Abstract
Folic acid and its derivatives (e.g., folinic acid) are a group of water-soluble compounds collectively known as vitamin B9. Synthetic folic acid is a component of dietary supplements, medications and other pharmaceuticals and fortified foods. Folinic acid (5-formyltetrahydrofolic acid) is the active metabolite of folic acid. It is used to treat vitamin B9 deficiency and as an adjunct to various combination therapies. Hypersensitivity reactions to folic acid or folinic acid are rare and occur following exposure to synthetic folic acid or its derivatives but not on natural folates. In people allergic to folates, cross-reactions are possible following exposure to folic acid analogues (including antifolates, e.g., methotrexate). The mechanism of hypersensitivity to folic acid and/or folinic acid has not been clearly established. Both IgE-dependent and non-IgE-dependent hypersensitivity reactions are likely. It is possible that folic or folinic acid is either an immunogen or a hapten. Diagnosing hypersensitivity to folic/folinic acid is difficult. There are no validated in vitro or in vivo diagnostic tests. The basophil activation test (BAT) appears to be a promising tool for diagnosing folate allergy. The aims of the manuscript were to review published clinical cases of hypersensitivity reactions to folic or folinic acid, potential mechanisms of these reactions and possible cross-allergies, and current diagnostic possibilities of folate hypersensitivity.
1. Introduction
Folates (including folic acid and folinic acid) are classified as water-soluble B vitamins. The term “folates” is a general term used to describe a number of vitamins from the B9 family (sometimes also called vitamin B11). The B9 vitamin group includes both synthetic folic acid and its naturally occurring derivatives. Folic acid is a form of vitamin B9 that, due to its chemical stability, is used in vitamin supplement tablets and cell culture media [1]. Folic acid plays a key role in many metabolic processes, including nucleic acid synthesis [2]. As a substance not produced by animals, it must be supplied to the body through food (folates) or supplemented with pharmaceuticals, dietary supplements or vitamin supplements (folic acid) [3,4,5]. Folic acid is also used as a carrier for various drugs and chemotherapeutics, enabling the delivery of folate-bound substances into cells via endocytosis via the folate receptor (FR) [6,7,8,9].
Vitamin B9, especially of natural origin, does not appear to pose a significant risk of inducing a hypersensitivity reaction and is not generally considered a primary cause of allergy. However, it may be a substance worth considering in the diagnostic process for hypersensitivity reactions with a difficult-to-identify causative factor [10].
The aims of the manuscript were to review published clinical cases of hypersensitivity reactions to folic or folinic acid, to present potential etiological mechanisms of these reactions and possible cross-allergies, clinical implications of these reactions and to analyze current diagnostic possibilities of folate hypersensitivity.
This narrative review included publications on hypersensitivity/allergy to folinic acid and/or folic acid published up to July 2025 (in English and non-English). Publications were searched for keywords in PubMed, Google Scholar, etc., and in popular search engines (e.g., Google) using the terms “hypersensitivity” or “allergy” or “anaphylaxis” and “folic acid” or “folinic acid” and by analyzing the references of the reviewed publications. No artificial intelligence (AI) was used for data retrieval and analysis.
2. Folic Acid and Folinic Acid
2.1. History of Discovery and General Information
The discovery of folate is owed to Lucy Wills’s (1888–1964) long-standing research into the cause of severe anemia in pregnant women. Between 1929 and 1931, Wills investigated the cause of severe anemia in pregnant women in Bombay, India. Clinical symptoms of pernicious anemia in pregnancy included swelling of the face, ankles and feet, weakness, hypotension, intermittent fevers, sore mouth and tongue, and diarrhea. In extreme cases, it led to death. Microscopic examination of blood smears revealed macrocytosis [11,12,13,14]. Although the symptoms and appearance of erythrocytes resembled the recently described pernicious anemia [15,16,17], hematological disorders in pregnancy were considered a distinct disease entity [12]. Due to the climatic and sanitary conditions in India, Wills initially focused on the infectious etiology of this disease. After ruling out this possibility, she turned her attention to the nutritional causes of anemia. The concept of nutritional deficiencies causing anemia was linked both to the analogy to the effects of vitamin B12 deficiency and to the results of analysis of women’s diets, which were deficient in both caloric value and quality of the consumed foods. L. Wills’s research led to the discovery of an essential factor of dietetic origin, the deficiency of which results in the occurrence of anemia during pregnancy. Wills identified this factor as present in yeast and liver, which, when introduced into the diet of affected women, effectively treated the symptoms of anemia [18]. Wills published her observations, research results and descriptions of effective therapy in 1930–1931 in a series of papers [19,20,21,22,23,24,25].
This then-new hematopoietic factor, present in yeast and liver, which treated tropical macrocytic anemia in humans, was initially called the “Wills factor.” Further studies of the chemical and biological properties of this substance led to its recognition as a vitamin, initially called vitamin M (from the English word “monkey”) or vitamin B(c) (from the English word “chick”). These names stemmed from the observation that this factor was effective in preventing nutritional pancytopenia in monkeys and in treating experimental anemia in chickens. Folic acid was isolated in 1941 by Herschel Kenworthy Mitchell from spinach leaves [26]. Mitchell then proposed the name folic acid, from the Latin word “folium,” meaning the leaf from which the vitamin was extracted [26,27,28]. Since then, folic acid has been produced by chemical synthesis, microbiological synthesis or metabolic engineering on an industrial scale [29,30,31].
2.2. Physical and Chemical Properties, Biological Function, Metabolism
Folic acid (pteroylglutamic acid) is an N-acylamino acid with a molecular weight of 441.4 g/mol. It is a complex chemical compound whose molecule contains three components: a pteridine derivative (2-amino-4-hydroxy-6-methylpteridine), p-aminobenzoic acid (PABA) and glutamic acid (Figure 1) [32,33].
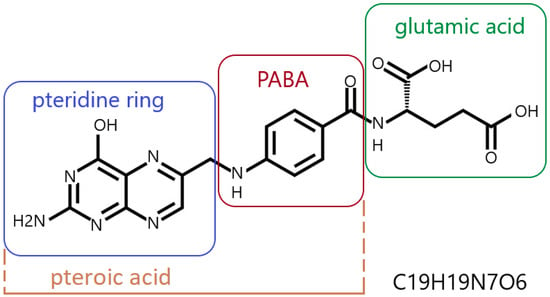
Figure 1.
Folic acid—structural formula and empirical formula (Hill notation); PABA—para-aminobenzoic acid; author’s own figure based on [3,7,34,35,36,37,38].
A folic acid molecule can contain more than one glutamic acid residue. It typically occurs as a polyglutamate conjugate, with 2 to 9 glutamic acid residues attached to a pteroyl acid residue (Figure 1) [7,34,35]. Pure folic acid is a synthetic compound, presenting as odorless, orange-yellow needles or platelets. It darkens and carbonizes at temperatures around 250 °C [32]. Under natural conditions, folic acid forms folates, which are derivatives of folic acid with varying degrees of pteridine ring oxidation and varying numbers of glutamic acid residues [6]. Folic acid is synthesized and converted into folates by higher plants, yeasts and some bacteria (including gastrointestinal bacteria). Animals do not synthesize folic acid because they do not produce PABA and do not have the ability to form a pteroyl residue link with glutamate [36,39,40].
Folic acid, regardless of its natural or synthetic origin, is not biologically active. It is only converted into active forms during metabolism. Active forms of folates are formed by the reduction of folic acid to dihydrofolate (DHF) (Figure 2A) and then to tetrahydrofolate (THF) (Figure 2B) in reactions catalyzed by dihydrofolate reductase (DHFR). The main folate found in blood is 5-methyltetrahydrofolate (5-methylTHF) (Figure 2C) [34,41,42,43,44].
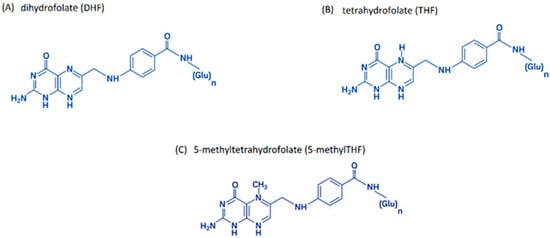
Figure 2.
The main biologically active folic acid derivatives: (A) dihydrofolate (DHF); (B) tetrahydrofolate (THF); (C) 5-methyltetrahydrofolate (5-methylTHF); author’s own figure based on [4,41,42,43,44].
Folate circulates in the blood as monoglutamate derivatives. Folate is transported into cells via several pathways: folate receptor (FR)-mediated endocytosis, reduced folate carrier (RFC) or proton-coupled folate transporter (PCFT) [7,9,35,41]. Active folate forms are essential coenzymes that play a key role in numerous metabolic processes ensuring the proper functioning of all body cells (Box 1) [40,45,46,47].
Box 1. Important processes in which tetrahydrofolic acid participates as a coenzyme involved in the transfer of one-carbon fragments [41,48].
- •
- Purine and pyrimidine base synthesis (essential for nucleic acid synthesis)
- •
- Nucleotide synthesis
- •
- Protein synthesis
- •
- Phospholipid synthesis
- •
- Protein methylation
- •
- Deoxyribonucleic acid (DNA) methylation
- •
- Remethylation of homocysteine to methionine (methionine cycle)
- •
- Conversion of histidine to glutamic acid
Folinic acid or levofolinic acid (leucovorin) (Figure 3) is an active form of folic acid (5-formyltetrahydrofolic acid) used in the treatment of anemia or as an adjunct to cancer therapy with some chemotherapeutic agents (e.g., methotrexate and 5-fluorouracil) [49,50].
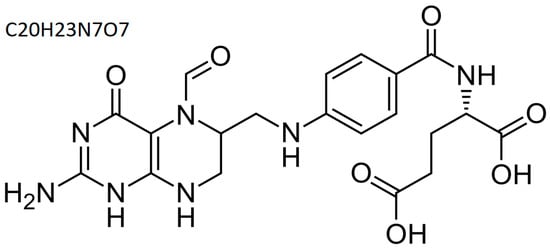
Figure 3.
Folinic acid—structural and summary formula; author’s own figure based on [51].
This strategy allows for the delivery of a biologically active form to the body, bypassing endogenous metabolic pathways that may be inactive or intentionally blocked during therapeutic procedures. The use of folinic acid in treatment or as adjunctive therapy accelerates the achievement of the intended therapeutic effects (e.g., in the treatment of anemia) and/or reduces the toxicity of the therapy used (e.g., in cancer treatment) [43,50,51].
Adequate availability of active forms of folate is particularly important during phases of rapid cell division, including periods of rapid growth and development, pregnancy and erythropoiesis [52]. Due to its participation in metabolic processes essential for cell growth and proliferation, as well as metabolic changes occurring in cancer cells aimed at maximizing nutrient utilization (e.g., increased expression of the folate receptor on the cell surface), folic acid supports cancer cell proliferation, which promotes tumor growth and development. It is believed to play a protective role in cancer development [53,54,55]. Some therapeutic strategies used in the treatment of cancer utilize the overexpression of a receptor essential for folate endocytosis into the cell and poorly expressed on healthy cells to deliver drugs to cancer cells [7].
2.3. Sources, Requirement and Deficiency Symptoms
Humans are unable to synthesize folic acid endogenously, therefore vitamin B9 must be fully supplied with food or supplemented with vitamin preparations [4,37]. The recommended daily intake of folic acid (Table 1) depends on age and, in women, on the physiological status (e.g., pregnancy, lactation) [37]. The requirement for folic acid may increase in certain pathologies and during the use of specific therapies, especially those that disrupt folic acid metabolism (e.g., cytostatics) [37,56,57].

Table 1.
Recommended daily intake of folic acid according to the recommendations of the Food and Nutrition Board (FNB) at the National Academies of Sciences, Engineering, and Medicine [58].
Natural folic acid is commonly found (as various types of folates) in a wide range of foods (Table 2).

Table 2.
Content of natural folates in selected food products [59].
Natural dietary folates are less stable than synthetic folic acid (e.g., a component of drugs, supplements or fortified foods). Folic acid losses during harvesting, storage, distribution and culinary processing of food products can be significant. It is estimated that vitamin B9 loss during cooking of vegetables and fruit reaches as much as 50–80%. Dietary folates are also absorbed more slowly than synthetic folic acid, and their bioavailability depends largely on the type of food, meal composition and its general physicochemical characteristics (e.g., pH). It is estimated that 170 μg of folate is equivalent to 100 μg of synthetic folic acid. This means that the dietary equivalent of folate is its content in food multiplied by 1.7 [60]. Due to the widespread occurrence of folates in staple foods, despite their low stability and variable bioavailability in natural sources, it is believed that a diet containing at least three servings of fresh green vegetables per day should meet the daily requirement for vitamin B9 under physiological conditions. The exceptions are women who are pregnant, lactating or preparing for pregnancy [61].
Due to the widespread presence of folates in staple foods, folic acid deficiency is a rare phenomenon, although it is suspected that its scale may be underestimated. Most often, this condition results from insufficient intake (unbalanced diet), malabsorption, increased physiological demand or increased demand resulting from pharmacotherapy [38,62,63]. Folic acid deficiency induces inhibition of proliferation and interruption of the cell cycle. This leads to impaired cell proliferation and maturation and accelerates the aging process [48]. Clinically, folic acid deficiency leads to megaloblastic anemia [64,65,66], pancytopenia [67,68] and neuropsychiatric symptoms [69,70], including depressed mood [71,72] and cognitive impairment [73]. Folic acid deficiency may also contribute to complicated pregnancy, including neural tube defects in the fetus [74,75,76].
3. Hypersensitivity to Folic Acid and/or Folinic Acid
The first published clinical description of a hypersensitivity reaction to folic acid dates back to 1949 [77]. Since then, fewer than forty documented cases of allergic hypersensitivity to folic acid (Table 3) or folinic acid (Table 4) have been reported, with varying degrees of severity and symptom manifestations, including anaphylactic shock. Interestingly, most of these reactions occurred in women. Reactions were usually associated with folic or folinic acid supplementation for prophylactic purposes (in pregnant women and those planning a pregnancy), for the treatment of anemia or during cancer chemotherapy. No case of hypersensitivity to folates naturally occurring in food has been reported to date.

Table 3.
Hypersensitivity to folic acid in clinical cases (based on available data, reference numbers are in the table).

Table 4.
Hypersensitivity to folinic acid in clinical cases—leucovorin was administered intravenously (i.v.) as a supplement to cancer treatment with chemotherapeutic agents (based on available data, reference numbers are in the table).
According to reported case reports, hypersensitivity to folic acid (Table 3) or folinic acid (Table 4) can have a variety of clinical presentations. Mild dyspeptic symptoms, a feeling of heat, hives of varying degrees of severity, localized (usually facial) or generalized edema, as well as severe anaphylactic reactions are possible.
An analysis of the reported folic acid hypersensitivity reactions (Table 3) highlights the fact that all events occurred after exposure to synthetic folic acid administered orally or intravenously in the form of various vitamin medications, vitamin supplements or foods fortified with synthetic vitamin B9. There are no reports of hypersensitivity to folates naturally occurring in food products. There have also been no reports of hypersensitivity following local exposure or allergic reactions associated with occupational exposure to folic acid (e.g., during industrial synthesis).
Folinic acid (Table 4) caused hypersensitivity reactions following intravenous administration. No such situation has been described after oral administration of this substance or after local exposure. Furthermore, all described cases (Table 4) involved folinic acid (leucovorin) administered during the treatment of cancer, usually colon cancer, and hypersensitivity reactions always required differentiation from a possible allergy to the chemotherapeutic agents used. It is also worth noting that, in addition to the symptoms typical of this type of clinical event (such as shortness of breath, swelling, redness or hives), symptoms associated with the nervous system (e.g., numbness in the extremities), pain limited to certain areas of the body (e.g., the back) or generalized pain were also observed.
The cause of the hypersensitivity reaction appears to be synthetic folic acid, which is a component of dietary supplements, medications or added to foods to enhance their nutritional value. Interestingly, however, folates naturally occurring in food products likely do not have allergenic properties.
4. Probable Mechanisms of Folic Acid or Folinic Acid Hypersensitivity
No definitive mechanism for hypersensitivity to folic acid or its derivatives (including folinic acid) has yet been described. The type and nature of reported clinical events (Table 3 and Table 4) suggest that more than one pathogenetic pathway may be involved in vitamin B9 allergy. Mixed mechanisms of folate hypersensitivity cannot be ruled out either [10,85,92,107].
4.1. Folic Acid
For example, Nucera et al. [92] described three different cases of folic acid hypersensitivity caused by oral supplementation with this vitamin (details in Table 3). The intensity and type of clinical symptoms, the course of the reaction and the results of diagnostic tests suggest different pathogenetic pathways for these events. It is likely that two of these cases were IgE-mediated, while the third was non-IgE-mediated and was classified by Nucera et al. [92] as a persistent drug eruption.
Analyzing the clinical cases of hypersensitivity reactions to folic acid presented in Table 3, it can be seen that in many of them, both the clinical course of the reaction and the results of the diagnostic tests performed indicate with a high degree of probability the IgE-dependent nature of these reactions (Figure 4).
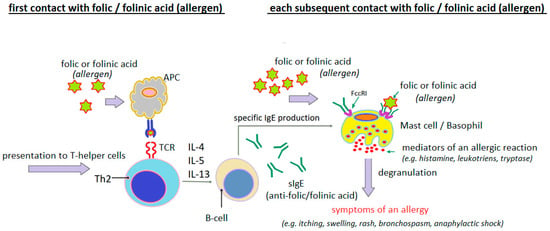
Figure 4.
Probable mechanism of IgE-dependent hypersensitivity reaction to folic acid or folinic acid as a complete allergen (author’s own figure).
The presence of specific IgE antibodies to folic acid was demonstrated by Dykiewicz et al. [85] and Gaeta et al. [93]. Dykiewicz et al. [85] first detected IgE antibodies specific to the folic acid/human serum albumin (FA/HSA) conjugate in a woman who had experienced anaphylaxis twice after taking various multivitamin mixtures with folic acid. They then performed inhibition tests of the sIgE antibodies detected in the patient’s serum using HSA, FA and FA/HSA. Only the FA/HSA conjugate blocked the detected antibodies. Neither FA nor HSA used separately were effective. This suggests that FA acts as a hapten, acquiring the properties of a full antigen (allergen) only after binding to an endogenous protein (usually albumin) (Figure 5).
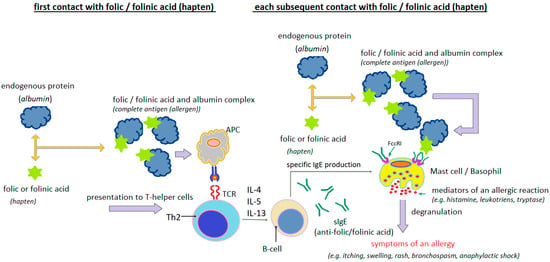
Figure 5.
Probable mechanism of IgE-dependent hypersensitivity reaction to folic acid or folinic acid as a hapten (author’s own figure).
Albumin is a transporter of folic acid in the blood. Various types of folic acid–albumin bonds are possible (Figure 6). The type and site of binding influence the stability of the entire complex and the conformation of albumin [110,111,112,113,114,115,116].
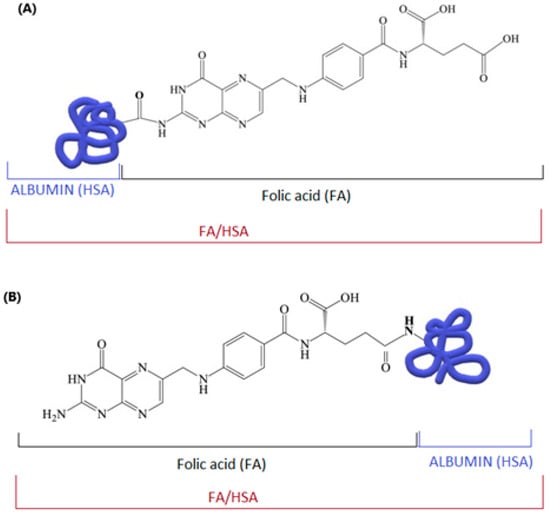
Figure 6.
Possible structures of the “folic acid–albumin” conjugate: (A) via the amino group of folic acid and the carboxyl group of albumin; (B) via an amide bond between the carboxyl group of the folic acid residue and the amino group of albumin; author’s own figure based on [113,117,118].
The hapten theory of folic acid is plausible, although there are also counterintuitive factors. According to Gaeta et al. [93], allergy to folic acid and/or its derivatives is likely associated with an IgE-dependent mechanism, in which the sensitizing factor is free (unbound to protein) folic acid or its derivatives. These researchers point to the lack of clear data confirming the phenomenon of endogenous haptenization of folic acid or its derivatives in human metabolism and the fact that free folic acid stimulates histamine release from basophils. Furthermore, as reported by Gaeta et al. [93], drugs with a similar molecular weight to folic acid can cause sensitization via an IgE-dependent mechanism without prior haptenization.
It is also noteworthy that, despite the widespread natural occurrence of folates in food, reactions to this group of compounds present in natural products have never been reported. Reactions that developed after consuming food were associated with the consumption of foods artificially enriched with synthetic folic acid [10,86,87,93]. It has also been noted that people sensitive to folic acid supplements can also consume high-folate foods (e.g., spinach) without any adverse reactions [91]. This situation is likely due to the fact that pteroylglutamic acid (folic acid) is almost always naturally present as polyglutamic acid, while pharmaceutical products, supplements and fortified foods use the monoglutamine form (pteroylmonoglutamate) [84,119].
Multi-stage folate metabolism occurs within enterocytes, where monoglutamate folic acid is reduced, methylated and released into the bloodstream as 5-methyltetrahydrofolate monoglutamate. This causes folates from natural sources to be absorbed very slowly (their bioavailability is low), while free folic acid (monoglutamate) does not penetrate into the plasma. Therefore, the concentration of folates entering the blood from natural sources may be too low to trigger such reactions [79]. Synthetic folic acid has a significantly higher bioavailability than natural folates and can be rapidly absorbed by enterocytes [120]. Monoglutamates delivered rapidly in high concentrations may exhaust the body’s capacity for methylation and reduction to 5-ethyltetrahydrofolate (due to saturation of the enzymatic systems involved in this process), resulting in the absorption of pteroylglutamic acid monoglutamates into the circulation [79]. Studies show that unmetabolized synthetic folic acid can be detected in the blood even at doses as low as 200 μg [10]. Assuming a hapten role for folic acid, these absorbed, unmetabolized monoglutamine molecules bind to endogenous proteins, becoming immunogenic and potentially causing immediate allergic reactions [84]. This mechanism seems particularly likely in the case of intensive supplementation with synthetic folic acid due to the saturation of gastrointestinal enzymes following excessive exposure to a high dose of folic acid [10].
4.2. Folinic Acid
The probable mechanisms of hypersensitivity to folinic acid (5-formyltetrahydrofolic acid, leucovorin) were analyzed by Apraxine et al. [107]. These researchers based their findings on the analysis of two clinical cases they reported and previously published reports (Table 4). They noted that hypersensitivity to folinic acid can likely occur via both IgE-dependent and -independent mechanisms. A mixed nature of hypersensitivity to leucovorin cannot be ruled out. Regardless of the pathogenetic mechanism of folinic acid hypersensitivity, the reaction rate may also be caused by the route of exposure. Folinic acid is usually administered intravenously, which means it is not subject to intestinal folic acid metabolism and is delivered rapidly at high concentrations, which may be an additional factor predisposing to the development of various adverse reactions, including hypersensitivity reactions [107]. It should be noted that investigating the mechanisms of hypersensitivity to folinic acid is significantly hampered by the fact that these reactions have so far occurred in individuals treated for cancer, primarily colorectal cancer (Table 4). Both the underlying disease and the treatment, which is very burdensome for the patient’s body, may influence the development of the immune response and predispose to the occurrence of various complications, including hypersensitivity reactions [121,122,123,124]. Furthermore, it should be noted that in the context of hypersensitivity reactions during cancer treatment, the therapeutic team’s attention is usually focused on the chemotherapeutic agents or biological drugs used [103], while other, less obvious drugs and adjuvants are often overlooked as unlikely factors that could cause allergy [107,125,126,127,128]. It also cannot be ruled out that hypersensitivity reactions develop independently to both the chemotherapy agent and folinic acid [109].
5. Hypersensitivity to Folic or Folinic Acid—Cross-Allergies to Folic Acid Analogues
Folic acid analogues (antifolates) are chemical compounds that have a structure similar to folic acid and different biological activity. Antifolates are classified as classic (e.g., methotrexate, pemetrexed and raltitrexed) and non-classic (e.g., sulfonamides) (Figure 7).
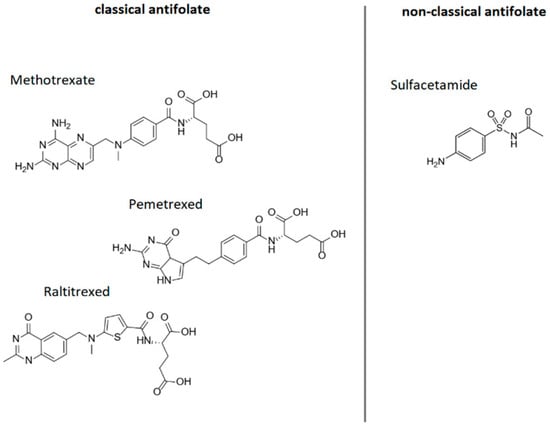
Figure 7.
Folic acid analogues; author’s own figure based on [42,129,130,131,132].
These substances are also called antimetabolites. Antimetabolites inhibit enzymes involved in the synthesis of nitrogenous bases (e.g., dihydrofolate reductase (DHFR), a key enzyme in folic acid metabolism) by competing with folic acid. Blocking DHFR stops the conversion of folic acid to its active form, tetrahydrofolic acid, which ultimately results in the inhibition of DNA and RNA synthesis. Interrupting DNA replication prevents further cell division and results in, among other things, inhibiting the proliferation of cancerous tissue. In medicine, folic acid analogues are mainly used as anticancer and immunosuppressive drugs [42,129].
Available literature data indicate that antifolates, especially the classic ones, should be considered as a probable factor causing hypersensitivity reactions, in the mechanism of cross-reactivity, in people allergic to folic acid or its derivatives, which results from the similarity in the structure of a significant fragment of the molecule [10,85,90,93].
Nishitani et al. [90] described the case of a 42-year-old woman who developed generalized urticaria and shortness of breath 2 h after taking a folic acid supplement. The patient had never experienced such symptoms before, although she had taken folic acid preparations approximately 2 years earlier. The patient underwent skin prick testing with the suspected supplement and its components: folic acid (5 mg/mL), riboflavin, biotin, niacin, thiamine, pyridoxine hydrochloride, calcium pantothenate and yeast. Skin prick testing was also performed with folic acid analogues: methotrexate (2 mg/mL) and folinic acid (1 mg/mL). Positive reactions were found to the vitamin supplement, folic acid and methotrexate, but not to folinic acid. These researchers also performed a histamine release test (HRT) with folic acid, folinic acid and methotrexate, obtaining positive results for folic acid and methotrexate, but not for folinic acid. It therefore appears that cross-hypersensitivity reactions between folic acid and its analogues are possible, although not necessarily with all derivatives. Similar conclusions are provided by the results of inhibition tests performed by Gaeta et al. [93], who observed that IgE antibodies specific for folic acid are blocked by both folic acid (strong inhibition) and methotrexate (moderate inhibition). Studies by other authors [10,85,93] also seem to confirm that the phenomenon of allergic cross-reactivity to methotrexate in individuals initially allergic to folic acid is possible and may have broad clinical implications. Gaeta et al. [93] suggest that patients with a history of folic acid or folate allergy should be considered at increased risk of hypersensitivity to methotrexate and other classic antifolates (e.g., pemetrexed), while patients with immediate-type reactions to methotrexate and pemetrexed may be at increased risk of reaction to synthetic folic acid found in dietary supplements and fortified foods.
There is no data on cross-sensitivity to non-classic antifolates (e.g., sulfonamides). This group of drugs can cause hypersensitivity reactions [133,134]; however, allergy to folic acid has not yet been considered as a potential cause of allergic response after exposure to sulfonamides.
6. Hypersensitivity to Folic and/or Folinic Acid—Current Diagnostic Options
Diagnosing hypersensitivity to folic acid or folinic acid is fraught with difficulties, stemming in part from the fact that these reactions are rare. This means that folates are not considered as possible causative factors at the beginning of the diagnostic process.
A significant methodological problem in the diagnosis of folate hypersensitivity is the lack of standardized tests for both in vitro and in vivo testing. Although the presence of sIgE antibodies to folic acid has been described, these tests were performed using proprietary immuno(dot)blot and ELISA (enzyme-linked immunosorbent assay) assays [85,93]. Commercial, validated kits for detecting folic acid-specific IgE are not available.
It is noticeable that the diagnostic process in clinical cases reported by various authors (Table 3 and Table 4) typically involved various types of skin tests (prick and intradermal) with available pharmaceutical preparations of folic acid and/or its derivatives. This strategy appears to be the most accessible diagnostic test when folate hypersensitivity is suspected based on a carefully collected clinical history.
The basophil activation test (BAT) appears to be a good solution for diagnosing allergy to folic acid or its derivatives. This procedure was successfully used by Gouveia et al. [96]. This technique appears to be very promising; however, the lack of a folic acid solution validated for the BAT test and the lack of reference values significantly limits its use in routine diagnosis of hypersensitivity to folic acid or its derivatives.
It appears that the introduction of standardized diagnostic procedures and the development of validated diagnostic tools (e.g., folic and folinic acid solutions) for in vivo diagnostics (such as skin prick tests, epidermal patch tests, provocation tests) and in vitro diagnostic kits (e.g., determination of folate-specific IgE and BAT) are necessary to significantly improve and enhance the diagnostics of sensitivity to folic acid or folinic acid. This, in turn, may increase the diagnostic effectiveness of folate hypersensitivity, favorably impacting appropriate therapeutic management in individuals who require folate supplementation, and support understanding of the pathogenic mechanisms of vitamin B9 hypersensitivity.
7. Summary
According to available data, hypersensitivity to folic acid and/or folinic acid is a relatively rare phenomenon. Clinically, these reactions have a very diverse range of manifestations. They range from self-limiting, mild symptoms (usually generalized or localized sensations of heat, hives or itching) to severe anaphylactic reactions, which occur sporadically and are more likely to occur after intravenous administration of vitamin B7. The spectrum of symptoms of folinic acid hypersensitivity has also included neurological symptoms (e.g., impaired sensation in the legs) or pain (e.g., back pain). It is worth noting that reactions can occur after both oral and intravenous administration. Intravenous administration is more likely to involve folinic acid, but this may be due to the specific therapy in which this derivative is used (often in combination with chemotherapy in cancer treatment). It is also worth noting that folates, naturally found in food, are unlikely to cause hypersensitivity. Reactions that have developed as a result of consuming various food products have always been associated with the consumption of foods fortified with synthetic folic acid or multivitamin drinks.
The mechanism of hypersensitivity reactions to folic or folinic acid is not entirely clear. Both IgE-dependent immediate reactions and other non-IgE-mediated pathomechanisms have been implicated. It is also unclear whether folic acid is a standalone allergen or whether it functions as a hapten, acquiring the characteristics of a full-fledged antigen only after binding to endogenous proteins. It appears that intensive intake of synthetic folic or folinic acid during supplementation may be a significant predisposing factor to hypersensitivity to these substances.
Hypersensitivity to folic or folinic acid should be considered when diagnosing unclear allergic reactions with a difficult-to-determine causative agent, associated with the use of vitamin preparations, vitamin-fortified foods, combination therapies involving folates and in situations suggesting the possibility of a hypersensitivity reaction due to cross-reactivity (e.g., during methotrexate therapy).
Diagnosing folate hypersensitivity is difficult primarily due to the lack of standardized diagnostic tests for detecting folate-specific IgE antibodies and the lack of commercially available pure folic acid solutions for skin testing or allergen challenge in BAT. BAT appears to be a very promising tool in diagnosing folate hypersensitivity, but the stimulation solutions, the procedure and the clinical interpretation of results require standardization.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef]
- Stover, P.J. Folate biochemical pathways and their regulation. In Folate in Health and Disease, 2nd ed.; Bailey, L.B., Ed.; CRC Press, Taylor & Francis Group: Gainesville, FL, USA, 2009; pp. 49–74. [Google Scholar]
- Kurowska, K.; Kobylińska, M.; Antosik, K. Folic acid—Importane for human health and its role in COVID-19 therapy. Ann. Natl. Inst. Hyg. 2023, 74, 131–141. [Google Scholar] [CrossRef]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef]
- Merrell, B.J.; McMurry, J.P. Folic Acid [Updated 8 August 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554487/ (accessed on 16 June 2025).
- Zhao, R.B.; Matherly, L.H.; Goldman, I.D. Membrane transporters and folate homeostasis: Intestinal absorption and transport into systemic compartments and tissues. Expert Rev. Mol. Med. 2009, 11, e4. [Google Scholar] [CrossRef] [PubMed]
- Danenberg, P.V.; Gustavsson, B.; Johnston, P.; Lindberg, P.; Moser, R.; Odin, E.; Peters, G.J.; Petrelli, N. Folates as adjuvants to anticancer agents: Chemical rationale and mechanism of action. Crit. Rev. Oncol. Hematol. 2016, 106, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Progress of Folic Acid-Folate Receptor as Drug Carriers in Targeted Drug Delivery System. SHS Web Conf. 2022, 144, 01002. [Google Scholar] [CrossRef]
- Ge, Y.; Kwon, M.H.; Kou, F.; Uthamapriya, R.A.; Zhang, P.; Lee, D.J.; Yang, R.; Bao, H.; Palanisamy, S.; You, S.G. Folic-acid-targeted drug delivery system implementing Angelica gigas polysaccharide: A potential strategy for colorectal cancer treatment. Int. J. Biol. Macromol. 2024, 283, 137653. [Google Scholar] [CrossRef]
- Schrijvers, R.; Chiriac, A.M.; Demoly, P. Allergy Workup for Suspected Folic Acid Hypersensitivity. J. Investig. Allergol. Clin. Immunol. 2015, 25, 233–236. [Google Scholar]
- Lynch, L. Treatment of Anemia during Pregnancy, by Lucy Wills; Embryo Project Encyclopedia (2017-04-20); 1931. ISSN: 1940-5030. Available online: https://hdl.handle.net/10776/11484 (accessed on 16 July 2025).
- Franklin, J.L. Marmite: Its place in medical history, Lucy Wills, and the discovery of folic acid. Hektoen Int. 2022, 14. Available online: https://hekint.org/2022/05/26/marmite-its-place-in-medical-history-lucy-wills-and-the-discovery-of-folic-acid/ (accessed on 16 July 2025).
- Firkin, B.G. Historical Review: Some Women Pioneers in Haematology. Br. J. Haematol. 2000, 108, 6–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roe, D.A. Lucy Wills (1888–1964) A Biographical Sketch. J. Nutr. 1978, 108, 1377–1383. [Google Scholar] [CrossRef]
- Castle, W.; Locke, E. Observations on the etiological relationship of achylia gastrica to pernicious anemia (abstract). J. Clin. Investig. 1928, 6, 2–3. [Google Scholar]
- Kass, L.; William, B. Castle and intrinsic factor. Ann. Intern. Med. 1978, 89, 983–991. [Google Scholar] [CrossRef] [PubMed]
- John, M.S.; Molloy, A.M. The Discovery of Vitamin B12. Ann. Nutr. Metab. 2012, 61, 239–245. [Google Scholar] [CrossRef]
- Bastian, H. Lucy Wills (1888–1964), the life and research of an adventurous independent woman. Commentaries on the history of treatment evaluation. JLL Bull. 2007. Available online: https://www.jameslindlibrary.org/articles/lucy-wills-1888-1964-the-life-and-research-of-an-adventurous-independent-woman/ (accessed on 10 July 2025).
- Wills, L.; Mechta, M.M. Studies in ‘pernicious anaemia’ of pregnancy. Part I Preliminary report. Indian J. Med. Res. 1930, 17, 777–792. [Google Scholar]
- Wills, L.; Talpade, S.N. Studies in ‘pernicious anaemia’ of pregnancy. Part II A survey of dietetic and hygienic conditions of women in Bombay. Indian J. Med. Res. 1930, 18, 283–306. [Google Scholar]
- Wills, L.; Mechta, M.M. Studies in ‘pernicious anaemia’ of pregnancy. Part III Determination of normal blood standards for the nutritional laboratory’s stock albino rat. Indian J. Med. Res. 1930, 18, 307–317. [Google Scholar]
- Wills, L.; Mechta, M.M. Studies in ‘pernicious anaemia’ of pregnancy. Part IV The production of pernicious anaemia (Bartonella anaemia) in intact albino rats by deficient feeding. Indian J. Med. Res. 1930, 18, 663–683. [Google Scholar]
- Wills, L. Treatment of “pernicious amaemia of pregnancy” and “tropi cal anemia. Br. Med. J. 1931, 1, 1059–1064. [Google Scholar] [CrossRef]
- Wills, L. Treatment of “pernicious anaemia of pregnancy” and “tropical anaemia” with special reference to yeast extract as a curative agent. Natl. Med. J. India 1931, 26, 117–122. [Google Scholar]
- Wills, L.; Evans, B.D.F. Tropical Macrocytic Anaemia: Its Relation to Pernicious Anaemia. Lancet 1938, 2, 416–421. Available online: http://www.sciencedirect.com/science/article/pii/S0140673600416156 (accessed on 10 July 2025). [CrossRef]
- Mitchell, H.K.; Snell, E.E.; Williams, R.J. The concentration of “folic acid”. Nutr. Rev. 1988, 46, 324–325. [Google Scholar] [CrossRef]
- Rosenberg, I.H. A history of the isolation and identification of folic acid (folate). Ann. Nutr. Metab. 2012, 61, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Hoffbrand, A.V.; Weir, D.G. The history of folic acid. Br. J. Haematol. 2001, 113, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Amatriain, C.; Ledesma-Amaro, R.; López-Nicolás, R.; Ros, G.; Jiménez, A.; Revuelta, J.L. Folic Acid Production by Engineered Ashbya gossypii. Metab. Eng. 2016, 38, 473–482. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, X.; Liu, Y.; Liu, L.; Li, J.; Du, G.; Chen, J. Synthetic biology-driven microbial production of folates: Advances and perspectives. Bioresour. Technol. 2021, 324, 124624. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Raimondi, S.; Costantino, L.; Amaretti, A. Folate: Relevance of Chemical and Microbial Production. In Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants; Vandamme, E.J., Revuelta, J.L., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2016; pp. 103–128. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Folic-Acid (accessed on 12 July 2025).
- Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:27470 (accessed on 10 July 2025).
- Mc Carron, P.; Crowley, A.; O’Shea, D.; McCann, M.; Howe, O.; Hunt, M.; Devereux, M. Targeting the Folate Receptor: Improving Efficacy in Inorganic Medicinal Chemistry. Curr. Med. Chem. 2018, 25, 2675–2708. [Google Scholar] [CrossRef]
- Kok, D.E.; van Duijnhoven, F.J.; Lubberman, F.J.; McKay, J.A.; Lanen, A.V.; Winkels, R.M.; Wesselink, E.; van Halteren, H.K.; de Wilt, J.H.; Ulrich, C.M.; et al. Intake and biomarkers of folate and folic acid as determinants of chemotherapy-induced toxicities in patients with colorectal cancer: A cohort study. Am. J. Clin. Nutr. 2024, 119, 294–301. [Google Scholar] [CrossRef]
- Czeczot, H. Folic acid in physiology and pathology. Postep. Hig. Med. Dosw. (Online) 2008, 62, 405–419. [Google Scholar]
- Siatka, T.; Mát’uš, M.; Moravcová, M.; Harčárová, P.; Lomozová, Z.; Matoušová, K.; Suwanvecho, C.; Krčmová, L.K.; Mladěnka, P. Biological, dietetic and pharmacological properties of vitamin B9. NPJ Sci. Food 2025, 9, 30. [Google Scholar] [CrossRef]
- Warzyszynska, J.E.; Kim, Y.-I. Folate in Human Health and Disease. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2014. [Google Scholar] [CrossRef]
- Watanabe, H.; Miyake, T. Folic and Folate Acid. In Functional Food—Improve Health through Adequate Food; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Stover, P.J. Physiology of folate and vitamin B12 in health and disease. Nutr. Rev. 2004, 62 Pt 2, S3–S12; discussion S13. [Google Scholar] [CrossRef]
- Zheng, Y.; Cantley, L.C. Toward a better understanding of folate metabolism in health and disease. J. Exp. Med. 2019, 216, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Villa, D.; Aguilar, M.R.; Rojo, L. Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications. Int. J. Mol. Sci. 2019, 20, 4996. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef]
- Rezk, B.M.; Haenen, G.R.; van der Vijgh, W.J.; Bast, A. Tetrahydrofolate and 5-methyltetrahydrofolate are folates with high antioxidant activity. Identification of the antioxidant pharmacophore. FEBS Lett. 2003, 555, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.yeastgenome.org/chemical/folic_acid (accessed on 9 July 2025).
- Greenberg, J.A.; Bell, S.J.; Guan, Y.; Yu, Y.H. Folic Acid supplementation and pregnancy: More than just neural tube defect prevention. Rev. Obstet. Gynecol. 2011, 4, 52–59. [Google Scholar]
- Cieślik, E.; Cieślik, I. Occurrence and significance of folic acid. Pteridines 2018, 29, 187–195. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Kang, Y.J.; Sung, B.; Jang, J.Y.; Hwang, N.L.; Oh, H.J.; Ahn, Y.R.; Kim, H.J.; Shin, J.H.; Yoo, M.A.; et al. Folic acid is necessary for proliferation and differentiation of C2C12 myoblasts. J. Cell. Physiol. 2018, 233, 736–747. [Google Scholar] [CrossRef]
- Budak, M.Ş.; Oğlak, S.C.; Akgöl, S.; Can, B.; Arkan, K.; Erkmen, A.D.; Halisçelik, M.A.; Budak, A.; Tunç, Ş.; Bolluk, G.; et al. Folic acid versus folinic acid during methotrexate treatment for low-risk gestational trophoblastic neoplasia. Ginekol. Pol. 2025, 96, 200–205. [Google Scholar] [CrossRef]
- Gristan, Y.D.; Patel, P.; Moosavi, L. Folinic Acid [Updated 28 February 2024]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545232/ (accessed on 9 July 2025).
- Merzel, R.L.; Boutom, S.M.; Chen, J.; Frey, C.; Shedden, K.; Marsh, E.N.; Banaszak Holl, M.M. Folate binding protein: Therapeutic natural nanotechnology for folic acid, methotrexate, and leucovorin. Nanoscale 2017, 9, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Kim, Y.I.; Refsum, H. Is folic acid good for everyone? Am. J. Clin. Nutr. 2008, 87, 517–533. [Google Scholar] [CrossRef]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and Its Impact on Cancer Risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef]
- Thabet, R.H.; Alessa, R.E.M.; Al-Smadi, Z.K.K.; Alshatnawi, B.S.G.; Amayreh, B.M.I.; Al-Dwaaghreh, R.B.A.; Salah, S.K.A. Folic acid: Friend or foe in cancer therapy. J. Int. Med. Res. 2024, 52, 3000605231223064. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I. Folate and carcinogenesis: Evidence, mechanisms, and implications. J. Nutr. Biochem. 1999, 10, 66–88. [Google Scholar] [CrossRef] [PubMed]
- Seremak-Mrozikiewicz, A.; Bomba-Opoń, D.; Drews, K.; Kaczmarek, P.; Wielgoś, M.; Sieroszewski, P. Expert position of the Polish Society of Gynecologists and Obstetricians on folate supplementation and the conditions for the use of additional choline and vitamin B6 and B12 supplementation during the preconception period, pregnancy and postpartum period. Pract. Gynaecol. Perinatol. (GIPP) 2024, 9, 154–156. [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinionon the substantiation of a health claim related to increasing maternal folate status by supplemental folate intake and reducedrisk of neural tube defects pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2013, 11, 3328. [Google Scholar] [CrossRef][Green Version]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and Its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press (US): Washington, DC, USA, 1998. [Google Scholar]
- Borucki, M.; Dziedziński, M.; Kobus-Cisowski, J. Charakteristics of folates and processes that shape kontent of folates and folic acid In plants and Animals products and their importance in human nutrition. Issues Agric. Advis. Serv. 2023, 113, 21–36. [Google Scholar][Green Version]
- Suitor, C.W.; Bailey, L.B. Dietary folate equivalents: Interpretation and application. J. Am. Diet. Assoc. 2000, 100, 88–94. [Google Scholar] [CrossRef]
- WHO Library Cataloguing-in-Publication Data Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; Vitamin and Mineral Requirements in Human Nutrition: Report of a Joint FAO/WHO Expert Consultation; WHO: Geneva, Switzerland, 1998; pp. 289–303. [Google Scholar]
- Baddam, S.; Khan, K.M.; Jialal, I. Folic Acid Deficiency [Updated 25 June 2025]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535377/ (accessed on 14 July 2025).
- Allen, L.H. Causes of vitamin B12 and folate deficiency. Food. Nutr. Bull. 2008, 29 (Suppl. 2), S20–S34; discussion S35–S37. [Google Scholar] [CrossRef]
- Socha, D.S.; DeSouza, S.I.; Flagg, A.; Sekeres, M.; Rogers, H.J. Severe megaloblastic anemia: Vitamin deficiency and other causes. Cleve. Clin. J. Med. 2020, 87, 153–164. [Google Scholar] [CrossRef]
- Dutta, T.K. Megaloblastic Anemia. Ann. Clin. Med. Case. Rep. 2023, V10, 1–5. [Google Scholar]
- Hariz, A.; Bhattacharya, P.T. Megaloblastic Anemia [Updated 3 April 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537254/ (accessed on 9 July 2025).
- Hansen, P.B.; Jørgensen, L.M. Pancytopenia—A rare manifestation of folic acid deficiency. J. Intern. Med. 1989, 225, 143–144. [Google Scholar] [CrossRef]
- Abdullah, A.; Shakoor, E.U.; Sarwar, S.; Jamil, T.R.; Hina, A. A Rare Case of Severe Folate Deficiency-Induced Pancytopenia. Cureus 2024, 16, e65858. [Google Scholar] [CrossRef]
- Massoni, L. Folic Acid in Neuropsychiatric Disorders. Med. Discov. 2024, 3, 1133. [Google Scholar] [CrossRef]
- Reynolds, E.H. Chapter 61—The neurology of folic acid deficiency. In Handbook of Clinical Neurology; Biller, J., Ferro, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 120, pp. 927–943. [Google Scholar] [CrossRef]
- Liwinski, T.; Lang, U.E. Folate and Its Significance in Depressive Disorders and Suicidality: A Comprehensive Narrative Review. Nutrients 2023, 15, 3859. [Google Scholar] [CrossRef]
- Lazarou, C.; Kapsou, M. The role of folic acid in prevention and treatment of depression: An overview of existing evidence and implications for practice. Complement. Ther. Clin. Pr. 2010, 16, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.H. Folic acid, ageing, depression, and dementi. BMJ 2002, 324, 1512. [Google Scholar] [CrossRef] [PubMed]
- Steegers-Theunissen, R.P. Folate metabolism and neural tube defects: A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 61, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Beaudin, A.E.; Stover, P.J. Folate-mediated one-carbon metabolism and neural tube defects: Balancing genome synthesis and gene expression. Birth Defects Res. C. Embryo Today 2007, 81, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Pulikkunnel, S.T.; Thomas, S.V. Neural tube defects: Pathogenesis and folate metabolism. J. Assoc. Physicians India 2005, 53, 127–135. [Google Scholar] [PubMed]
- Mitchell, D.C.; Vilter, R.W.; Vilter, C.F. Hypersensitivity to folic acid. Ann. Intern. Med. 1949, 31, 1102–1105. [Google Scholar] [CrossRef]
- Rausch, F. Anaphylactic shock after folic acid therapy. Ther. Ggw. 1956, 95, 53–55. [Google Scholar] [PubMed]
- Chanarin, I.; Fenton, J.C.; Mollin, D.L. Sensitivity to folic acid. Br. Med. J. 1957, 1, 1162–1163. [Google Scholar] [CrossRef]
- Levander-Lindgren, M. Hypersensitivity to folic acid in a case of erythroblastomatosis. Acta Med. Scand. 1957, 157, 233–234. [Google Scholar] [CrossRef]
- Woodliff, H.J.; Davis, R.E. Allergy to folic acid. Med. J. Aust. 1966, 21, 351–352. [Google Scholar] [CrossRef]
- Mathur, B.P. Sensitivity of folic acid: A case report. Indian J. Med. Sci. 1966, 20, 133–134. [Google Scholar]
- Sesin, G.P.; Kirschenbaum, H. Folic acid hypersensitivity and fever: A case report. Am. J. Hosp. Pharm. 1979, 36, 1565–1567. [Google Scholar] [CrossRef]
- Sparling, R.; Abela, M. Hypersensitivity to folic acid therapy. Clin. Lab. Haematol. 1985, 7, 184–185. [Google Scholar]
- Dykewicz, M.S.; Orfan, N.A.; Sun, W. In vitro demonstration of IgE antibody to folate-albumin in anaphylaxis from folic acid. J. Allergy Clin. Immunol. 2000, 106, 386–389. [Google Scholar] [CrossRef]
- Sanders, G.M.; Fritz, S.B. Allergy to natural and supplemental folic acid as a cause of chronic, intermittent urticaria and angioedema. Ann. Allergy Asthma Immunol. 2004, 93, S51–S52. [Google Scholar] [CrossRef]
- Smith, J.; Empson, M.; Wall, C. Recurrent anaphylaxis to synthetic folic acid. Lancet 2007, 370, 652. [Google Scholar] [CrossRef]
- Pfab, F.; Willi, R.; Albert, A.; Huss-Marp, J.; Athanasiadis, G.I.; Jakob, T.; Ollert, M.; Ring, J.; Darsow, U. Anaphylactic reaction to folic acid verified by provocational testing. Allergy 2007, 62, 823–824. [Google Scholar] [CrossRef]
- Valdivieso, R.; Cevallos, F.; Caballero, M.T.; Quirce, S. Chronic urticaria caused by folic acid. Ann. Allergy Asthma Immunol. 2009, 103, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, N.; Adachi, A.; Fukumoto, T.; Ueno, M.; Fujiwara, N.; Ogura, K.; Horikawa, T. Folic acid-induced anaphylaxis showing cross-reactivity with methotrexate: A case report and review of the literature. Int. J. Dermatol. 2009, 48, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Roy, M. A case of folic Acid allergy in pregnancy. J. Obstet. Gynaecol. India 2012, 62 (Suppl. 1), 33–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nucera, E.; Aruanno, A.; Mezzacappa, S.; Pascolini, L.; Buonomo, A.; Schiavino, D. Hypersensitivity reactions to folic acid: Three case reports and a review of the literature. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418817704. [Google Scholar] [CrossRef]
- Gaeta, F.; Romano, A.; Guéant-Rodriguez, R.M.; Guéant, J.L. IgE-mediated anaphylactic reaction against free synthetic folic acid and methyl folate. J. Allergy Clin. Immunol. Pr. 2020, 8, 809–811. [Google Scholar] [CrossRef]
- Schnebert, B.; Saïd, B.B.; Dupire, G. IgE-mediated allergy to folic acid: A case report. Eur. J. Dermatol. 2023, 33, 41–42. [Google Scholar] [CrossRef]
- Nonhoff, J.; Jafari, R.; Tronnier, M. Folate malady—Anaphylaxis after folic acid substitution. JDDG 2023, 21 (Suppl. 2), 13–14. [Google Scholar] [CrossRef]
- Gouveia, J.; Mendes, A.; Neves, E.; Falcão, H.; Gomes, E. Folic acid allergy and the role of the basophil activation test. Rev. Port. Imunoalergologia 2024, 32, 151–154. [Google Scholar] [CrossRef]
- Benchalal, M.; Yahchouchy-Chouillard, E.; Fouere, S.; Fingerhut, A. Anaphylactic shock secondary to intravenous administration of folinic acid: A first report. Ann. Oncol. 2002, 13, 480–481. [Google Scholar] [CrossRef]
- Vermeulen, C.; Mathelier-Fusade, P.; Gaouar, H.; Leynadier, F. Two cases of allergy to leucovorin. Rev. Fr. D’Allergologie 2003, 43, 342–343. [Google Scholar] [CrossRef]
- Prabu, R.; Bakhshi, S. Systemic reaction to leucovorin in a child with lymphoblastic lymphoma suggestive of hypersensitivity. Pediatr. BloodCancer 2009, 52, 148. [Google Scholar] [CrossRef]
- Katirtzoglou, N.A.; Hotchkiss, S.; Gambaccini, M.; Kaley, K.; Syrigos, K.N.; Saif, M.W. Anaphylactic reaction associated with intravenous administration of folinic acid in a patient with colon cancer. In Vivo 2011, 25, 995–996. [Google Scholar]
- Damaske, A.; Ma, N.; Williams, R. Leucovorin-induced hypersensitivity reaction. J. Oncol. Pharm. Pr. 2012, 18, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Tavera, A.; Zamora-Verduga, M.; Madrigal-Burgaleta, R.; Angel-Pereira, D.; Berges-Gimeno, M.P.; Alvarez-Cuesta, E. Hypersensitivity reactions to racemic calcium folinate (leucovorin) during FOLFOX and FOLFIRI chemotherapy administrations. J. Allergy. Clin. Immunol. 2015, 135, 1066–1067. [Google Scholar] [CrossRef]
- Florit-Sureda, M.; Conde-Estévez, D.; Vidal, J.; Montagut, C. Hypersensitivity reaction caused by folinic acid administration: A case report and literature review. J. Chemother. 2016, 28, 500–505. [Google Scholar] [CrossRef]
- Mathew, A.A.; Chandran, C.; Prabhu, R.; Reghu, R. Leucovorin-induced hypersensitivity reaction in acute lymphoblastic leukemia—A case report. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 1007–1008. [Google Scholar] [CrossRef]
- Nesbitt, P.; Storer, H.; Vo, P.-U.; Sevilla Berrios, R. Leucovorin: A rarely reported causa of hypersensitivity. Chest 2019, 156, A1525. [Google Scholar] [CrossRef]
- Gudimetla, V.; Daw, J.; Almhana, F.; Haddad, A. Delayed Hypersensitivity Reaction to Leucovorin. Am. J. Ther. 2019, 26, e533–e534. [Google Scholar] [CrossRef]
- Apraxine, M.; Van den Eynde, M.; De Cuyper, A.; Pirson, F. Hypersensitivity reactions to folinic acid: Mechanisms involved based on two case reports and a literature review. Allergy Asthma Clin. Immunol. 2022, 18, 107. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, D.H.; Lee, J.Y.; Nam, Y.H.; Lee, J.H. Leucovorin-induced Hypersensitivity Reaction in a Patient with Metastatic Colorectal Cancer Treated with Cetuximab Plus FOLFOX Chemotherapy: A Case Report. Korean J. Gastroenterol. 2023, 82, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, A.; Alvarez Arango, S. A case of chemotherapy hypersensitivity to oxaliplatin and leucovorin. Ann. Allergy Asthma Immunol. 2024, 133, S106. [Google Scholar] [CrossRef]
- Chilom, C.G.; Bacalum, M.; Stanescu, M.M.; Florescu, M. Insight into the interaction of human serum albumin with folic acid: A biophysical study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 204, 648–656. [Google Scholar] [CrossRef]
- Chilom, C.G.; David, M.; Florescu, M. Monitoring biomolecular interaction between folic acid and bovine serum albumin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 230, 118074. [Google Scholar] [CrossRef]
- Nosrati, H.; Abbasi, R.; Charmi, J.; Rakhshbahar, A.; Aliakbarzadeh, F.; Danafar, H.; Davaran, S. Folic acid conjugated bovine serum albumin: An efficient smart and tumor targeted biomacromolecule for inhibition folate receptor positive cancer cells. Int. J. Biol. Macromol. 2018, 117, 1125–1132. [Google Scholar] [CrossRef]
- Gorobets, M.G.; Toroptseva, A.V.; Abdullina, M.I.; Pokrovsky, V.S.; Khachatryan, D.S.; Bychkova, A.V. Folic acid conjugated with serum albumin for nano- and submicron delivery systems for applications in therapy and diagnostics. Explor. Drug Sci. 2025, 3, 1008101. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Zhang, C.; Lin, K.; Yang, J.; Zhang, Y.; Hao, J.; Tian, F. Folic acid-coupled bovine serum albumin-modified magnetic nanocomposites from quantum-sized Fe3O4 and layered double hydroxide for actively targeted delivery of 5-fluorouracil. Int. J. Biol. Macromol. 2024, 256 Pt 1, 128385. [Google Scholar] [CrossRef]
- Bourassa, P.; Hasni, I.; Tajmir-Riahi, H.A. Folic acid complexes with human and bovine serum albumins. Food Chem. 2011, 129, 1148–1155. [Google Scholar] [CrossRef]
- Śliwińska-Hill, U.; Wiglusz, K. Multispectroscopic studies of the interaction of folic acid with glycated human serum albumin. J. Biomol. Struct. Dyn. 2019, 37, 3731–3739. [Google Scholar] [CrossRef]
- Lian, B.; Wu, M.; Feng, Z.; Deng, Y.; Zhong, C.; Zhao, X. Folate-conjugated human serum albumin-encapsulated resveratrol nanoparticles: Preparation, characterization, bioavailability and targeting of liver tumors. Artif. Cells Nanomed. Biotechnol. 2019, 47, 154–165. [Google Scholar] [CrossRef]
- Ma, N.; Liu, J.; He, W.; Li, Z.; Luan, Y.; Song, Y.; Garg, S. Folic acid-grafted bovine serum albumin decorated graphene oxide: An efficient drug carrier for targeted cancer therapy. J. Colloid Interface Sci. 2017, 490, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Konings, E.J.; Roomans, H.H.; Dorant, E.; Goldbohm, R.A.; Saris, W.H.; van den Brandt, P.A. Folate intake of the Dutch population according to newly established liquid chromatography data for foods. Am. J. Clin. Nutr. 2001, 73, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, P.; McNulty, H.; Mastroiacovo, P.; McDowell, I.F.; Melse-Boonstra, A.; Finglas, P.M.; Gregory, J.F. 3rd. Folate bioavailability: UK Food Standards Agency workshop report. Br. J. Nutr. 2003, 90, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M. Is cancer a severe delayed hypersensitivity reaction and histamine a blueprint? Clin. Transl. Med. 2016, 5, 35. [Google Scholar] [CrossRef]
- Jang, H.Y.; Choi, B.; Kim, I.W.; Kang, H.R.; Oh, J.M. Risk Factors of Hypersensitivity Reactions to Carboplatin: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pr. 2025, 13, 610–618.e10. [Google Scholar] [CrossRef]
- Kawarada, Y.; Miyazaki, M.; Itoh, A.; Araki, R.; Iwamizu, H.; Kataoka, T.; Kumakura, Y.; Ota, A.; Nagai, T.; Yamada, K. Incidence of and risk factors associated with nedaplatin-related hypersensitivity reactions. Int. J. Clin. Oncol. 2017, 22, 593–599. [Google Scholar] [CrossRef]
- Vega, A.; Jimenez-Rodriguez, T.W.; Barranco, R.; Bartra, J.; Diéguez, M.C.; Doña, I.; Fernández-Rivas, M.; Gandolfo-Cano, M.; Gastaminza-Lasarte, G.; González-Mancebo, E.; et al. Hypersensitivity Reactions to Cancer Chemotherapy: Practical Recommendations of ARADyAL for Diagnosis and Desensitization. J. Investig. Allergol. Clin. Immunol. 2021, 31, 364–384. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Du, Q.; Ye, X.; Yu, S.; Luo, X.; Zhai, Q. Meta-analysis of risk factors associated with oxaliplatin hypersensitivity reactions in cancer patients. Int. J. Clin. Oncol. 2021, 26, 2194–2204. [Google Scholar] [CrossRef]
- Pagani, M.; Bavbek, S.; Alvarez-Cuesta, E.; Berna Dursun, A.; Bonadonna, P.; Castells, M.; Cernadas, J.; Chiriac, A.; Sahar, H.; Madrigal-Burgaleta, R.; et al. Hypersensitivity reactions to chemotherapy: An EAACI Position Paper. Allergy 2022, 77, 388–403. [Google Scholar] [CrossRef]
- Bulut, İ.; Yegin Katran, Z. Hypersensitivity Reaction and Rapid Drug Desensitization with Chemotherapeutics: A Tertiary Reference Center Experiences. Int. Arch. Allergy Immunol. 2023, 184, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Seghers, S.; Teuwen, L.A.; Beyens, M.; De Blick, D.; Sabato, V.; Ebo, D.G.; Prenen, H. Immediate hypersensitivity reactions to antineoplastic agents—A practical guide for the oncologist. Cancer Treat. Rev. 2023, 116, 102559. [Google Scholar] [CrossRef]
- Kovalev, I.S.; Zyryanov, G.V.; Santra, S.; Majee, A.; Varaksin, M.V.; Charushin, V.N. Folic Acid Antimetabolites (Antifolates): A Brief Review on Synthetic Strategies and Application Opportunities. Molecules 2022, 27, 6229. [Google Scholar] [CrossRef]
- Montemurro, M.; De Zan, M.M.; Robles, J.C. Optimized high performance liquid chromatography-ultraviolet detection method using core-shell particles for the therapeutic monitoring of methotrexate. J. Pharm. Anal. 2016, 6, 103–111. [Google Scholar] [CrossRef] [PubMed]
- de Rouw, N.; Piet, B.; Derijks, H.J.; van den Heuvel, M.M.; Ter Heine, R. Mechanisms, Management and Prevention of Pemetrexed-Related Toxicity. Drug Saf. 2021, 44, 1271–1281. [Google Scholar] [CrossRef]
- Kandeel, M.; Suganuma, K. The Broad-Spectrum Antitrypanosomal Inhibitory Efficiency of the Antimetabolite/Anticancer Drug Raltitrexed. Processes 2022, 10, 2158. [Google Scholar] [CrossRef]
- Wulf, N.R.; Matuszewski, K.A. Sulfonamide cross-reactivity: Is there evidence to support broad cross-allergenicity? Am. J. Health Syst. Pharm. 2013, 70, 1483–1494. [Google Scholar] [CrossRef]
- Serrano-Arias, B.; Araya-Zúñiga, A.; Waterhouse-Garbanzo, J.; Rojas-Barrantes, Z.; Arguedas-Chacón, S.; Zavaleta-Monestel, E. A Comprehensive Review of Sulfonamide Hypersensitivity: Implications for Clinical Practice. Clin. Rev. Allergy Immunol. 2023, 65, 433–442. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).