Evodiamine Boosts AR Expression to Trigger Senescence and Halt Proliferation in OSCC Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Processing
2.3. Sample Collection
2.4. Data Download and Analysis Using the Public Database
2.5. Target Screening in TCMSP Database
2.6. CellAge Database Analysis
2.7. Pan-Cancer Correlation Analysis
2.8. Survival Analysis
2.9. Expression Analysis of the AR in Cell Lines in the High-Throughput Omics-Based Tumor Roadmap on the Shiny (ShinyTHOR) App
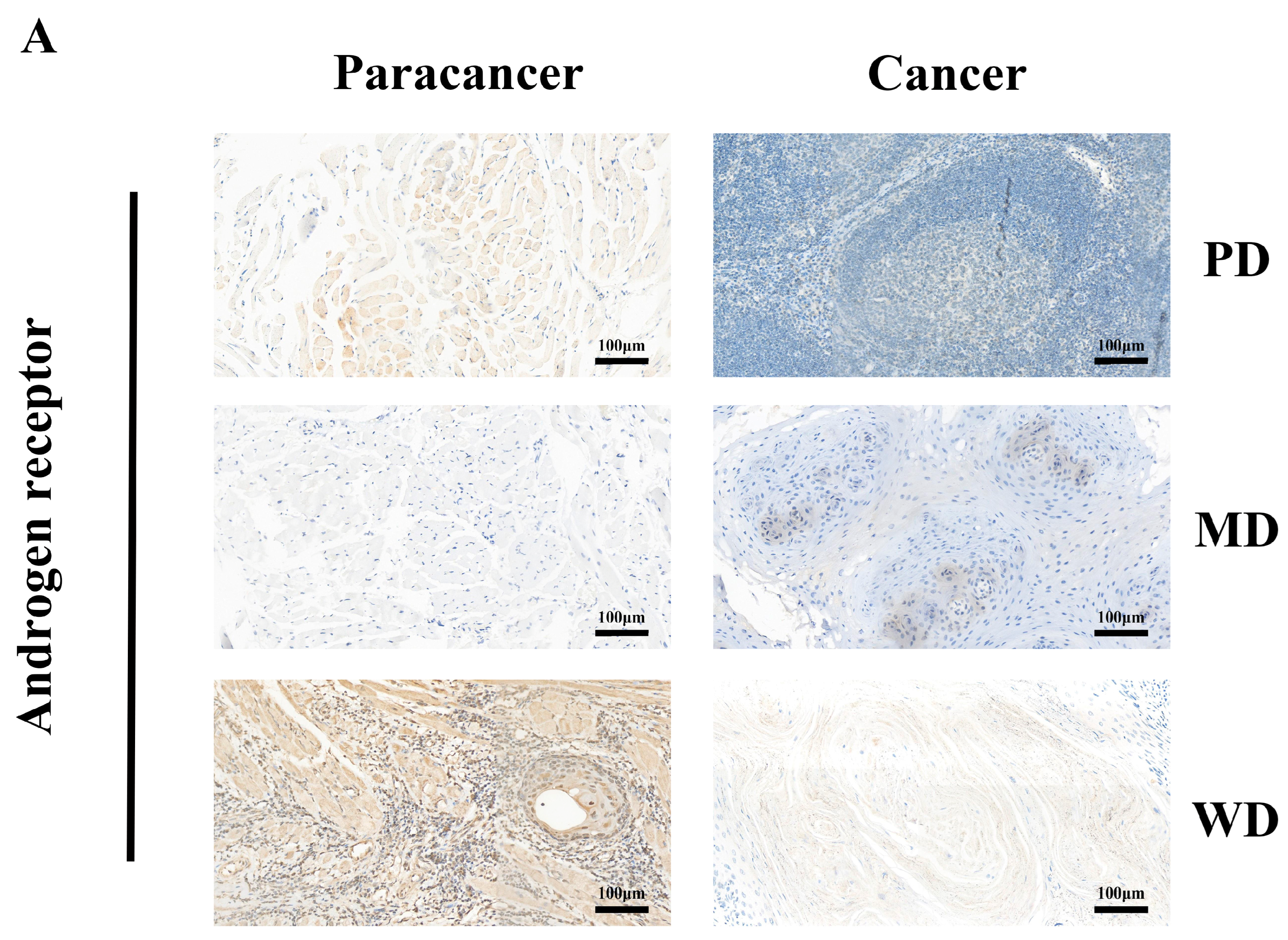
2.10. Immunohistochemistry (IHC)
2.11. Cell Counting Kit-8 (CCK8) Assay
2.12. Cell Cycle and Apoptosis Examination
2.13. Cell Colony Formation
2.14. 5-Ethynyl-2’-deoxyuridine (EdU) Detection
2.15. β-Galactosidase Detection
2.16. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
2.17. Protein Extraction, Protein Concentration Measurement, and Western Blotting
2.18. Statistics and Analysis
3. Results
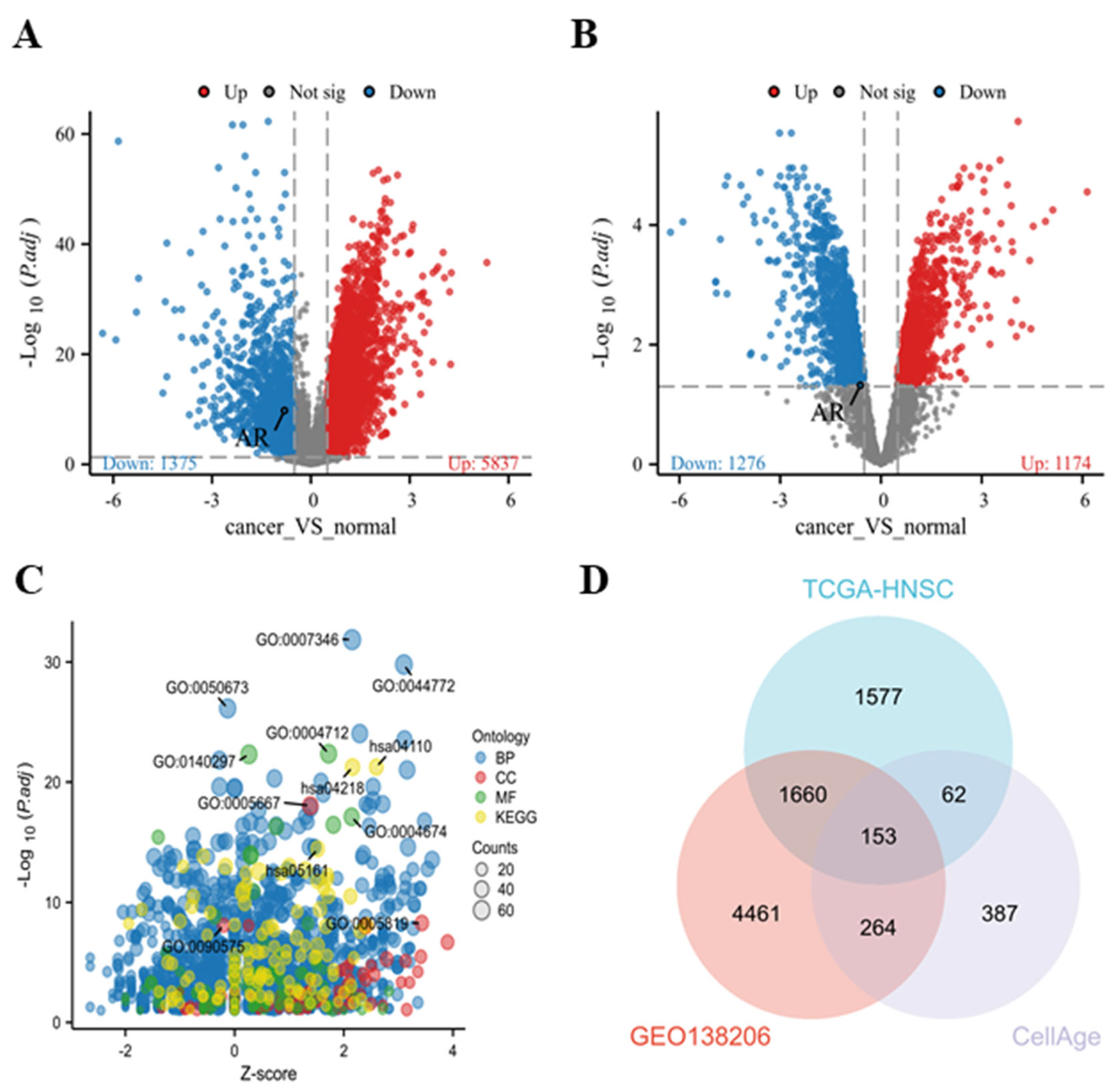
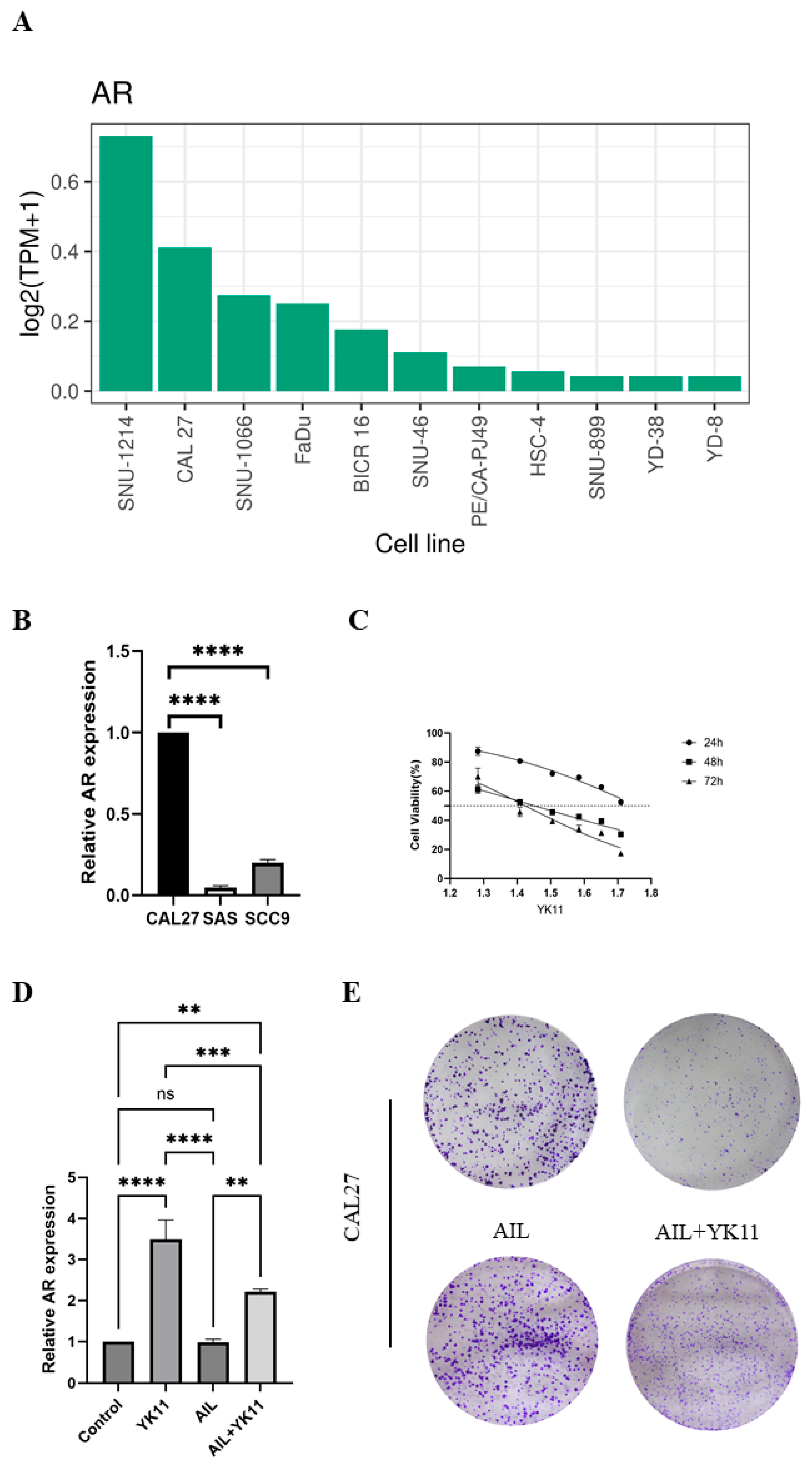
3.1. Screening of Differential Genes and Selection of Key Genes in HNSCC
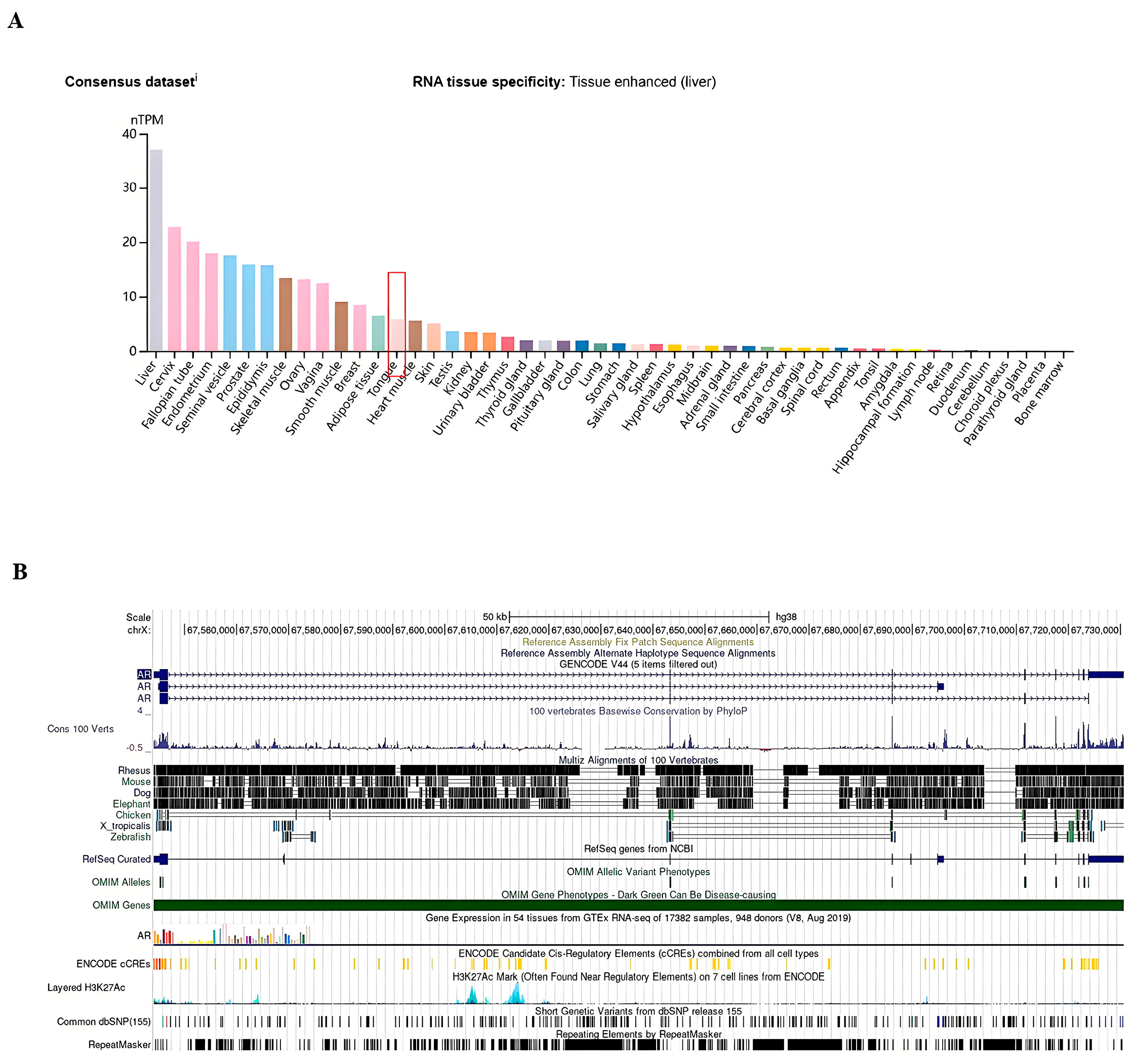
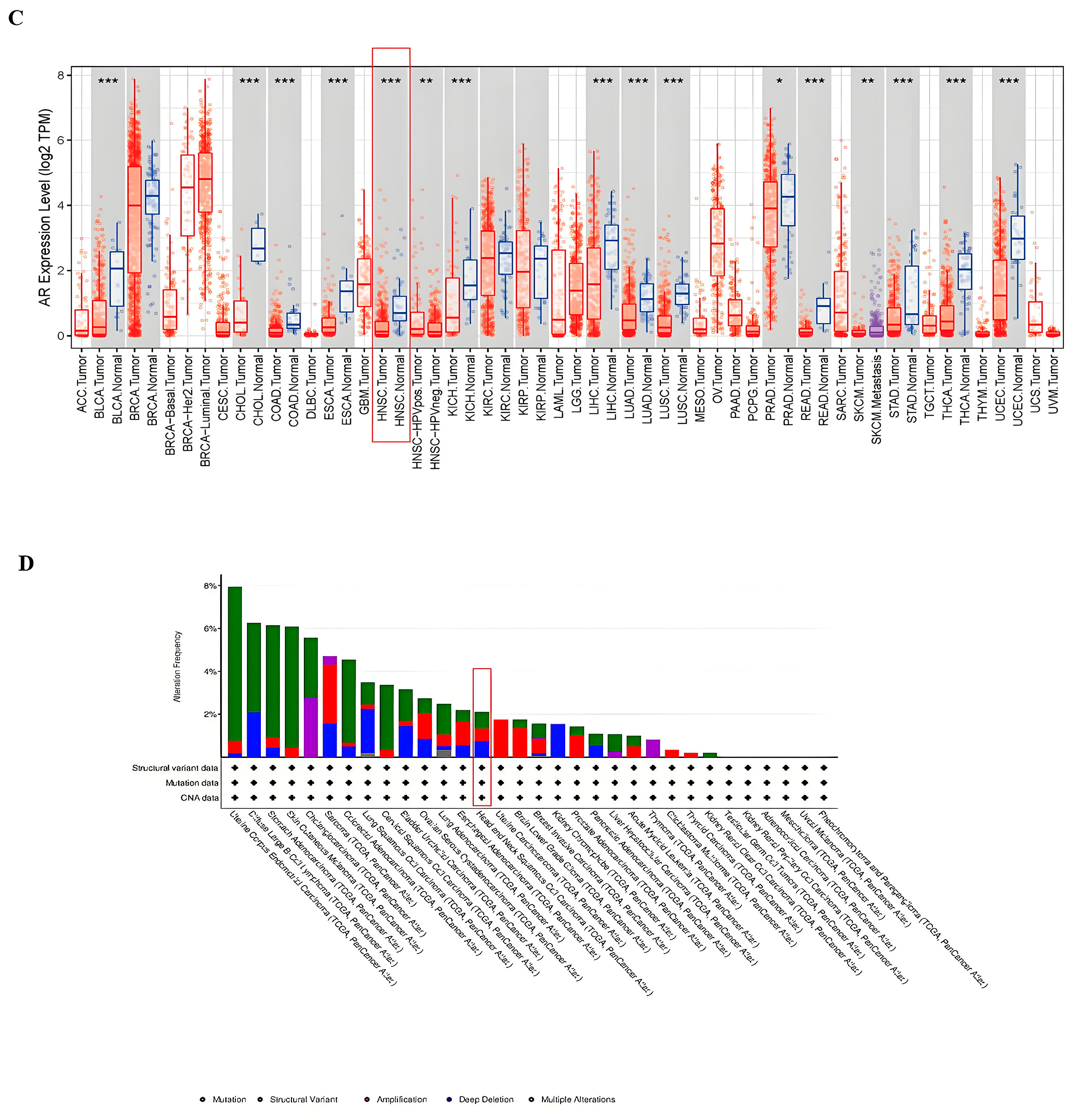
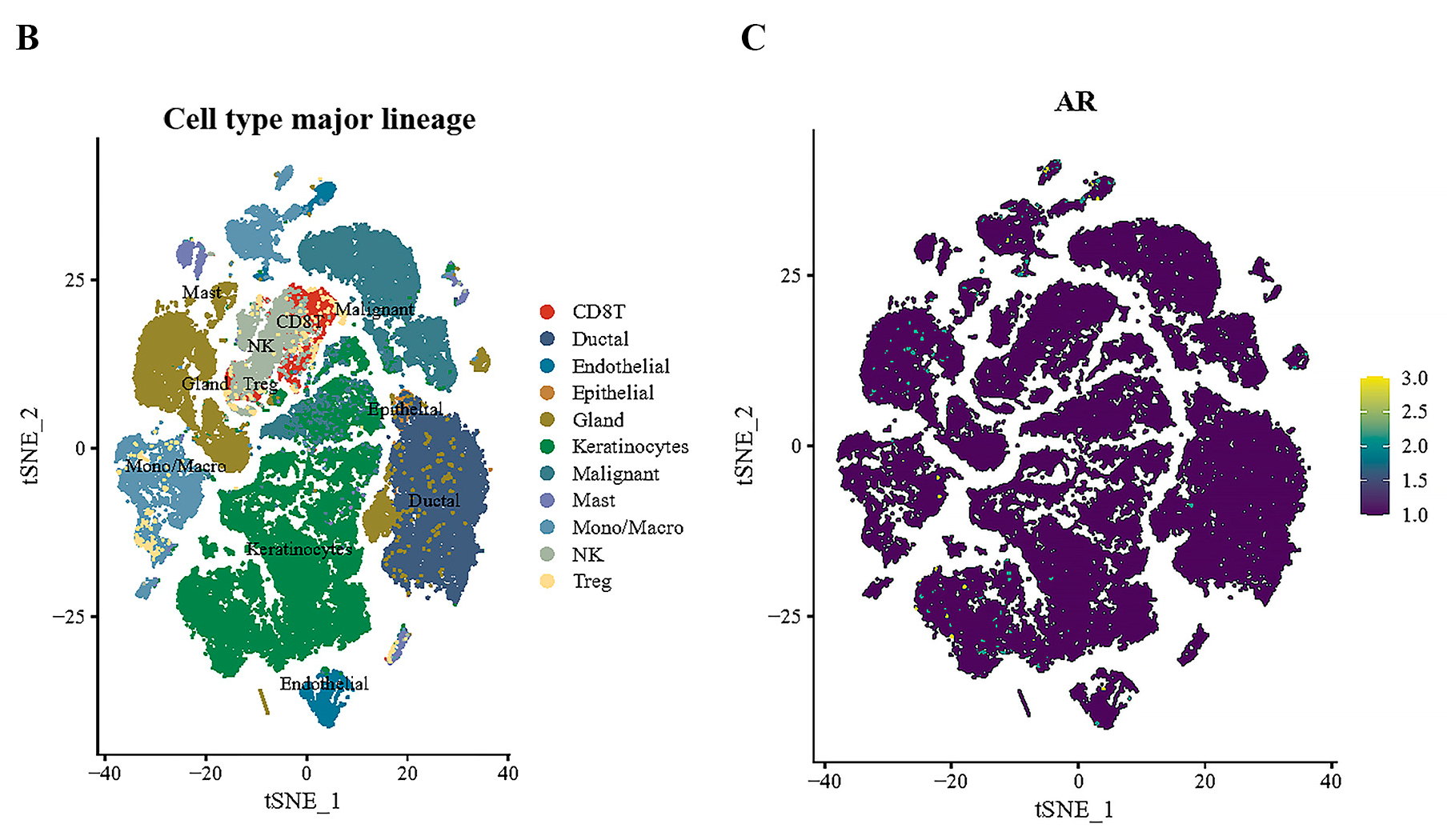
3.2. Tissue-Specific Expression of AR in Pan-Cancers
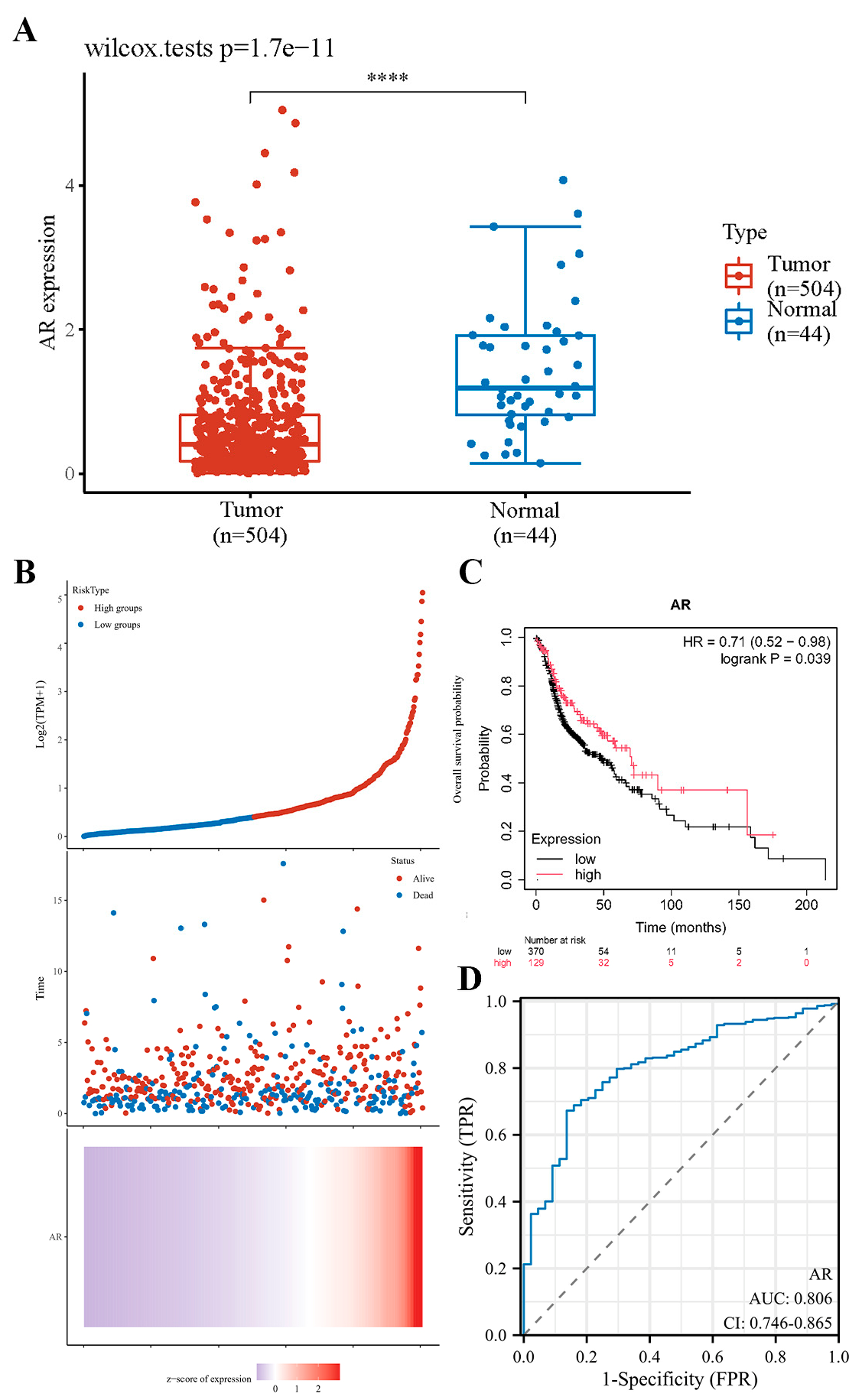
3.3. Downregulated Expression of AR Is Associated with the Survival Rate of HNSCC Patients
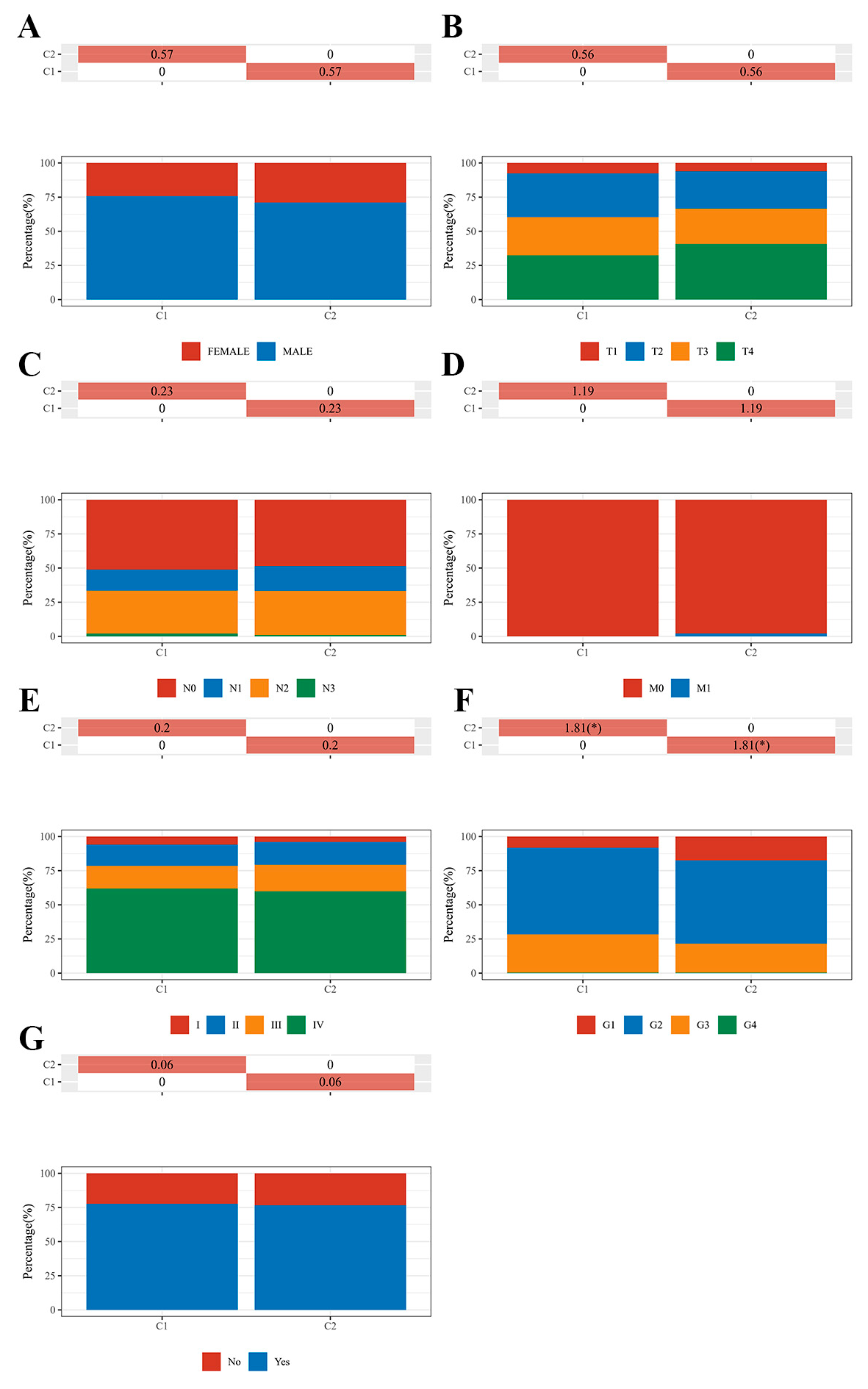
3.4. Correlational Analysis of AR Expression Aberrations with Clinical Characteristics in HNSCC
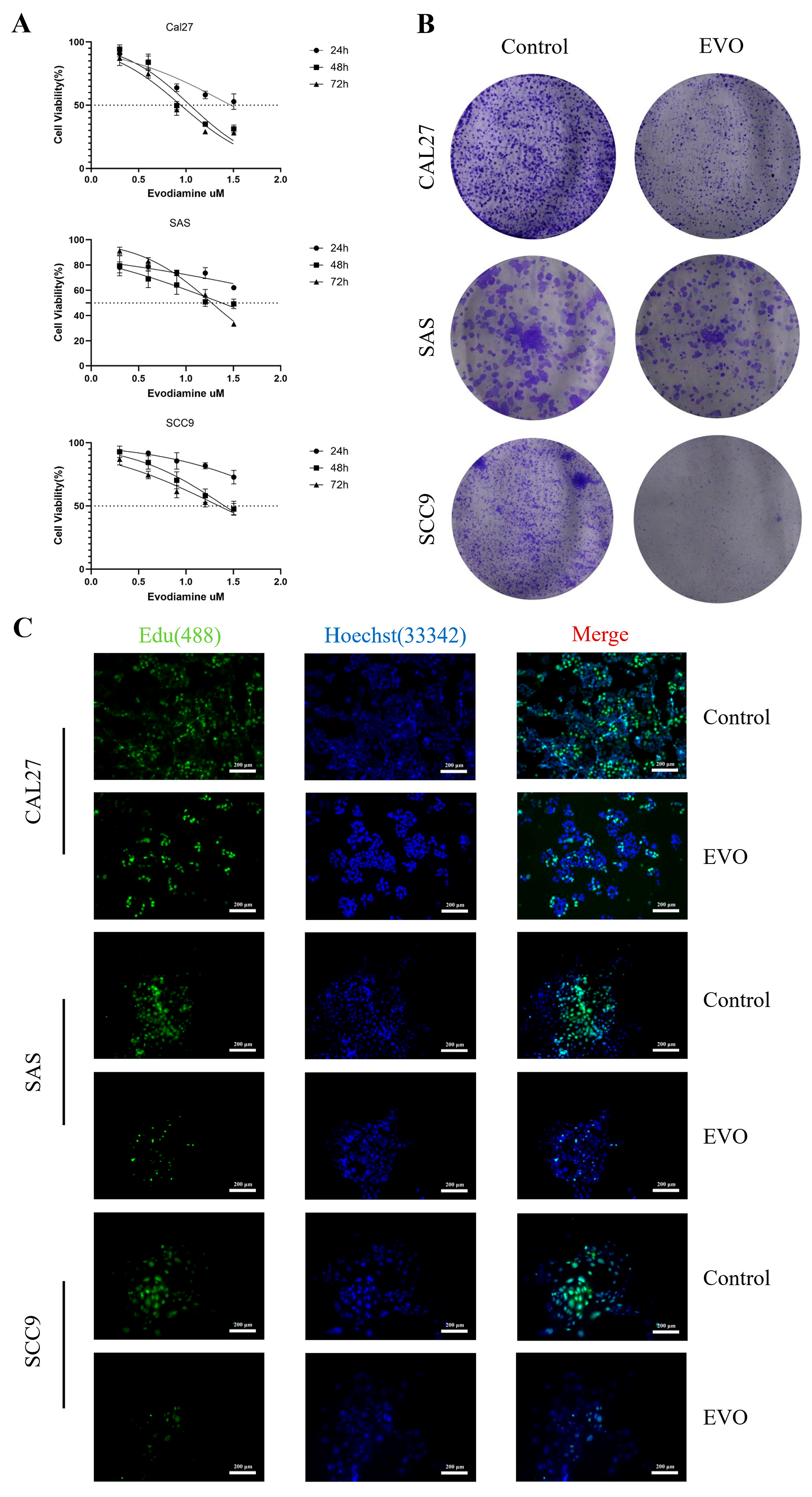
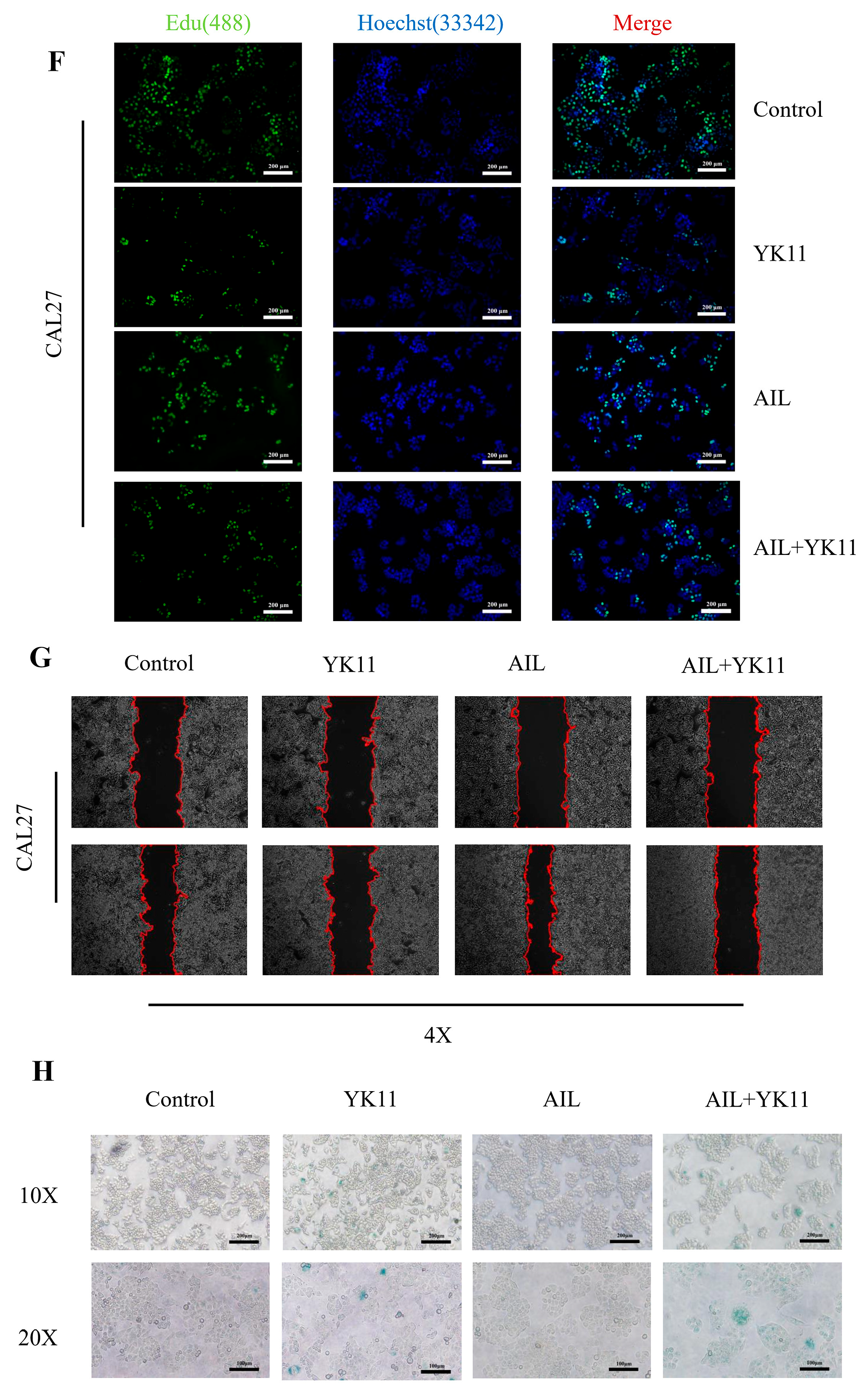
3.5. Evodiamine Suppresses OSCC Cell Proliferation In Vitro and Induces G2/M Arrest and Apoptosis
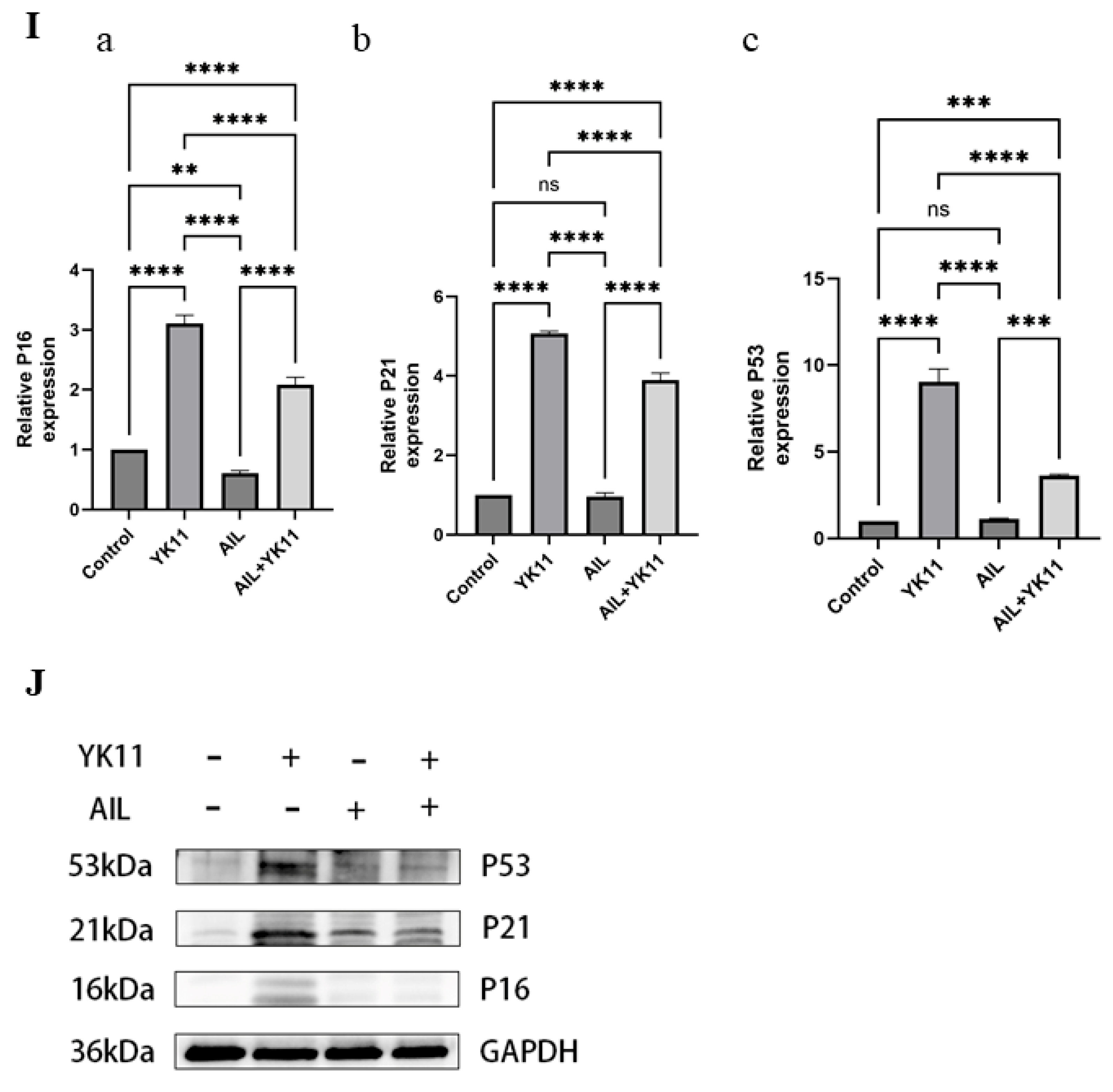
3.6. Stimulated AR Overexpression in OSCC Cells Promotes Senescence and Halts Cell Activities
3.7. Evodiamine Triggers Senescence of OSCC Cells by Enhancing Expression of AR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, L.; Liu, M.; Jiang, H.; Wu, Y. Oral squamous cell carcinoma: Insights into cellular heterogeneity, drug resistance, and evolutionary trajectories. Cell Biol. Toxicol. 2025, 41, 101. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.H.; Tokheim, C.; Porta-Pardo, E.; Sengupta, S.; Bertrand, D.; Weerasinghe, A.; Colaprico, A.; Wendl, M.C.; Kim, J.; Reardon, B.; et al. Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell 2018, 173, 371–385.e318. [Google Scholar] [CrossRef]
- Panda, M.; Tripathi, S.K.; Zengin, G.; Biswal, B.K. Evodiamine as an anticancer agent: A comprehensive review on its therapeutic application, pharmacokinetic, toxicity, and metabolism in various cancers. Cell Biol. Toxicol. 2023, 39, 1–31. [Google Scholar] [CrossRef]
- Meng, T.; Fu, S.; He, D.; Hu, G.; Gao, X.; Zhang, Y.; Huang, B.; Du, J.; Zhou, A.; Su, Y.; et al. Evodiamine Inhibits Lipopolysaccharide (LPS)-Induced Inflammation in BV-2 Cells via Regulating AKT/Nrf2-HO-1/NF-κB Signaling Axis. Cell. Mol. Neurobiol. 2021, 41, 115–127. [Google Scholar] [CrossRef]
- Deng, J.-D.; Lei, S.; Jiang, Y.; Zhang, H.-H.; Hu, X.-L.; Wen, H.-X.; Tan, W.; Wang, Z. A concise synthesis and biological study of evodiamine and its analogues. Chem. Commun. 2019, 55, 3089–3092. [Google Scholar] [CrossRef]
- Yang, S.; Chen, J.; Tan, T.; Wang, N.; Huang, Y.; Wang, Y.; Yuan, X.; Zhang, P.; Luo, J.; Luo, X. Evodiamine Exerts Anticancer. Effects Against 143B and MG63 Cells Through the Wnt/β-Catenin Signaling Pathway. Cancer Manag. Res. 2020, 12, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, Y.; Wang, T.; Li, K.; Lu, J.; Huang, M.; Dong, G.; Sheng, C. Evodiamine-Inspired Topoisomerase-Histone Deacetylase Dual Inhibitors: Novel Orally Active Antitumor Agents for Leukemia Therapy. J. Med. Chem. 2022, 65, 4818–4831. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Chen, X.; Yuan, S.; Xu, T.; Zhao, W.; Li, M.; Wang, F.; Hoffman, R.M.; Jia, L. Evodiamine inhibits ESCC by inducing M-phase cell-cycle arrest via CUL4A/p53/p21 axis and activating noxa-dependent intrinsic and DR4-dependent extrinsic apoptosis. Phytomedicine 2023, 108, 154493. [Google Scholar] [CrossRef]
- Ren, L.; Lou, Y.; Sun, M. The anti-tumor effects of evodiamine on oral squamous cell carcinoma (OSCC) through regulating advanced glycation end products (AGE)/receptor for advanced glycation end products (RAGE) pathway. Bioengineered 2021, 12, 5985–5995. [Google Scholar] [CrossRef]
- Lin, L.; Lv, S.; Ren, H.; Li, R.; Li, L.; Pang, Y.; Wang, J. Evodiamine inhibits EPRS expression to regulate glutamate metabolism and proliferation of oral squamous cell carcinoma cells. Kaohsiung J. Med. Sci. 2024, 40, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Bousset, L.; Gil, J. Targeting senescence as an anticancer therapy. Mol. Oncol. 2022, 16, 3855–3880. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Jiang, L.; Cao, J. Senotherapies: A novel strategy for synergistic anti-tumor therapy. Drug Discov. Today 2022, 27, 103365. [Google Scholar] [CrossRef]
- Wang, C.; Vegna, S.; Jin, H.; Benedict, B.; Lieftink, C.; Ramirez, C.; de Oliveira, R.L.; Morris, B.; Gadiot, J.; Wang, W.; et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature 2019, 574, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Takayama, K.-I. The biological and clinical advances of androgen receptor function in age-related diseases and cancer [Review]. Endocr. J. 2017, 64, 933–946. [Google Scholar] [CrossRef]
- Li, D.; Zhou, W.; Pang, J.; Tang, Q.; Zhong, B.; Shen, C.; Xiao, L.; Hou, T. A magic drug target: Androgen receptor. Med. Res. Rev. 2019, 39, 1485–1514. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Q.; Li, D.; Li, Y.; Jiang, X. Evodiamine: A Privileged Structure with Broad-ranging Biological Activities. Mini Rev. Med. Chem. 2022, 22, 2680–2701. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Wu, H.-T.; Shan, Y.-S.; Wang, C.-T.; Shieh, G.-S.; Wu, C.-L.; Ou, H.-Y. Evodiamine Exhibits Anti-Bladder Cancer Activity by Suppression of Glutathione Peroxidase 4 and Induction of Ferroptosis. Int. J. Mol. Sci. 2023, 24, 6021. [Google Scholar] [CrossRef]
- Gao, W.; Bohl, C.E.; Dalton, J.T. Chemistry and structural biology of androgen receptor. Chem. Rev. 2005, 105, 3352–3370. [Google Scholar] [CrossRef]
- Knerr, J.; Werner, R.; Schwan, C.; Wang, H.; Gebhardt, P.; Grötsch, H.; Caliebe, A.; Spielmann, M.; Holterhus, P.-M.; Grosse, R.; et al. Formin-mediated nuclear actin at androgen receptors promotes transcription. Nature 2023, 617, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Vellano, C.P.; White, M.G.; Andrews, M.C.; Chelvanambi, M.; Witt, R.G.; Daniele, J.R.; Titus, M.; McQuade, J.L.; Conforti, F.; Burton, E.M.; et al. Androgen receptor blockade promotes response to BRAF/MEK-targeted therapy. Nature 2022, 606, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Bencomo, E.; Segura, A.V.C.; Sevillano, O.R.; Mayanga, A.; Sono, J.L.B.; Rojas, C.A.O.; Carrasco, A.G.M.; Lengauer, T. ShinyTHOR app: Shiny-built tumor high-throughput omics-based roadmap. Bioinform. Adv. 2025, 5, vbaf061. [Google Scholar] [CrossRef]
- Xia, N.; Xue, H.; Li, Y.; Liu, J.; Lou, Y.; Li, S.; Wang, Y.; Lu, J.; Chen, X. Potential Mechanisms and Effects of Dai Bai Jie Ethanol Extract in Preventing Acute Alcoholic Liver Injury. Curr. Issues Mol. Biol. 2024, 47, 3. [Google Scholar] [CrossRef]
- Hu, Y.; Liao, M.; Ding, J.; Zhou, J.; Xu, K. Co-Deletion of both p15/p16 Genes Correlates with Poor Prognosis Non-Small Cell Lung Cnacer. Chin. J. Cancer Res. 2002, 14, 216–219. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Huang, Z.; Su, Q.; Li, W.; Ren, H.; Huang, H.; Wang, A. Suppressed mitochondrial respiration via NOX5-mediated redox imbalance contributes to the antitumor activity of anlotinib in oral squamous cell carcinoma. J. Genet. Genom. 2021, 48, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zhao, R.; Li, H.; Xu, X.; Gao, J.; Chen, L.; Wei, B. Inhibition of androgen receptor enhanced the anticancer effects of everolimus through targeting glucose transporter 12. Int. J. Biol. Sci. 2023, 19, 104–119. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Liu, C.-X.; Pan, W.-D.; Shang, L.-R.; Zheng, J.-L.; Huang, B.-Y.; Chen, J.-Y.; Zheng, L.; Fang, J.-H.; Zhuang, S.-M. Dual and opposing roles of the androgen receptor in VETC-dependent and invasion-dependent metastasis of hepatocellular carcinoma. J. Hepatol. 2021, 75, 900–911. [Google Scholar] [CrossRef]
- Hickey, T.E.; Selth, L.A.; Chia, K.M.; Laven-Law, G.; Milioli, H.H.; Roden, D.; Jindal, S.; Hui, M.; Finlay-Schultz, J.; Ebrahimie, E.; et al. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat. Med. 2021, 27, 310–320. [Google Scholar] [CrossRef]
- Zhang, F.; Biswas, M.; Massah, S.; Lee, J.; Lingadahalli, S.; Wong, S.; Wells, C.; Foo, J.; Khan, N.; Morin, H.; et al. Dynamic phase separation of the androgen receptor and its coactivators key to regulate gene expression. Nucleic Acids Res. 2023, 51, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ying, N.; Rao, X.; Chen, X. MiR-942-3p as a Potential Prognostic Marker of Gastric Cancer Associated with AR and MAPK/ERK Signaling Pathway. Curr. Issues Mol. Biol. 2022, 44, 3835–3848. [Google Scholar] [CrossRef]
- Mirochnik, Y.; Veliceasa, D.; Williams, L.; Maxwell, K.; Yemelyanov, A.; Budunova, I.; Volpert, O.V.; Blagosklonny, M.V. Androgen receptor drives cellular senescence. PLoS ONE 2012, 7, e31052. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Gao, H.; Yu, J.; Zhang, H.; Nguyen, T.T.L.; Gu, Y.; Passow, M.R.; Carter, J.M.; Qin, B.; Boughey, J.C.; et al. Pharmacological Targeting of Androgen Receptor Elicits Context-Specific Effects in Estrogen Receptor-Positive Breast Cancer. Cancer Res. 2023, 83, 456–470. [Google Scholar] [CrossRef]
- Fan, Q.; Huang, T.; Sun, X.; Yang, X.; Wang, J.; Liu, Y.; Ni, T.; Gu, S.; Li, Y.; Wang, Y. miR-130a-3p promotes cell proliferation and invasion by targeting estrogen receptor α and androgen receptor in cervical cancer. Exp. Ther. Med. 2021, 21, 414. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; O’Connell, T.J.; Zhao, H.; Darzynkiewicz, Z.; Gupta, A.; Buchsbaum, J.; Shin, E.; Iacob, C.; Suslina, N.; Moscatello, A.; et al. Androgen receptor activation decreases proliferation in thyroid cancer cells. J. Cell. Biochem. 2021; in press. [Google Scholar]
- Hayflick, L. The Limited In Vitro Lifetime of Human Diploid Cell Strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Wang, T.; Wu, X.; Al Rudaisat, M.; Song, Y.; Cheng, H. Curcumin induces G2/M arrest and triggers autophagy, ROS generation and cell senescence in cervical cancer cells. J. Cancer 2020, 11, 6704–6715. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, K.H.; Na Kim, Y.; Jeong, B.Y.; Park, C.G.; Hur, G.M.; Lee, H.Y. Correction to: Resveratrol attenuates norepinephrine-induced ovarian cancer invasiveness through downregulating hTERT expression. Arch. Pharm. Res. 2018, 41, 938–940. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, X.; Zhao, M.; Wei, Z.; Li, X.; Zhang, X.; Liu, Z.; Gong, Y.; Shao, C. Berberine induces senescence of human glioblastoma cells by downregulating the EGFR-MEK-ERK signaling pathway. Mol. Cancer Ther. 2015, 14, 355–363. [Google Scholar] [CrossRef]
- Samad, M.A.; Saiman, M.Z.; Abdul Majid, N.; Karsani, S.A.; Yaacob, J.S. Berberine Inhibits Telomerase Activity and Induces Cell Cycle Arrest and Telomere Erosion in Colorectal Cancer Cell Line, HCT 116. Molecules 2021, 26, 376. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | GTGGAGTCCACTGGCGTCT | GTCGAGGAGGCATTGCTGAT |
| AR | GACGACCAGATGGCTGTCATT | GGGCGAAGTAGAGCATCC |
| p16 | TGGCTCTGACCATTCTGT | AGCTTTGGAAGCTCTCAG |
| p21 | GGGATGAGTTGGGAGGAGG | CGGCGTTTGGAGTGGTAG |
| p53 | CGCTTCGAGATGTTCCGAGA | CTGGGACCCAATGAGATGGG |
| ID | Molecule Name | Target Name |
|---|---|---|
| 1 | Evodiamine | Gamma-aminobutyric acid receptor subunit alpha-1 |
| 2 | Beta-2 adrenergic receptor | |
| 3 | mRNA of PKA catalytic subunit C-alpha | |
| 4 | Nuclear receptor coactivator 1 | |
| 5 | Calcium-activated potassium channel subunit alpha 1 | |
| 6 | Muscarinic acetylcholine receptor M3 | |
| 7 | Retinoic acid receptor RXR-alpha | |
| 8 | Coagulation factor VII | |
| 9 | Carbonic anhydrase II | |
| 10 | 5-hydroxytryptamine receptor 3A | |
| 11 | Trypsin-1 | |
| 12 | Nitric-oxide synthase, endothelial | |
| 13 | Prostaglandin G/H synthase 2 | |
| 14 | Muscarinic acetylcholine receptor M5 | |
| 15 | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit, gamma isoform | |
| 16 | Coagulation factor Xa | |
| 17 | Sodium channel protein type 5 subunit alpha | |
| 18 | Prostaglandin G/H synthase 1 | |
| 19 | Heat shock protein HSP 90 | |
| 20 | Androgen receptor | |
| 21 | Muscarinic acetylcholine receptor M1 | |
| 22 | Potassium voltage-gated channel subfamily H member 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Du, H.-L.; Liu, J.-N.; Cheng, J.; Chen, J.; Yin, X.-Y.; Wei, H.-L.; Wang, J. Evodiamine Boosts AR Expression to Trigger Senescence and Halt Proliferation in OSCC Cells. Curr. Issues Mol. Biol. 2025, 47, 558. https://doi.org/10.3390/cimb47070558
Chen G, Du H-L, Liu J-N, Cheng J, Chen J, Yin X-Y, Wei H-L, Wang J. Evodiamine Boosts AR Expression to Trigger Senescence and Halt Proliferation in OSCC Cells. Current Issues in Molecular Biology. 2025; 47(7):558. https://doi.org/10.3390/cimb47070558
Chicago/Turabian StyleChen, Gang, Hong-Liang Du, Jia-Nan Liu, Jie Cheng, Jing Chen, Xiao-Yang Yin, Hu-Lai Wei, and Jing Wang. 2025. "Evodiamine Boosts AR Expression to Trigger Senescence and Halt Proliferation in OSCC Cells" Current Issues in Molecular Biology 47, no. 7: 558. https://doi.org/10.3390/cimb47070558
APA StyleChen, G., Du, H.-L., Liu, J.-N., Cheng, J., Chen, J., Yin, X.-Y., Wei, H.-L., & Wang, J. (2025). Evodiamine Boosts AR Expression to Trigger Senescence and Halt Proliferation in OSCC Cells. Current Issues in Molecular Biology, 47(7), 558. https://doi.org/10.3390/cimb47070558





