Next-Generation Sequencing Reveals the Potential Role of RET Protooncogene in Metastasis Progression in Medullary Thyroid Cancer
Abstract
1. Introduction
2. Material and Methods
2.1. Institutional Review Board Statement
2.2. Tissue Samples and Patient
2.3. Next-Generation Sequencing: Cancer Hotspot Panel of MTC
2.4. Comparison with Gene Data Banks
3. Results
3.1. RET Protooncogene Is the Predominant Mutation in Cancer Hotspot Panel Analysis of Mtc Primary Tumour Tissue
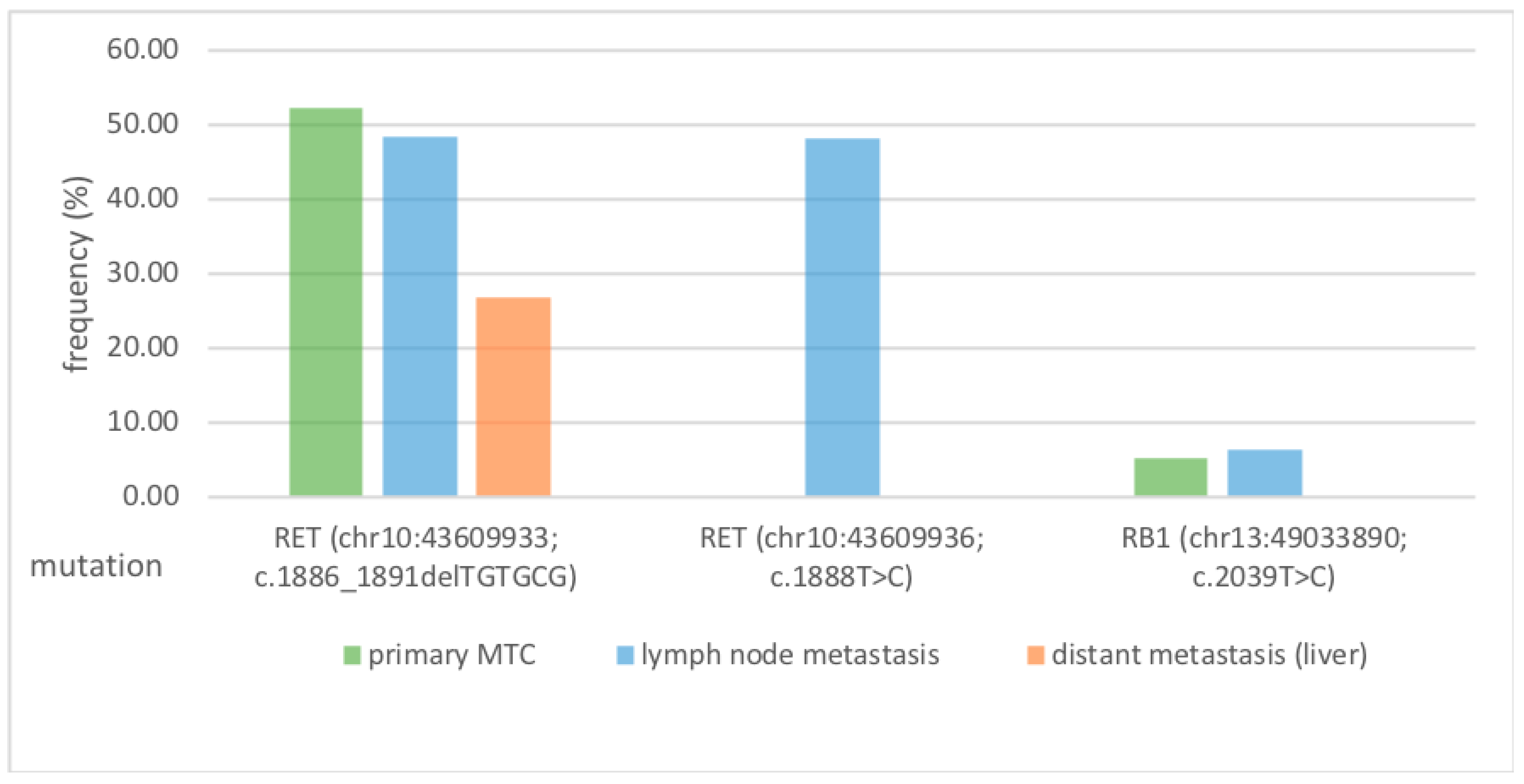
3.2. Mutational Profile Between the Primarius Tumour and the Lymph Node Metastases: RET Protooncogene Is the Predominant Mutation
3.3. RET Protooncogene Is Independent of the Metastasis Localization
3.4. Summary of Results
4. Discussion
4.1. Evaluation of the Pathogenicity of the Mutations
4.2. RET Protooncogene Is the Predominant Mutation from Primary Tumour to Lymph Node Metastasis to Distant Metastasis—What Therapeutic Relevance Does This Have?
4.3. Strengths and Weaknesses of This Study
5. Conclusions
Highlights
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ClinVar | ClinVar Databank |
| COSMIC | catalogue of somatic mutations in cancer; coverage |
| dbSNP | reference Single nucleotide polymorphism |
| DM | distant metastasis |
| EMA | European Medicines Agency |
| FDA | U.S. Food and Drug Administration |
| LNM | lymph node metastasis |
| MTC | medullary thyroid cancer |
| MEN | multiple endocrine neoplasia |
| NRTK | neurotropic tyrosine receptor kinase |
| RB1 | Tumour suppressor Retinoblastom Protein 1 |
| RET | Receptor tyrosine kinase RET |
| SIFT | sorting intolerant from tolerant |
| SNP | single nucleotide polymorphism |
| PolyPhen | polymorphism phenotyping |
References
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid Cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Q.; Zhang, K.; Liang, Y.; Ren, F.; Zhang, J.; Kan, C.; Han, F.; Sun, X. NTRK Fusions in Thyroid Cancer: Pathology and Clinical Aspects. Crit. Rev. Oncol./Hematol. 2023, 184, 103957. [Google Scholar] [CrossRef] [PubMed]
- Ceolin, L.; Siqueira, D.R.; Ferreira, C.V.; Romitti, M.; Maia, S.C.; Leiria, L.; Crispim, D.; Prolla, P.A.; Maia, A.L. Additive Effect of RET Polymorphisms on Sporadic Medullary Thyroid Carcinoma Susceptibility and Tumor Aggressiveness. Eur. J. Endocrinol. 2012, 166, 847–854. [Google Scholar] [CrossRef]
- Modigliani, E.; Cohen, R.; Campos, J.; Conte-Devolx, B.; Maes, B.; Boneu, A.; Schlumberger, M.; Bigorgne, J.; Dumontier, P.; Leclerc, L.; et al. Prognostic Factors for Survival and for Biochemical Cure in Medullary Thyroid Carcinoma: Results in 899 Patients. Clin. Endocrinol. 1998, 48, 265–273. [Google Scholar] [CrossRef]
- Khatami, F.; Tavangar, S.M. Multiple Endocrine Neoplasia Syndromes from Genetic and Epigenetic Perspectives. Biomark Insights 2018, 13, 117727191878512. [Google Scholar] [CrossRef]
- Leboulleux, S.; Baudin, E.; Travagli, J.; Schlumberger, M. Medullary Thyroid Carcinoma. Clin. Endocrinol. 2004, 61, 299–310. [Google Scholar] [CrossRef]
- Scollo, C.; Baudin, E.; Travagli, J.-P.; Caillou, B.; Bellon, N.; Leboulleux, S.; Schlumberger, M. Rationale for Central and Bilateral Lymph Node Dissection in Sporadic and Hereditary Medullary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2003, 88, 2070–2075. [Google Scholar] [CrossRef]
- Machens, A.; Hauptmann, S.; Dralle, H. Increased Risk of Lymph Node Metastasis in Multifocal Hereditary and Sporadic Medullary Thyroid Cancer. World J. Surg. 2007, 31, 1960–1965. [Google Scholar] [CrossRef]
- Agrawal, N.; Jiao, Y.; Sausen, M.; Leary, R.; Bettegowda, C.; Roberts, N.J.; Bhan, S.; Ho, A.S.; Khan, Z.; Bishop, J.; et al. Exomic Sequencing of Medullary Thyroid Cancer Reveals Dominant and Mutually Exclusive Oncogenic Mutations in RET and RAS. J. Clin. Endocrinol. Metab. 2013, 98, E364–E369. [Google Scholar] [CrossRef]
- Elisei, R.; Schlumberger, M.J.; Müller, S.P.; Schöffski, P.; Brose, M.S.; Shah, M.H.; Licitra, L.; Jarzab, B.; Medvedev, V.; Kreissl, M.C.; et al. Cabozantinib in Progressive Medullary Thyroid Cancer. J. Clin. Oncol. 2013, 31, 3639–3646. [Google Scholar] [CrossRef]
- Wells, S.A.; Robinson, B.G.; Gagel, R.F.; Dralle, H.; Fagin, J.A.; Santoro, M.; Baudin, E.; Elisei, R.; Jarzab, B.; Vasselli, J.R.; et al. Vandetanib in Patients with Locally Advanced or Metastatic Medullary Thyroid Cancer: A Randomized, Double-Blind Phase III Trial. J. Clin. Oncol. 2012, 30, 134–141. [Google Scholar] [CrossRef]
- Grüllich, C. Martens, U.M., Ed.; Cabozantinib: Multi-Kinase Inhibitor of MET, AXL, RET, and VEGFR2. In Small Molecules in Oncology; Recent Results in Cancer Research; Springer International Publishing: Cham, Switzerland, 2018; Volume 211, pp. 67–75. ISBN 978-3-319-91441-1. [Google Scholar]
- Wirth, L.J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N. Engl. J. Med. 2020, 383, 825–835. [Google Scholar] [CrossRef]
- Hadoux, J.; Elisei, R.; Brose, M.S.; Hoff, A.O.; Robinson, B.G.; Gao, M.; Jarzab, B.; Isaev, P.; Kopeckova, K.; Wadsley, J.; et al. Phase 3 Trial of Selpercatinib in Advanced RET-Mutant Medullary Thyroid Cancer. N. Engl. J. Med. 2023, 389, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Hadoux, J.; Schlumberger, M. Chemotherapy and Tyrosine-Kinase Inhibitors for Medullary Thyroid Cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C. SIFT: Predicting Amino Acid Changes That Affect Protein Function. Nucleic Acids Res. 2003, 31, 3812–3814. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A Method and Server for Predicting Damaging Missense Mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Hong, D.; Ye, L.; Gagel, R.; Chintala, L.; El Naggar, A.K.; Wright, J.; Kurzrock, R. Medullary Thyroid Cancer: Targeting the RET Kinase Pathway with Sorafenib/Tipifarnib. Mol. Cancer Ther. 2008, 7, 1001–1006. [Google Scholar] [CrossRef]
- Salvatore, D.; Santoro, M.; Schlumberger, M. The Importance of the RET Gene in Thyroid Cancer and Therapeutic Implications. Nat. Rev. Endocrinol. 2021, 17, 296–306. [Google Scholar] [CrossRef]
- Sahakian, N.; Castinetti, F.; Romanet, P. Molecular Basis and Natural History of Medullary Thyroid Cancer: It Is (Almost) All in the RET. Cancers 2023, 15, 4865. [Google Scholar] [CrossRef]
- Gild, M.L.; Clifton-Bligh, R.J.; Wirth, L.J.; Robinson, B.G. Medullary Thyroid Cancer: Updates and Challenges. Endocr. Rev. 2023, 44, 934–946. [Google Scholar] [CrossRef]
- Elisei, R.; Romei, C. Looking for RET Alterations in Thyroid Cancer: Clinical Relevance, Methodology and Timing. Endocrine 2023, 81, 206–215. [Google Scholar] [CrossRef]
- Elisei, R.; Ciampi, R.; Matrone, A.; Prete, A.; Gambale, C.; Ramone, T.; Simeakis, G.; Materazzi, G.; Torregrossa, L.; Ugolini, C.; et al. Somatic RET Indels in Sporadic Medullary Thyroid Cancer: Prevalence and Response to Selpercatinib. J. Clin. Endocrinol. Metab. 2022, 107, 2195–2202. [Google Scholar] [CrossRef]
- Krampitz, G.W.; Norton, J.A. RET Gene Mutations (Genotype and Phenotype) of Multiple Endocrine Neoplasia Type 2 and Familial Medullary Thyroid Carcinoma. Cancer 2014, 120, 1920–1931. [Google Scholar] [CrossRef]
| Distant Metastasis MTC | Lymph Node Metastasis MTC | Primary Tumour MTC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locus | Potential Targets | Coding | Exon | Transcript | Amino Acid Change | Coverage | % Frequency | % Frequency | % Frequency | Variant ID | ClinVar | Type | Length | Variant Effect |
| chr2:212812097 | ERBB4 | c.421+58A>G | NM_005235.2 | p.? | 1999 | 99.90 | 100.00 | 100.00 | SNV | 1 | unknown | |||
| chr4:1807894 | FGFR3 | c.1953G>A | 14 | NM_000142.4 | p.(=) | 1999 | 99.75 | 99.90 | 99.85 | SNV | 1 | synonymous | ||
| chr4:55141050 | PDGFRA | c.1701A>G | 12 | NM_006206.4 | p.(=) | 1984 | 99.90 | 100.00 | 99.95 | COSM12417 | SNV | 1 | synonymous | |
| chr4:55980239 | KDR | c.798+54G>A | NM_002253.2 | p.? | 1329 | 99.70 | 100.00 | 99.90 | SNV | 1 | unknown | |||
| chr5:112175769 | APC | c.4479G>A | 16 | NM_000038.5 | p.(=) | 1989 | 45.55 | 48.48 | 53.19 | COSM19714; COSM19626; COSM19349; COSM19674; COSM23598 | SNV | 1 | synonymous | |
| chr5:149433596 | CSF1R, HMGXB3 | c.*1841TG>GA, c.2954_2955delCAinsTC | NM_014983.2, NM_005211.3 | p.?, p.? | 1847 | 99.78 | 99.93 | 100.00 | MNV | 2 | unknown, unknown | |||
| chr7:55249063 | EGFR, EGFR-AS1 | c.2361G>A | 20 | NM_005228.3, NR_047551.1 | p.(=) | 1592 | 49.25 | 53.82 | 53.41 | Benign, Likely benign | SNV | 1 | synonymous | |
| chr10:43609933 | RET | c.1886_1891delTGTGCG | 11 | NM_020975.4 | p.Leu629_Asp631delinsHis | 1983 | 26.83 | 48.32 | 52.20 | COSM27040 | INDEL | 6 | non- frameshift deletion | |
| chr10:43609936 | RET | c.1888T>C | 11 | NM_020975.4 | p.Cys630Arg | 1999 | n.d. | 48.22 | n.d. | 1237917:964:29806 | Pathogenic: Likely pathogenic | SNV | 1 | missense |
| chr10:43613843 | RET | c.2307G>T | 13 | NM_020975.4 | p.(=) | 1998 | 100.00 | 100.00 | 99.90 | Benign | SNV | 1 | synonymous | |
| chr11:534242 | HRAS | c.81T>C | 2 | NM_001130442.2 | p.(=) | 2000 | 45.70 | 52.65 | 55.53 | COSM249860 | Benign | SNV | 1 | synonymous |
| chr13:28610183 | FLT3 | c.1310-3T>C | NM_004119.2 | p.? | 2000 | 68.75 | 57.05 | 50.10 | SNV | 1 | unknown | |||
| chr13:49033890 | RB1 | c.2039T>C | 20 | NM_000321.2 | p.Ile680Thr | 1985 | n.d. | 6.30 | 5.33 | 870 | SNV | 1 | missense | |
| chr17:7579472 | TP53 | c.215C>G | 4 | NM_000546.5 | p.Pro72Arg | 2000 | 99.25 | 99.97 | 99.22 | COSM45985 | Benign. Uncertain significance, drug response | SNV | 1 | missense |
| chr18:48586344 | SMAD4 | c.955+58C>T | NM_005359.5 | p.? | 1997 | 50.13 | 48.47 | 50.70 | SNV | 1 | unknown | |||
| chr19:1220321 | STK11 | c.465-51T>C | NM_000455.4 | p.? | 1707 | 47.51 | 50.39 | 47.72 | SNV | 1 | unknown | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, M.; Klein, A.J.C.; Raem, A.M.; Garrelfs, N.; Fischer, H.J.; Hölzle, F.; Wermker, K. Next-Generation Sequencing Reveals the Potential Role of RET Protooncogene in Metastasis Progression in Medullary Thyroid Cancer. Curr. Issues Mol. Biol. 2025, 47, 560. https://doi.org/10.3390/cimb47070560
Klein M, Klein AJC, Raem AM, Garrelfs N, Fischer HJ, Hölzle F, Wermker K. Next-Generation Sequencing Reveals the Potential Role of RET Protooncogene in Metastasis Progression in Medullary Thyroid Cancer. Current Issues in Molecular Biology. 2025; 47(7):560. https://doi.org/10.3390/cimb47070560
Chicago/Turabian StyleKlein, Maurice, Anna Julia Claudia Klein, Arnold M. Raem, Nicklas Garrelfs, Henrike J. Fischer, Frank Hölzle, and Kai Wermker. 2025. "Next-Generation Sequencing Reveals the Potential Role of RET Protooncogene in Metastasis Progression in Medullary Thyroid Cancer" Current Issues in Molecular Biology 47, no. 7: 560. https://doi.org/10.3390/cimb47070560
APA StyleKlein, M., Klein, A. J. C., Raem, A. M., Garrelfs, N., Fischer, H. J., Hölzle, F., & Wermker, K. (2025). Next-Generation Sequencing Reveals the Potential Role of RET Protooncogene in Metastasis Progression in Medullary Thyroid Cancer. Current Issues in Molecular Biology, 47(7), 560. https://doi.org/10.3390/cimb47070560





