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, D.; Rui, L.; Gao, X.; Furness, M.S.; Wu, C. Analysis of Regulatory Botanical Submission Profile for Cancer Management from the U.S. FDA Perspectives. Ther. Innov. Regul. Sci. 2025. [Google Scholar] [CrossRef]
- Klingemann, H. Viscum album (mistletoe) extract for dogs with cancer? Front. Vet. Sci. 2023, 10, 1285354. [Google Scholar] [CrossRef]
- Biegel, U.; Mevissen, M.; Schuller, S.; Ruess, K.; Christen, O.; Ayrle, H.; Koch, C.; Walkenhorst, M. Viscum album L., a Therapeutic Option for Neoplastic Diseases in Companion Animals? A Systematic Review. Complement. Med. Res. 2022, 29, 465–482. [Google Scholar] [CrossRef]
- Wu, C.; Lee, S.L.; Taylor, C.; Li, J.; Chan, Y.M.; Agarwal, R.; Temple, R.; Throckmorton, D.; Tyner, K. Scientific and Regulatory Approach to Botanical Drug Development: A U.S. FDA Perspective. J. Nat. Prod. 2020, 83, 552–562. [Google Scholar] [CrossRef]
- Gravelin, M.; Wright, J.; Holbein, M.E.B.; Berro, M.; Brown, J.S.; Mashour, G.A.; Weatherwax, K.J. Role of CTSA institutes and academic medical centers in facilitating preapproval access to investigational agents and devices during the COVID-19 pandemic. J. Clin. Transl. Sci. 2021, 5, e94. [Google Scholar] [CrossRef]

| Year | Discovery | Cancer Type/Model | Key Finding | Mechanism | Ref. |
|---|---|---|---|---|---|
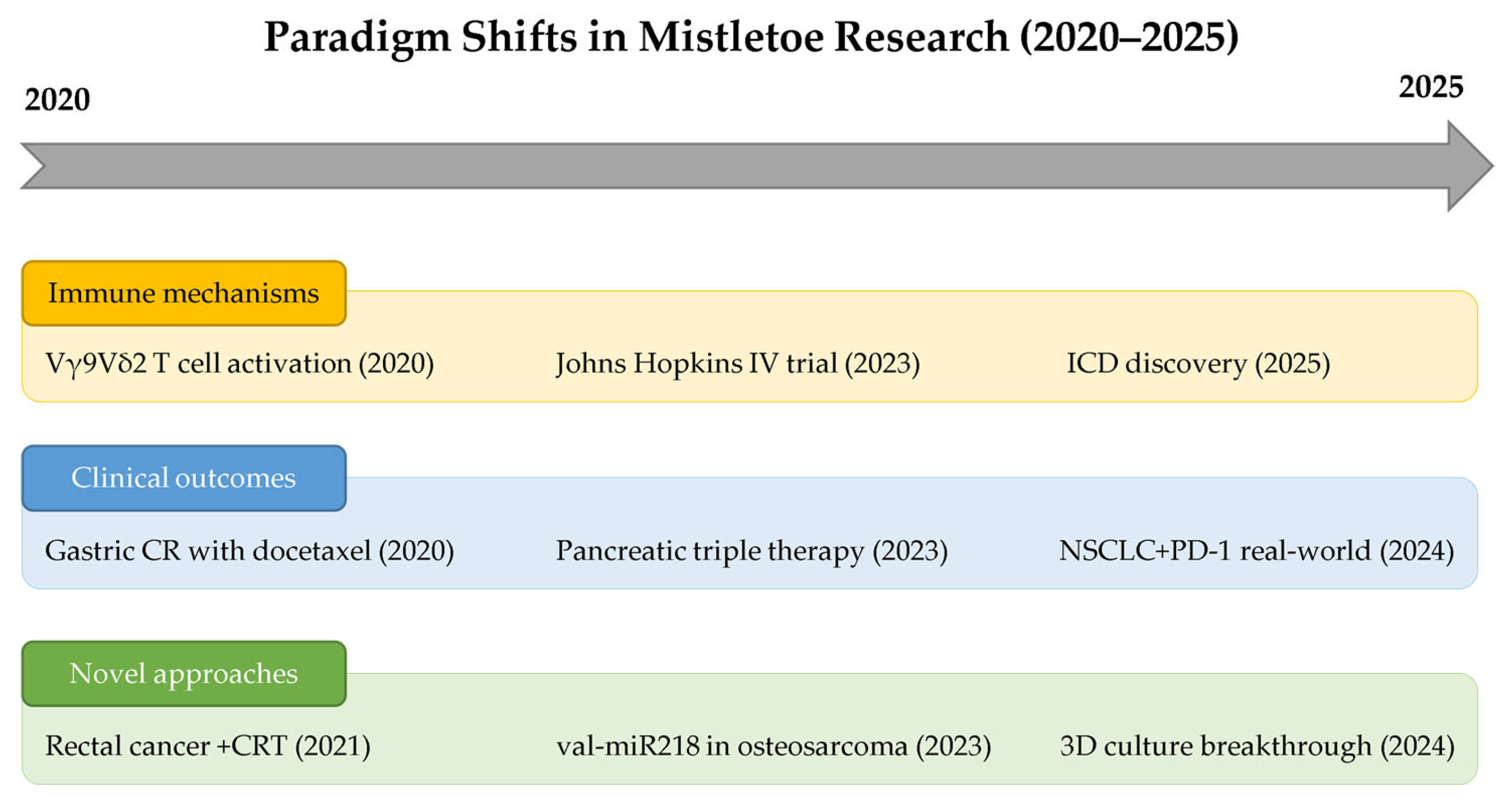
| 2025 | First evidence of mistletoe-induced ICD markers | Breast (SKBR3, MDA-MB-231, MCF-7), melanoma (B16F10) | ER stress (p-eIF2α↑), DAMPs (CRT, HSP70, HSP90), ROS↑, ATP release | Fermented VAE triggers ER stress-mediated ICD | [34] |
| 2024 | VAD30 shows selective cytotoxicity | HepG2 vs. MSCs | Decreased HepG2 viability, no MSC damage | Selective cancer cell targeting | [47] |
| 2023 | Plant miRNA inhibits osteosarcoma | Osteosarcoma | val-miR218 targets 61 cell cycle genes | Cross-kingdom miRNA regulation | [48] |
| Triple therapy extends pancreatic cancer survival | Pancreatic cancer (n = 206) | OS: chemo 8.6 mo → +ML 11.2 mo → +ML+HT 18.9 mo | Multi-modal synergy | [49] | |
| 2022 | ML targets Myc-amplified SCLC | Small-cell lung cancer | Myc overexpression enhances sensitivity | Direct Myc protein downregulation | [50] |
| 2021 | Mistletoe enhances chemoradiotherapy response | Rectal cancer (n = 52) | pCR: 53.3% vs. 21.6% (p = 0.044) | Enhanced apoptosis, caspase-3 activation | [51] |
| 2020 | Non-fermented mistletoe activates Vγ9Vδ2 T cells | Human T cells | AbnobaViscum induces specific expansion, IFNγ/TNFα production | BTN3A-dependent, phosphoantigen-independent | [52] |
| Docetaxel + mistletoe achieves complete remission | Recurrent gastric cancer | Complete response sustained >60 months | Synergistic cytotoxicity + immune modulation | [53] |
| Immune Cell Type | Effects Observed | Key Findings | Reference |
|---|---|---|---|
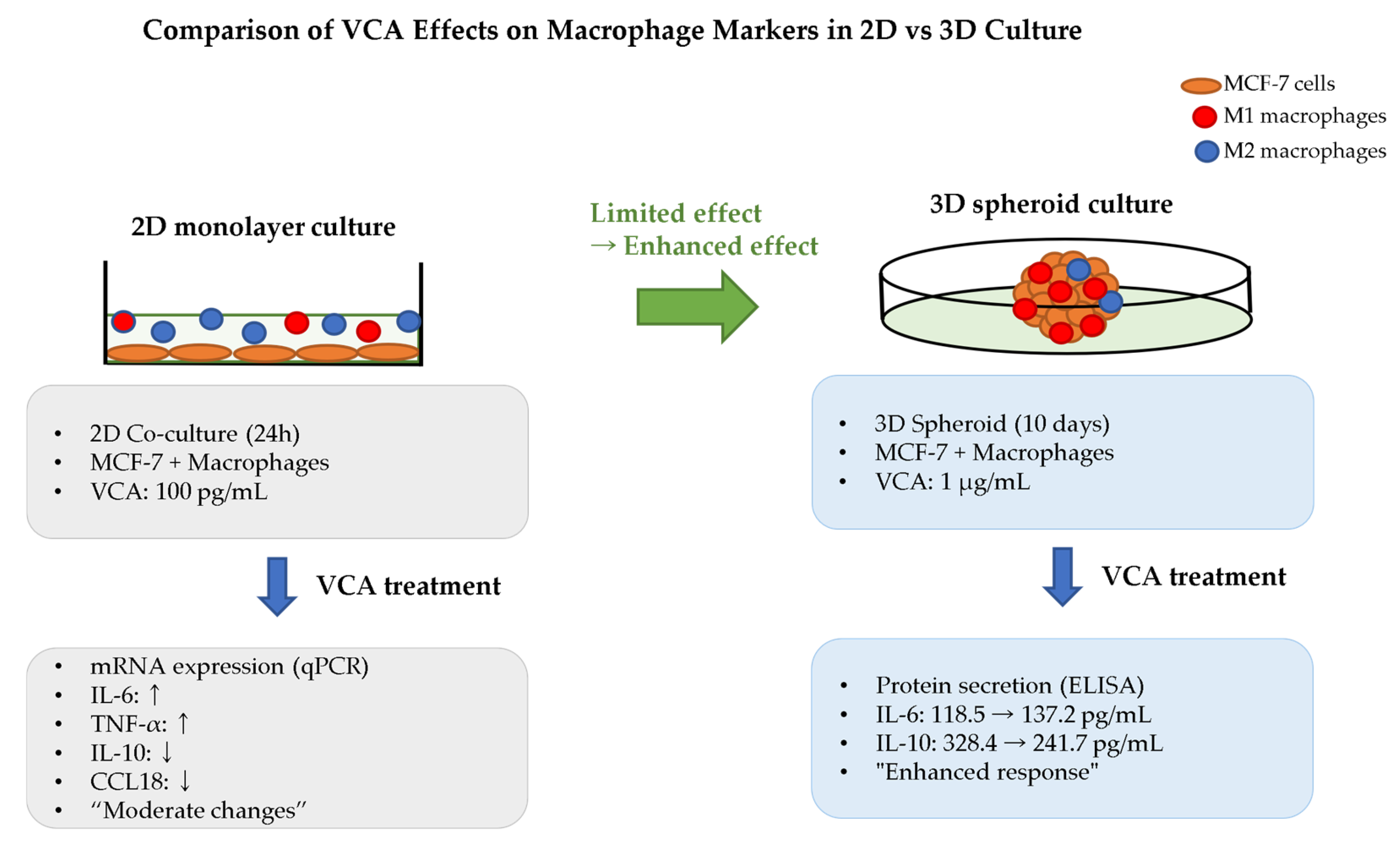
| Macrophages (TAMs) | M2→M1 reprogramming | -IL-6↑ 15.8% in M1 -IL-10↓ 26.4% in M2 -Enhanced in 3D cultures | [36] |
| γδ T cells | Activation and expansion | -Vγ9Vδ2 T cell expansion -IFN-γ and TNF-α production -BTN3A-dependent | [52] |
| CTLs | Enhanced responses | -Increased with mEHT -IFN-γ and granzyme↑ | [64] |
| Dendritic cells | Limited direct data | -ICD provides maturation signals -Recent direct studies lacking | [34] |
| Cytokine network | Preliminary modulation | -CXCL9, CXCL10↑ -Comprehensive profiling needed | [43] |
| Cancer Type | Combination Therapy | N | Key Outcome | Reference |
|---|---|---|---|---|
| NSCLC | VA + PD-1/PD-L1 inhibitors | 300 | Mistletoe + PD-1 | [84] |
| Pancreatic | VA + Chemo → +HT | 206 | OS: 8.6→11.2→18.9 months | [49] |
| Rectal | VA + CRT | 52 | pCR: 21.6%→53.3% (p = 0.044) | [51] |
| Gastric | VA + Docetaxel | 1 | CR sustained >60 months | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, C.-E.; Lyu, S.-Y. Mistletoe in Cancer Cell Biology: Recent Advances. Curr. Issues Mol. Biol. 2025, 47, 672. https://doi.org/10.3390/cimb47080672
Hong C-E, Lyu S-Y. Mistletoe in Cancer Cell Biology: Recent Advances. Current Issues in Molecular Biology. 2025; 47(8):672. https://doi.org/10.3390/cimb47080672
Chicago/Turabian StyleHong, Chang-Eui, and Su-Yun Lyu. 2025. "Mistletoe in Cancer Cell Biology: Recent Advances" Current Issues in Molecular Biology 47, no. 8: 672. https://doi.org/10.3390/cimb47080672
APA StyleHong, C.-E., & Lyu, S.-Y. (2025). Mistletoe in Cancer Cell Biology: Recent Advances. Current Issues in Molecular Biology, 47(8), 672. https://doi.org/10.3390/cimb47080672






