Fyn Kinase: A Potential Target in Glucolipid Metabolism and Diabetes Mellitus
Abstract
1. Introduction
2. Effects of Fyn on Glucolipid Metabolism
3. Role of Fyn in the Development of Diabetic Complications
4. Role of Fyn in Signaling Pathways Associated with Glucolipid Metabolism
4.1. Mechanisms of Fyn in Lipid Metabolism
4.1.1. Role of Fyn in Signaling Pathways Directly Affecting Lipid Metabolism
4.1.2. Role of Fyn in Mechanisms of CD36 Affecting Lipid Metabolism
4.1.3. Role of Fyn in Adipocyte Differentiation
4.2. Role of Fyn in Insulin Signaling
4.2.1. Direct Interactions Between Fyn and Insulin Signaling Components
4.2.2. Role of Fyn in Insulin Signaling Through Lipid Raft
4.3. Role of Fyn in the State of Inflammation Associated with Hyperglycemia and Hyperlipidemia
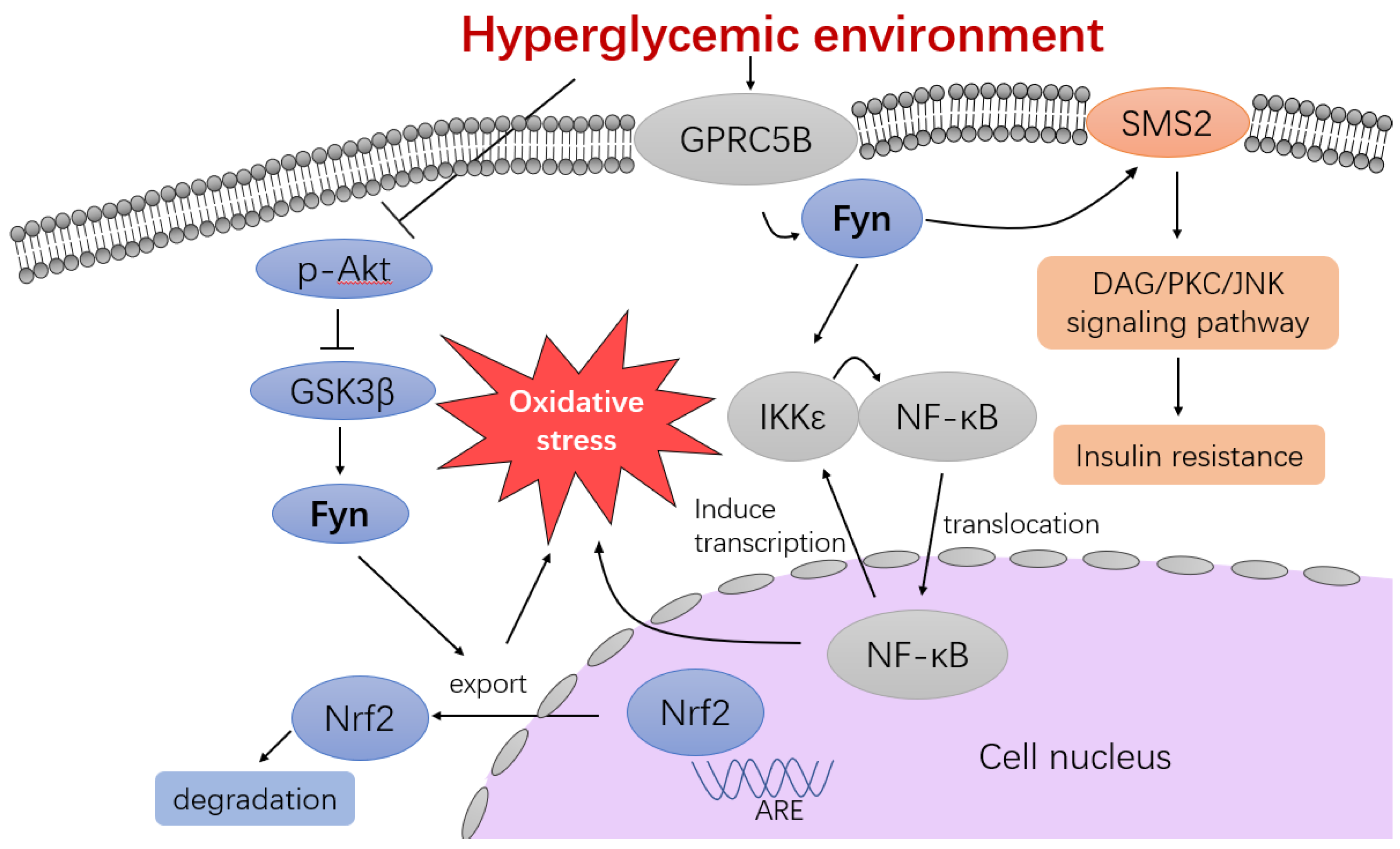
4.3.1. Akt/GSK3β/Fyn/Nrf2 Signaling Pathway
4.3.2. GPRC5B Signaling Pathway
4.3.3. Insulin-Mediated Mast Cell Response
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef]
- Nauck, M.A.; Wefers, J.; Meier, J.J. Treatment of type 2 diabetes: Challenges, hopes, and anticipated successes. Lancet Diabetes Endocrinol. 2021, 9, 525–544. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas 2025. Available online: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (accessed on 30 April 2025).
- Trefts, E.; Shaw, R.J. AMPK: Restoring metabolic homeostasis over space and time. Mol. Cell 2021, 81, 3677–3690. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.J.; Parsons, J.T. Src family kinases, key regulators of signal transduction. Oncogene 2004, 23, 7906–7909. [Google Scholar] [CrossRef] [PubMed]
- Semba, K.; Nishizawa, M.; Miyajima, N.; Yoshida, M.C.; Sukegawa, J.; Yamanashi, Y.; Sasaki, M.; Yamamoto, T.; Toyoshima, K. yes-related protooncogene, syn, belongs to the protein-tyrosine kinase family. Proc. Natl. Acad. Sci. USA 1986, 83, 5459–5463. [Google Scholar] [CrossRef]
- Boggon, T.J.; Eck, M.J. Structure and regulation of Src family kinases. Oncogene 2004, 23, 7918–7927. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.; Fournel, M.; Veillette, A. Oncogenic activation of p59fyn tyrosine protein kinase by mutation of its carboxyl-terminal site of tyrosine phosphorylation, tyrosine 528. J. Biol. Chem. 1994, 269, 10956–10963. [Google Scholar] [CrossRef]
- Goldsmith, J.F.; Hall, C.G.; Atkinson, T.P. Identification of an alternatively spliced isoform of the fyn tyrosine kinase. Biochem. Biophys. Res. Commun. 2002, 298, 501–504. [Google Scholar] [CrossRef]
- Resh, M.D. Fyn, a Src family tyrosine kinase. Int. J. Biochem. Cell Biol. 1998, 30, 1159–1162. [Google Scholar] [CrossRef]
- Palacios, E.H.; Weiss, A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 2004, 23, 7990–8000. [Google Scholar] [CrossRef]
- Peng, S.; Fu, Y. FYN: Emerging biological roles and potential therapeutic targets in cancer. J. Transl. Med. 2023, 21, 84. [Google Scholar] [CrossRef]
- Matrone, C.; Petrillo, F.; Nasso, R.; Ferretti, G. Fyn tyrosine kinase as harmonizing factor in neuronal functions and dysfunctions. Int. J. Mol. Sci. 2020, 21, 4444. [Google Scholar] [CrossRef]
- Bastie, C.C.; Zong, H.; Xu, J.; Busa, B.; Judex, S.; Kurland, I.J.; Pessin, J.E. Integrative metabolic regulation of peripheral tissue fatty acid oxidation by the SRC kinase Family member Fyn. Cell Metab. 2007, 5, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Yamada, E.; Pessin, J.E.; Kurland, I.J.; Schwartz, G.J.; Bastie, C.C. Fyn-Dependent regulation of energy expenditure and body weight is mediated by tyrosine phosphorylation of LKB1. Cell Metab. 2010, 11, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.A.; Kwon, H.; Zong, H.; Yamada, E.; Vatish, M.; Pessin, J.E.; Bastie, C.C. FYN deficiency promotes a preferential increase in subcutaneous adipose tissue mass and decreased visceral adipose tissue inflammation. Diabetes 2013, 62, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Yamada, E.; Bastie, C.C. Disruption of Fyn SH3 domain interaction with a proline-rich motif in liver kinase B1 Results in activation of AMP-activated protein kinase. PLoS ONE 2014, 9, e89604. [Google Scholar] [CrossRef]
- Uehara, R.; Yamada, E.; Okada, S.; Bastie, C.C.; Maeshima, A.; Ikeuchi, H.; Horiguchi, K.; Yamada, M. Fyn phosphorylates transglutaminase 2 (Tgm2) and modulates autophagy and p53 expression in the development of diabetic kidney disease. Cells 2023, 12, 1197. [Google Scholar] [CrossRef]
- Lv, Z.; Hu, M.; Ren, X.; Fan, M.; Zhen, J.; Chen, L.; Lin, J.; Ding, N.; Wang, Q.; Wang, R. FYN mediates high Glucose-Induced actin cytoskeleton reorganization of podocytes via promoting ROCK activation in vitro. J. Diabetes Res. 2016, 2016, 5671803. [Google Scholar] [CrossRef]
- Hsu, M.-F.; Ito, Y.; Afkarian, M.; Haj, F.G. Deficiency of the Src homology phosphatase 2 in podocytes is associated with renoprotective effects in mice under hyperglycemia. Cell. Mol. Life Sci. 2022, 79, 516. [Google Scholar] [CrossRef]
- Xie, Y.; Yuan, Q.; Cao, X.; Qiu, Y.; Zeng, J.; Cao, Y.; Xie, Y.; Meng, X.; Huang, K.; Yi, F.; et al. Deficiency of nuclear receptor coactivator 3 aggravates diabetic kidney disease by impairing podocyte autophagy. Adv. Sci. 2024, 11, e2308378. [Google Scholar] [CrossRef]
- Chen, W.; Jump, D.B.; Esselman, W.J.; Busik, J.V. Inhibition of cytokine signaling in human retinal endothelial cells through modification of Caveolae/lipid rafts by docosahexaenoic acid. Investig. Ophthalmol. Vis. Sci. 2006, 48, 18–26. [Google Scholar] [CrossRef]
- Ho, N.; Gendron, R.L.; Grozinger, K.; Whelan, M.A.; Hicks, E.A.; Tennakoon, B.; Gardiner, D.; Good, W.V.; Paradis, H. Tubedown regulation of retinal endothelial permeability signaling pathways. Biol. Open 2015, 4, 970–979. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Liu, S.; Liu, J.; Li, X. NORAD lentivirus shRNA mitigates fibrosis and inflammatory responses in diabetic cardiomyopathy via the ceRNA network of NORAD/miR-125a-3p/Fyn. Inflamm. Res. 2021, 70, 1113–1127. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Jaiswal, A.K. GSK-3Β acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J. Biol. Chem. 2007, 282, 16502–16510. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.S.; Al-Mulla, F. A defect in Nrf2 signaling constitutes a mechanism for cellular stress hypersensitivity in a genetic rat model of type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1119–E1129. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, X.; Tan, Y.; Li, B.; Miao, X.; Jin, L.; Shi, X.; Zhang, X.; Miao, L.; Li, X.; et al. Diabetes-induced hepatic pathogenic damage, inflammation, oxidative stress, and insulin resistance was exacerbated in zinc deficient mouse model. PLoS ONE 2012, 7, e49257. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Sano, T.; Nabetani, T.; Asano, Y.; Hirabayashi, Y. GPRC5B activates obesity-associated inflammatory signaling in adipocytes. Sci. Signal. 2012, 5, ra85. [Google Scholar] [CrossRef]
- Fukunishi, S.; Tsuda, Y.; Takeshita, A.; Fukui, H.; Miyaji, K.; Fukuda, A.; Higuchi, K. p59fyn is associated with the development of hepatic steatosis due to chronic ethanol consumption. J. Clin. Biochem. Nutr. 2011, 49, 20–24. [Google Scholar] [CrossRef]
- Yang, Y.; Tarabra, E.; Yang, G.S.; Vaitheesvaran, B.; Palacios, G.; Kurland, I.J.; Pessin, J.E.; Bastie, C.C. Alteration of de novo glucose production contributes to fasting hypoglycaemia in fyn deficient mice. PLoS ONE 2013, 8, e81866. [Google Scholar] [CrossRef][Green Version]
- Thompson, W.R.; Guilluy, C.; Xie, Z.; Sen, B.; Brobst, K.E.; Yen, S.S.; Uzer, G.; Styner, M.; Case, N.; Burridge, K.; et al. Mechanically activated fyn utilizes mTORC2 to regulate RhoA and adipogenesis in mesenchymal stem cells. Stem Cells 2013, 31, 2528–2537. [Google Scholar] [CrossRef]
- Dorotea, D.; Jiang, S.; Pak, E.S.; Son, J.B.; Choi, H.G.; Ahn, S.M.; Ha, H. Pan-Src kinase inhibitor treatment attenuates diabetic kidney injury via inhibition of Fyn kinase-mediated endoplasmic reticulum stress. Exp. Mol. Med. 2022, 54, 1086–1097. [Google Scholar] [CrossRef]
- Han, Y.P.; Liu, L.J.; Yan, J.L.; Chen, M.Y.; Meng, X.F.; Zhou, X.R.; Qian, L.B. Autophagy and its therapeutic potential in diabetic nephropathy. Front. Endocrinol. 2023, 14, 1139444. [Google Scholar] [CrossRef]
- Li, S.; Lin, Z.; Xiao, H.; Xu, Z.; Li, C.; Zeng, J.; Xie, X.; Deng, L.; Huang, H. Fyn deficiency inhibits oxidative stress by decreasing c-Cbl-mediated ubiquitination of Sirt1 to attenuate diabetic renal fibrosis. Metabolism 2022, 139, 155378. [Google Scholar] [CrossRef]
- Du, J.; Yang, J.; Meng, L. Screening and identification of differentially expressed genes between diabetic nephropathy glomerular and normal glomerular via bioinformatics technology. Chem. High Throughput Screen. 2020, 24, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, B.; Chen, L.; Jiang, H. Identification and validation of immune-related biomarkers and potential regulators and therapeutic targets for diabetic kidney disease. BMC Med. Genom. 2023, 16, 90. [Google Scholar] [CrossRef]
- Ma, F.; Sun, T.; Wu, M.; Wang, W.; Xu, Z. Identification of key genes for diabetic kidney disease using biological informatics methods. Mol. Med. Rep. 2017, 16, 7931–7938. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Galgani, G.; Bray, G.; Martelli, A.; Calderone, V.; Citi, V. In Vitro models of diabetes: Focus on diabetic retinopathy. Cells 2024, 13, 1864. [Google Scholar] [CrossRef] [PubMed]
- Martín, E.D.; Sánchez-Perez, A.; Trejo, J.L.; Martin-Aldana, J.A.; Jaimez, M.C.; Pons, S.; Umanzor, C.A.; Menes, L.; White, M.F.; Burks, D.J. IRS-2 deficiency impairs NMDA receptor-dependent long-term potentiation. Cereb. Cortex 2011, 22, 1717–1727. [Google Scholar] [CrossRef]
- Suo, M.; Wang, P.; Zhang, M. Role of Fyn-mediated NMDA receptor function in prediabetic neuropathy in mice. J. Neurophysiol. 2016, 116, 448–455. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, Y.C.; Huang, J.; Chen, K.Y.; McGarrigle, D.K.; Huang, X.Y. Requirement of SRC-family tyrosine kinases in fat accumulation. Biochemistry 2005, 44, 14455–14462. [Google Scholar] [CrossRef]
- Ross, F.A.; Hawley, S.A.; Auciello, F.R.; Gowans, G.J.; Atrih, A.; Lamont, D.J.; Hardie, D.G. Mechanisms of paradoxical activation of AMPK by the kinase inhibitors SU6656 and sorafenib. Cell Chem. Biol. 2017, 24, 813–824.e4. [Google Scholar] [CrossRef]
- Tse, M.C.L.; Liu, X.; Yang, S.; Ye, K.; Chan, C.B. FYN regulates adipogenesis by promoting PIKE-A/STAT5A interaction. Mol. Cell. Biol. 2013, 33, 1797–1808. [Google Scholar] [CrossRef]
- Woeller, C.F.; O’Loughlin, C.W.; Pollock, S.J.; Thatcher, T.H.; Feldon, S.E.; Phipps, R.P. Thy1 (CD90) controls adipogenesis by regulating activity of the Src family kinase, Fyn. FASEB J. 2014, 29, 920–931. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Yang, G.; Xu, K.; Yin, Y.; Brecchia, G.; Yin, J. CD36 favours fat sensing and transport to govern lipid metabolism. Prog. Lipid Res. 2022, 88, 101193. [Google Scholar] [CrossRef] [PubMed]
- Samovski, D.; Sun, J.; Pietka, T.; Gross, R.W.; Eckel, R.H.; Su, X.; Stahl, P.D.; Abumrad, N.A. Regulation of AMPK activation by CD36 links fatty acid uptake to Β-Oxidation. Diabetes 2014, 64, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, C.; Luo, X.; Wang, P.; Zhou, W.; Zhong, S.; Xie, Y.; Jiang, Y.; Yang, P.; Tang, R.; et al. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J. Hepatol. 2018, 69, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zeng, H.; Tan, W.; Luo, X.; Zheng, E.; Zhao, L.; Wei, L.; Ruan, X.Z.; Chen, Y.; Chen, Y. Loss of CD36 impairs hepatic insulin signaling by enhancing the interaction of PTP1B with IR. FASEB J. 2020, 34, 5658–5672. [Google Scholar] [CrossRef]
- Schleh, M.W.; Ryan, B.J.; Ahn, C.; Ludzki, A.C.; Van Pelt, D.W.; Pitchford, L.M.; Chugh, O.K.; Luker, A.T.; Luker, K.E.; Samovski, D.; et al. Impaired suppression of fatty acid release by insulin is a strong predictor of reduced whole-body insulin-mediated glucose uptake and skeletal muscle insulin receptor activation. Acta Physiol. 2025, 241, e14249. [Google Scholar] [CrossRef]
- Sun, S.; Tan, P.; Huang, X.; Zhang, W.; Kong, C.; Ren, F.; Su, X. Ubiquitinated CD36 sustains insulin-stimulated Akt activation by stabilizing insulin receptor substrate 1 in myotubes. J. Biol. Chem. 2017, 293, 2383–2394. [Google Scholar] [CrossRef]
- Yang, X.; Lu, X.; Wang, L.; Bai, L.; Yao, R.; Jia, Z.; Ma, Y.; Chen, Y.; Hao, H.; Wu, X.; et al. Stearic acid promotes lipid synthesis through CD36/Fyn/FAK/mTORC1 axis in bovine mammary epithelial cells. Int. J. Biol. Macromol. 2023, 253, 127324. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, Y.; Wang, J.; Lang, L.; Liang, S.; Zhang, S.; Wang, L.; Gao, P.; Shu, G.; Zhu, C.; et al. Conjugated linoleic acid (CLA) reduces intestinal fatty acid uptake and chylomicron formation in HFD-fed mice associated with the inhibition of DHHC7-mediated CD36 palmitoylation and the downstream ERK pathway. Food Funct. 2024, 15, 5000–5011. [Google Scholar] [CrossRef]
- El-Yassimi, A.; Hichami, A.; Besnard, P.; Khan, N.A. Linoleic acid induces calcium signaling, SRC kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J. Biol. Chem. 2008, 283, 12949–12959. [Google Scholar] [CrossRef]
- Subramaniam, S.; Ozdener, M.H.; Abdoul-Azize, S.; Saito, K.; Malik, B.; Maquart, G.; Hashimoto, T.; Marambaud, P.; Aribi, M.; Tordoff, M.G.; et al. ERK1/2 activation in human taste bud cells regulates fatty acid signaling and gustatory perception of fat in mice and humans. FASEB J. 2016, 30, 3489–3500. [Google Scholar] [CrossRef]
- Chen, K.; Febbraio, M.; Li, W.; Silverstein, R.L. A specific CD36-Dependent signaling pathway is required for platelet activation by oxidized Low-Density lipoprotein. Circ. Res. 2008, 102, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, W.; Major, J.; Rahaman, S.O.; Febbraio, M.; Silverstein, R.L. Vav guanine nucleotide exchange factors link hyperlipidemia and a prothrombotic state. Blood 2011, 117, 5744–5750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, D.; Xu, X.; He, H.; Zhu, Y.; Lei, T.; Ou, H. Oxidized high-density lipoprotein promotes CD36 palmitoylation and increases lipid uptake in macrophages. J. Biol. Chem. 2022, 298, 102000. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, C.; Wang, R.; Xu, J.; Chi, Y.; Qin, J.; Liu, Q. The ω-carboxyl group of 7-ketocholesteryl-9-carboxynonanoate mediates the binding of oxLDL to CD36 receptor and enhances caveolin-1 expression in macrophages. Int. J. Biochem. Cell Biol. 2017, 90, 121–135. [Google Scholar] [CrossRef]
- Luo, X.; Li, Y.; Yang, P.; Chen, Y.; Wei, L.; Yu, T.; Xia, J.; Ruan, X.Z.; Zhao, L.; Chen, Y. Obesity induces preadipocyte CD36 expression promoting inflammation via the disruption of lysosomal calcium homeostasis and lysosome function. eBioMedicine 2020, 56, 102797. [Google Scholar] [CrossRef]
- Panicker, N.; Sarkar, S.; Harischandra, D.S.; Neal, M.; Kam, T.I.; Jin, H.; Saminathan, H.; Langley, M.; Charli, A.; Samidurai, M.; et al. Fyn kinase regulates misfolded α-synuclein uptake and NLRP3 inflammasome activation in microglia. J. Exp. Med. 2019, 216, 1411–1430. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, X.; Shan, C.; Xia, J.; Zhang, Z.; Shi, H.; Leng, K.; Wu, Y.; Ji, C.; Zhong, T. Src family kinases involved in the differentiation of human preadipocytes. Mol. Cell. Endocrinol. 2021, 533, 111323. [Google Scholar] [CrossRef]
- Mastick, C.C.; Saltiel, A.R. Insulin-stimulated tyrosine phosphorylation of caveolin is specific for the differentiated adipocyte phenotype in 3T3-L1 cells. J. Biol. Chem. 1997, 272, 20706–20714. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; DeYoung, S.M.; Zhang, M.; Dold, L.H.; Saltiel, A.R. The Stomatin/Prohibitin/Flotillin/HFLK/C domain of Flotillin-1 contains distinct sequences that direct plasma membrane localization and protein interactions in 3T3-L1 adipocytes. J. Biol. Chem. 2005, 280, 16125–16134. [Google Scholar] [CrossRef]
- Chen, M.; Dong, Y.; Tian, L.; Zhou, J.; Zhu, E.; Yuan, H.; Li, X.; Wang, B. Metastasis suppressor 1 interacts with protein tyrosine phosphatase receptor-δ to regulate adipogenesis. FASEB J. 2023, 37, e22857. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.J.; Pons, S.; Asano, T.; Myers, M.G.; Glasheen, E.; White, M.F. The Fyn tyrosine kinase binds Irs-1 and forms a distinct signaling complex during insulin stimulation. J. Biol. Chem. 1996, 271, 10583–10587. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Dhe-Paganon, S.; Melendez, P.A.; Lee, J.; Shoelson, S.E. Two new substrates in insulin signaling, IRS5/DOK4 and IRS6/DOK5. J. Biol. Chem. 2003, 278, 25323–25330. [Google Scholar] [CrossRef]
- Desbuquois, B.; Carré, N.; Burnol, A. Regulation of insulin and type 1 insulin-like growth factor signaling and action by the Grb10/14 and SH2B1/B2 adaptor proteins. FEBS J. 2012, 280, 794–816. [Google Scholar] [CrossRef]
- Langlais, P.; Dong, L.Q.; Hu, D.; Liu, F. Identification of Grb10 as a direct substrate for members of the Src tyrosine kinase family. Oncogene 2000, 19, 2895–2903. [Google Scholar] [CrossRef][Green Version]
- Ribon, V.; Saltiel, A.R. Insulin stimulates tyrosine phosphorylation of the proto-oncogene product of c-Cbl in 3T3-L1 adipocytes. Biochem. J. 1997, 324, 839–846. [Google Scholar] [CrossRef]
- Hunter, S.; Burton, E.A.; Wu, S.C.; Anderson, S.M. Fyn associates with Cbl and phosphorylates Tyrosine 731 in Cbl, a binding site for phosphatidylinositol 3-kinase. J. Biol. Chem. 1999, 274, 2097–2106. [Google Scholar] [CrossRef]
- Rao, N.; Ghosh, A.K.; Zhou, P.; Douillard, P.; Andoniou, C.E.; Band, H. An essential role of ubiquitination in Cbl-mediated negative regulation of the Src-family kinase Fyn. Signal Transduct. 2002, 2, 29–39. [Google Scholar] [CrossRef]
- Yamamoto, M.; Toya, Y.; Schwencke, C.; Lisanti, M.P.; Myers, M.G.; Ishikawa, Y. Caveolin is an activator of insulin receptor signaling. J. Biol. Chem. 1998, 273, 26962–26968. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, J.; Parpal, S.; Karlsson, M.; Ramsing, C.; Thorn, H.; Borg, M.; Lindroth, M.; Peterson, K.H.; Magnusson, K.; Strålfors, P. Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J. 1999, 13, 1961–1971. [Google Scholar] [CrossRef]
- Mastick, C.C.; Brady, M.J.; Saltiel, A.R. Insulin stimulates the tyrosine phosphorylation of caveolin. J. Cell Biol. 1995, 129, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Couet, J.; Lisanti, M.P. SRC tyrosine kinases, GA subunits, and H-RaS share a common membrane-anchored scaffolding protein, caveolin. J. Biol. Chem. 1996, 271, 29182–29190. [Google Scholar] [CrossRef]
- Hanwei, H.; Zhao, H. FYN-dependent muscle–immune interaction after sciatic nerve injury. Muscle Nerve 2010, 42, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Wandelmer, J.; Dávalos, A.; De La Peña, G.; Cano, S.; Giera, M.; Canfrán-Duque, A.; Bracher, F.; Martín-Hidalgo, A.; Fernández-Hernando, C.; Lasunción, M.A.; et al. Haloperidol disrupts lipid rafts and impairs insulin signaling in SH-SY5Y cells. Neuroscience 2010, 167, 143–153. [Google Scholar] [CrossRef]
- Sánchez-Wandelmer, J.; Dávalos, A.; Herrera, E.; Giera, M.; Cano, S.; De La Peña, G.; Lasunción, M.A.; Busto, R. Inhibition of cholesterol biosynthesis disrupts lipid raft/caveolae and affects insulin receptor activation in 3T3-L1 preadipocytes. Biochim. Biophys. Acta 2009, 1788, 1731–1739. [Google Scholar] [CrossRef]
- Alland, L.; Peseckis, S.M.; Atherton, R.E.; Berthiaume, L.; Resh, M.D. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J. Biol. Chem. 1994, 269, 16701–16705. [Google Scholar] [CrossRef]
- Sato, I.; Obata, Y.; Kasahara, K.; Nakayama, Y.; Fukumoto, Y.; Yamasaki, T.; Yokoyama, K.K.; Saito, T.; Yamaguchi, N. Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. J. Cell Sci. 2009, 122, 965–975. [Google Scholar] [CrossRef]
- Gottlieb-Abraham, E.; Gutman, O.; Pai, G.M.; Rubio, I.; Henis, Y.I. The residue at position 5 of the N-terminal region of Src and Fyn modulates their myristoylation, palmitoylation, and membrane interactions. Mol. Biol. Cell 2016, 27, 3926–3936. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Nazarian, A.; Erdjument-Bromage, H.; Bornmann, W.; Tempst, P.; Resh, M.D. Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction. J. Biol. Chem. 2001, 276, 30987–30994. [Google Scholar] [CrossRef] [PubMed]
- Biedi, C.; Panetta, D.; Segat, D.; Cordera, R.; Maggi, D. Specificity of insulin-like growth factor I and insulin on Shc phosphorylation and Grb2 recruitment in caveolae. Endocrinology 2003, 144, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Haddad, D.; Madhoun, A.A.; Nizam, R.; Al-Mulla, F. Role of Caveolin-1 in diabetes and its complications. Oxid. Med. Cell. Longev. 2020, 2020, 1–20. [Google Scholar] [CrossRef]
- Newcomb, L.F.; Mastick, C.C. SRC family kinase-dependent phosphorylation of a 29-kDa Caveolin-Associated protein. Biochem. Biophys. Res. Commun. 2002, 290, 1447–1453. [Google Scholar] [CrossRef]
- Kimura, A.; Mora, S.; Shigematsu, S.; Pessin, J.E.; Saltiel, A.R. The insulin receptor catalyzes the tyrosine phosphorylation of caveolin-1. J. Biol. Chem. 2002, 277, 30153–30158, Erratum in J. Biol. Chem. 2002, 277, 40167. [Google Scholar] [CrossRef]
- Sanguinetti, A.R.; Cao, H.; Mastick, C.C. Fyn is required for oxidative- and hyperosmotic-stress-induced tyrosine phosphorylation of caveolin-1. Biochem. J. 2003, 376, 159–168. [Google Scholar] [CrossRef]
- Okada, S.; Yamada, E.; Saito, T.; Ohshima, K.; Hashimoto, K.; Yamada, M.; Uehara, Y.; Tsuchiya, T.; Shimizu, H.; Tatei, K.; et al. CDK5-dependent phosphorylation of the RHO family GTPASE TC10A regulates insulin-stimulated GLUT4 translocation. J. Biol. Chem. 2008, 283, 35455–35463. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Niture, S.K.; Kaspar, J.W.; Shen, J.; Jaiswal, A.K. Nrf2 signaling and cell survival. Toxicol. Appl. Pharmacol. 2009, 244, 37–42. [Google Scholar] [CrossRef]
- Uruno, A.; Furusawa, Y.; Yagishita, Y.; Fukutomi, T.; Muramatsu, H.; Negishi, T.; Sugawara, A.; Kensler, T.W.; Yamamoto, M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol. Cell. Biol. 2013, 33, 2996–3010. [Google Scholar] [CrossRef]
- Dodson, M.; Shakya, A.; Anandhan, A.; Chen, J.; Garcia, J.G.N.; Zhang, D.D. NRF2 and diabetes: The good, the bad, and the complex. Diabetes 2022, 71, 2463–2476. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Anton, M.I.; Floria, M.; Seritean Isac, P.N.; Hurjui, L.L.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Oxidative stress and NRF2/KEAP1/ARE pathway in diabetic kidney disease (DKD): New perspectives. Biomolecules 2022, 12, 1227. [Google Scholar] [CrossRef]
- Tarabra, E.; Lee, T.W.A.; Zammit, V.A.; Vatish, M.; Yamada, E.; Pessin, J.E.; Bastie, C.C. Differential activation of Fyn kinase distinguishes saturated and unsaturated fats in mouse macrophages. Oncotarget 2017, 8, 86634–86645. [Google Scholar] [CrossRef][Green Version]
- Yan, X.; Su, Y.; Fan, X.; Chen, H.; Lu, Z.; Liu, Z.; Li, Y.; Yi, M.; Zhang, G.; Gu, C.; et al. Liraglutide improves the angiogenic capability of EPC and promotes ischemic angiogenesis in mice under diabetic conditions through an Nrf2-dependent mechanism. Cells 2022, 11, 3821. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, X.; Niu, C.; Huang, X.; An, N.; Sun, J.; Huang, S.; Ye, W.; Li, S.; Shen, Y.; et al. Baicalin alleviates hyperglycemia-induced endothelial impairment via Nrf2. J. Endocrinol. 2018, 240, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Yazici, E.; Sahin, E.; Sahin, N.; Tuzcu, M.; Sahin, K.; Orhan, C. Mango ginger (Curcuma amada Roxb.) may alleviate the effect of high-fat diet/streptozotocin-induced diabetes by activation of the GSK-3β/Fyn/Nrf2 pathway. Food Sci. Nutr. 2023, 11, 6041–6051. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Yan, X.; Zeng, J.; Chen, J.; Wang, Y.; Chen, J.; Li, Y.; Barati, M.T.; Wintergerst, K.A.; Pan, K.; et al. Elevating CXCR7 improves angiogenic function of EPCs via AKT/GSK-3Β/Fyn-Mediated NRF2 activation in diabetic limb ischemia. Circ. Res. 2017, 120, e7–e23. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Guo, W.; Li, F.; Sun, W.; Chen, J.; Zhang, C.; Lu, X.; Tan, Y.; Feng, W.; et al. Up-regulation of Nrf2 is involved in FGF21-mediated fenofibrate protection against type 1 diabetic nephropathy. Free Radic. Biol. Med. 2016, 93, 94–109. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Ma, F.; Sun, W.; Wang, W.; Yu, J.; Shi, Y.; Cai, L.; Xu, Z. The role of Akt2 in the protective effect of Fenofibrate against diabetic nephropathy. Int. J. Biol. Sci. 2020, 16, 553–567. [Google Scholar] [CrossRef]
- Shang, G.; Tang, X.; Gao, P.; Guo, F.; Liu, H.; Zhao, Z.; Chen, Q.; Jiang, T.; Zhang, N.; Li, H. Sulforaphane attenuation of experimental diabetic nephropathy involves GSK-3 beta/Fyn/Nrf2 signaling pathway. J. Nutr. Biochem. 2015, 26, 596–606. [Google Scholar] [CrossRef]
- Li, Z.; Guo, H.; Li, J.; Ma, T.; Zhou, S.; Zhang, Z.; Miao, L.; Cai, L. Sulforaphane prevents type 2 diabetes-induced nephropathy via AMPK-mediated activation of lipid metabolic pathways and Nrf2 antioxidative function. Clin. Sci. 2020, 134, 2469–2487. [Google Scholar] [CrossRef]
- Li, B.; Cui, W.; Tan, Y.; Luo, P.; Chen, Q.; Zhang, C.; Qu, W.; Miao, L.; Cai, L. Zinc is essential for the transcription function of Nrf2 in human renal tubule cells in vitro and mouse kidney in vivo under the diabetic condition. J. Cell Mol. Med. 2014, 18, 895–906. [Google Scholar] [CrossRef]
- Xuan, Y.; Wang, J.; Zhang, X.; Wang, J.; Li, J.; Liu, Q.; Lu, G.; Xiao, M.; Gao, T.; Guo, Y.; et al. Resveratrol attenuates high glucose-induced osteoblast dysfunction via AKT/GSK3Β/FYN-mediated NRF2 activation. Front. Pharmacol. 2022, 13, 862618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Sun, B.; Ma, L.; Ma, Y. Catalpol promotes osseointegration of Titanium implants under conditions of type 2 diabetes via AKT/GSK3β/FYN pathway-mediated NRF2 activation. ACS Omega 2024, 9, 5761–5771. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Amisten, S.; Rorsman, P.; Salehi, A. GPRC5B a putative glutamate-receptor candidate is negative modulator of insulin secretion. Biochem. Biophys. Res. Commun. 2013, 441, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Freundt, G.V.; Von Samson-Himmelstjerna, F.A.; Nitz, J.T.; Luedde, M.; Waltenberger, J.; Wieland, T.; Frey, N.; Preusch, M.; Hippe, H.J. The orphan receptor GPRC5B activates pro-inflammatory signaling in the vascular wall via Fyn and NFκB. Biochem. Biophys. Res. Commun. 2022, 592, 60–66. [Google Scholar] [CrossRef]
- Kim, Y.J.; Greimel, P.; Hirabayashi, Y. GPRC5B-Mediated Sphingomyelin synthase 2 phosphorylation plays a critical role in insulin resistance. iScience 2018, 8, 250–266. [Google Scholar] [CrossRef]
- Masini, M.; Suleiman, M.; Novelli, M.; Marselli, L.; Marchetti, P.; De Tata, V. Mast cells and the pancreas in human type 1 and type 2 diabetes. Cells 2021, 10, 1875. [Google Scholar] [CrossRef]
- Kempuraj, D.; Caraffa, A.; Ronconi, G.; Lessiani, G.; Conti, P. Are mast cells important in diabetes? Pol. J. Pathol. 2016, 67, 199–206. [Google Scholar] [CrossRef]
- Kettner, A.; Di Matteo, M.; Santoni, A. Insulin potentiates FcɛRI-mediated signaling in mouse bone marrow-derived mast cells. Mol. Immunol. 2009, 47, 1039–1046. [Google Scholar] [CrossRef]


| Drug (or Receptor) | Species | The Effect of the Drug (or Receptor) via the Akt/GSK-3β/Fyn/Nrf2 Signal Pathway | Intermediate Links Between the Drug (or Receptor) And the Akt/GSK-3β/Fyn/Nrf2 Pathway | The Main Action of the Drug (or Receptor) | References |
|---|---|---|---|---|---|
| Liraglutide | Mouse | Ameliorates the impairment of endothelial progenitor cell functions under diabetic condition | - | Treats T2DM | [96] |
| Baicalin | Mouse and human | Ameliorates the oxidative stress state in vascular endothelial cells | - | Antibacterial, anti-inflammatory, cholesterol-lowering, antithrombotic, etc. | [97] |
| Mango ginger (Curcuma amada Roxb.) | Rat | Antioxidant, alleviates insulin resistance | - | Anti-inflammatory, antioxidant, antibacterial, anti-cancer, antihyperglycemic | [98] |
| CXC chemokine receptor 7 | Mouse | Improves the angiogenic capability of endothelial progenitor cells in T2DM | - | Involved in cell proliferation | [99] |
| Fenofibrate | Mouse | Ameliorates DKD | Upregulates fibroblast growth factor 21 | Reduces blood lipid | [100,101] |
| Sulforaphane | Rat and mouse | Ameliorates DKD | Through AMPK-α2 | Antioxidant, anti-cancer, etc. | [102,103] |
| Zinc | Mouse and human | Alleviates the liver impairment induced by diabetes mellitus, ameliorates DKD | - | Promotes growth and immune function, maintains cell membrane structure, etc. | [27,104] |
| Resveratrol | Mouse | Alleviates osteoblast dysfunction induced by hyperglycemia | - | Antioxidant, anti-cancer, cardiovascular-protective, etc. | [105] |
| Catalpol | Mouse | Ameliorates osteoblast dysfunction induced by hyperglycemia, promotes osseointegration of titanium implants | - | Anti-inflammatory, anti-cancer, hypoglycemic, etc. | [106] |
| Type of Cell | Signal Pathway | Effect |
|---|---|---|
| Adipocytes | Inhibits AMPK activation | Reduces the oxidation of FAs and promotes lipogenesis |
| Participates in Thy1-PPARγ pathway | Promotes lipogenesis | |
| Activates STAT5a | Promotes lipogenesis and adipocyte differentiation | |
| Participates in MTSS1-PTPRD pathway | Promotes adipocyte differentiation | |
| Participates in insulin-induced GLUT4 translocation | Promotes glucose uptake | |
| Participates in GPRC5B-NFκB pathway | Promotes inflammation | |
| Skeletal muscle cells | Inhibits AMPK activation through CD36 | Reduces the oxidation of FAs and promotes lipid synthesis |
| Prevents IRS1 degradation | Delays but sustains insulin signaling | |
| Hepatocytes | Inhibits AMPK activation through CD36 | Reduces the oxidation of FAs and promotes lipid synthesis |
| Inhibits Nrf2 | Promotes inflammation in diabetes mellitus | |
| Mast cells | Enhances IgE-mediated mast cell degranulation | Promotes inflammation in diabetes mellitus |
| Macrophages | Inhibits Nrf2 | Promotes inflammation in hyperlipidemia |
| Vascular endothelial cells | Inhibits Nrf2 | Promotes inflammation in diabetes mellitus |
| Vascular smooth muscle cells | Participates in GPRC5B-NFκB pathway | Promotes inflammation in hyperglycemia |
| Renal tubule cells | Inhibits Nrf2; through mTOR pathway; through Transglutaminase2 pathway | Promotes inflammation in DKD |
| Podocytes | Activates Rho-associated coiled-coil-forming protein kinase pathway; participates in NCOA3 pathway | Promotes inflammation in DKD |
| Mesangial cells | Inhibits Nrf2; negatively regulates Sirt1/Foxo3a pathway | Promotes inflammation in DKD |
| Retinal vascular endothelial cells | Promotes TNF-α-VCAM-1 pathway; participates in role of tubedown | Promotes inflammation in diabetic retinopathy |
| Gustatory cells | Participates in CD36-mediated calcium ion influx | Increases perception of fat taste |
| Long bone marrow mesenchymal stem cells | Activates mTORC2 | Inhibits adipocyte formation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, R.; Shen, C.; Shen, W.; Wu, X.; Deng, X.; Jia, J.; Yuan, G. Fyn Kinase: A Potential Target in Glucolipid Metabolism and Diabetes Mellitus. Curr. Issues Mol. Biol. 2025, 47, 623. https://doi.org/10.3390/cimb47080623
Xiao R, Shen C, Shen W, Wu X, Deng X, Jia J, Yuan G. Fyn Kinase: A Potential Target in Glucolipid Metabolism and Diabetes Mellitus. Current Issues in Molecular Biology. 2025; 47(8):623. https://doi.org/10.3390/cimb47080623
Chicago/Turabian StyleXiao, Ruifeng, Cong Shen, Wen Shen, Xunan Wu, Xia Deng, Jue Jia, and Guoyue Yuan. 2025. "Fyn Kinase: A Potential Target in Glucolipid Metabolism and Diabetes Mellitus" Current Issues in Molecular Biology 47, no. 8: 623. https://doi.org/10.3390/cimb47080623
APA StyleXiao, R., Shen, C., Shen, W., Wu, X., Deng, X., Jia, J., & Yuan, G. (2025). Fyn Kinase: A Potential Target in Glucolipid Metabolism and Diabetes Mellitus. Current Issues in Molecular Biology, 47(8), 623. https://doi.org/10.3390/cimb47080623







