DJ-1 Serves as a Central Regulator of Diabetes Complications
Abstract
1. Introduction
2. Global Trends and Pathogenesis of Diabetes
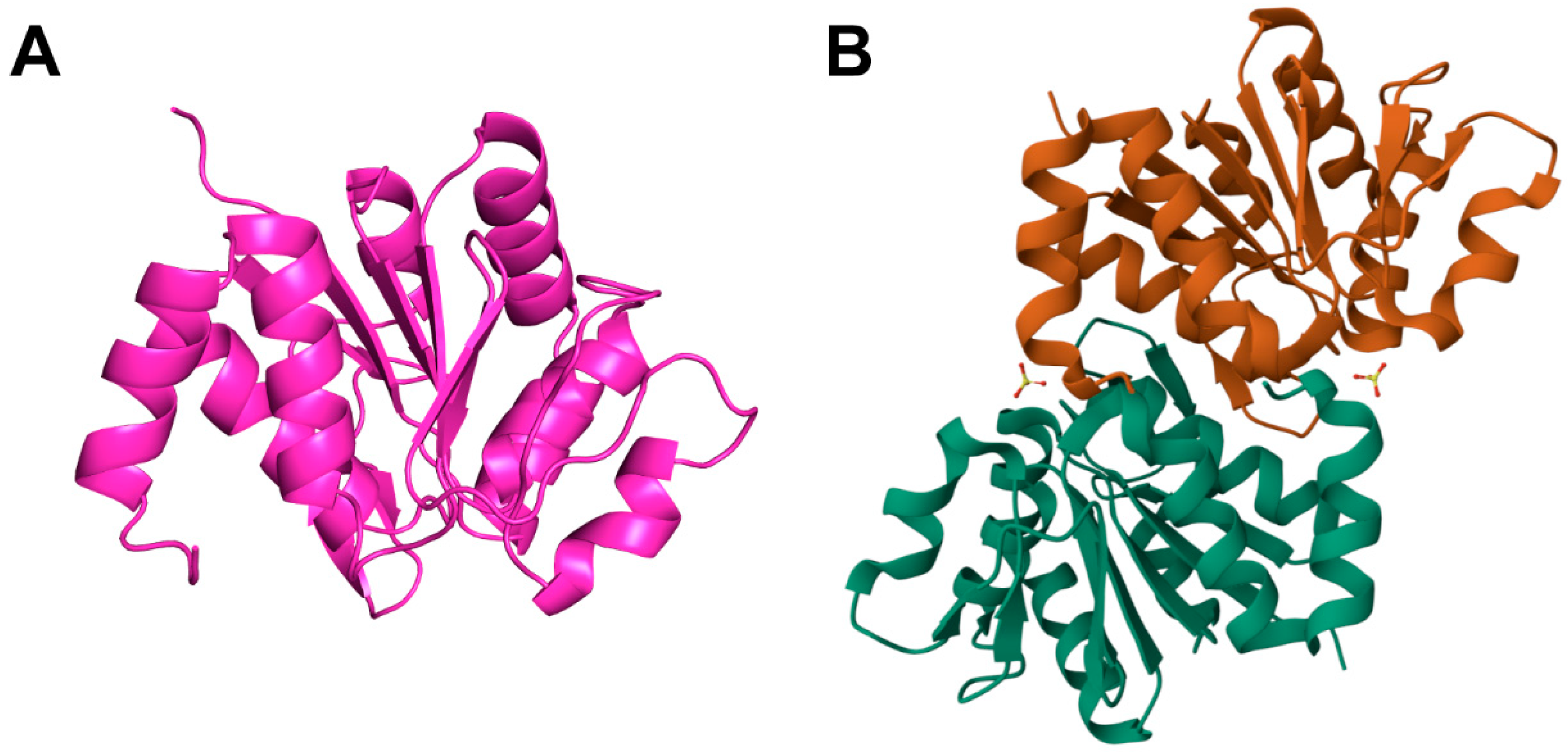
3. Structure and Function of DJ-1
3.1. Structure of DJ-1
3.2. Functions of DJ-1
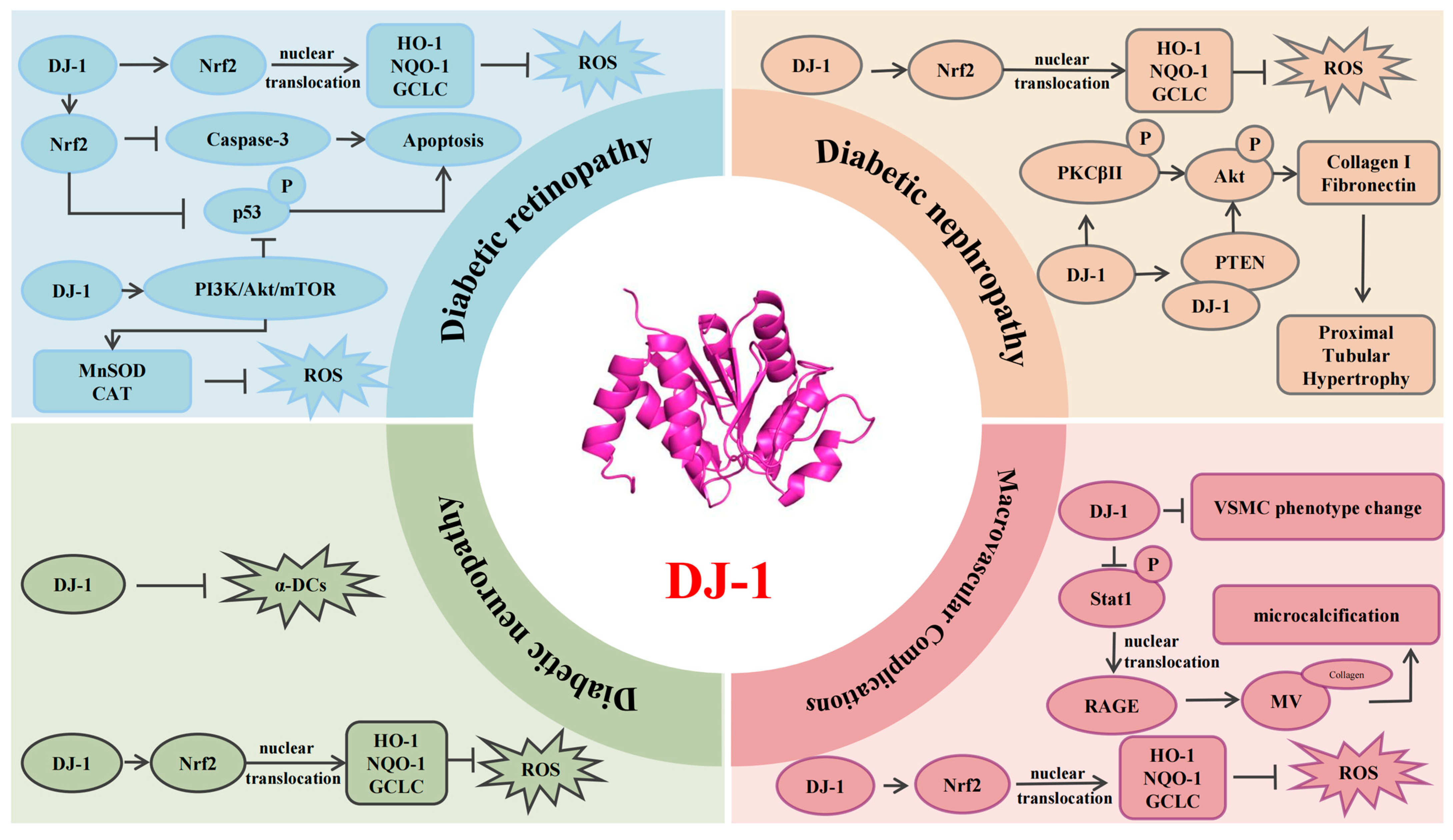
4. DJ-1 and the Pathological Mechanisms of Diabetic Complications
4.1. Diabetic Retinopathy
4.2. Diabetic Nephropathy
4.3. Diabetic Neuropathy
4.4. Macrovascular Complications of Diabetes
5. Clinical Prospects and Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cole, J.B.; Florez, J.C. Genetics of Diabetes Mellitus and Diabetes Complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 Diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Zhang, H.; Tan, J.; Wang, C. Neuroprotective Role and Mechanistic Insights of DJ-1 Dimerization in Parkinson’s Disease. Cell Commun. Signal. CCS 2025, 23, 129. [Google Scholar] [CrossRef]
- Parfitt, G.M.; Coccia, E.; Goldman, C.; Whitney, K.; Reyes, R.; Sarrafha, L.; Nam, K.H.; Sohail, S.; Jones, D.R.; Crary, J.F.; et al. Disruption of lysosomal proteolysis in astrocytes facilitates midbrain organoid proteostasis failure in an early-onset Parkinson’s disease model. Nat. Commun. 2024, 15, 447. [Google Scholar]
- Sun, Z.; Li, L.; Wu, Y.; Zhang, L.; Zang, G.; Qian, Y.; Yao, H.; Mao, X.; Wang, Z. Acetylation-ubiquitination crosstalk of DJ-1 mediates microcalcification formation in diabetic plaques via collagen-matrix vesicles interaction. Cardiovasc. Res. 2024, 121, 296–310. [Google Scholar] [CrossRef]
- Yan, Z.; Li, Y.; Wang, M.; Xu, K.; Liu, Y.; Wang, L.; Luo, H.; Chen, Z.; Liu, X. O-GlcNAcylation of DJ-1 suppresses ferroptosis in renal cell carcinoma by affecting the transsulfuration pathway. Int. Immunopharmacol. 2025, 148, 114098. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, H.; Ma, B.; Sun, X.; Chen, B. DJ-1 DJ-1 regulates mitochondrial function and promotes retinal ganglion cell survival under high glucose-induced oxidative stress. Front. Pharmacol. 2024, 15, 1455439. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, H.; Chen, B. DJ-1 protects retinal pericytes against high glucose-induced oxidative stress through the Nrf2 signaling pathway. Sci. Rep. 2020, 10, 2477. [Google Scholar] [CrossRef]
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Jia, W.; Chan, J.C.; Wong, T.Y.; Fisher, E.B. Diabetes in China: Epidemiology, pathophysiology and multi-omics. Nat. Metab. 2025, 7, 16–34. [Google Scholar] [CrossRef]
- Wong, T.Y.; Cheung, C.; Larsen, M.; Sharma, S.; Simó, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef]
- Das, F.; Ghosh-Choudhury, N.; Maity, S.; Kasinath, B.S.; Choudhury, G.G. Oncoprotein DJ-1 interacts with mTOR complexes to effect transcription factor Hif1α-dependent expression of collagen I (α2) during renal fibrosis. J. Biol. Chem. 2022, 298, 102246. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, J.; Li, M.; Wang, T.; Wang, K.; Cao, Q.; Ding, Y.; Xiang, Y.; Wang, S.; Yang, Q.; et al. Diabetes in China part 1: Epidemiology and risk factors. Lancet Public Health 2024, 9, e1089–e1097. [Google Scholar] [CrossRef] [PubMed]
- Defronzo, R.A. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 dia-betes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Wang, J.; Yang, B.; He, Q.; Weng, Q. Role of DJ-1 in Immune and Inflammatory Diseases. Front. Immunol. 2020, 11, 994. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, X.-B.; Wu, Q.; Zhu, H.-Y.; Du, C.-Y.; Ying, M.-D.; He, Q.-J.; Zhu, H.; Yang, B.; Cao, J. The C terminus of DJ-1 determines its homodimerization, MGO detoxification activity and suppression of ferroptosis. Acta Pharmacol. Sin. 2020, 42, 1150–1159. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, J.; Zhang, X.; Xu, X.; Luo, D.; Lin, Y.; Yan, M.; Song, Y. The antioxidant effect of tetrahedral framework nucleic acid-based delivery of small activating RNA targeting DJ-1 on retinal oxidative stress injury. Cell Prolif. 2024, 57, e13635. [Google Scholar] [CrossRef]
- Huang, M.; Chen, S. DJ-1 in neurodegenerative diseases: Pathogenesis and clinical application. Prog. Neurobiol. 2021, 204, 102114. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-B.; Zhu, H.-Y.; Bao, K.; Jiang, L.; Zhu, H.; Ying, M.-D.; He, Q.-J.; Yang, B.; Sheng, R.; Cao, J. Bis-isatin derivatives: Design, synthesis, and biological activity evaluation as potent dimeric DJ-1 inhibitors. Acta Pharmacol. Sin. 2021, 42, 1160–1170. [Google Scholar] [CrossRef]
- Ji, Y.-W.; Wen, X.-Y.; Tang, H.-P.; Jin, Z.-S.; Su, W.-T.; Zhou, L.; Xia, Z.-Y.; Lei, S.-Q. DJ-1: Potential target for treatment of myocardial ischemia-reperfusion injury. Biomed. Pharmacother. 2024, 179, 117383. [Google Scholar] [CrossRef]
- Chan, J.Y.; Chan, S.H. Activation of endogenous antioxidants as a common therapeutic strategy against cancer, neurodegeneration and cardiovascular diseases: A lesson learnt from DJ-1. Pharmacol. Ther. 2015, 156, 69–74. [Google Scholar] [CrossRef]
- Olivo, E.; La Chimia, M.; Ceramella, J.; Catalano, A.; Chiaradonna, F.; Sinicropi, M.S.; Cuda, G.; Iacopetta, D.; Scumaci, D. Moving beyond the Tip of the Iceberg: DJ-1 Implications in Cancer Metabolism. Cells 2022, 11, 1432. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.A. The Role of Cysteine Oxidation in DJ-1 Function and Dysfunction. Antioxid. Redox Signal. 2011, 15, 111–122. [Google Scholar] [CrossRef]
- Cao, J.; Chen, X.; Jiang, L.; Lu, B.; Yuan, M.; Zhu, D.; Zhu, H.; He, Q.; Yang, B.; Ying, M. DJ-1 suppresses ferroptosis through preserving the activity of S-adenosyl homocysteine hydrolase. Nat. Commun. 2020, 11, 1251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C. Quantitative reactive cysteinome profiling reveals a functional link between ferroptosis and proteasome-mediated degradation. Cell Death Differ. 2022, 30, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Mencke, P.; Boussaad, I.; Romano, C.D.; Kitami, T.; Linster, C.L.; Krüger, R. The Role of DJ-1 in Cellular Metabolism and Pathophysiological Implications for Parkinson’s Disease. Cells 2021, 10, 347. [Google Scholar] [CrossRef]
- Susarla, G.; Kataria, P.; Kundu, A.; D’Silva, P. Saccharomyces cerevisiae DJ-1 paralogs maintain genome integrity through glycation repair of nucleic acids and proteins. eLife 2023, 12, e88875. [Google Scholar] [CrossRef]
- Neves, M.; Grãos, M.; Anjo, S.I.; Manadas, B. Modulation of signaling pathways by DJ-1: An updated overview. Redox Biol. 2022, 51, 102283. [Google Scholar] [CrossRef]
- Zeng, J.; Zhao, H.; Chen, B. DJ-1/PARK7 inhibits high glucose-induced oxidative stress to prevent retinal pericyte apoptosis via the PI3K/AKT/mTOR signaling pathway. Exp. Eye Res. 2019, 189, 107830. [Google Scholar] [CrossRef]
- Jain, D.; Jain, R.; Eberhard, D.; Eglinger, J.; Bugliani, M.; Piemonti, L.; Marchetti, P.; Lammert, E. Age- and diet-dependent requirement of DJ-1 for glucose homeostasis in mice with implications for human type 2 diabetes. J. Mol. Cell Biol. 2012, 4, 221–230. [Google Scholar] [CrossRef]
- Eberhard, D.; Lammert, E. The Role of the Antioxidant Protein DJ-1 in Type 2 Diabetes Mellitus. Adv. Exp. Med. Biol. 2017, 1037, 173–186. [Google Scholar]
- Zhang, X.; Qiu, J.; Huang, F.; Shan, K.; Zhang, C. Type 2 Diabetes Mellitus Makes Corneal Endothelial Cells Vulnerable to Ultraviolet A-Induced Oxidative Damage Via Decreased DJ-1/Nrf2/NQO1 Pathway. Investig. Ophthalmol. Vis. Sci. 2022, 63, 25. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Lyon, C.J.; Xia, X.; Liu, J.Z.; Tangirala, R.K.; Yin, F.; Boyadjian, R.; Bikineyeva, A.; Praticò, D.; Harrison, D.G.; et al. Age-Accelerated Atherosclerosis Correlates with Failure to Upregulate Antioxidant Genes. Circ. Res. 2009, 104, e42–e54. [Google Scholar] [CrossRef]
- Sun, Q.; Shen, Z.-Y.; Meng, Q.-T.; Liu, H.-Z.; Duan, W.-N.; Xia, Z.-Y. The role of DJ-1/Nrf2 pathway in the pathogenesis of diabetic nephropathy in rats. Ren. Fail. 2016, 38, 294–304. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Leng, Y.; Xiong, Y.; Xue, R.; Chen, R.; Xia, Z. Mechanism of N-acetylcysteine in alleviating diabetic myocardial ischemia reperfusion injury by regulating PTEN/Akt pathway through promoting DJ-1. Biosci. Rep. 2020, 40, BSR20192118. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Leng, Y.; Xiong, Y.; Li, W.; Cai, Y.; Xue, R.; Chen, R.; Lei, S.; Xia, Z.; Xia, Z. DJ-1 preserves ischemic postconditioning-induced cardioprotection in STZ-induced type 1 diabetic rats: Role of PTEN and DJ-1 subcellular translocation. Cell Commun. Signal. 2024, 22, 252. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhou, B.; Xia, Z.-Y.; Zhao, B.; Lei, S.-Q.; Yang, Q.-J.; Xue, R.; Leng, Y.; Xu, J.-J.; Xia, Z. Hyperglycemia-Induced Inhibition of DJ-1 Expression Compromised the Effectiveness of Ischemic Postconditioning Cardioprotection in Rats. Oxidative Med. Cell. Longev. 2013, 2013, 564902. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, Z.; Xia, P.; Shi, A.; FuChen, X.; Zhang, J.; Yu, P. Ferroptosis and ferritinophagy in diabetes complications. Mol. Metab. 2022, 60, 101470. [Google Scholar] [CrossRef]
- Hayashi, T.; Ishimori, C.; Takahashi-Niki, K.; Taira, T.; Kim, Y.-C.; Maita, H.; Maita, C.; Ariga, H.; Iguchi-Ariga, S.M.M. DJ-1 binds to mitochondrial complex I and maintains its activity. Biochem. Biophys. Res. Commun. 2009, 390, 667–672. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Kochevar, I.E.; Jurkunas, U.V. Decreased DJ-1 Leads to Impaired Nrf2-Regulated Antioxidant Defense and Increased UV-A–Induced Apoptosis in Corneal Endothelial Cells. Investig. Opthalmology Vis. Sci. 2014, 55, 5551–5560. [Google Scholar] [CrossRef]
- Das, F.; Ghosh-Choudhury, N.; Kasinath, B.S.; Sharma, K.; Choudhury, G.G.; Murugan, A.K. High glucose couples DJ-1 with PTEN to activate PDGFRβ for renal proximal tubular cell injury. PLoS ONE 2025, 20, e0311828. [Google Scholar] [CrossRef]
- Wu, F.; Li, S.; Zhang, N.; Huang, W.; Li, X.; Wang, M.; Bai, D.; Han, B. Hispidulin alleviates high-glucose-induced podocyte injury by regulating protective autophagy. Biomed. Pharmacother. 2018, 104, 307–314. [Google Scholar] [CrossRef]
- Asgari, M.; Salehi, I.; Ranjbar, K.; Khosravi, M.; Zarrinkalam, E. Interval training and Crataegus persica ameliorate diabetic nephropathy via miR-126/Nrf-2 mediated inhibition of stress oxidative in rats with diabetes after myocardial ischemia-reperfusion injury. Biomed. Pharmacother. 2022, 153, 113411. [Google Scholar] [CrossRef] [PubMed]
- Das, F.; Dey, N.; Venkatesan, B.; Kasinath, B.S.; Ghosh-Choudhury, N.; Choudhury, G.G. High glucose upregulation of early-onset Parkinson’s disease protein DJ-1 integrates the PRAS40/TORC1 axis to mesangial cell hypertrophy. Cell Signal. 2011, 23, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-Y.; Sun, Q.; Xia, Z.-Y.; Meng, Q.-T.; Lei, S.-Q.; Zhao, B.; Tang, L.-H.; Xue, R.; Chen, R. Overexpression of DJ-1 reduces oxidative stress and attenuates hypoxia/reoxygenation injury in NRK-52E cells exposed to high glucose. Int. J. Mol. Med. 2016, 38, 729–736. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Glyoxalase in diabetes, obesity and related disorders. Semin. Cell Dev. Biol. 2011, 22, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, J.; Bose, N.; Gong, J.; Hall, D.; Rifkind, A.; Bhaumik, D.; Peiris, T.H.; Chamoli, M.; Le, C.H.; Liu, J.; et al. A Caenorhabditis elegans Model Elucidates a Conserved Role for TRPA1-Nrf Signaling in Reactive α-Dicarbonyl Detoxification. Curr. Biol. 2016, 26, 3014–3025. [Google Scholar] [CrossRef]
- Hashemi, M.; Zandieh, M.A.; Ziaolhagh, S.; Mojtabavi, S.; Sadi, F.H.; Koohpar, Z.K.; Ghanbarirad, M.; Haghighatfard, A.; Behroozaghdam, M.; Khorrami, R.; et al. Nrf2 signaling in diabetic nephropathy, cardiomyopathy and neuropathy: Therapeutic targeting, challenges and future prospective. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166714. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, J.; Liu, B.; Xie, F.; Li, C.; Qiao, W.; Lu, Q.; Wang, Y.; Zhang, M. Protein deglycase DJ-1 deficiency induces phenotypic switching in vascular smooth muscle cells and exacerbates atherosclerotic plaque instability. J. Cell. Mol. Med. 2021, 25, 2816–2827. [Google Scholar] [CrossRef]
- Gan, L.; Zhao, J.; Yao, P.; Christopher, T.A.; Lopez, B.; Lau, W.B.; Koch, W.; Gao, E.; Ma, X.; Wang, Y. Adipocyte-derived small extracellular vesicles exacerbate diabetic ischemic heart injury by promoting oxidative stress and mitochondrial-mediated cardiomyocyte apoptosis. Redox Biol. 2024, 79, 103443. [Google Scholar] [CrossRef]
- Zhou, B.; Lei, S.; Xue, R.; Leng, Y.; Xia, Z.; Xia, Z.-Y. DJ-1 overexpression restores ischaemic post-conditioning-mediated cardioprotection in diabetic rats: Role of autophagy. Clin. Sci. 2017, 131, 1161–1178. [Google Scholar] [CrossRef]
- De Lazzari, F.; Prag, H.A.; Gruszczyk, A.V.; Whitworth, A.J.; Bisaglia, M. DJ-1: A promising therapeutic candidate for ischemia-reperfusion injury. Redox Biol. 2021, 41, 101884. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, Q.A. Redox modulatory role of DJ-1 in Parkinson’s disease. Biogerontology 2025, 26, 81. [Google Scholar] [CrossRef] [PubMed]
| DJ-1 Protein Partners | Experimental Species | Biological Effect | References |
| Nuclear factor erythropoiesis-related factor 2 (NRF2) | Mus musculus Rattus norvegicus | DJ-1 facilitates NRF2 release from KEAP1, promoting its nuclear translocation. NRF2 then binds AREs, enhancing transcription of oxidative stress-responsive genes such as HO-1 and NQO1, thereby mitigating hyperglycemia-induced oxidative stress. | [8,33,34] |
| Mus musculus | NRF2 translocation reduces caspase-3 activity and p53 phosphorylation, decreasing the susceptibility of corneal endothelial cells to UVA-induced oxidative injury. | [32] | |
| Phosphatase and tensin homolog (PTEN) | Rattus norvegicus | NAC alleviates diabetic myocardial I/R injury via DJ-1-mediated activation of the PTEN/Akt pathway. | [35] |
| Rattus norvegicus | DJ-1 overexpression re-establishes IPostC-induced cardioprotection in diabetes through mitochondrial and nuclear translocation, suppressing PTEN and influencing cell survival and immune regulation. | [36] | |
| Phosphatidylinositol 3-kinase (PI3K) | Rattus norvegicus | DJ-1 may exert cytoprotective effects by activating the PI3K/Akt pathway, which governs key cellular functions such as migration, metabolism, and survival. | [37] |
| Signal transducer and activator of transcription 1 (STAT1) | Mus musculus Homo sapiens | The interplay between NAT10-mediated acetylation and TRIM32-mediated ubiquitination drives the proteasomal degradation of DJ-1. This activates the DJ-1/STAT1/RAGE axis, promoting coronary microcalcification via osteogenic transdifferentiation. | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Zhou, J.-B.; Wei, T.-P.; Wu, D.; Wang, R.-X. DJ-1 Serves as a Central Regulator of Diabetes Complications. Curr. Issues Mol. Biol. 2025, 47, 613. https://doi.org/10.3390/cimb47080613
Zhou F, Zhou J-B, Wei T-P, Wu D, Wang R-X. DJ-1 Serves as a Central Regulator of Diabetes Complications. Current Issues in Molecular Biology. 2025; 47(8):613. https://doi.org/10.3390/cimb47080613
Chicago/Turabian StyleZhou, Feng, Jia-Bin Zhou, Tian-Peng Wei, Dan Wu, and Ru-Xing Wang. 2025. "DJ-1 Serves as a Central Regulator of Diabetes Complications" Current Issues in Molecular Biology 47, no. 8: 613. https://doi.org/10.3390/cimb47080613
APA StyleZhou, F., Zhou, J.-B., Wei, T.-P., Wu, D., & Wang, R.-X. (2025). DJ-1 Serves as a Central Regulator of Diabetes Complications. Current Issues in Molecular Biology, 47(8), 613. https://doi.org/10.3390/cimb47080613





