The Aging Lung: Exploring Multimorbidity Patterns and Their Clinical Implications: A Narrative Review
Abstract
1. Introduction
2. Mechanisms of Aging and Multimorbidity
2.1. Aging and Changes in Body Composition and Metabolism
2.2. Aging and the Cardiovascular System
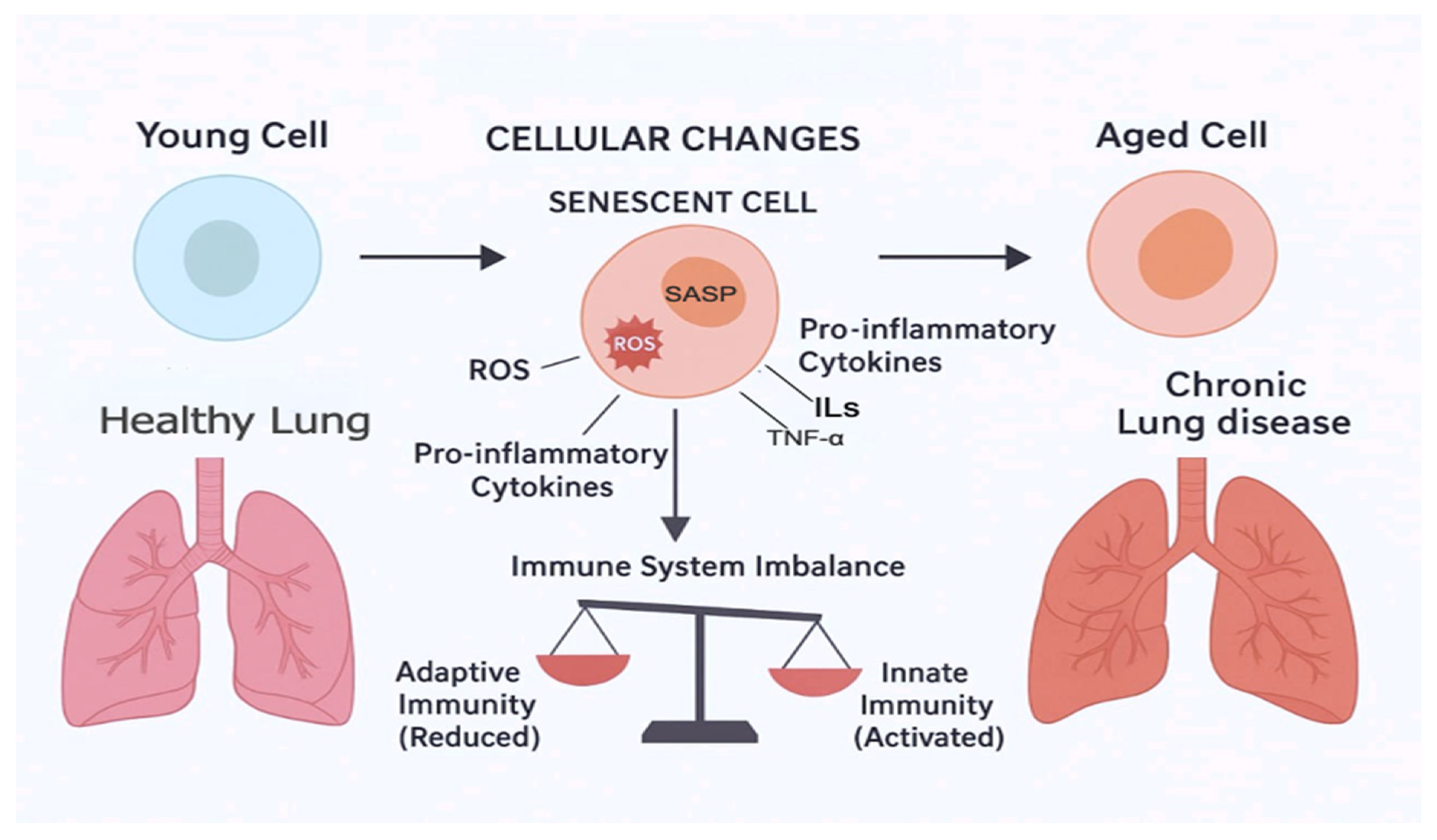
2.3. Aging and the Respiratory System
2.4. Aging and the Central Nervous System
3. Interlink Between Aging, Frailty, and the Lung
4. Treatment of Aging and Multimorbidity
4.1. Pharmacotherapy
4.2. Non-Pharmacotherapy
4.2.1. Exercise and Physical Conditioning
4.2.2. Calorie Control
5. Expert Opinion and Practical Recommendations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vetrano, D.; Calderón-Larrañaga, A.; Marengoni, A.; Onder, G.; Bauer, J.M.; Cesari, M.; Ferrucci, L.; Fratiglioni, L. An International Perspective on Chronic Multimorbidity: Approaching the Elephant in the Room. J. Gerontol. Ser. A 2018, 73, 1350–1356. [Google Scholar] [CrossRef]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multiple chronic conditions and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef]
- Vetrano, D.L.; Palmer, K.; Marengoni, A.; Marzetti, M.; Lattanzio, F.; Roller-Wirnsberger, R.; Samaniego, L.; Rodríguez-Mañas, L.; Bernabei, R.; Onder, G. Frailty and Multimorbidity: A Systematic Review and Meta-analysis. J. Gerontol. Ser. A 2019, 74, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Ornstein, S.M.; Nietert, P.J.; Jenkins, R.G.; Litvin, C.B. The Prevalence of Chronic Diseases and Multimorbidity in Primary Care Practice: A PPRNet Report. J. Am. Board Fam. Med. 2013, 26, 518–524. [Google Scholar] [CrossRef]
- Fabbri, L.; Boyd, C.; Boschetto, P.; Rabe, K.; Buist, A.; Yawn, B.; Leff, B.; Kent, D.; Schünemann, H.; ATS/ERS Ad Hoc Committee on Integrating and Coordinating Efforts in COPD Guideline Development. How to integrate multiple comorbidities in guideline development: Article 10 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report. Proc. Am. Thorac. Soc. 2012, 9, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Jin, K. Modern Biological Theories of Aging. Aging Dis. 2010, 1, 72–74. [Google Scholar]
- North, B.J.; Sinclair, D.A. The Intersection Between Aging and Cardiovascular Disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Temby, O.F.; Smith, K.R. The association between adult mortality risk and family history of longevity: The moderating effects of socioeconomic status. J. Biosoc. Sci. 2014, 46, 703–716. [Google Scholar] [CrossRef]
- Argentieri, M.A.; Amin, N.; Nevado-Holgado, A.J.; Sproviero, W.; Collister, J.A.; Keestra, S.M.; Kuilman, M.M.; Ginos, B.N.R.; Ghanbari, M.; Doherty, A.; et al. Integrating the environmental and genetic architectures of aging and mortality. Nat. Med. 2025, 31, 1016–1025. [Google Scholar] [CrossRef]
- Herskind, A.M.; McGue, M.; Holm, N.V.; Sørensen, T.I.A.; Harvald, B.; Vaupel, J.W. The heritability of human longevity: A population-based study of 2872 Danish twin pairs born 1870–1900. Hum. Genet. 1996, 97, 319–323. [Google Scholar] [CrossRef]
- Ljungquist, B.; Berg, S.; Lanke, J.; McClearn, G.E.; Pedersen, N.L. The effect of genetic factors for longevity: A comparison of identical and fraternal twins in the Swedish Twin Registry. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1998, 53, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.J.; Craig, J.M.; Mather, K.A.; Sachdev, P.S.; Saffery, R.; Sung, J.; Tan, Q.; Thalamuthu, A.; Milne, R.L.; Giles, G.G.; et al. Genetic and environmental causes of variation in epigenetic aging across the lifespan. Clin. Epigenetics 2020, 12, 158. [Google Scholar]
- Bhardwaj, R.; Amiri, S.; Buchwald, D.; Amram, O. Environmental Correlates of Reaching a Centenarian Age: Analysis of 144,665 Deaths in Washington State for 2011–2015. Int. J. Environ. Res. Public Health 2020, 17, 2828. [Google Scholar] [CrossRef] [PubMed]
- Mucherino, S.; Gimeno-Miguel, A.; Carmona-Pirez, J.; Gonzalez-Rubio, F.; Ioakeim-Skoufa, I.; Moreno-Juste, A.; Orlando, V.; Aza-Pascual-Salcedo, M.; Poblador-Plou, B.; Menditto, E.; et al. Changes in Multimorbidity and Polypharmacy Patterns in Young and Adult Population over a 4-Year Period: A 2011-2015 Comparison Using Real-World Data. Int. J. Environ. Res. Public Health 2021, 18, 4422. [Google Scholar] [CrossRef]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef]
- de Oliveira Neto, L.; Tavares, V.D.d.O.; Agrícola, P.M.D.; de Oliveira, L.P.; Sales, M.C.; de Sena-Evangelista, K.C.M.; Gomes, I.C.; Galvão-Coelho, N.L.; Pedrosa, L.F.C. Factors associated with inflamm-aging in institutionalized older people. Sci. Rep. 2021, 11, 18333. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Luo, G.; Xu, Y.; Deng, X.; Lin, Y.; Wang, Z.; Zhou, S.; Wang, S.; Chen, H.; et al. The aging lung: Microenvironment, mechanisms, and diseases. Front. Immunol. 2024, 15, 1383503. [Google Scholar] [CrossRef]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The cellular aging-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Van Remoortel, H.; Hornikx, M.; Langer, D.; Burtin, C.; Everaerts, S.; Verhamme, P.; Boonen, S.; Gosselink, R.; Decramer, M.; Troosters, T.; et al. Risk factors and comorbidities in the preclinical stages of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 189, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Weinberger, B.; Grubeck-Loebenstein, B. The aging of the immune system. Transpl. Int. 2009, 22, 1041–1050. [Google Scholar] [CrossRef]
- Barnes, P.J.; Baker, J.; Donnelly, L.E. Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am. J. Respir. Crit. Care Med. 2019, 200, 556–564. [Google Scholar] [CrossRef]
- Papaconstantinou, J. Insulin/IGF-1 and ROS signaling pathway cross-talk in aging and longevity determination. Mol. Cell. Endocrinol. 2009, 299, 89–100. [Google Scholar] [CrossRef]
- De Martinis, M.; Franceschi, C.; Monti, D.; Ginaldi, L. Inflammation markers predicting frailty and mortality in the elderly. Exp. Mol. Pathol. 2006, 80, 219–227. [Google Scholar] [CrossRef]
- Hughes, M.J.; McGettrick, H.M.; Sapey, E. Shared mechanisms of multiple chronic conditions in COPD, atherosclerosis and type-2 diabetes: The neutrophil as a potential inflammatory target. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2020, 29, 190102. [Google Scholar] [CrossRef]
- Brown, C.D.; Higgins, M.; Donato, K.A.; Rohde, F.C.; Garrison, R.; Obarzanek, E.; Ernst, N.D.; Horan, M. Body Mass Index and the Prevalence of Hypertension and Dyslipidemia. Obes. Res. 2000, 8, 605–619. [Google Scholar] [CrossRef]
- Gosker, H.R.; Zeegers, M.P.; Wouters, E.F.; Schols, A.M. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: A systematic review and meta-analysis. Thorax 2007, 62, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Barberà, J.A.; Wouters, E.F.M.; Peinado, V.I.; Jeffery, P.K. Lungs, bone marrow and adipose tissue. A network approach to the pathobiology of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef]
- Haight, T.; Tager, I.; Sternfeld, B.; Satariano, W.; van der Laan, M. Effects of Body Composition and Leisure-time Physical Activity on Transitions in Physical Functioning in the Elderly. Am. J. Epidemiol. 2005, 162, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.W.; Montgomery, P.S. Differences in exercise performance and leisure-time physical activity in older men ans women. Clin. Med. Geriatr. 2008, 2008, 9–15. [Google Scholar]
- Alter, P.; Kahnert, K.; Trudzinski, F.C.; Bals, R.; Watz, H.; Speicher, T.; Söhler, S.; Andreas, S.; Welte, T.; Rabe, K.F.; et al. Disease Progression and Age as Factors Underlying Multimorbidity in Patients with COPD: Results from COSYCONET. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 1703–1713. [Google Scholar] [CrossRef]
- Schubert, C.; Rogers, N.; Remsberg, K.; Sun, S.; Chumlea, W.; Demerath, E.; Czerwinski, S.; Towne, B.; Siervogel, R. Lipids, lipoproteins, lifestyle, adiposity and fat-free mass during middle age: The Fels Longitudinal Study. Int. J. Obes. Relat. Metab. Disord. 2005, 30, 251–260. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Lakatta, E.G. Age-associated Cardiovascular Changes in Health: Impact on Cardiovascular Disease in Older Persons. Heart Fail. Rev. 2002, 7, 29–49. [Google Scholar] [CrossRef]
- Lauder, L.; Mahfoud, F.; Azizi, M.; Bhatt, D.L.; Ewen, S.; Kario, K.; Parati, G.; Rossignol, P.; Schlaich, M.P.; Teo, K.K.; et al. Hypertension management in patients with cardiovascular comorbidities. Eur. Heart J. 2023, 44, 2066–2077. [Google Scholar] [CrossRef]
- Pencina, M.; D’Agostino, R.; Larson, M.; Massaro, J.; Vasan, R. Predicting the 30-year risk of cardiovascular disease: The Framingham Heart Study. Circulation 2009, 119, 3078–3084. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Brandes, R.; Fleming, I.; Busse, R. Endothelial aging. Cardiovasc. Res. 2005, 66, 286–294. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.; Newman, A.B.; Kritchevsky, S.B.; Nicklas, B.J.; Sutton-Tyrrell, K.; Rubin, S.M.; Ding, J.; Simonsick, E.M.; Harris, T.B. Inflammatory markers and onset of cardiovascular events results from the Health ABC Study. Circulation 2003, 108, 2317–2322. [Google Scholar] [CrossRef]
- Rodríguez-Mañas, L.; El-Assar, M.; Vallejo, S.; López-Dóriga, P.; Solís, J.; Petidier, R.; Montes, M.; Nevado, J.; Castro, M.; Gómez-Guerrero, C.; et al. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell 2009, 8, 226–238. [Google Scholar] [CrossRef]
- Derlin, T.; Tóth, Z.; Papp, L.; Wisotzki, C.; Apostolova, I.; Habermann, C.R.; Mester, J.; Klutmann, S. Correlation of Inflammation Assessed by 18F-FDG PET, Active Mineral Deposition Assessed by 18F-Fluoride PET, and Vascular Calcification in Atherosclerotic Plaque: A Dual-Tracer PET/CT Study. J. Nucl. Med. 2011, 52, 1020–1027. [Google Scholar] [CrossRef]
- Abdelbaky, A.; Corsini, E.; Figueroa, A.L.; Fontanez, S.; Subramanian, S.; Ferencik, M.; Brady, T.J.; Hoffmann, U.; Tawakol, A. Focal Arterial Inflammation Precedes Subsequent Calcification in the Same Location A Longitudinal FDG-PET/CT Study. Circ. Cardiovasc. Imaging 2013, 6, 747–754. [Google Scholar] [CrossRef]
- Vanfleteren, L.E.; Spruit, M.A.; Groenen, M.; Gaffron, S.; van Empel, V.P.; Bruijnzeel, P.L.; Rutten, E.P.; Op ’t Roodt, J.; Wouters, E.F.; Franssen, F.M. Clusters of Comorbidities Based on Validated Objective Measurements and Systemic Inflammation in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.R.; Vestbo, J.; Anzueto, A.; Locantore, N.; Mullerova, H.; Tal-Singer, R.; Miller, B.; Lomas, D.A.; Agusti, A.; MacNee, W.; et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 2010, 363, 1128–1138. [Google Scholar] [CrossRef]
- Feary, J.R.; Rodrigues, L.C.; Smith, C.J.; Hubbard, R.B.; Gibson, J.E. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: A comprehensive analysis using data from primary care. Thorax 2010, 65, 956–962. [Google Scholar] [CrossRef]
- Van Eeden, S.; Leipsic, J.; Paul Man, S.F.; Sin, D.D. The relationship between lung inflammation and cardiovascular disease. Am. J. Respir. Crit. Care Med. 2012, 186, 11–16. [Google Scholar] [CrossRef]
- Thomsen, M.; Dahl, M.; Lange, P.; Vestbo, J.; Nordestgaard, B.G. Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 186, 982–988. [Google Scholar] [CrossRef]
- Brandstetter, R.; Kazemi, H. Aging and the respiratory system. Med. Clin. N. Am. 1983, 67, 419. [Google Scholar] [CrossRef]
- Dyer, C. The interaction of ageing and lung disease. Chronic Respir. Dis. 2012, 9, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Hanania, N.A.; Shim, Y.M. The aging immune system and its relationship to the development of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2009, 6, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Desler, C.; Hansen, T.L.; Frederiksen, J.B.; Marcker, M.L.; Singh, K.K.; Juel Rasmussen, L. Is there a link between mitochondrial reserve respiratory capacity and aging? J. Aging Res. 2012, 2012, 192503. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, I., Jr.; Oliveira, L.M.; Mayer, A.F.; Jardim, J.R.; Natour, J. Evaluation of pulmonary function and quality of life in women with osteoporosis. Osteoporos. Int. 2005, 16, 1247–1253. [Google Scholar] [CrossRef]
- Brown, M.; Hasser, E.M. Complexity of age-related change in skeletal muscle. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1996, 51, B117–B123. [Google Scholar] [CrossRef]
- Miniño, A.M.; Murphy, S.L.; Xu, J.; Kochanek, K.D. Deaths: Final Data for 2008. Natl. Vital Stat. Rep. 2011, 59. [Google Scholar]
- de Oliveira-Maul, J.P.; de Carvalho, H.B.; Goto, D.M.; Maia, R.M.; Flo, C.; Barnabe, V.; Franco, D.R.; Benabou, S.; Perracini, M.R.; Jacob-Filho, W. Aging, diabetes, and hypertension are associated with decreased nasal mucociliary clearance. CHEST J. 2013, 143, 1091–1097. [Google Scholar] [CrossRef]
- Svartengren, M.; Falk, R.; Philipson, K. Long-term clearance from small airways decreases with age. Eur. Respir. J. 2005, 26, 609–615. [Google Scholar] [CrossRef]
- Barnes, P.; Shapiro, S.; Pauwels, R. Chronic obstructive pulmonary disease: Molecular and cellularmechanisms. Eur. Respir. J. 2003, 22, 672–688. [Google Scholar] [CrossRef] [PubMed]
- Grolleau-Julius, A.; Harning, E.K.; Abernathy, L.M.; Yung, R.L. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. 2008, 68, 6341–6349. [Google Scholar] [CrossRef]
- Salthouse, T. Consequences of age-related cognitive declines. Annu. Rev. Psychol. 2012, 63, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Dag, E.; Bulcun, E.; Turkel, Y.; Ekici, A.; Ekici, M. Factors influencing cognitive function in subjects with COPD. Respir. Care 2016, 61, 1044–1050. [Google Scholar] [CrossRef]
- López-Torres, I.; Valenza, M.C.; Torres-Sánchez, I.; Cabrera-Martos, I.; Rodriguez-Torres, J.; Moreno-Ramírez, M.P. Changes in cognitive status in COPD patients across clinical stages. COPD 2016, 13, 327–332. [Google Scholar] [CrossRef]
- Cleutjens, F.A.H.M.; Spruit, M.A.; Ponds, R.W.H.M.; Dijkstra, J.B.; Franssen, F.M.E.; Wouters, E.F.M.; Janssen, D.J.A. Cognitive functioning in obstructive lung disease: Results from the United Kingdom biobank. J. Am. Med. Diractory Assoc. 2014, 15, 214–219. [Google Scholar] [CrossRef]
- Engelhart, M.J.; Geerlings, M.I.; Meijer, J.; Kiliaan, A.; Ruitenberg, A.; van Swieten, J.C.; Stijnen, T.; Hofman, A.; Witteman, J.C.M.; Breteler, M.M.B. Inflammatory proteins in plasma and the risk of dementia: The Rotterdam study. Arch. Neurol. 2004, 61, 668–672. [Google Scholar] [CrossRef]
- Federman, A.D.; Wolf, M.S.; Sheng, T.; O’cOnor, R.; Martynenko, M.; Wisnivesky, J. Diminished Cognitive Function Among Chronic Obstructive Pulmonary Disease Patients During Periods of Acute Illness Exacerbation. J. Gerontol. 2016, 71, 279–280. [Google Scholar] [CrossRef]
- Dodd, J.W. Lung disease as a determinant of cognitive decline and dementia. Alzheimers Res. Ther. 2015, 7, 32. [Google Scholar] [CrossRef]
- Ortapamuk, H.; Naldoken, S. Brain perfusion abnormalities in chronic obstructive pulmonary disease: Comparison with cognitive impairment. Ann. Nucular Med. 2006, 20, 99–106. [Google Scholar] [CrossRef]
- Dal Negro, R.W.; Bonadiman, L.; Bricolo, F.P.; Tognella, S.; Turco, P. Cognitive dysfunction in severe chronic obstructive pulmonary disease (COPD) with or without long-term oxygen therapy (LTOT). Multidiscipilanry Respir. Med. 2015, 10, 17. [Google Scholar] [CrossRef]
- Dodd, J.W.; Chung, A.W.; van den Broek, M.D.; Barrick, T.R.; Charlton, R.A.; Jones, P.W. Brain structure and function in chronic obstructive pulmonary disease: A multimodal cranial magnetic resonance imaging study. Am. J. Respir. Crit. Care Med. 2012, 186, 240–245. [Google Scholar] [CrossRef]
- Herigstad, M.; Hayen, A.; Evans, E.; Hardinge, F.M.; Davies, R.J.; Wiech, K.; Pattinson, K.T.S. Dyspnea-related cues engage the prefrontal cortex: Evidence from functional brain imaging in COPD. Chest 2015, 148, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Lin, J.; Sun, Y.; Huang, Y.; Yang, T.; Zheng, S.; Fan, M.; Zhang, J. Grey and white matter abnormalities in chronic obstructive pulmonary disease: A case–control study. BMJ Open 2012, 2, e000844. [Google Scholar] [CrossRef]
- Yin, M.; Wang, H.; Hu, X.; Li, X.; Fei, G.; Yu, Y. Patterns of brain structural alteration in COPD with different levels of pulmonary function impairment and its association with cognitive deficits. BMC Pulm. Med. 2019, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Xue, Q.-L.; Cappola, A.R.; Ferrucci, L.; Chaves, P.; Varadhan, R.; Guralnik, J.M.; Leng, S.X.; Semba, R.D.; Walston, J.D. Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 1049–1057. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin. Geriatr. Med. 2011, 27, 17–26. [Google Scholar] [CrossRef]
- Song, X.; Mitnitski, A.; Rockwood, K. Prevalence and 10-Year Outcomes of Frailty in Older Adults in Relation to Deficit Accumulation. J. Am. Geriatr. Soc. 2010, 58, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Elsa, D.; Ian, C.; Stuart, H.; Cynthia, P.; Renuka, V. Frailty and functional decline indices predict poor outcomes in hospitalised older people. Age Ageing 2013, 43, 477–484. [Google Scholar]
- Gale, N.S.; Albarrati, A.M.; Munnery, M.M.; Hubbard, R.E.; Tal-Singer, R.; Cockcroft, J.R.; Shale, D.J. Frailty: A global measure of the multisystem impact of COPD. Chronic Respir. Dis. 2018, 15, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Marengoni, A.; Vetrano, D.L.; Manes-Gravina, E.; Bernabei, R.; Onder, G.; Palmer, K. The relationship between COPD and frailty: A systematic review and meta-analysis of observational studies. Chest 2018, 154, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Mora, R.; García-Guillamón, G.; Valera-Novella, E.; Giménez-Giménez, L.M.; Escolar-Reina, P.; Medina-Mirapeix, F. Frailty is a predictive factor of readmission within 90 days of hospitalization for acute exacerbations of chronic obstructive pulmonary disease: A longitudinal study. Ther. Adv. Respir. Dis. 2017, 11, 383–392. [Google Scholar] [CrossRef]
- Gale, N.S.; Duckers, J.M.; Enright, S.; Cockcroft, J.R.; Shale, D.J.; Bolton, C.E. Does pulmonary rehabilitation address cardiovascular risk factors in patients with COPD? BMC Pulm. Med. 2011, 11, 20. [Google Scholar] [CrossRef]
- Maddocks, M.; Kon, S.S.C.; Canavan, J.L.; E Jones, S.; Nolan, C.M.; Labey, A.; I Polkey, M.; Man, W.D.-C. Physical frailty and pulmonary rehabilitation in COPD: A prospective cohort study. Thorax 2016, 71, 988–995. [Google Scholar] [CrossRef]
- Gephine, S.; Saey, D.; Grosbois, J.M.; Maltais, F.; Mucci, P. Home-based pulmonary rehabilitation is effective in frail COPD patients with chronic respiratory failure. Chronic Obstr. Pulm. Dis. 2022, 9, 15–25. [Google Scholar] [CrossRef]
- Mittal, N.; Raj, R.; Islam, E.; Nugent, K. Pulmonary rehabilitation improves frailty and gait speed in some ambulatory patients with chronic lung diseases. Southwest Respir. Crit. Care Chronic 2015, 3, 2–10. [Google Scholar]
- Maddocks, M.; Brighton, L.J.; Alison, J.A.; Ter Beek, L.; Bhatt, S.P.; Brummel, N.E.; Burtin, C.; Cesari, M.; Evans, R.A.; Ferrante, L.E.; et al. Rehabilitation for People with Respiratory Disease and Frailty: An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2023, 20, 767–780. [Google Scholar] [CrossRef]
- Kojima, T.; Mizokami, F.; Akishita, M. Geriatric management of older patients with multiple chronic conditions. Geriatr. Gerontol. Int. 2020, 20, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Menditto, E.; Gimeno Miguel, A.; Moreno Juste, A.; Poblador Plou, B.; Aza Pascual-Salcedo, M.; Orlando, V.; González Rubio, F.; Prados Torres, A. Patterns of multiple chronic conditions and polypharmacy in young and adult population: Systematic associations among chronic diseases and drugs using factor analysis. PLoS ONE 2019, 14, e0210701. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet 2009, 373, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chang, R.; Yao, J.; Xu, X.; Teng, Y.; Cheng, N. Effectiveness of long-term using statins in COPD—A network meta-analysis. Respir. Res. 2019, 20, 17–26. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D. Hsp90 inhibitors as senolytic drugs to extend healthy aging. Cell Cycle 2018, 17, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.-J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132. [Google Scholar] [CrossRef]
- Thapa, R.K.; Nguyen, H.T.; Jeong, J.-H.; Kim, J.R.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Progressive slowdown/prevention of cellular senescence by CD9-targeted delivery of rapamycin using lactose-wrapped calcium carbonate nanoparticles. Sci. Rep. 2017, 7, 43299. [Google Scholar] [CrossRef]
- Baar, M.P.; Brandt, R.M.; Putavet, D.A.; Klein, J.D.; Derks, K.W.; Bourgeois, B.R.; de Keizer, P.L. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 2017, 169, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Baker, D.J.; Petersen, R.C. Emerging senotherapies for age-related diseases. Nat. Rev. Drug Discov. 2024, 23, 200–218. [Google Scholar]
- Elmer, P.J.; Obarzanek, E.; Vollmer, W.M.; Simons-Morton, D.; Stevens, V.J.; Young, D.R.; Lin, P.-H.; Champagne, C.; Harsha, D.W.; Svetkey, L.P.; et al. Effects of Comprehensive Lifestyle Modification on Diet, Weight, Physical Fitness, and Blood Pressure Control: 18-Month Results of a Randomized Trial. Ann. Intern. Med. 2006, 144, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Haggerty, J.; Almirall, J.; Bouhali, T.; Sasseville, M.; Lemieux, M. Lifestyle factors and multiple chronic conditions: A cross sectional study. BMC Public Health 2014, 14, 686. [Google Scholar] [CrossRef]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Meyer, T.E.; Klein, S.; Holloszy, J.O. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 6659–6663. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; de Jonge, L.; Frisard, M.I.; DeLany, J.P.; Larson-Meyer, D.E.; Rood, J.; Nguyen, T.; Martin, C.K.; Volaufova, J.; Most, M.M. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: A randomized controlled trial. JAMA 2006, 295, 1539–1548. [Google Scholar] [CrossRef]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il’yasova, D.; Ravussin, E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018, 27, 805–815.e4. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Bhapkar, M.; Huffman, K.M.; Pieper, C.F.; Krupa Das, S.; Redman, L.M.; Villareal, D.T.; Rochon, J.; Roberts, S.B.; Ravussin, E.; et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): Exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Das, S.K.; Redman, L.M.; Kraus, W.E.; Huffman, K.M.; Bales, C.W.; Ravussin, E. Calorie restriction modulates the transcription of genes related to stress response and longevity in human muscle: The CALERIE study. Aging Cell 2023, 22, e13891. [Google Scholar]
- Aversa, Z.; White, T.A.; Heeren, A.A.; Hulshizer, C.A.; Saul, D.; Zhang, X.; Molina, A.J.A.; Redman, L.M.; Martin, C.K.; Racette, S.B.; et al. Calorie restriction reduces biomarkers of cellular cellular aging in humans. Aging Cell 2024, 23, e14038. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Heilbronn, L.K.; Redman, L.M.; Newcomer, B.R.; Frisard, M.I.; Anton, S.; Smith, S.R.; Alfonso, A.; Ravussin, E. Effect of calorie restriction with or without exercise on insulin sensitivity, β-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 2006, 29, 1337–1344. [Google Scholar] [CrossRef]
- Lefevre, M.; Redman, L.M.; Heilbronn, L.K.; Smith, J.V.; Martin, C.K.; Rood, J.C.; Greenway, F.L.; Williamson, D.A.; Smith, S.R.; Ravussin, E. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis 2009, 203, 206–213. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albarrati, A.; Gale, N.S. The Aging Lung: Exploring Multimorbidity Patterns and Their Clinical Implications: A Narrative Review. Curr. Issues Mol. Biol. 2025, 47, 561. https://doi.org/10.3390/cimb47070561
Albarrati A, Gale NS. The Aging Lung: Exploring Multimorbidity Patterns and Their Clinical Implications: A Narrative Review. Current Issues in Molecular Biology. 2025; 47(7):561. https://doi.org/10.3390/cimb47070561
Chicago/Turabian StyleAlbarrati, Ali, and Nichola S. Gale. 2025. "The Aging Lung: Exploring Multimorbidity Patterns and Their Clinical Implications: A Narrative Review" Current Issues in Molecular Biology 47, no. 7: 561. https://doi.org/10.3390/cimb47070561
APA StyleAlbarrati, A., & Gale, N. S. (2025). The Aging Lung: Exploring Multimorbidity Patterns and Their Clinical Implications: A Narrative Review. Current Issues in Molecular Biology, 47(7), 561. https://doi.org/10.3390/cimb47070561






