Brassinosteroid Coordinates with ROS, Auxin and Gibberellin to Promote Mesocotyl Elongation and Deep-Sowing Tolerance in Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Growth Conditions
2.2. Treatment Assays
2.3. Paraffin Sections of Mesocotyl
2.4. Measurement of Cellulose, Hemicellulose, and Total Pectin Contents
2.5. Measurement of Pectinase, Cellulase, and Acidic Xylanase Activities
2.6. NBT and DAB Staining Assays
2.7. Measurement of POD and SOD Activities
2.8. Measurement of Mesocotyl After Deep Sowing of Maize
2.9. Statistical Analysis
2.10. Principal Component Analysis
2.11. Correlation Analysis
3. Results
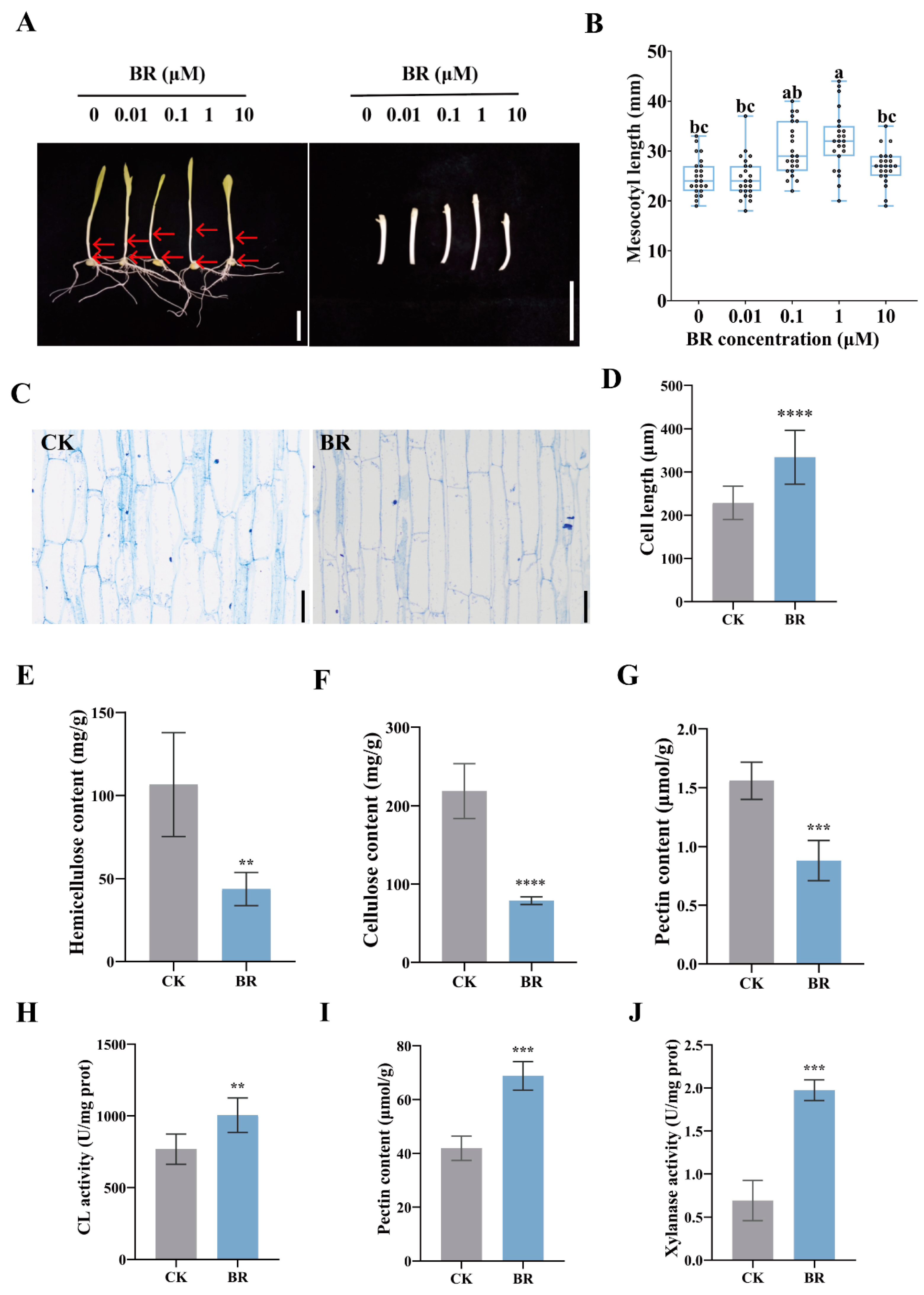
3.1. BR Regulates Cell-Wall Metabolism to Promote Cell Elongation in Maize Mesocotyls
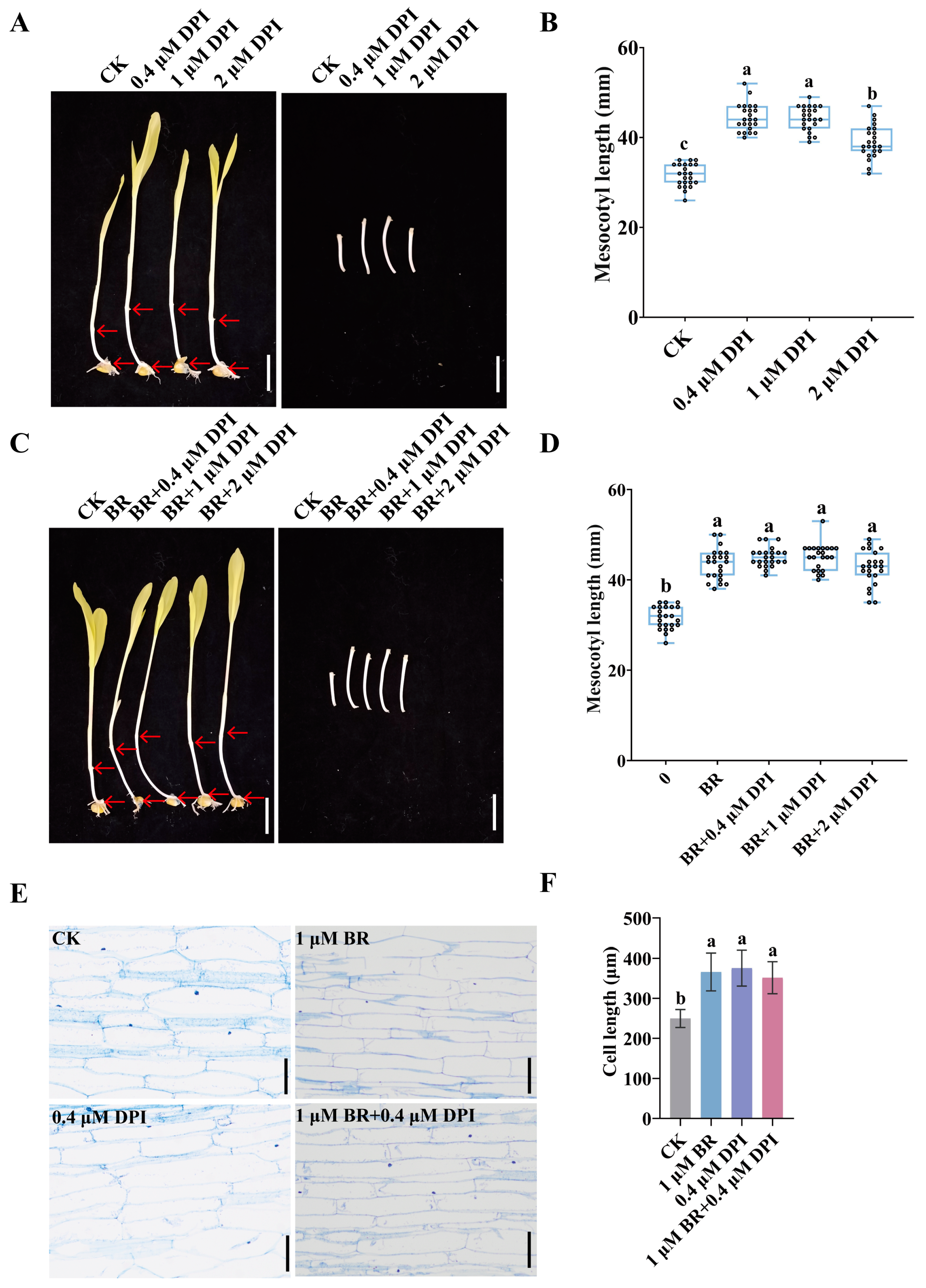
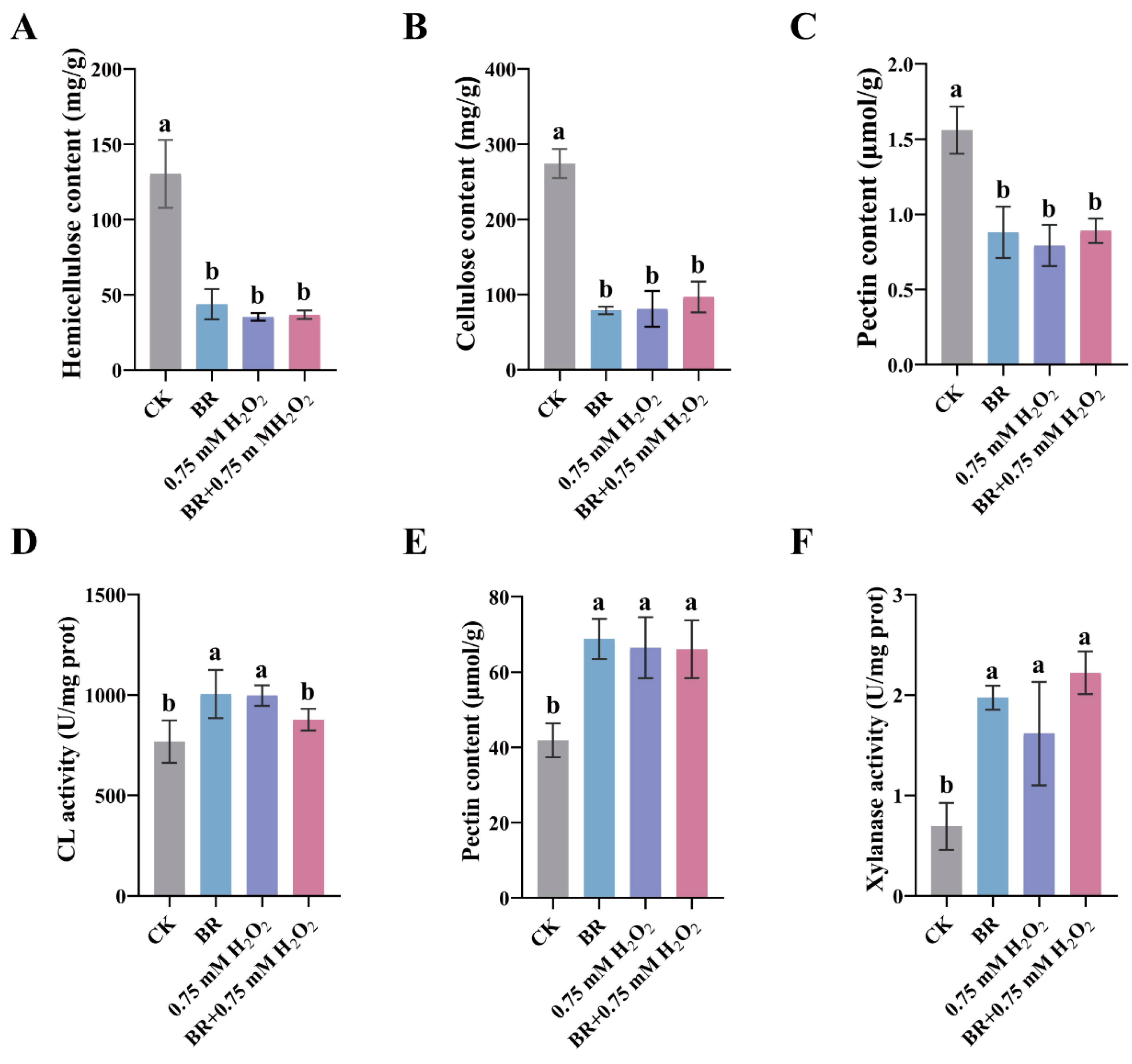
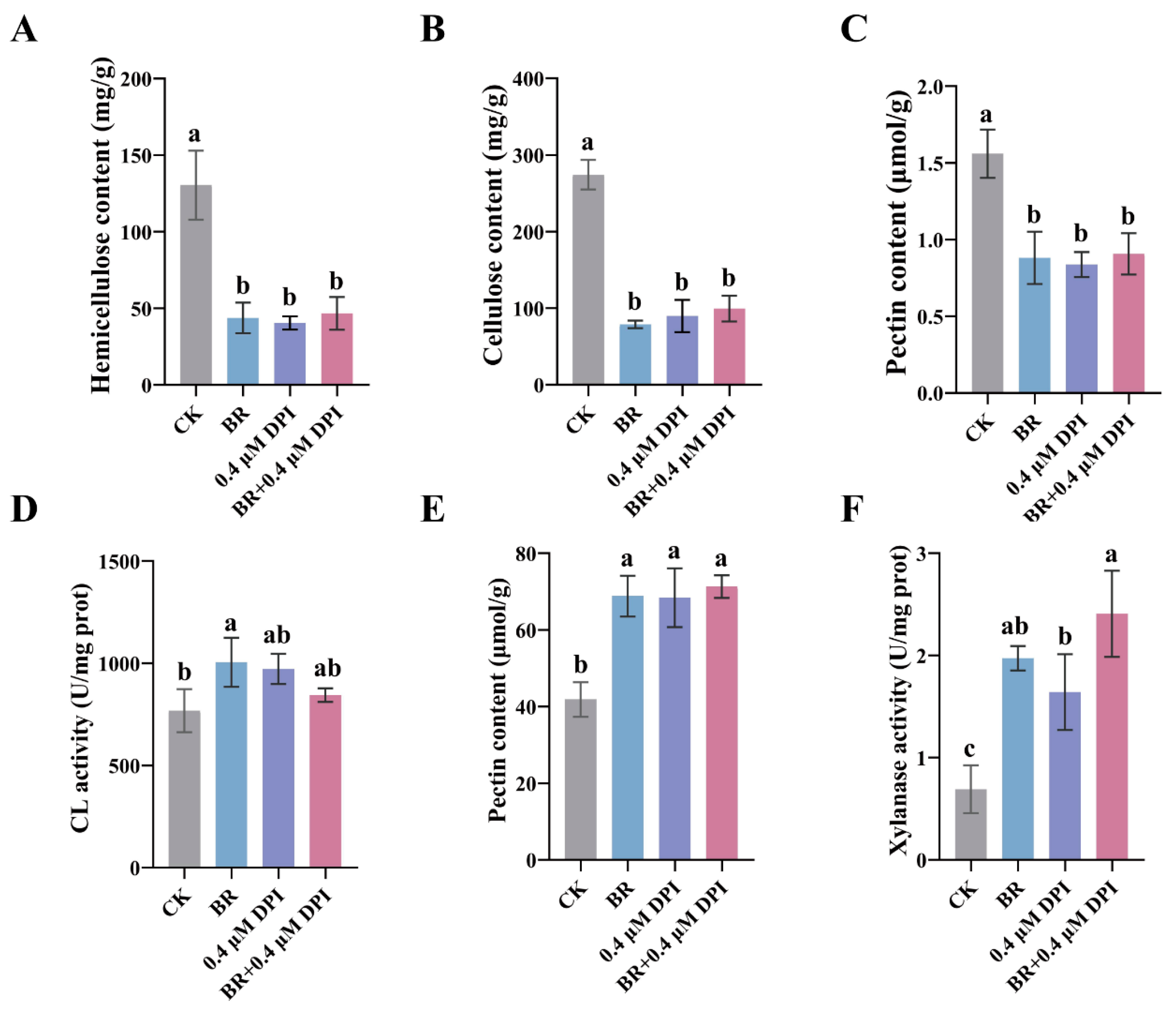
3.2. BR Synergizes with ROS to Promote Mesocotyl Cell Elongation in Maize
3.3. BR and ROS Decrease Cell-Wall Components by Enhancing Loosening Enzyme Activity in the Maize Mesocotyl
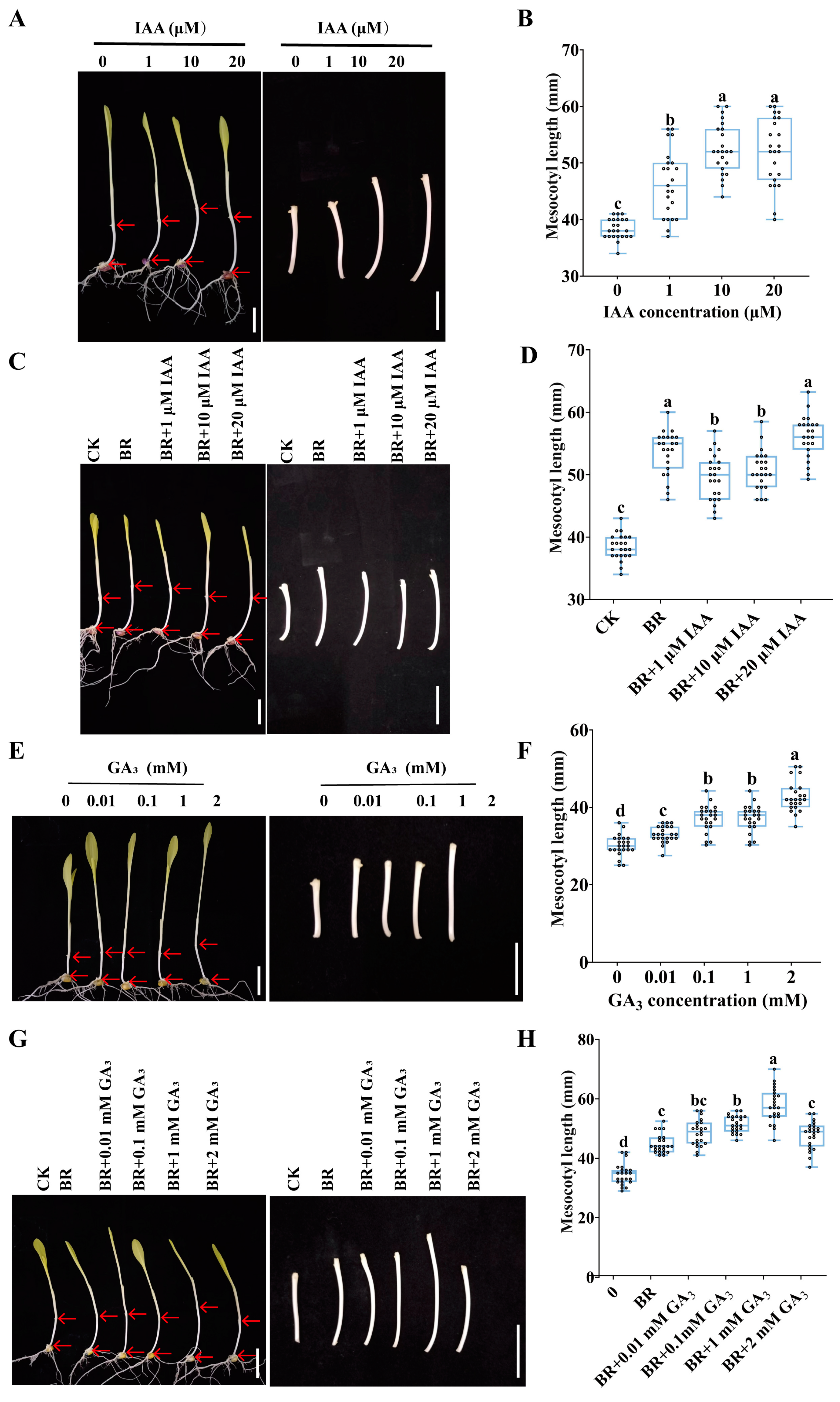
3.4. BR Coordinates with IAA and GA to Promote Mesocotyl Cell Elongation in Maize
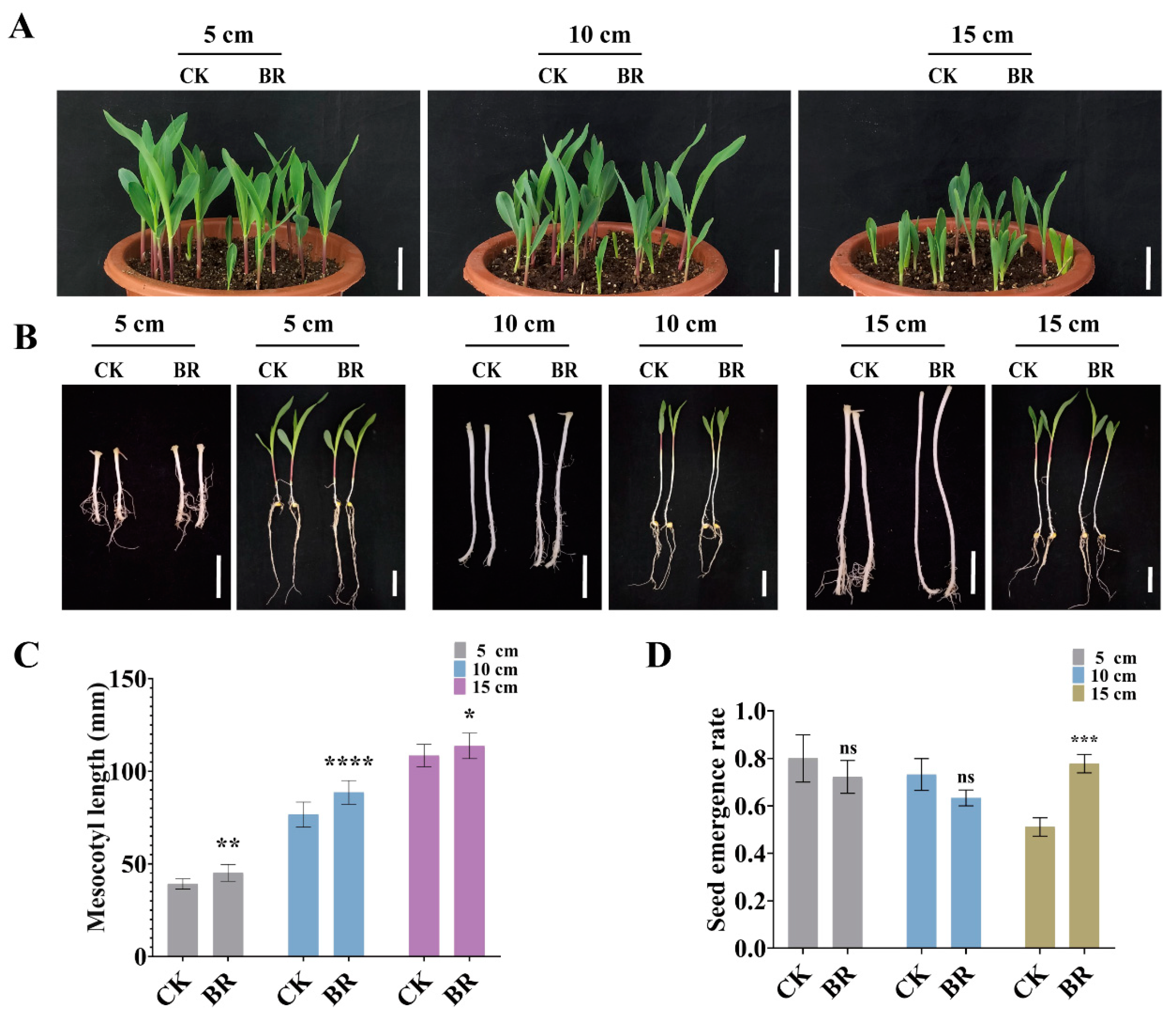
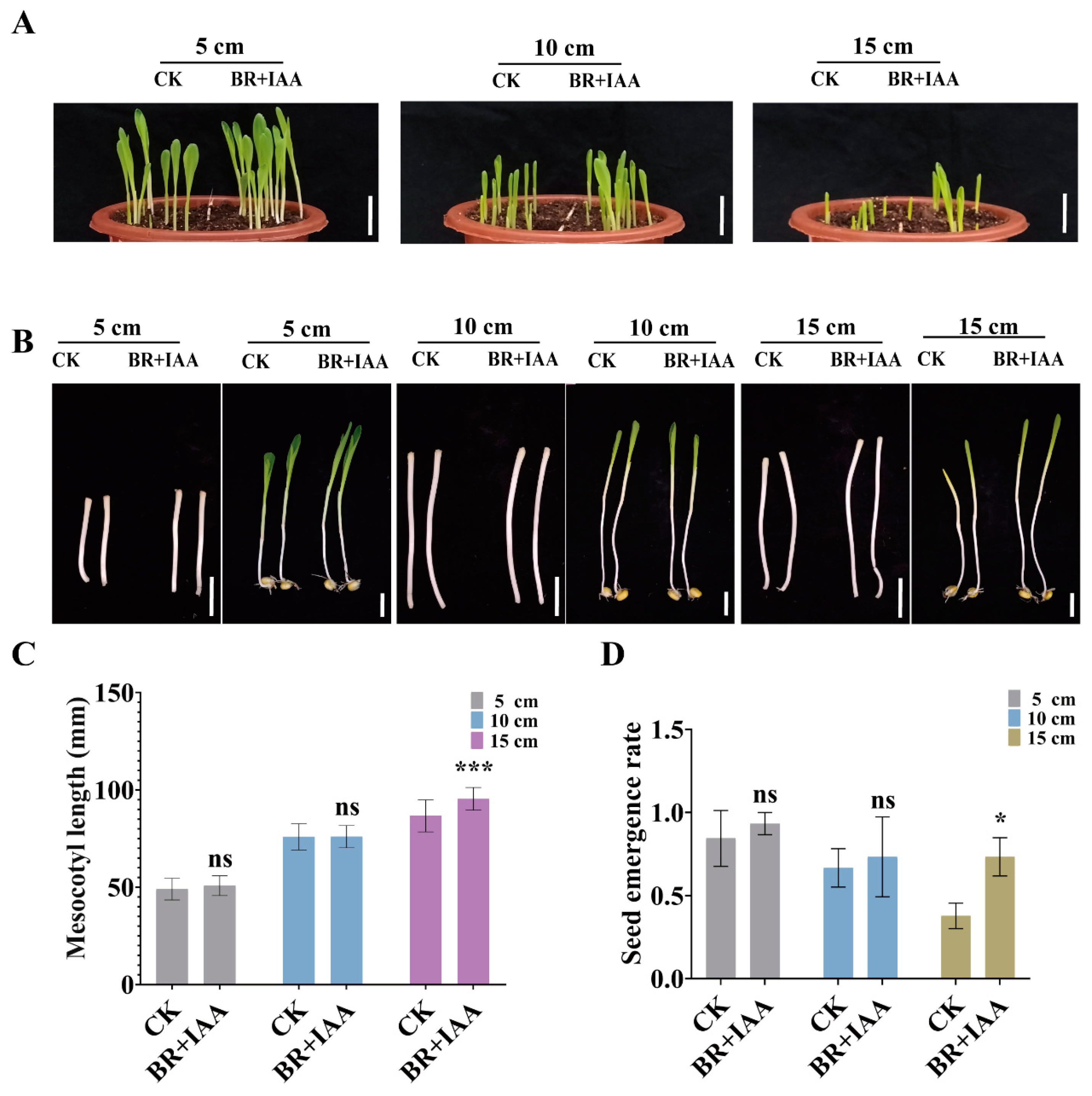
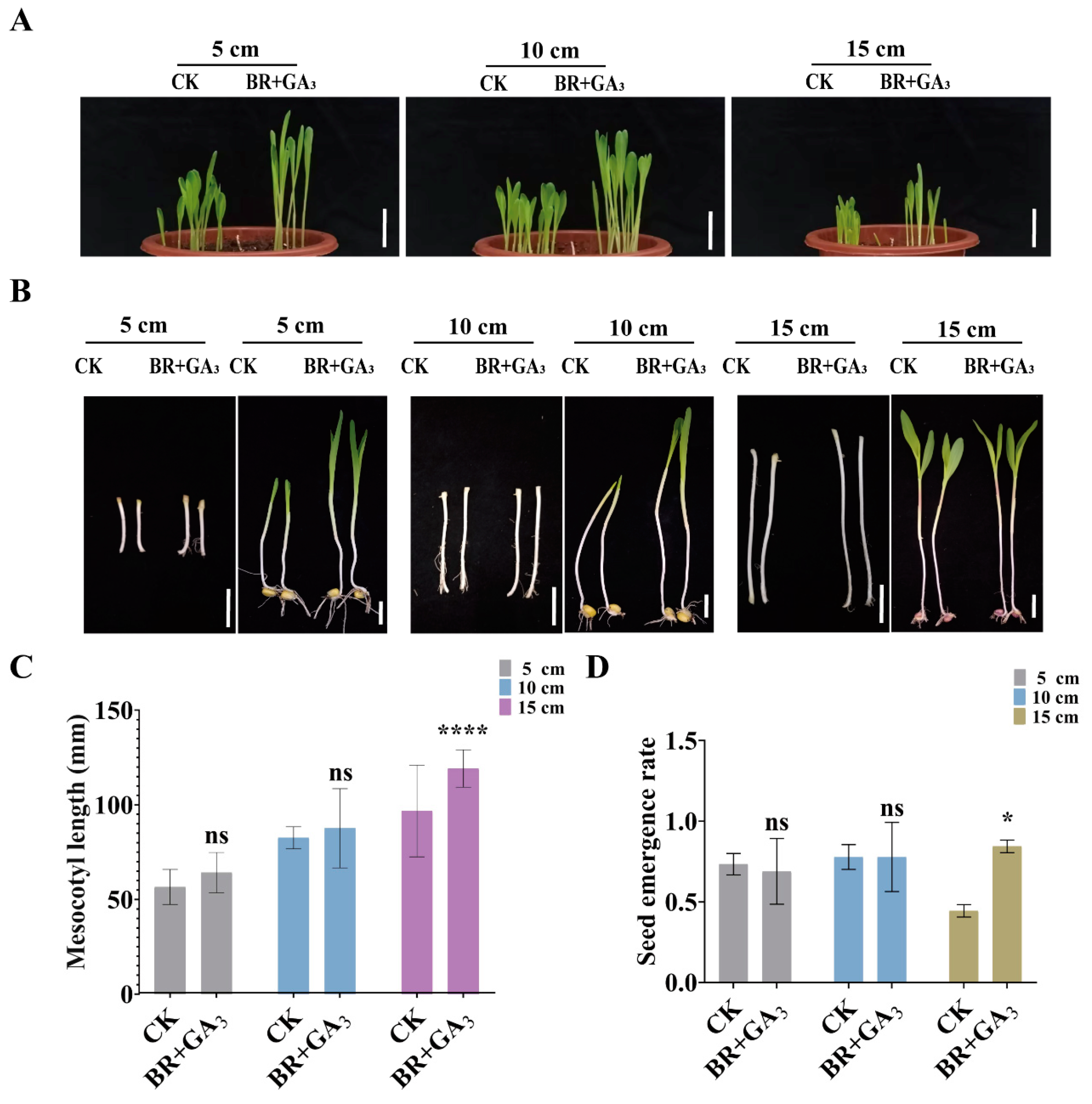
3.5. BR Synergizes with IAA and GA to Promote Maize Deep-Sowing Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Tanumihardjo, S.A.; McCulley, L.; Roh, R.; Lopez-Ridaura, S.; Palacios-Rojas, N.; Gunaratna, N.S. Maize agro-food systems to ensure food and nutrition security in reference to the Sustainable Development Goals. Glob. Food Secur. 2020, 25, 100327. [Google Scholar] [CrossRef]
- Niu, L.; Hao, R.; Wu, X.; Wang, W. Maize mesocotyl: Role in response to stress and deep-sowing tolerance. Plant Breed. 2020, 139, 466–473. [Google Scholar] [CrossRef]
- Sáenz Rodríguez, M.N.; Cassab, G.I. Primary root and mesocotyl elongation in maize seedlings: Two organs with antagonistic growth below the soil surface. Plants 2021, 10, 1274. [Google Scholar] [CrossRef]
- Sun, M.; Pu, M.; Zheng, G.; Tian, Z.; Zhang, M.; He, X.; Zhao, Y.; Zhao, X.; Zhang, X.; Yang, X.; et al. Enhanced antioxidant activity improves deep-sowing tolerance in maize. BMC Plant Biol. 2024, 24, 1229. [Google Scholar] [CrossRef]
- Qin, L.; Kong, F.; Wei, L.; Cui, M.; Li, J.; Zhu, C.; Liu, Y.; Xia, G.; Liu, S. Maize ZmSRO1e promotes mesocotyl elongation and deep sowing tolerance by inhibiting the activity of ZmbZIP61. J. Integr. Plant Biol. 2024, 66, 1571–1586. [Google Scholar] [CrossRef]
- Zhan, J.; Lu, X.; Liu, H.; Zhao, Q.; Ye, G. Mesocotyl elongation, an essential trait for dry-seeded rice (Oryza sativa L.): A review of physiological and genetic basis. Planta 2019, 251, 27. [Google Scholar] [CrossRef]
- Chen, F.; Ji, X.; Bai, M.; Zhuang, Z.; Peng, Y. Network analysis of different exogenous hormones on the regulation of deep sowing tolerance in maize seedlings. Front. Plant Sci. 2021, 12, 739101. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2019, 32, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Seto, Y.; Kameoka, H.; Yamaguchi, S.; Kyozuka, J. Recent advances in strigolactone research: Chemical and biological aspects. Plant Cell Physiol. 2012, 53, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Xu, H.; Zhang, P.; Su, X.; Zhao, H. Effects of 2,4-epibrassinolide on photosynthesis and Rubisco activase gene expression in Triticum aestivum L. seedlings under a combination of drought and heat stress. Plant Growth Regul. 2017, 81, 377–384. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Chang, J.; Wei, H.; Li, H.; Li, C.; Yang, J.; Song, Z.; Wang, Z.; Lun, J.; et al. Foliar application of 24-epibrassinolide enhances leaf nicotine content under low temperature conditions during the mature stage of flue-cured tobacco by regulating cold stress tolerance. BMC Plant Biol. 2025, 25, 77. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, Y.; Zhou, W. Molecular mechanisms of mesocotyl elongation induced by brassinosteroid in maize under deep-seeding stress by RNA-sequencing, microstructure observation, and physiological metabolism. Genomics 2021, 113, 3565–3581. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Xu, X.; Wang, R.; Liu, Y.; Huang, S.; Wei, H.; Wei, Z. Molecular Mechanisms of Diverse Auxin Responses during Plant Growth and Development. Int. J. Mol. Sci. 2022, 23, 12495. [Google Scholar] [CrossRef]
- Du, M.; Spalding, E.P.; Gray, W.M. Rapid auxin-mediated cell expansion. Annu. Rev. Plant Biol. 2020, 71, 379–402. [Google Scholar] [CrossRef]
- Majda, M.; Robert, S. The role of auxin in cell wall expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef]
- Zhao, G.-W.; Wang, J.-H. Effect of auxin on mesocotyl elongation of dark-grown maize under different seeding depths. Russ. J. Plant Physl 2010, 57, 79–86. [Google Scholar] [CrossRef]
- Yang, R.; Li, K.; Wang, M.; Sun, M.; Li, Q.; Chen, L.; Xiao, F.; Zhang, Z.; Zhang, H.; Jiao, F.; et al. ZmNAC17 regulates mesocotyl elongation by mediating auxin and ROS biosynthetic pathways in maize. Int. J. Mol. Sci. 2024, 25, 4585. [Google Scholar] [CrossRef] [PubMed]
- Castro-Camba, R.; Sánchez, C.; Vidal, N.; Vielba, J.M. Plant development and crop yield: The role of gibberellins. Plants 2022, 11, 2650. [Google Scholar] [CrossRef]
- Leng, B.; Li, M.; Mu, C.; Yan, Z.; Yao, G.; Kong, X.; Ma, C.; Zhang, F.; Liu, X. Molecular mechanism of gibberellins in mesocotyl elongation response to deep-sowing stress in sweet maize. Curr. Issues Mol. Biol. 2022, 45, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.M.; Muhlemann, J.K.; Gayomba, S.R.; Muday, G.K. RBOH-dependent ROS synthesis and ROS scavenging by plant specialized metabolites to modulate plant development and stress responses. Chem. Res. Toxicol. 2019, 32, 370–396. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.; Takeda, S.; Kobayashi, M.J.; Kimura, S.; Iizuka, A.; Imai, A.; Hishinuma, H.; Kawarazaki, T.; Mori, K.; Yamamoto, Y.; et al. Comparative analysis of the reactive oxygen species-producing enzymatic activity of Arabidopsis NADPH oxidases. Plant J. 2019, 98, 291–300. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Liu, H.; Mu, Y.; Xuan, Y.; Wu, X.; Wang, W.; Zhang, H. Hydrogen peroxide signaling in the maintenance of plant root apical meristem activity. Antioxidants 2024, 13, 554. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.C.; Han, C.; Wang, S.; Bai, M.Y.; Song, C.P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Kärkönen, A.; Kuchitsu, K. Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 2015, 112, 22–32. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2014, 5, 771. [Google Scholar] [CrossRef]
- Fry, S.C.; Dumville, J.C.; Miller, J.G. Fingerprinting of polysaccharides attacked by hydroxyl radicals in vitro and in the cell walls of ripening pear fruit. Biochem. J. 2001, 357, 729–737. [Google Scholar] [CrossRef]
- Liszkay, A.; van der Zalm, E.; Schopfer, P. Production of reactive oxygen intermediates O2−, H2O2, and OH by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 2004, 136, 3114–3123. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Structure and growth of plant cell walls. Nat. Rev. Mol. Cell Bio 2024, 25, 340–358. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Ofoedu, C.E.; You, L.; Osuji, C.M.; Iwouno, J.O.; Kabuo, N.O.; Ojukwu, M.; Agunwah, I.M.; Chacha, J.S.; Muobike, O.P.; Agunbiade, A.O.; et al. Hydrogen peroxide effects on natural-sourced polysacchrides: Free radical formation/production, degradation process, and reaction mechanism—A critical synopsis. Foods 2021, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. Methods Mol. Biol. 2010, 639, 292–298. [Google Scholar] [PubMed]
- Freitas, C.D.T.; Costa, J.H.; Germano, T.A.; de, O.; Rocha, R.; Ramos, M.V.; Bezerra, L.P. Class III plant peroxidases: From classification to physiological functions. Int. J. Biol. Macromol. 2024, 263, 130306. [Google Scholar] [CrossRef]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Sekmen Cetinel, A.H.; Yalcinkaya, T.; Akyol, T.Y.; Gokce, A.; Turkan, I. Pretreatment of seeds with hydrogen peroxide improves deep-sowing tolerance of wheat seedlings. Plant Physiol. Biochem. 2021, 167, 321–336. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Bae, H. ROS interplay between plant growth and stress biology: Challenges and future perspectives. Plant Physiol. Biochem. 2023, 203, 108032. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, Y.; Ma, Y.; Wu, X.; Wang, W.; Liu, H.; Li, X. Brassinosteroid Coordinates with ROS, Auxin and Gibberellin to Promote Mesocotyl Elongation and Deep-Sowing Tolerance in Maize. Curr. Issues Mol. Biol. 2025, 47, 668. https://doi.org/10.3390/cimb47080668
Wang Y, Li Y, Ma Y, Wu X, Wang W, Liu H, Li X. Brassinosteroid Coordinates with ROS, Auxin and Gibberellin to Promote Mesocotyl Elongation and Deep-Sowing Tolerance in Maize. Current Issues in Molecular Biology. 2025; 47(8):668. https://doi.org/10.3390/cimb47080668
Chicago/Turabian StyleWang, Yahui, Ying Li, Yuze Ma, Xiaolin Wu, Wei Wang, Hui Liu, and Xiaoming Li. 2025. "Brassinosteroid Coordinates with ROS, Auxin and Gibberellin to Promote Mesocotyl Elongation and Deep-Sowing Tolerance in Maize" Current Issues in Molecular Biology 47, no. 8: 668. https://doi.org/10.3390/cimb47080668
APA StyleWang, Y., Li, Y., Ma, Y., Wu, X., Wang, W., Liu, H., & Li, X. (2025). Brassinosteroid Coordinates with ROS, Auxin and Gibberellin to Promote Mesocotyl Elongation and Deep-Sowing Tolerance in Maize. Current Issues in Molecular Biology, 47(8), 668. https://doi.org/10.3390/cimb47080668






