Protective Potential of Satureja montana-Derived Polyphenols in Stress-Related Central Nervous System Disorders, Including Dementia
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Bioactive Compounds of Satureja montana
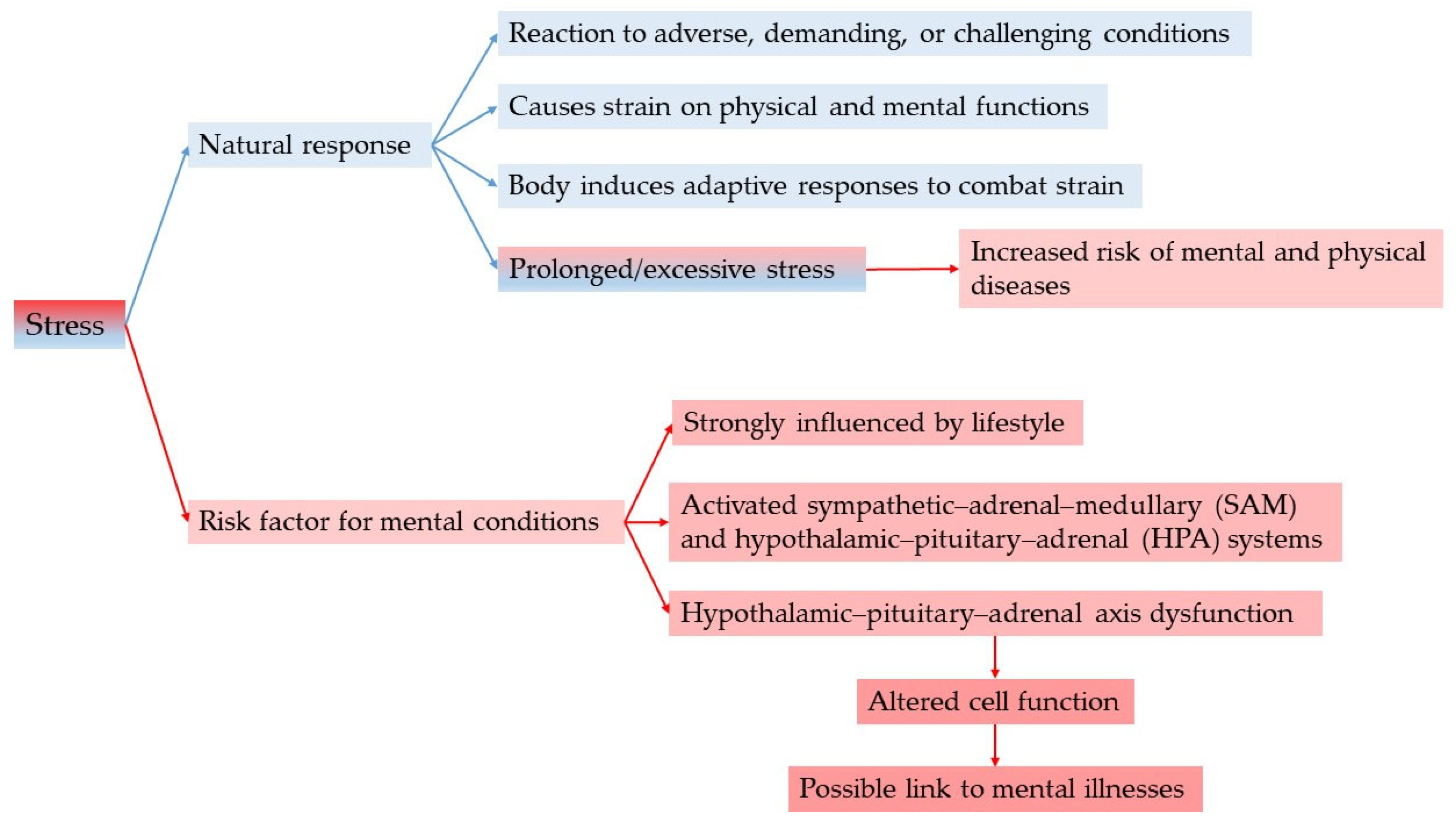
3.2. Relation of Stress to the Pathogenesis of Certain Mental Disorders
3.3. CNS-Activity of Satureja montana
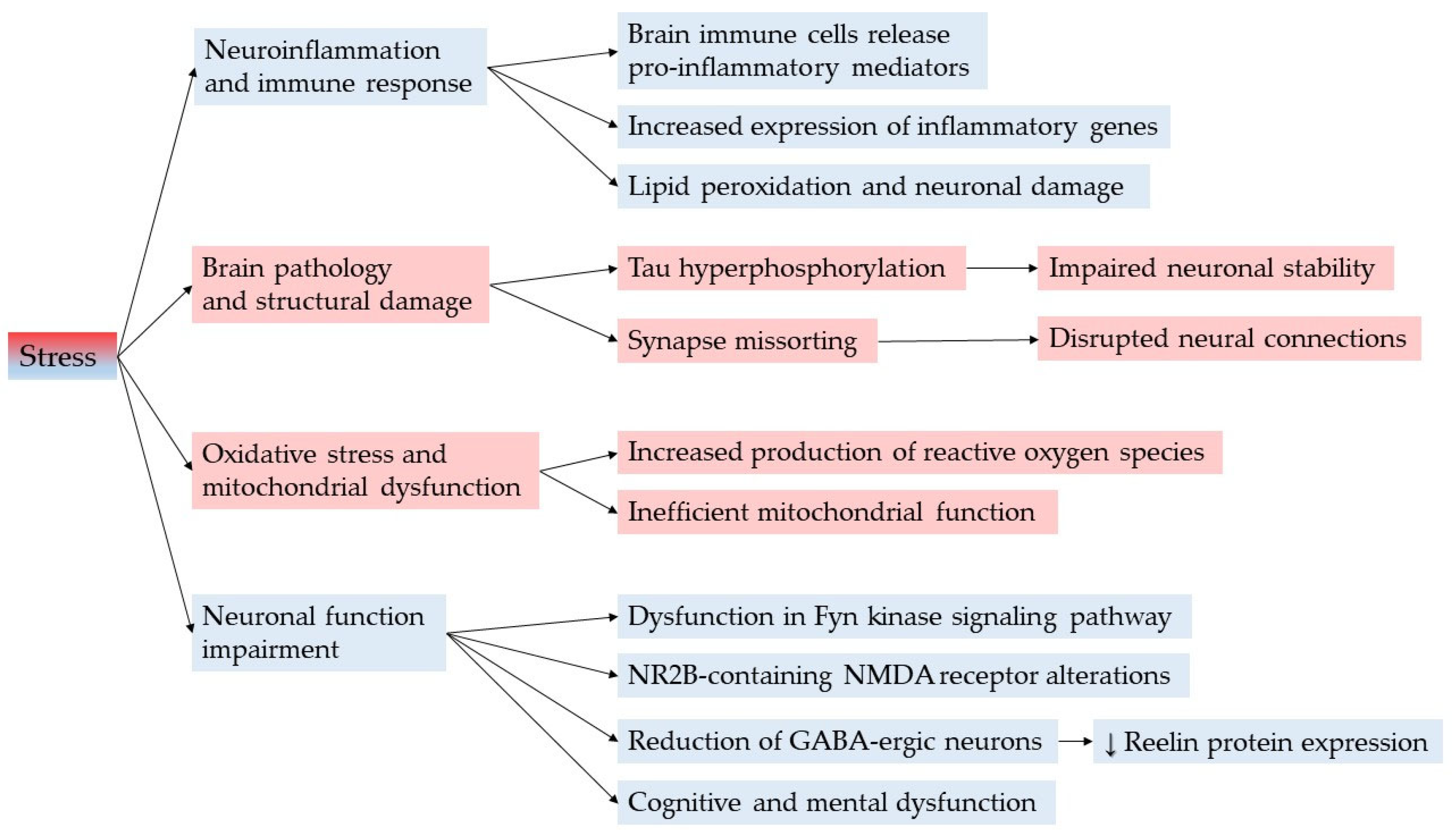
3.4. Neuroprotective Mechanisms of Satureja montana
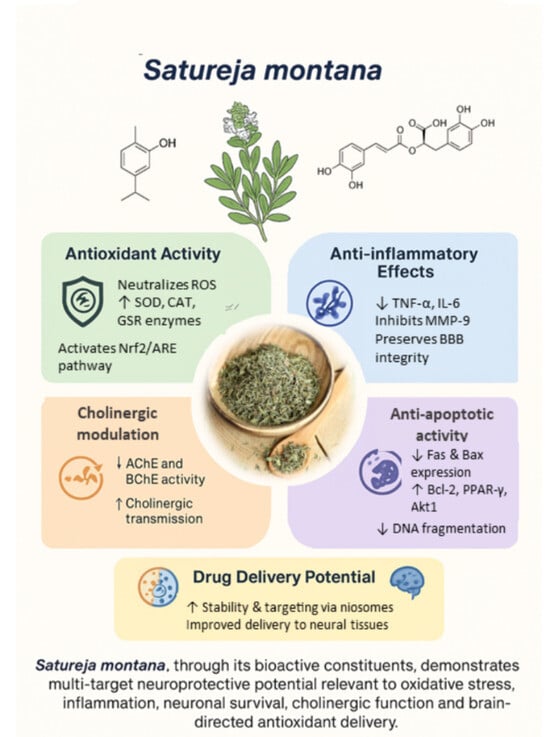
3.4.1. Antioxidant Activity
| Mechanism | Description | Ref. |
|---|---|---|
| Direct Antioxidant Activity | Scavenging of free radicals, reducing oxidative stress | [176] |
| Enzyme Modulation | Increased activity of SOD, CAT, and GSR | [35,177] |
| Reduction in Lipid Peroxidation | Decreased lipid peroxidation, protecting cell membranes | [33,177] |
| Anti-inflammatory Effects | Decreased levels of TNF-α and IL-6 | [176] |
| Hepatoprotective Effects | Reduced oxidative stress enzymes and inflammatory cells in the liver | [33] |
| Neuroprotective Pathways | Activation of Nrf2/ARE pathway, enhancing antioxidant protein expression | [178,179] |
| Drug Delivery Potential | Use of niosomes for stable and targeted delivery of antioxidants | [180] |
3.4.2. Anti-Inflammatory Activity
3.4.3. Anti-Apoptotic Properties
3.4.4. Acetylcholinesterase Inhibition
4. Discussion
4.1. Neuroprotective and Psychotropic Potential of Satureja montana
4.2. Mechanistic Insights into CNS Activity of Satureja montana
4.3. Clinical Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| BBB | Blood–brain barrier |
| BChE | Butyrylcholinesterase |
| CAT | Catalase |
| CNS | Central nervous system |
| DI | Discrimination index |
| GABA | Gamma-aminobutyric acid |
| GSR | Glutathione reductase |
| MAPK | Mitogen-activated protein kinase |
| MCI | Mild cognitive impairment |
| MMP-9 | Matrix metalloproteinase-9 |
| NMDA | N-methyl-D-aspartate |
| PI3K | Phosphoinositide 3-kinase |
| PPAR-γ | Peroxisome proliferator-activated receptor-gamma |
| ROS | Reactive oxygen species |
| SM | Satureja montana |
| SOD | Superoxide dismutase |
References
- Tugume, P.; Nyakoojo, C. Ethno-pharmacological survey of herbal remedies used in the treatment of paediatric diseases in Buhunga parish, Rukungiri District, Uganda. BMC Complement. Altern. Med. 2019, 19, 353. [Google Scholar] [CrossRef] [PubMed]
- Herbal Medicine Market. 2021. Available online: https://www.insightslice.com/herbal-medicine-market (accessed on 23 December 2024).
- Jakovljević, M.; Vladić, J.; Vidović, S.; Pastor, K.; Jokić, S.; Molnar, M.; Jerković, I. Application of Deep Eutectic Solvents for the Extraction of Rutin and Rosmarinic Acid from Satureja montana L. and Evaluation of the Extracts Antiradical Activity. Plants 2020, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Manchanda, R.; Goswami, R.; Chawla, S. Biodiversity and Therapeutic Potential of Medicinal Plants. In Environmental Concerns and Sustainable Development; Shukla, V., Kumar, N., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Davis, C.C.; Choisy, P. Medicinal plants meet modern biodiversity science. Curr. Biol. 2024, 34, R158–R173. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Trinh, T.T.; Akter, R.; Jeong, Y.J.; Goh, S.H.; Kim, C.-S.; Lee, K.-J. Therapeutic Potential of Natural Products in Treating Neurodegenerative Disorders and Their Future Prospects and Challenges. Molecules 2021, 26, 5327. [Google Scholar] [CrossRef] [PubMed]
- Aware, C.B.; Patil, D.N.; Suryawanshi, S.S.; Mali, P.R.; Rane, M.R.; Gurav, R.G.; Jadhav, J.P. Natural bioactive products as promising therapeutics: A review of natural product-based drug development. S. Afr. J. Bot. 2022, 151, 512–528. [Google Scholar] [CrossRef]
- Wangensteen, H.; Diallo, D.; Paulsen, B.S. Medicinal plants from Mali: Chemistry and biology. J. Ethnopharmacol. 2017, 176, 429–437. [Google Scholar] [CrossRef] [PubMed]
- de Rus Jacquet, A.; Timmers, M.; Ma, S.Y.; Thieme, A.; McCabe, G.P.; Vest, J.H.C.; Lila, M.A.; Rochet, J.C. Lumbee traditional medicine: Neuroprotective activities of medicinal plants used to treat Parkinson’s disease-related symptoms. J. Ethnopharmacol. 2017, 206, 408–425. [Google Scholar] [CrossRef] [PubMed]
- Veeren, B.; Ghaddar, B.; Bringart, M.; Khazaal, S.; Gonthier, M.P.; Meilhac, O.; Diotel, N.; Bascands, J.L. Phenolic Profile of Herbal Infusion and Polyphenol-Rich Extract from Leaves of the Medicinal Plant Antirhea borbonica: Toxicity Assay Determination in Zebrafish Embryos and Larvae. Molecules 2020, 25, 4482. [Google Scholar] [CrossRef] [PubMed]
- Ciupei, D.; Colişar, A.; Leopold, L.; Stănilă, A.; Diaconeasa, Z.M. Polyphenols: From Classification to Therapeutic Potential and Bioavailability. Foods 2024, 13, 4131. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Grumetto, L. Recent Advances in Natural Polyphenol Research. Molecules 2022, 27, 8777. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-L.; Mutie, F.M.; Xu, Y.-B.; Saleri, F.D.; Hu, G.-W.; Guo, M.-Q. Antioxidant, Anti-inflammatory Activities and Polyphenol Profile of Rhamnus prinoides. Pharmaceuticals 2020, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Bonner, M.Y.; Arbiser, J.L. Use of Polyphenolic Compounds in Dermatologic Oncology. Am. J. Clin. Dermatol. 2016, 17, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Moraga, A.; Argandoña, J.; Mota, B.; Pérez, J.; Verde, A.; Fajardo, J.; Gómez-Navarro, J.; Castillo-López, R.; Ahrazem, O.; Gómez-Gómez, L. Screening for polyphenols, antioxidant and antimicrobial activities of extracts from eleven Helianthemum taxa (Cistaceae) used in folk medicine in southeastern Spain. J. Ethnopharmacol. 2013, 148, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Benslama, A.; Harrar, A.; Gül, F.; Demirtaş, I. Phenolic compounds, antioxidant and antibacterial activities of Zizyphus lotus L. leaves extracts. Nat. Prod. J. 2017, 7, 316–322. [Google Scholar] [CrossRef]
- Zarshenas, M.M.; Krenn, L. Phytochemical and pharmacological aspects of Salvia mirzayanii Rech. f. & Esfand. J. Evid.-Based Complement. Altern. Med. 2015, 20, 65–72. [Google Scholar] [CrossRef]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef] [PubMed]
- Zawiślak, G.; Nurzyńska-Wierdak, R. Variation in winter savory (Satureja montana L.) yield and essential oil production as affected by different plant density and number of harvests. Acta Sci. Pol. Hortorum Cultus 2017, 16, 159–168. [Google Scholar] [CrossRef]
- Jafari, F.; Ghavidel, F.; Zarshenas, M.M. A critical overview on the pharmacological and clinical aspects of popular Satureja species. J. Acupunct. Meridian Stud. 2016, 9, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Beshkov, S. Contributions to the knowledge of the Geometridae fauna of the Balkan Peninsula with some new species for Bulgaria, Serbia, Albania, and Macedonia. Atalanta 2017, 48, 275–290. [Google Scholar]
- Šilić, Č. Monografija rodova Satureja L., Calamintha Miller, Micromeria Bentham, Acinos Miller i Clinopodium L. u Flori Jugoslavije; Zemaljski muzej BiH: Sarajevo, Bosnia and Herzegovina, 1979. [Google Scholar]
- World Checklist of Selected Plant Families. Available online: http://ww2.bgbm.org/EuroPlusMed (accessed on 11 November 2024).
- Hudz, N.; Makowicz, E.; Shanaida, M.; Białoń, M.; Jasicka-Misiak, I.; Yezerska, O.; Svydenko, L.; Wieczorek, P.P. Phytochemical evaluation of tinctures and essential oil obtained from Satureja montana herb. Molecules 2020, 25, 4763. [Google Scholar] [CrossRef] [PubMed]
- Čutović, N.; Batinić, P.; Marković, T.; Jovanović, A.A. Optimization of the extraction process from Satureja montana L.: Physicochemical characterization of the extracts. Hem. Ind. 2023, 77, 251–263. [Google Scholar] [CrossRef]
- Kremer, D.; Košir, I.J.; Končić, M.Z.; Čerenak, A.; Potočnik, T.; Srečec, S.; Randić, M.; Kosalec, I. Antimicrobial and antioxidant properties of Satureja montana L. and S. subspicata Vis. (Lamiaceae). Curr. Drug Targets 2015, 16, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Vilmosh, N.; Georgieva-Kotetarova, M.; Dimitrova, S.; Zgureva, M.; Atanassova, P.K.; Hrischev, P.I.; Kostadinova, I. Composition and chronic toxicity of dry methanol-aqueous extract of wild-growing Satureja montana. Folia Med. 2023, 65, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, H.D.; Hah, S.-A.A.; Abdelmohsen, M.M. Antioxidant polyphenolic constituents of Satureja montana L. growing in Egypt. Int. J. Pharm. Pharm. Sci. 2014, 6, 578–581. [Google Scholar]
- Fortini, P.; Di Marzio, P.; Guarrera, P.M.; Iorizzi, M. Ethnobotanical study on the medicinal plants in the Mainarde Mountains (central-southern Apennine, Italy). J. Ethnopharmacol. 2016, 184, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Puricelli, C.; Mikerezi, I.; Iriti, M. Plants, people, and traditions: Ethnobotanical survey in the Lombard Stelvio National Park and neighboring areas (Central Alps, Italy). J. Ethnopharmacol. 2015, 173, 435–458. [Google Scholar] [CrossRef] [PubMed]
- Milijašević, B.; Steinbach, M.; Mikov, M.; Rašković, A.; Čapo, I.; Živković, J.; Borišev, I.; Panić, J.Ć.; Teofilović, B.; Vujčić, M.; et al. Impact of winter savory extract (Satureja montana L.) on biochemical parameters in serum and oxidative status of liver with application of principal component analysis in extraction solvent selection. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4721–4734. [Google Scholar] [CrossRef] [PubMed]
- Vilmosh, N. Изследване на Фармакoлoгични Ефекти на Satureja montana. Ph.D. Thesis, Plovdiv Medical University, Plovdiv, Bulgaria, 2022. Available online: https://ras.nacid.bg/api/reg/FilesStorage?key=108d0d7c-d26f-44d4-a601-c58eaad770a9&mimeType=application/pdf&fileName=%D0%90%D0%B2%D1%82%D0%BE%D1%80%D0%B5%D1%84%D0%B5%D1%80%D0%B0%D1%82_%D0%B4%D1%80%20%D0%92%D0%B8%D0%BB%D0%BC%D0%BE%D1%88.pdf&dbId=1 (accessed on 10 July 2025).
- Sefidkon, F.; Bistgani, Z.E. Integrative review on ethnobotany, essential oil, phytochemical, agronomy, molecular, and pharmacological properties of Satureja species. J. Essent. Oil Res. 2021, 33, 114–132. [Google Scholar] [CrossRef]
- Demyashkin, G.; Sataieva, T.; Shevkoplyas, L.; Kuevda, T.; Ahrameeva, M.; Parshenkov, M.; Mimuni, A.; Pimkin, G.; Atiakshin, D.; Shchekin, V.; et al. Burn wound healing activity of hydroxyethylcellulose gels with different water extracts obtained from various medicinal plants in Pseudomonas aeruginosa-infected rabbits. Int. J. Mol. Sci. 2024, 25, 8990. [Google Scholar] [CrossRef] [PubMed]
- Vladimir-Knežević, S.; Blažeković, B.; Kindl, M.; Vladić, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant, and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.V.; Martins, A.; Salta, J.; Neng, N.R.; Nogueira, J.M.; Mira, D.; Gaspar, N.; Justino, J.; Grosso, C.; Urieta, J.S.; et al. Phytochemical profile and anticholinesterase and antimicrobial activities of supercritical versus conventional extracts of Satureja montana. J. Agric. Food Chem. 2009, 57, 11557–11563. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Galiffa, V.; Cásedas, G.; Moliner, C.; Maggi, F.; López, V.; Gómez-Rincón, C. Essential oils of two subspecies of Satureja montana L. against gastrointestinal parasite Anisakis simplex and acetylcholinesterase inhibition. Molecules 2024, 29, 4640. [Google Scholar] [CrossRef] [PubMed]
- Rezende, D.A.S.; Cardoso, M.; Konig, I.F.M.; Lunguinho, A.S.; Ferreira, V.R.F.; Brandão, R.M.; Gonçalves, R.R.P.; Caetano, A.R.S.; Nelson, D.L.; Remedio, R.N. Repellent effect on Rhipicephalus sanguineus and inhibition of acetylcholinesterase by volatile oils. Rev. Bras. Farmacogn. 2021, 31, 470–476. [Google Scholar] [CrossRef]
- Gomes, F.; Dias, M.I.; Lima, Â.; Barros, L.; Rodrigues, M.E.; Ferreira, I.C.F.R.; Henriques, M. Satureja montana L. and Origanum majorana L. decoctions: Antimicrobial activity, mode of action, and phenolic characterization. Antibiotics 2020, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Šovljanski, O.; Pezo, L.; Travičić, V.; Tomić, A.; Zheljazkov, V.D.; Ćetković, G.; Švarc-Gajić, J.; Brezo-Borjan, T.; Sofrenić, I. Variability in biological activities of Satureja montana subsp. montana and subsp. variegata based on different extraction methods. Antibiotics 2022, 11, 1235. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, L.; Maccelli, A.; Marazzato, M.; Scazzocchio, F.; Comanducci, A.; Fornarini, S.; Crestoni, M.E.; Filippi, A.; Fraschetti, C.; Rinaldi, F.; et al. Satureja montana L. essential oil and its antimicrobial activity alone or in combination with gentamicin. Microb. Pathog. 2019, 126, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Ezaouine, A.; Nouadi, B.; Sbaoui, Y.; Bennis, F. Use of the genus Satureja as a food supplement: Possible modulation of the immune system via intestinal microbiota during SARS-CoV-2 infection. Anti-Infect. Agents 2022, 20, e221221199259. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Long-Smith, C.; Kennedy, P.; Cryan, J.F.; Cowan, C.S.M.; Cenit, M.C.; van der Kamp, J.W.; Sanz, Y. Feeding melancholic microbes: MyNewGut recommendations on diet and mood. Clin. Nutr. 2019, 38, 1995–2001. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.I.; Gomez-Arbelaez, D.; Crujeiras, A.B.; Granero, R.; Aguera, Z.; Jimenez-Murcia, S.; Sajoux, I.; Lopez-Jaramillo, P.; Fernandez-Aranda, F.; Casanueva, F.F. Effect of a very low-calorie ketogenic diet on food and alcohol cravings, physical and sexual activity, sleep disturbances, and quality of life in obese patients. Nutrients 2018, 10, 1348. [Google Scholar] [CrossRef] [PubMed]
- Delpech, J.-C.; Madrigal, J.L.M.; Masto, G.; Pazos, R.; Dexter, J.; McLaughlin, B.; Sheridan, J.F.; Godbout, J.P. Microglia in neuronal plasticity: Influence of stress. Neuropharmacology 2015, 96, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Lépinay, A.L.; Larrieu, T.; Joffre, C.; Acar, N.; Garate, I.; Castanon, N.; Layé, S. Perinatal high-fat diet increases hippocampal vulnerability to the adverse effects of subsequent high-fat feeding. Psychoneuroendocrinology 2015, 53, 82–93. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, R.; de Sola, S.; Hernández, G.; Farré, M.; Pujol, J.; Rodríguez, J.; Espadaler, J.M.; Langohr, K.; Cuenca-Royo, A.; Principe, A.; et al. Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down’s syndrome (TESDAD): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016, 15, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.S.; Hiles, S.; Bisquera, A.; Hure, A.J.; McEvoy, M.; Attia, J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am. J. Clin. Nutr. 2014, 99, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 2019, 24, 965–986. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The effects of dietary improvement on symptoms of depression and anxiety: A meta-analysis of randomized controlled trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Gomez-Pinilla, F. ‘Metabolic syndrome’ in the brain: Deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J. Physiol. 2012, 590, 2485–2499. [Google Scholar] [CrossRef] [PubMed]
- Prenderville, J.A.; Kennedy, P.J.; Dinan, T.G.; Cryan, J.F. Adding fuel to the fire: The impact of stress on the ageing brain. Trends Neurosci. 2015, 38, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Dallman, M.F. Stress-induced obesity and the emotional nervous system. Trends Endocrinol. Metab. 2010, 21, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.L. Emotional influences on food choice: Sensory, physiological and psychological pathways. Physiol. Behav. 2006, 89, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G.; Wardle, J. Perceived effects of stress on food choice. Physiol. Behav. 1999, 66, 511–515. [Google Scholar] [CrossRef] [PubMed]
- El Aidy, S.; Dinan, T.G.; Cryan, J.F. Gut microbiota: The conductor in the orchestra of immune–neuroendocrine communication. Clin. Ther. 2015, 37, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. 2017, 179, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, H.; Finger, B.C.; Dinan, T.G.; Cryan, J.F. Ghrelin signalling and obesity: At the interface of stress, mood and food reward. Pharmacol. Ther. 2012, 135, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota–gut–brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef] [PubMed]
- de Wouw, M.V.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Microbiota-gut-brain axis: Modulator of host metabolism and appetite. J. Nutr. 2017, 147, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska, A.; Grzeszczuk, M.; Jadczak, D. Influence of harvest term on the content of carvacrol, p-cymene, γ-terpinene and β-caryophyllene in the essential oil of Satureja montana. Not. Bot. Horti. Agrobo. 2014, 42, 392–397. [Google Scholar] [CrossRef]
- Caprioli, G.; Lupidi, G.; Maggi, F. Comparison of chemical composition and antioxidant activities of two Winter savory subspecies (Satureja montana subsp. variegata and Satureja montana subsp. montana) cultivated in Northern Italy. Nat. Prod. Res. 2019, 33, 3143–3147. [Google Scholar] [CrossRef] [PubMed]
- Bojović, D.; Šoškić, M.; Tadic, V. Comparative study of chemical composition of the essential oils from Satureja cuneifolia Ten. and Satureja montana L., Lamiaceae collected at National park Lovćen, Montenegro. Stud. Univ. Babes-Bolyai Chem. 2018, 63, 167–180. [Google Scholar] [CrossRef]
- Bezić, N.; Skočibušić, M.; Dunkić, V. Phytochemical composition and antimicrobial activity of Satureja montana L. and Satureja cuneifolia Ten. essential oils. Acta Bot. Croat. 2005, 64, 313–322. [Google Scholar]
- Marin, M.; Novaković, M.; Tešević, V.; Marin, P.D. Antioxidative, antibacterial and antifungal activity of the essential oil of wild-growing Satureja montana L. from Dalmatia, Croatia. Flavour Fragr. J. 2012, 27, 216–223. [Google Scholar] [CrossRef]
- Skocibusić, M.; Bezić, N. Phytochemical analysis and in vitro antimicrobial activity of two Satureja species essential oils. Phytother. Res. 2004, 18, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Abdelshafeek, K.A.; Osman, A.F.; Mouneir, S.M.; Elhenawy, A.A.; Abdallah, W.E. Phytochemical profile, comparative evaluation of Satureja montana alcoholic extract for antioxidants, anti-inflammatory and molecular docking studies. BMC Complement. Med. Ther. 2023, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Rahbardar, M.G.; Hosseinzadeh, H. Effects of rosmarinic acid on nervous system disorders: An updated review. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1779–1795. [Google Scholar] [CrossRef] [PubMed]
- Mohammedi, Z. Carvacrol: An update of biological activities and mechanism of action. Open Access J. Chem. 2017, 1, 53–62. [Google Scholar] [CrossRef]
- Elufioye, T.O.; Habtemariam, S. Hepatoprotective effects of rosmarinic acid: Insight into its mechanisms of action. Biomed. Pharmacother. 2019, 112, 108600. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Adolfse, S.J.; Ahad, D.S.; Tersteeg-Zijderveld, M.H.; Jongerius-Gortemaker, B.G.; Post, J.A.; Brüggemann, H.; Santos, R.R. Cinnamaldehyde, carvacrol and organic acids affect gene expression of selected oxidative stress and inflammation markers in IPEC-J2 cells exposed to Salmonella typhimurium. Phytother. Res. 2016, 30, 1988–2000. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Simin, N.; Orcic, D.; Franciskovic, M.; Knezevic, P.; Beara, I.; Aleksic, V.; Svircev, E.; Buzas, K.; Mimica-Dukic, N. Binary and tertiary mixtures of Satureja hortensis and Origanum vulgare essential oils as potent antimicrobial agents against Helicobacter pylori. Phytother. Res. 2016, 30, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Rajput, J.D.; Bagul, S.D.; Pete, U.D.; Zade, C.M.; Padhye, S.B.; Bendre, R.S. Perspectives on medicinal properties of natural phenolic monoterpenoids and their hybrids. Mol. Divers. 2018, 22, 225–245. [Google Scholar] [CrossRef] [PubMed]
- El-Hagrassi, A.M.; Abdallah, W.E.; Osman, A.F.; Abdelshafeek, K.A. Phytochemical study of bioactive constituents from Satureja montana L. growing in Egypt and their antimicrobial and antioxidant activities. Asian J. Pharm. Clin. Res. 2018, 11, 142–148. [Google Scholar] [CrossRef]
- Serrano, C.; Matos, O.; Teixeira, B.; Ramos, C.; Neng, N.; Nogueira, J.; Nunes, M.L.; Marques, A. Antioxidant and antimicrobial activity of Satureja montana L. extracts. J. Sci. Food Agric. 2011, 91, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Vilmosh, N.; Delev, D.; Kostadinov, I.; Zlatanova, H.; Kotetarova, M.; Kandilarov, I.; Kostadinova, I. Anxiolytic effect of Satureja montana dry extract and its active compounds rosmarinic acid and carvacrol in acute stress experimental model. J. Integr. Neurosci. 2022, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Bhatia, G.; Sen, P.; Palit, G. Anti-stress effects of Ginkgo biloba and Panax ginseng: A comparative study. J. Pharmacol. Sci. 2003, 93, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Fonseca, J.A.; Gosselink, K.L. Tauopathy and neurodegeneration: A role for stress. Neurobiol. Stress 2018, 9, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Debora, S.; Jasmin, V.; Baba, V.; Gomathi, S. Impact of stress on health. Narayana Nurs. J. 2018, 5, 11–14. [Google Scholar]
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057. [Google Scholar] [CrossRef] [PubMed]
- Manganyi, M.C.; Gunya, B.; Mavundza, E.J.; Sibuyi, N.R.S.; Meyer, M.; Madiehe, A.M. A chewable cure “kanna”: Biological and pharmaceutical properties of Sceletium tortuosum. Molecules 2021, 26, 2557. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Z.; Wang, Y.-X.; Jiang, C.-L. Inflammation: The common pathway of stress-related diseases. Front. Hum. Neurosci. 2017, 11, 273283. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Yu, L.; Zhang, L.; Rudenko, A.; Hahn, K.; McLeod, F.; Brotherton, D.; Maguire, J.; Bading, H.; Frenguelli, B.G.; et al. Tau protein is essential for stress-induced brain pathology. Proc. Natl. Acad. Sci. USA 2016, 113, E3755–E3763. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Bockmühl, Y.; Patchev, A.V.; Bauer, M.; Almeida, O.F. Methylation at the CpG island shore region upregulates Nr3c1 promoter activity after early-life stress. Epigenetics 2015, 10, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Y.; Sibille, E.; McEwen, B.S. Early-life stress leads to impaired spatial learning and memory in middle-aged ApoE4-TR mice. Mol. Neurodegener. 2016, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Allostasis and the epigenetics of brain and body health over the life course: The brain on stress. JAMA Psychiatry 2017, 74, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Alcubierre, D.D.; Díaz-Rodríguez, L.; Cano, M.; Ortiz, J.; González, M.; Torres, A.; García, L.; Pacheco, D.; Luna, E. Glucocorticoids and cognitive function: A walkthrough in endogenous and exogenous alterations. J. Endocrinol. Investig. 2023, 46, 1961–1982. [Google Scholar] [CrossRef] [PubMed]
- Henckens, M.J.A.G.; Deussing, J.M.; Chen, A. Region-specific roles of the corticotropin-releasing factor–urocortin system in stress. Nat. Rev. Neurosci. 2016, 17, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Spannenburg, L.; Reed, H. Adverse cognitive effects of glucocorticoids: A systematic review of the literature. Steroids 2023, 2023, 109314. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, J.J.; Almeida, O.F.; Sousa, N. Corticosteroid status influences the volume of the rat cingulate cortex–a magnetic resonance imaging study. J. Psychiatr. Res. 2005, 39, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, J.J.; Mailliet, F.; Almeida, O.F.; Jay, T.M.; Sousa, N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J. Neurosci. 2005, 25, 7792–7800. [Google Scholar] [CrossRef] [PubMed]
- Liston, C.; Gan, W.-B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 16074–16079. [Google Scholar] [CrossRef] [PubMed]
- Magariños, A.M.; McEwen, B.S.; Flugge, G.; Fuchs, E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J. Neurosci. 1996, 16, 3534–3540. [Google Scholar] [CrossRef] [PubMed]
- Radley, J.J.; Rocher, A.B.; Martinez, A.; Vasquez, S.; Williams, S.; Bloom, F.E.; Hof, P.R.; Morrison, J.H. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb. Cortex 2006, 16, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Wellman, C.L. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 2001, 49, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.; Langiano, E.; Brango, T.D.; Vito, E.D.; Cioccio, L.D.; Bauco, C. Prevalence of stress, anxiety and depression in Alzheimer caregivers. Health Qual. Life Outcomes 2008, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Quick, J.C.; Henderson, D.F. Occupational stress: Preventing suffering, enhancing wellbeing. Int. J. Environ. Res. Public Health 2016, 13, 459. [Google Scholar] [CrossRef] [PubMed]
- Jope, R.S.; Cheng, Y.; Lowell, J.A.; Worthen, R.J.; Sitbon, Y.H.; Beurel, E. Stressed and inflamed, can GSK3 be blamed? Trends Biochem. Sci. 2017, 42, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Neuroimmune interactions: From the brain to the immune system and vice versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef] [PubMed]
- Hodes, G.E.; Kana, V.; Menard, C.; Merad, M.; Russo, S.J. Neuroimmune mechanisms of depression. Nat. Neurosci. 2015, 18, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Øines, E.; Murison, R.; Mrdalj, J.; Grønli, J.; Milde, A.M. Neonatal maternal separation in male rats increases intestinal permeability and affects behavior after chronic social stress. Physiol. Behav. 2012, 105, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Ströhle, A.; Schüle, C.; Breuer, A.; Kluge, M.; Müller, M.B. The Diagnosis and Treatment of Anxiety Disorders. Dtsch. Arztebl. Int. 2018, 155, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.P.; Haber, P.S.; Hall, W.D. Alcohol use disorders. Lancet 2016, 387, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Atasoy, S.; Johar, H.; Herder, C.; Peters, A.; Kruse, J.; Ladwig, K.-H. Anxiety boosts progression of prediabetes to type 2 diabetes: Findings from the prospective Cooperative Health Research in the Region of Augsburg F4 and FF4 studies. Diabet. Med. 2020, 37, 1737–1741. [Google Scholar] [CrossRef] [PubMed]
- Naicker, K.; Johnson, J.A.; Skogen, J.C.; Manuel, D.; Øverland, S.; Sivertsen, B.; Colman, I. Type 2 Diabetes and comorbid symptoms of depression and anxiety: Longitudinal associations with mortality risk. Diabetes Care 2017, 40, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Steffen, A.; Nübel, J.; Jacobi, F.; Bätzing, J.; Holstiege, J. Mental and somatic comorbidity of depression: A comprehensive cross-sectional analysis of 202 diagnosis groups using German nationwide ambulatory claims data. BMC Psychiatry 2020, 20, 142. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.E.; Fournier, A.A.; Sisitsky, T.; Pike, C.T.; Kessler, R.C. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J. Clin. Psychiatry 2015, 76, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Josephson, C.B.; Jette, N. Psychiatric comorbidities in epilepsy. Int. Rev. Psychiatry 2017, 29, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, N. Depression and diabetes. Dialogues Clin. Neurosci. 2018, 20, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Martini, L.; Hoffmann, F. Comorbidity of chronic back pain and depression in Germany: Results from the GEDA study, 2009 and 2010. Z. Evid. Fortbild. Qual. Gesundhwes. 2018, 137–138, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Suárez-González, A.; Crutch, S.J.; Franco-Macías, E.; Gil-Néciga, E. Neuropsychiatric symptoms in posterior cortical atrophy and Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2016, 29, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Connors, M.H.; Seeher, K.M.; Crawford, J.; Ames, D.; Woodward, M.; Brodaty, H. The stability of neuropsychiatric subsyndromes in Alzheimer’s disease. Alzheimers Dement. 2018, 14, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.; Lahmann, C.O.R.; Rücker, G.; Bauer, J.; Boeker, M. Anxiety as a risk factor of Alzheimer’s disease and vascular dementia. Br. J. Psychiatry 2018, 213, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.F.; Tan, L.; Wang, H.F.; Jiang, T.; Tan, M.S.; Tan, L.; Xu, W.; Li, J.Q.; Wang, J.; Lai, T.J.; et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J. Affect Disord. 2016, 190, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Asmer, M.S.; Kirkham, J.; Newton, H.; Ismail, Z.; Elbayoumi, H.; Leung, R.H.; Seitz, D.P. Meta-analysis of the prevalence of major depressive disorder among older adults with dementia. J. Clin. Psychiatry 2018, 70, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Guarino, P.D.; Dysken, M.W.; Pallaki, M.; Asthana, S.; Llorente, M.D.; Sano, M. Neuropsychiatric symptoms and caregiver burden in individuals with Alzheimer’s disease: The TEAM-AD VA cooperative study. J. Geriatr. Psychiatry Neurol. 2018, 31, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sanford, A.M. Mild cognitive impairment. Clin. Geriatr. Med. 2017, 33, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Lipnicki, D.M.; Kochan, N.A.; Crawford, J.D.; Thalamuthu, A.; Andrews, G.; Brayne, C.; Matthews, F.E.; Stephan, B.C.; Lipton, R.B.; et al. The prevalence of mild cognitive impairment in diverse geographical and ethnocultural regions: The COSMIC collaboration. PLoS ONE 2015, 10, e0142388. [Google Scholar] [CrossRef] [PubMed]
- Luis, C.A.; Loewenstein, D.A.; Acevedo, A.; Barker, W.W.; Duara, R. Mild cognitive impairment: Directions for future research. Neurology 2003, 61, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Bruscoli, M.; Lovestone, S. Is MCI really just early dementia? A systematic review of conversion studies. Int. Psychogeriatr. 2004, 16, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.; Iulio, F.D.; Varsi, A.E.; Gianni, W.; Sancesario, G.; Caltagirone, C.; Spalletta, G. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: The role of depression and apathy. J. Alzheimers Dis. 2010, 20, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Delrieu, J.; Desmidt, T.; Camus, V.; Sourdet, S.; Boutoleau-Bretonnière, C.; Mullin, E.; Vellas, B.; Payoux, P.; Lebouvier, T.; Alzheimer’s Disease Neuroimaging Initiative. Apathy as a feature of prodromal Alzheimer’s disease: An FDG-PET ADNI study. Int. J. Geriatr. Psychiatry 2015, 30, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.; Kim, S.; Park, Y.H.; Lim, J.S.; Youn, Y.C.; Kim, S.; Jang, J.W.; Alzheimer’s Disease Neuroimaging Initiative. Depressive symptoms are associated with progression to dementia in patients with amyloid-positive mild cognitive impairment. J. Alzheimers Dis. 2017, 58, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Sugarman, M.A.; Alosco, M.L.; Tripodis, Y.; Steinberg, E.G.; Stern, R.A. Neuropsychiatric symptoms and the diagnostic stability of mild cognitive impairment. J. Alzheimers Dis. 2018, 62, 1841–1855. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.R.; Lowery, N.; Ghormley, C.; Combs, D.; Purdie, R.; Neel, J.; Davis, M.; Bornstein, R. Comorbid anxiety corresponds with neuropsychological dysfunction in unipolar depression. Cogn. Neuropsychiatry 2007, 12, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.B.; Mielke, M.M.; Appleby, B.; Oh, E.; Leoutsakos, J.M.; Lyketsos, C.G. Neuropsychiatric symptoms in MCI subtypes: The importance of executive dysfunction. Int. J. Geriatr. Psychiatry 2011, 26, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.; Berger, A.K.; Monastero, R.; Winblad, B.; Bäckman, L.; Fratiglioni, L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology 2007, 68, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Potvin, O.; Forget, H.; Grenier, S.; Préville, M.; Hudon, C. Anxiety, depression, and 1-year incident cognitive impairment in community-dwelling older adults. J. Am. Geriatr. Soc. 2011, 59, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Elbayoumi, H.; Fischer, C.E.; Hogan, D.B.; Millikin, C.P.; Schweizer, T.; Mortby, M.E.; Smith, E.E.; Patten, S.B.; Fiest, K.M. Prevalence of depression in patients with mild cognitive impairment: A systematic review and meta-analysis. JAMA Psychiatry 2017, 74, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.C.; Yiu, K.K.L.; Kwok, T.C.Y.; Wong, S.Y.S.; Tsoi, K.K.F. Depression and antidepressants as potential risk factors in dementia: A systematic review and meta-analysis of 18 longitudinal studies. J. Am. Med. Dir. Assoc. 2019, 20, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Shiri-Feshki, M. Rate of progression of mild cognitive impairment to dementia–meta-analysis of 41 robust inception cohort studies. Acta Psychiatr. Scand. 2009, 119, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.J.; Lu, P.H.; Hua, X.; Lee, S.; Wu, S.; Nguyen, K.; Teng, E.; Leow, A.D.; Jack, C.R., Jr.; Toga, A.W.; et al. Depressive symptoms in mild cognitive impairment predict greater atrophy in Alzheimer’s disease-related regions. Biol. Psychiatry 2012, 71, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Lopez, O.; Jones, B.; Fitzpatrick, A.L.; Breitner, J.; DeKosky, S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA 2002, 288, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; Lam, L.C.; Tam, C.W.; Lui, V.W.; Leung, G.T.; Lee, A.T.; Chan, S.S.; Fung, A.W.; Chiu, H.F.; Chan, W.M. Neuropsychiatric symptoms are associated with increased risks of progression to dementia: A 2-year prospective study of 321 Chinese older persons with mild cognitive impairment. Age Aging 2011, 40, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Coen, R.; Kilroy, D.; Belinski, K.; Bruce, I.; Coakley, D.; Walsh, B.; Cunningham, C.; Lawlor, B.A. Anxiety and behavioural disturbance as markers of prodromal Alzheimer’s disease in patients with mild cognitive impairment. Int. J. Geriatr. Psychiatry 2011, 26, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Rozzini, L.; Chilovi, B.V.; Peli, M.; Conti, M.; Rozzini, R.; Trabucchi, M.; Padovani, A. Anxiety symptoms in mild cognitive impairment. Int. J. Geriatr. Psychiatry 2009, 24, 300–305. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The WHO Special Initiative for Mental Health (2019-2023): Universal Health Coverage for Mental Health. WHO. Available online: https://www.who.int/publications/i/item/special-initiative-for-mental-health-(2019-2023) (accessed on 5 December 2024).
- Pitman, A.; Suleman, S.; Hyde, N.; Hodgkiss, A. Depression and anxiety in patients with cancer. BMJ 2018, 361, k1415. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Holmes, E.A.; Harrison, P.J. Depression and anxiety disorders during the COVID-19 pandemic: Knowns and unknowns. Lancet 2021, 398, 1665–1666. [Google Scholar] [CrossRef] [PubMed]
- Fedotova, J.; Kubatka, P.; Büsselberg, D.; Shleikin, A.G.; Caprnda, M.; Dragasek, J.; Pohanka, M.; Kruzliak, P. Therapeutical strategies for anxiety and anxiety-like disorders using plant-derived natural compounds and plant extracts. Biomed. Pharmacother. 2017, 95, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Kenda, M.; Seliškar, A.; Kreft, S.; Janeš, D.; Štrukelj, B.; Bogataj, B.; Ograjšek, A. Medicinal plants used for anxiety, depression, or stress treatment: An update. Molecules 2022, 27, 6021. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Michaelis, S.; Wedekind, D. Treatment of anxiety disorders. Dialogues Clin. Neurosci. 2017, 19, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Craske, M.G.; Stein, M.B.; Eley, T.C.; Milad, M.R.; Holmes, A.; Rapee, R.M.; Wittchen, H.-U. Anxiety disorders. Nat. Rev. Dis. Primers 2017, 3, 17024. [Google Scholar] [CrossRef] [PubMed]
- File, S.E.; Seth, P. A review of 25 years of the social interaction test. Eur. J. Pharmacol. 2003, 463, 35–53. [Google Scholar] [CrossRef] [PubMed]
- La-Vu, M.; Tobias, B.C.; Schuette, P.J.; Adhikari, A. To approach or avoid: An introductory overview of the study of anxiety using rodent assays. Front. Behav. Neurosci. 2020, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.B.; McCabe, J.T. Measuring anxiety-like behaviors in rodent models of traumatic brain injury. Front. Behav. Neurosci. 2021, 15, 682935. [Google Scholar] [CrossRef] [PubMed]
- Slattery, D.A.; Cryan, J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012, 7, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Melo, F.H.; Venâncio, E.T.; de Sousa, D.P.; de França Fonteles, M.M.; de Vasconcelos, S.M.; Viana, G.S.; Sousa, F.C.F.D. Anxiolytic-like effect of Carvacrol (5-isopropyl-2-methylphenol) in mice: Involvement with GABAergic transmission. Fundam. Clin. Pharmacol. 2010, 24, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Costello, H.; Gould, R.L.; Abrol, E.; Howard, R. Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalized anxiety disorder. BMJ Open 2019, 9, e027925. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, L.; Montano, N.; Tobaldini, E.; Thayer, J.F.; Sgoifo, A. The contagion of social defeat stress: Insights from rodent studies. Neurosci. Biobehav. Rev. 2020, 111, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Imran, M.; Gondal, T.A.; Imran, A.; Shahbaz, M.; Amir, R.M.; Sajid, M.W.; Qaisrani, T.B.; Atif, M.; Hussain, G.; et al. Therapeutic potential of Rosmarinic acid: A comprehensive review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Man, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Slattery, D.A.; Cryan, J.F. The ups and downs of modelling mood disorders in rodents. ILAR J. 2014, 55, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Mirza, F.J.; Amber, S.; Sumera, H.; Hassan, D.; Ahmed, T.; Zahid, S. Rosmarinic acid and ursolic acid alleviate deficits in cognition, synaptic regulation, and adult hippocampal neurogenesis in an Aβ1-42-induced mouse model of Alzheimer’s disease. Phytomedicine 2021, 83, 153490. [Google Scholar] [CrossRef] [PubMed]
- Fonteles, A.A.; de Souza, C.M.; de Sousa Neves, J.C.; Menezes, A.P.; do Carmo, M.R.S.; Fernandes, F.D.; de Araújo, P.R.; de Andrade, G.M. Rosmarinic acid prevents against memory deficits in ischemic mice. Behav. Brain Res. 2016, 297, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Peng, Z.; Lao, N.; Wang, H.; Chen, Y.; Fang, Z.; Hou, W.; Gao, F.; Li, X.; Xiong, L.; et al. Rosmarinic acid ameliorates PTSD-like symptoms in a rat model and promotes cell proliferation in the hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 51, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Elhady, M.A.; Khalaf, A.A.A.; Kamel, M.M.; Noshy, P.A. Carvacrol ameliorates behavioral disturbances and DNA damage in the brain of rats exposed to propiconazole. Neurotoxicology 2019, 70, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Inhibitory effect of carvacrol on lipopolysaccharide-induced memory impairment in rats. Korean J. Physiol. Pharmacol. 2020, 24, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Lataliza, A.A.B.; de Assis, P.M.; da Rocha Laurindo, L.; Gonçalves, E.C.D.; Raposo, N.R.B.; Dutra, R.C. Antidepressant-like effect of rosmarinic acid during LPS-induced neuroinflammatory model: The potential role of cannabinoid receptors/PPAR-γ signaling pathway. Phytother. Res. 2021, 35, 6974–6989. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S.; Guo, H.; Li, Y.; Jiang, Z.; Gu, T.; Su, B.; Hou, W.; Zhong, H.; Cheng, D.; et al. Rosmarinic acid protects rats against post-stroke depression after transient focal cerebral ischemic injury through enhancing antioxidant response. Brain Res. 2021, 1757, 147336. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.E.; Lobo, M.K. The molecular and cellular mechanisms of depression: A focus on reward circuitry. Mol. Psychiatry 2019, 24, 1798–1815. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; El Omri, A.; Han, J.; Isoda, H. Antidepressant-like effects of rosmarinic acid through mitogen-activated protein kinase phosphatase-1 and brain-derived neurotrophic factor modulation. J. Funct. Foods 2015, 14, 758–766. [Google Scholar] [CrossRef]
- Polli, F.S.; Gomes, J.N.; Ferreira, H.S.; Santana, R.C.; Fregoneze, J.B. Inhibition of salt appetite in sodium-depleted rats by carvacrol: Involvement of noradrenergic and serotonergic pathways. Eur. J. Pharmacol. 2019, 854, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Noshy, P.A.; Elhady, M.A.; Khalaf, A.A.A.; Kamel, M.M.; Hassanen, E.I. Ameliorative effect of carvacrol against propiconazole-induced neurobehavioral toxicity in rats. Neurotoxicology 2018, 67, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ćetković, G.S.; Čanadanović-Brunet, J.M.; Djilas, S.M.; Tumbas, V.T.; Markov, S.L.; Cvetković, D.D. Antioxidant Potential, Lipid Peroxidation Inhibition and Antimicrobial Activities of Satureja montana L. subsp. kitaibelii Extracts. Int. J. Mol. Sci. 2007, 8, 1013–1027. [Google Scholar] [CrossRef]
- Vilmosh, N.; Georgieva-Kotetarova, M.; Kandilarov, I.; Zlatanova-Tenisheva, H.; Murdjeva, M.; Kirina, V.; Dimitrova, S.; Katsarova, M.; Denev, P.; Kostadinova, I. Anti-inflammatory and in vitro antioxidant activities of Satureja montana dry extract. Folia Med. 2024, 66, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Šućur, J.; Popović, A.; Petrović, M.; Anačkov, G.T.; Bursic, V.; Kiprovski, B.; Prvulović, D. Alleloppathic effects and insecticidal activity of the aqueous extract of Satureja montana L. J. Serbian Chem. Soc. 2015, 80, 475–484. [Google Scholar] [CrossRef]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Costa, L.G. Chapter 1—Nutraceuticals in Central Nervous System Diseases: Potential Mechanisms of Neuroprotection, 2nd ed.; Gupta, R.C., Lall, R., Srivastava, A., Eds.; Nutraceuticals; Academic Press: Cambridge, MA, USA, 2021; pp. 3–15. ISBN 9780128210383. [Google Scholar] [CrossRef]
- Sadeghian, Z.; Bigdeli, M.R.; Moghadam, F.M.; Jahanfar, M.; Samavat, S. Niosome: A Stable Antioxidant Drug Delivery System and Improvement Effect on Neurological Defects in Animal Model of Stroke. BioNanoSci. 2025, 15, 117. [Google Scholar] [CrossRef]
- Abbasloo, E.; Khaksari, M.; Sanjari, M.; Kobeissy, F.; Thomas, T.C. Carvacrol decreases blood-brain barrier permeability post-diffuse traumatic brain injury in rats. Sci. Rep. 2023, 13, 14546. [Google Scholar] [CrossRef] [PubMed]
- Tadijan, A.; Vlašić, I.; Vlainić, J.; Đikić, D.; Oršolić, N.; Jembrek, M.J. Intracellular Molecular Targets and Signaling Pathways Involved in Antioxidative and Neuroprotective Effects of Cannabinoids in Neurodegenerative Conditions. Antioxidants 2022, 11, 2049. [Google Scholar] [CrossRef] [PubMed]
- Tawab, A.M.A.E.; Shahin, N.N.; AbdelMohsen, M.M. Protective effect of Satureja montana extract on cyclophosphamide-induced testicular injury in rats. Chem. Biol. Interact. 2014, 224, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Mihajilov-Krstev, T.; Radnović, D.; Kitić, D.; Jovanović, V.S.; Mitić, V.; Stojanović-Radić, Z.; Zlatković, B. Chemical composition, antimicrobial, antioxidative and anticholinesterase activity of Satureja montana L. ssp. montana essential oil. Cent. Eur. J. Biol. 2014, 9, 668–677. [Google Scholar] [CrossRef]
- Öztürk, M. Anticholinesterase and antioxidant activities of Savoury (Satureja thymbra L.) with identified major terpenes of the essential oil. Food Chem. 2012, 134, 48–54. [Google Scholar] [CrossRef]
- Kiziltas, H. Determination of LC-HRMS profiling, antioxidant activity, cytotoxic effect and enzyme inhibitory properties of Satureja avromanica using in vitro and in silico methods. Process Biochem. 2022, 116, 157–172. [Google Scholar] [CrossRef]


| Component | Class | Notes | Ref. |
|---|---|---|---|
| Carvacrol | Phenolic component | Phenolic monoterpene | [68,69] |
| Thymol | Phenolic component | Phenolic monoterpene | [26,64,65] |
| Rosmarinic acid | Phenolic component | Phenolic acid | [28,29,41] |
| Caffeic acid | Phenolic component | Phenolic acid | [26,28,70] |
| Chlorogenic acid | Phenolic component | Phenolic acid | [26,28,70] |
| Ellagic acid | Phenolic component | Phenolic acid | [26,28,70] |
| Quercetin | Flavonoid | Flavonol | [41] |
| Quercetin-3-O-α-L-rhamnopyranoside | Flavonoid | Quercetin glycoside | [41] |
| Quercetin-7-O-glucopyranoside | Flavonoid | Quercetin glycoside | [41] |
| Luteolin-7-rhamnoside-4′-O-β-glucopyranoside | Flavonoid | Luteolin derivative | [41] |
| Luteolin-7-O-glucopyranoside | Flavonoid | Luteolin derivative | [41] |
| Rutin | Flavonoid | Quercetin glycoside | [28] |
| Component | Percentage/Presence | Activity | Ref. |
|---|---|---|---|
| Carvacrol | 44.5–45.7% | Antimicrobial, Antioxidant | [64,65] |
| Anxiolytic, Antidepressant | [71,72] | ||
| p-Cymene | 12.6–16.9% | Antimicrobial | [64,65] |
| γ-Terpinene | 8.1–8.7% | Antioxidant | [64,65] |
| Thymol | Up to 81.79% | Antimicrobial, Antioxidant | [64,65] |
| Rosmarinic Acid | Major phenolic compound | Antioxidant, Anti-inflammatory | [28,29,41,71] |
| Anxiolytic, Antidepressant | [71,72,78] | ||
| Caffeic Acid | Present | Antioxidant | [28,70] |
| Chlorogenic Acid | Present | Antioxidant | [28,70] |
| Ellagic Acid | Present | Antioxidant, Anti-inflammatory | [28,70] |
| Quercetin | Present | Antioxidant, Antimicrobial | [41] |
| Luteolin | Present | Antioxidant | [41] |
| Rutin | Present | Antioxidant | [26,28] |
| Mechanism | Description | Ref. |
|---|---|---|
| Antioxidant Activity | Scavenges free radicals and reduces ROS | [42,181] |
| Cytokine Modulation | Decreases pro-inflammatory cytokines (TNF-α, IL-6) | [42] |
| BBB Protection | Reduces brain edema and prevents BBB permeability | [181] |
| MMP-9 Inhibition | Suppresses MMP-9 expression, preserving BBB integrity | [181] |
| Signaling Pathways | Activates PI3K/Akt and MAPK pathways for cell survival | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragomanova, S.; Tancheva, L.; Abarova, S.; Grigorova, V.B.; Gavazova, V.; Stanciu, D.; Tzonev, S.; Prandjev, V.; Kalfin, R. Protective Potential of Satureja montana-Derived Polyphenols in Stress-Related Central Nervous System Disorders, Including Dementia. Curr. Issues Mol. Biol. 2025, 47, 556. https://doi.org/10.3390/cimb47070556
Dragomanova S, Tancheva L, Abarova S, Grigorova VB, Gavazova V, Stanciu D, Tzonev S, Prandjev V, Kalfin R. Protective Potential of Satureja montana-Derived Polyphenols in Stress-Related Central Nervous System Disorders, Including Dementia. Current Issues in Molecular Biology. 2025; 47(7):556. https://doi.org/10.3390/cimb47070556
Chicago/Turabian StyleDragomanova, Stela, Lyubka Tancheva, Silviya Abarova, Valya B. Grigorova, Valentina Gavazova, Dana Stanciu, Svetlin Tzonev, Vladimir Prandjev, and Reni Kalfin. 2025. "Protective Potential of Satureja montana-Derived Polyphenols in Stress-Related Central Nervous System Disorders, Including Dementia" Current Issues in Molecular Biology 47, no. 7: 556. https://doi.org/10.3390/cimb47070556
APA StyleDragomanova, S., Tancheva, L., Abarova, S., Grigorova, V. B., Gavazova, V., Stanciu, D., Tzonev, S., Prandjev, V., & Kalfin, R. (2025). Protective Potential of Satureja montana-Derived Polyphenols in Stress-Related Central Nervous System Disorders, Including Dementia. Current Issues in Molecular Biology, 47(7), 556. https://doi.org/10.3390/cimb47070556