IGFBP5 Promotes Atherosclerosis in APOE−/− Mice Through Phenotypic Transformation of VSMCs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Serum Lipid Level Detection
2.3. Quantification of Atherosclerotic Lesions
2.4. Western Blot Assay
2.5. RNA Isolation and RT-qPCR Analysis
2.6. RNA-Seq Analysis
2.7. Flow Cytometry Detection of Cell Cycle Distribution
2.8. CCK-8 Assay for Cell Proliferation
2.9. Cell Migration Assays
2.10. Statistical Analysis
3. Results
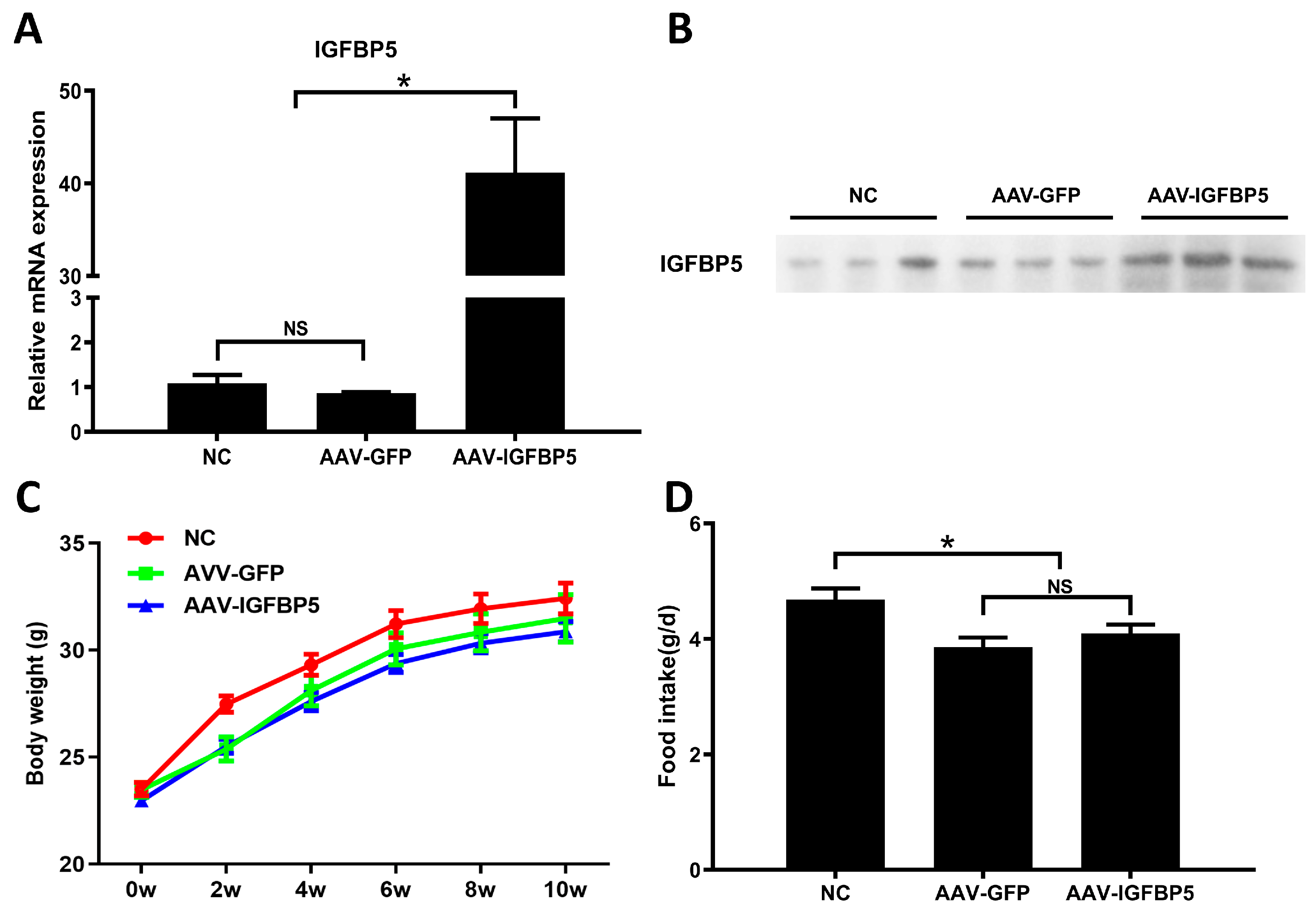
3.1. Overexpression of IGFBP5 Does Not Affect Food Intake by and the Body Weight of ApoE−/− Mice
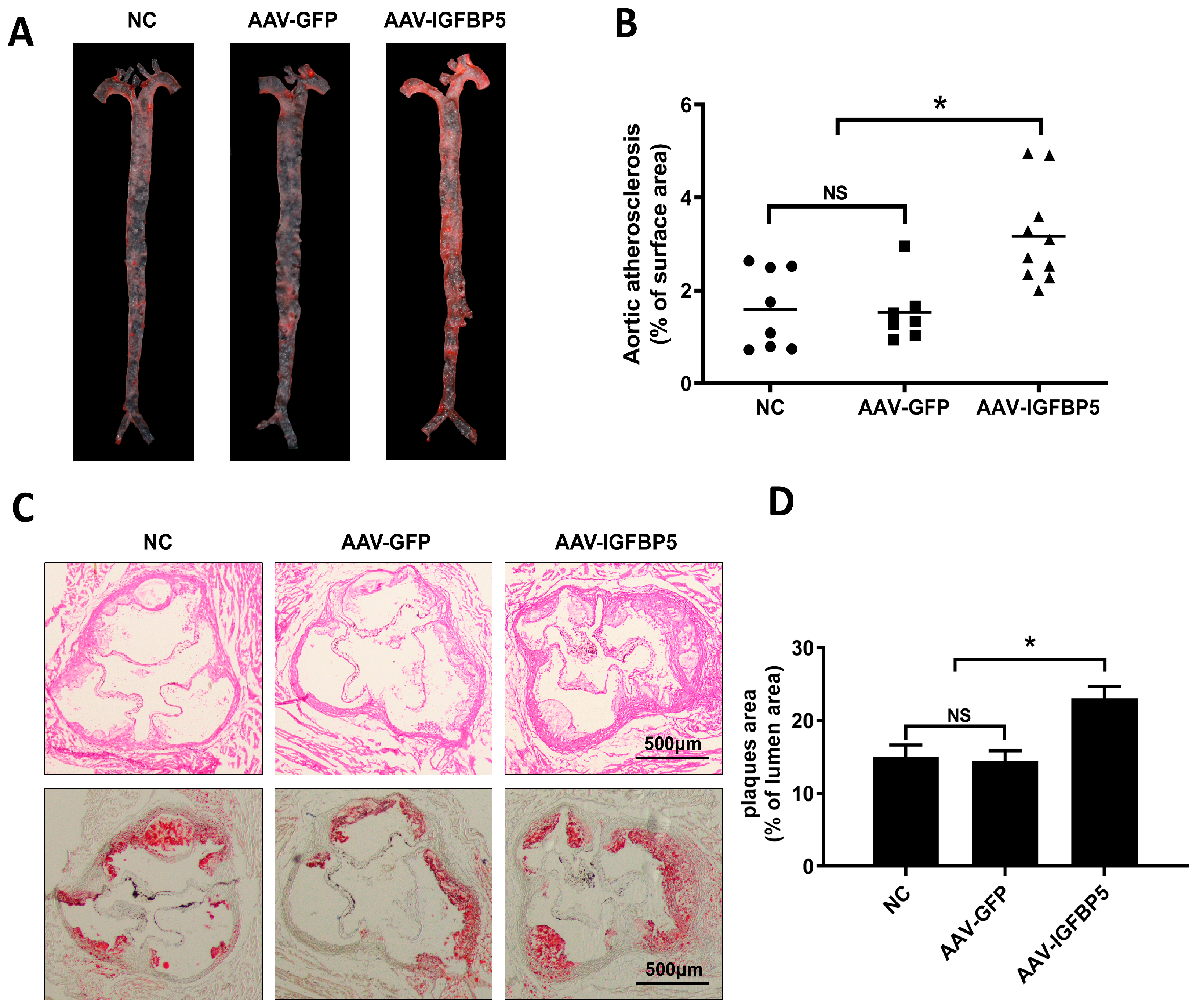
3.2. Overexpression of IGFBP5 Increases Atherosclerotic Plaque Formation in ApoE−/− Mice
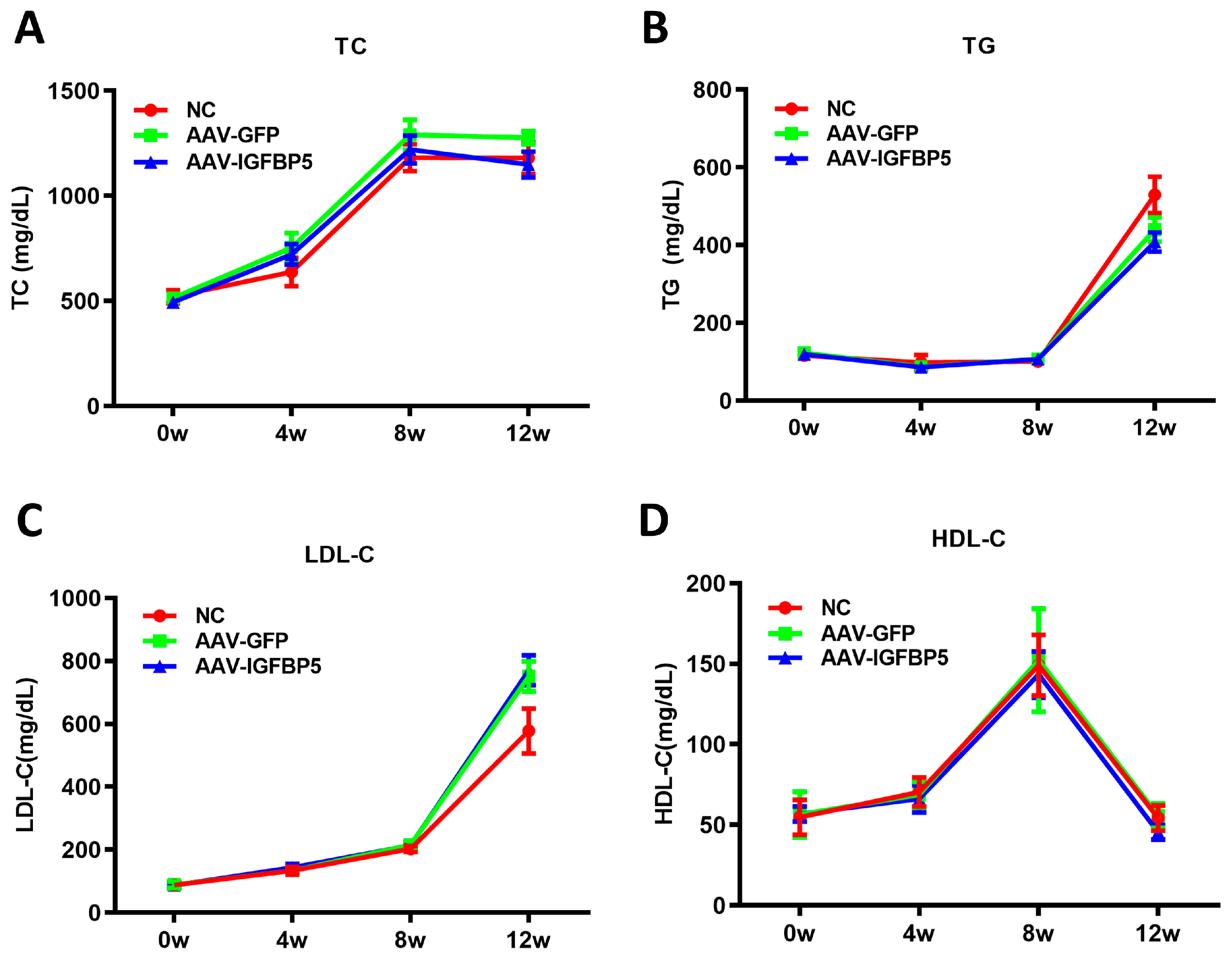
3.3. Overexpression of IGFBP5 Has No Significant Effect on Blood Lipids in ApoE−/− Mice
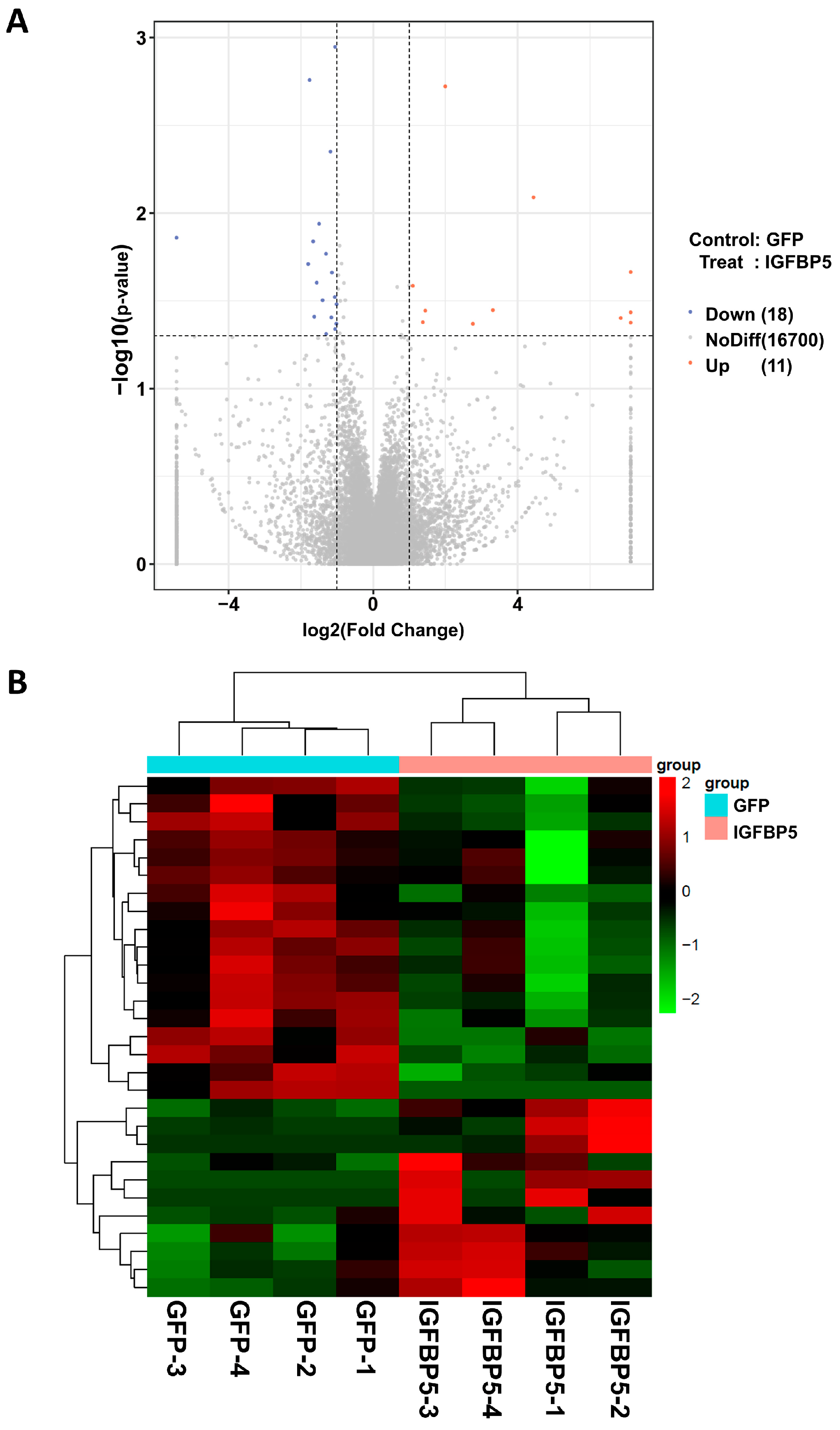
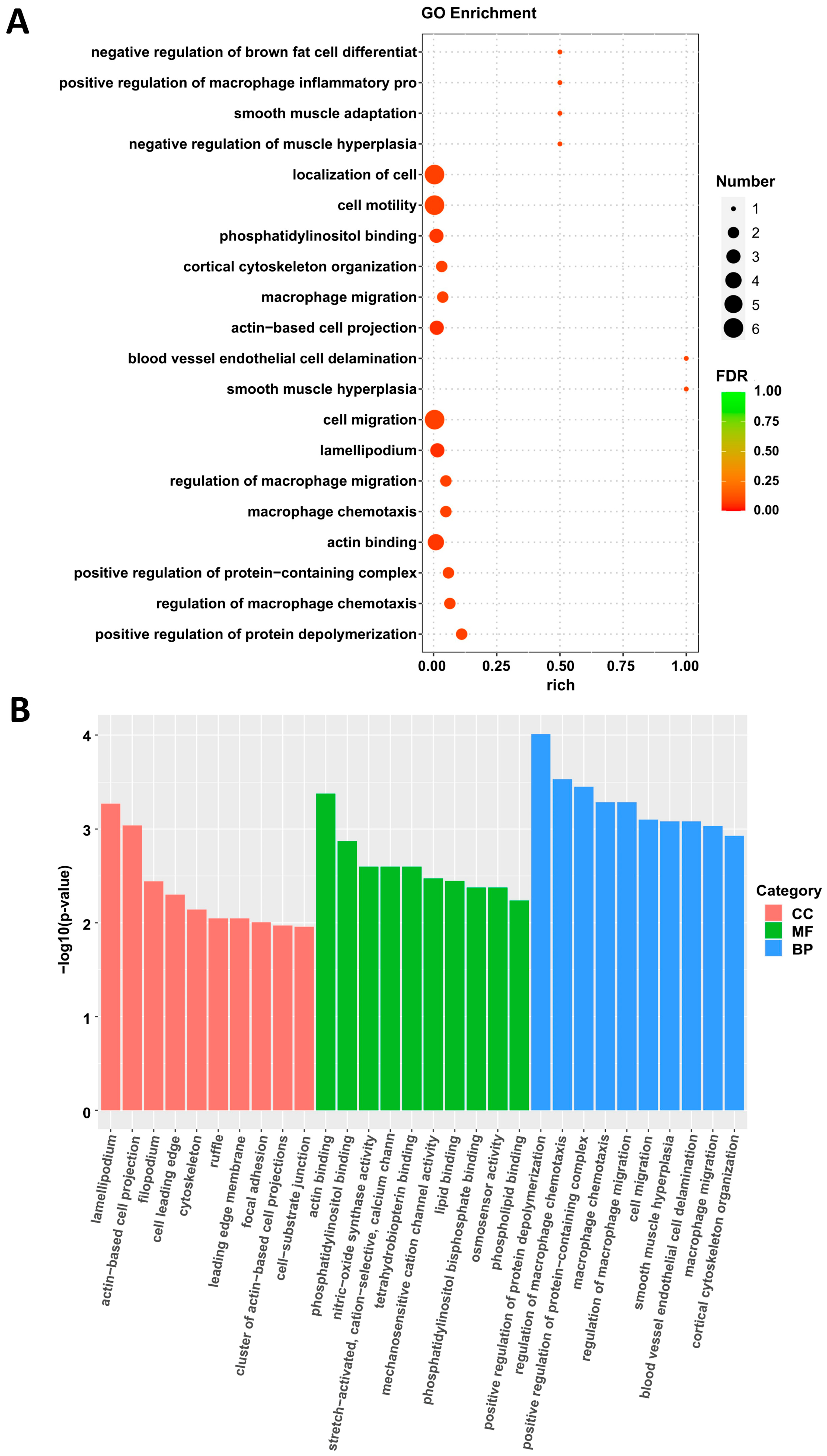
3.4. Sequencing Analysis of Aortic Transcriptome of ApoE−/− Mice After Overexpression of IGFBP5
3.5. Overexpression of IGFBP5 Promotes the Transformation of VSMCs to a Proliferative Phenotype
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VSMCs | Vascular smooth muscle cells |
| IGFBP5 | Insulin-like growth factor binding protein 5 |
| ApoE | Apolipoprotein E |
| GO | Gene Ontology |
| AAV | Adeno-associated virus |
| TC | Total cholesterol |
| TG | Total triglyceride |
| HDL-C | High-density lipoprotein cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| DEGs | Differentially expressed genes |
| H&E | Hematoxylin-eosin |
| GFP | Green fluorescent protein |
| CCK-8 | Cell counting kit-8 |
| DMEM | Dulbecco’s modified Eagle medium |
| FBS | Fetal bovine serum |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| PI | Propidium iodide |
| ACTA2 | Actin alpha 2 |
| MYH11 | Myosin heavy chain 11 |
| TAGLN | Transgelin |
| CNN1 | Calponin 1 |
| KLF4 | Kruppel-like factor 4 |
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis-from experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun. Signal. 2022, 20, 180. [Google Scholar] [CrossRef]
- Yu, Y.; Cai, Y.; Yang, F.; Yang, Y.; Cui, Z.; Shi, D.; Bai, R. Vascular smooth muscle cell phenotypic switching in atherosclerosis. Heliyon 2024, 10, e37727. [Google Scholar] [CrossRef]
- Duan, C.; Allard, J.B. Insulin-Like Growth Factor Binding Protein-5 in Physiology and Disease. Front. Endocrinol. 2020, 11, 100. [Google Scholar] [CrossRef]
- Bach, L.A. Insulin-like growth factor binding proteins 4–6. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 713–722. [Google Scholar] [CrossRef]
- Xiao, Z.; Chu, Y.; Qin, W. IGFBP5 modulates lipid metabolism and insulin sensitivity through activating AMPK pathway in non-alcoholic fatty liver disease. Life Sci. 2020, 256, 117997. [Google Scholar] [CrossRef]
- Xiang, A.; Chu, G.; Zhu, Y.; Ma, G.; Yang, G.; Sun, S. IGFBP5 suppresses oleate-induced intramyocellular lipids deposition and enhances insulin signaling. J. Cell. Physiol. 2019, 234, 15288–15298. [Google Scholar] [CrossRef]
- Hsieh, T.; Gordon, R.E.; Clemmons, D.R.; Busby, W.H., Jr.; Duan, C. Regulation of vascular smooth muscle cell responses to insulin-like growth factor (IGF)-I by local IGF-binding proteins. J. Biol. Chem. 2003, 278, 42886–42892. [Google Scholar] [CrossRef]
- Kuemmerle, J.F.; Zhou, H. Insulin-like growth factor-binding protein-5 (IGFBP-5) stimulates growth and IGF-I secretion in human intestinal smooth muscle by Ras-dependent activation of p38 MAP kinase and Erk1/2 pathways. J. Biol. Chem. 2002, 277, 20563–20571. [Google Scholar] [CrossRef]
- Fischer, F.; Schulte, H.; Mohan, S.; Tataru, M.C.; Kohler, E.; Assmann, G.; von Eckardstein, A. Associations of insulin-like growth factors, insulin-like growth factor binding proteins and acid-labile subunit with coronary heart disease. Clin. Endocrinol. 2004, 61, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chi, J.; Zeng, W.; Xu, F.; Li, X.; Wang, Z.; Qu, M. Discovery of crucial cytokines associated with deep vein thrombus formation by protein array analysis. BMC Cardiovasc. Disord. 2024, 24, 374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Cheang, I.; Guo, Q.; Lu, X.; Li, Y.; Yao, W.; Zhang, H.; Li, X. Serum IGFBP5 as a predictor of major adverse cardiac events in patients with acute myocardial infarction. Int. J. Cardiol. 2024, 411, 132268. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Duan, C.; Clemmons, D.R. The effect of extracellular matrix proteins on porcine smooth muscle cell insulin-like growth factor (IGF) binding protein-5 synthesis and responsiveness to IGF-I. J. Biol. Chem. 1998, 273, 8994–9000. [Google Scholar] [CrossRef]
- Kim, K.S.; Seu, Y.B.; Baek, S.H.; Kim, M.J.; Kim, K.J.; Kim, J.H.; Kim, J.R. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol. Biol. Cell 2007, 18, 4543–4552. [Google Scholar] [CrossRef]
- Sanada, F.; Muratsu, J.; Otsu, R.; Shimizu, H.; Koibuchi, N.; Uchida, K.; Taniyama, Y.; Yoshimura, S.; Rakugi, H.; Morishita, R. Local Production of Activated Factor X in Atherosclerotic Plaque Induced Vascular Smooth Muscle Cell Senescence. Sci. Rep. 2017, 7, 17172. [Google Scholar] [CrossRef]
- Xu, S.; Xu, Y.; Yin, M.; Zhang, S.; Liu, P.; Koroleva, M.; Si, S.; Little, P.J.; Pelisek, J.; Jin, Z.G. Flow-dependent epigenetic regulation of IGFBP5 expression by H3K27me3 contributes to endothelial anti-inflammatory effects. Theranostics 2018, 8, 3007–3021. [Google Scholar] [CrossRef]
- Guan, H.; Liu, T.; Liu, M.; Wang, X.; Shi, T.; Guo, F. SFRP4 Reduces Atherosclerosis Plaque Formation in ApoE Deficient Mice. Cardiol. Res. Pract. 2023, 2023, 8302289. [Google Scholar] [CrossRef]
- Su, P.; Tian, Y.; Yin, C.; Wang, X.; Li, D.; Yang, C.; Pei, J.; Deng, X.; King, S.; Li, Y.; et al. MACF1 promotes osteoblastic cell migration by regulating MAP1B through the GSK3beta/TCF7 pathway. Bone 2022, 154, 116238. [Google Scholar] [CrossRef] [PubMed]
- Mlynarska, E.; Czarnik, W.; Fularski, P.; Hajdys, J.; Majchrowicz, G.; Stabrawa, M.; Rysz, J.; Franczyk, B. From Atherosclerotic Plaque to Myocardial Infarction-The Leading Cause of Coronary Artery Occlusion. Int. J. Mol. Sci. 2024, 25, 7295. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, Y.J.; Guo, K.; Zhou, X.Q.; Abulaiti, A.; Olatunji, O.J.; Ji, C.L.; Zuo, J. Interaction with IGF1 overrides ANXA2-mediated anti-inflammatory functions of IGFBP5 in vivo. Front. Immunol. 2024, 15, 1539317. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.M.; Nam, J.O.; Kang, Y.J. Pitavastatin Regulates Ang II Induced Proliferation and Migration via IGFBP-5 in VSMC. Korean J. Physiol. Pharmacol. 2015, 19, 499–506. [Google Scholar] [CrossRef][Green Version]
- Lu, Q.B.; Wan, M.Y.; Wang, P.Y.; Zhang, C.X.; Xu, D.Y.; Liao, X.; Sun, H.J. Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFkappaB/mTOR/P70S6K signaling cascade. Redox Biol. 2018, 14, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, F.; Wang, X.; Gong, J.; Xian, Y.; Wang, G.; Zheng, Z.; Shang, C.; Wang, B.; He, Y.; et al. MiR-145 alleviates Hcy-induced VSMC proliferation, migration, and phenotypic switch through repression of the PI3K/Akt/mTOR pathway. Histochem. Cell Biol. 2020, 153, 357–366. [Google Scholar] [CrossRef]
- Pan, J.; Li, K.; Huang, W.; Zhang, X. MiR-137 inhibited cell proliferation and migration of vascular smooth muscle cells via targeting IGFBP-5 and modulating the mTOR/STAT3 signaling. PLoS ONE 2017, 12, e0186245. [Google Scholar] [CrossRef]

| Names | Sequence |
|---|---|
| IGFBP5 | F: AGATGAGACAGGAATCCGAACAAG R: GAAGGCGTGGCACTGAAAG |
| ACAT2 | F: GTCCCAGACATCAGGGAGTAA R: TCGGATACTTCAGCGTCAGGA |
| MYH11 | F: AAGCTGCGGCTAGAGGTCA R: CCCTCCCTTTGATGGCTGAG |
| TAGLN | F: CAACAAGGGTCCATCCTACGG R: ATCTGGGCGGCCTACATCA |
| CNN1 | F: TCTGCACATTTTAACCGAGGTC R: GCCAGCTTGTTCTTTACTTCAGC |
| KLF4 | F: GTGCCCCGACTAACCGTTG R: GTCGTTGAACTCCTCGGTCT |
| GAPDH | F: TCGCTCCTGGAAGATGGTGAT R: CAGTGGCAAAGTGGAGATTGTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, A.; Guan, H.; Su, P.; Zhang, L.; Chen, X.; Yu, Q. IGFBP5 Promotes Atherosclerosis in APOE−/− Mice Through Phenotypic Transformation of VSMCs. Curr. Issues Mol. Biol. 2025, 47, 555. https://doi.org/10.3390/cimb47070555
Xiang A, Guan H, Su P, Zhang L, Chen X, Yu Q. IGFBP5 Promotes Atherosclerosis in APOE−/− Mice Through Phenotypic Transformation of VSMCs. Current Issues in Molecular Biology. 2025; 47(7):555. https://doi.org/10.3390/cimb47070555
Chicago/Turabian StyleXiang, Aoqi, Hua Guan, Peihong Su, Lusha Zhang, Xiaochang Chen, and Qi Yu. 2025. "IGFBP5 Promotes Atherosclerosis in APOE−/− Mice Through Phenotypic Transformation of VSMCs" Current Issues in Molecular Biology 47, no. 7: 555. https://doi.org/10.3390/cimb47070555
APA StyleXiang, A., Guan, H., Su, P., Zhang, L., Chen, X., & Yu, Q. (2025). IGFBP5 Promotes Atherosclerosis in APOE−/− Mice Through Phenotypic Transformation of VSMCs. Current Issues in Molecular Biology, 47(7), 555. https://doi.org/10.3390/cimb47070555







