Bioinformatics Analysis and Functional Verification of Phytoene Synthase Gene PjPSY1 of Panax japonicus C. A. Meyer
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Methods
2.2.1. Total RNA Extraction and cDNA Synthesis of Panax japonicus Fruits
2.2.2. Amplification of PjPSY1 Gene
2.2.3. Bioinformatics Analysis of the PjPSY1 Gene
2.2.4. Construction and Transformation of PjPSY1 Plant Expression Vector
Construction of pBI121-PjPSY1 Recombinant Vector
Transformation of Arabidopsis thaliana with pBI121-PjPSY1 Recombinant Vector
Quantitative Fluorescence Analysis and Carotenoid Content of Trans-PjPSY1 Arabidopsis thaliana
3. Results
3.1. Successful Cloning of the PjPSY1 Gene of Panax japonicus
3.2. Biological Information About the PjPSY1 Gene
3.2.1. Physicochemical Properties of PjPSY1
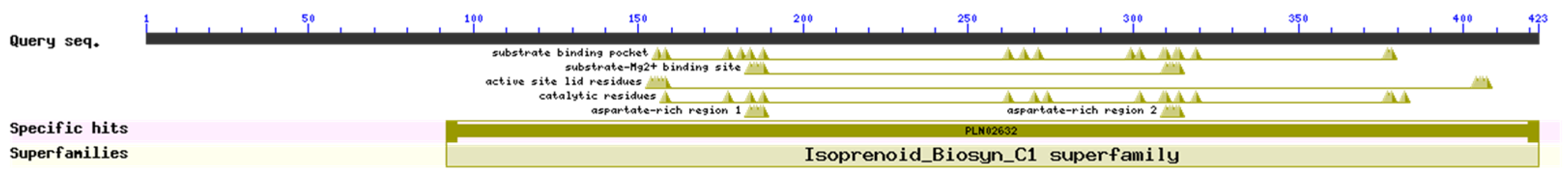
3.2.2. PjPSY1 Conserved Domains
3.2.3. Amino Acid Hydrophilicity, Signal Peptide and Subcellular Localization
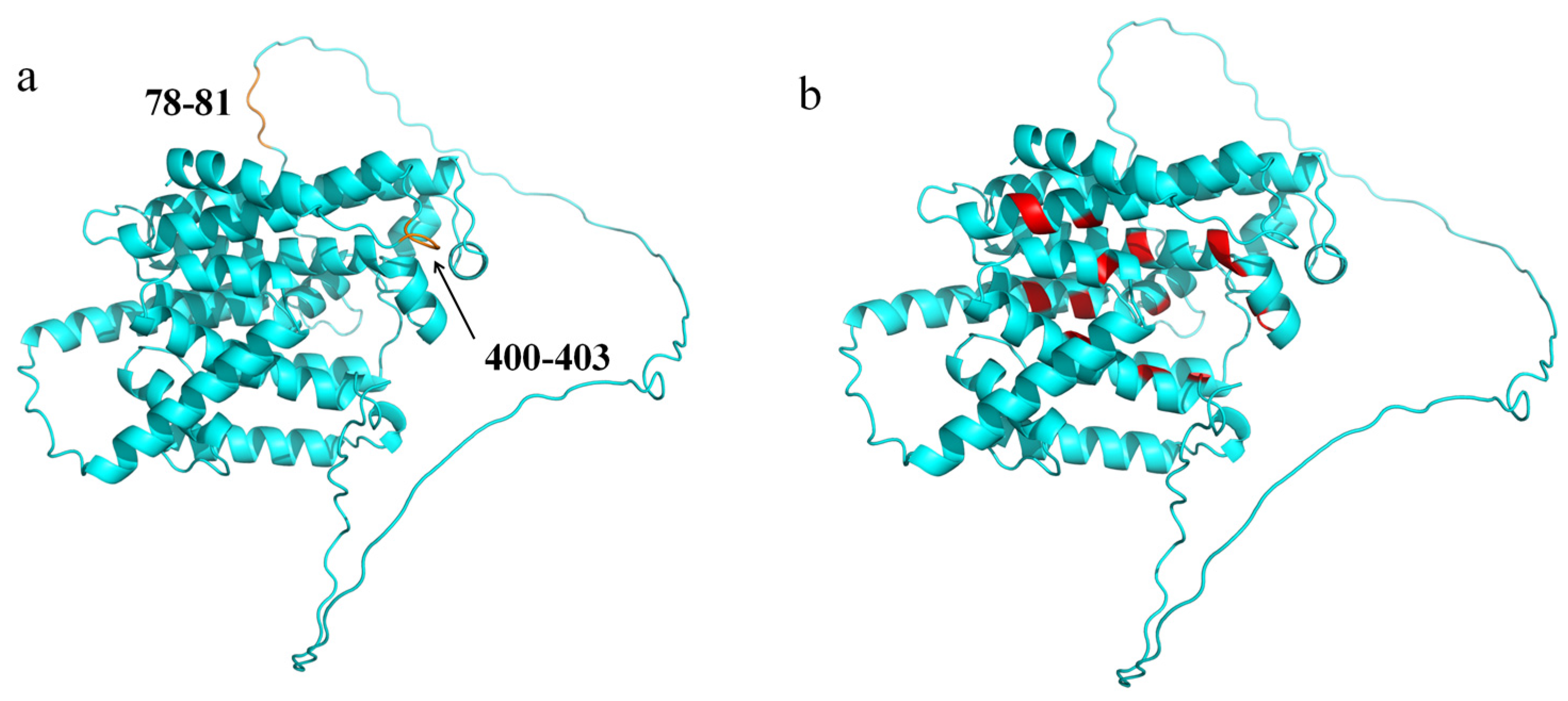
3.2.4. Protein Secondary Structure and Tertiary Structure
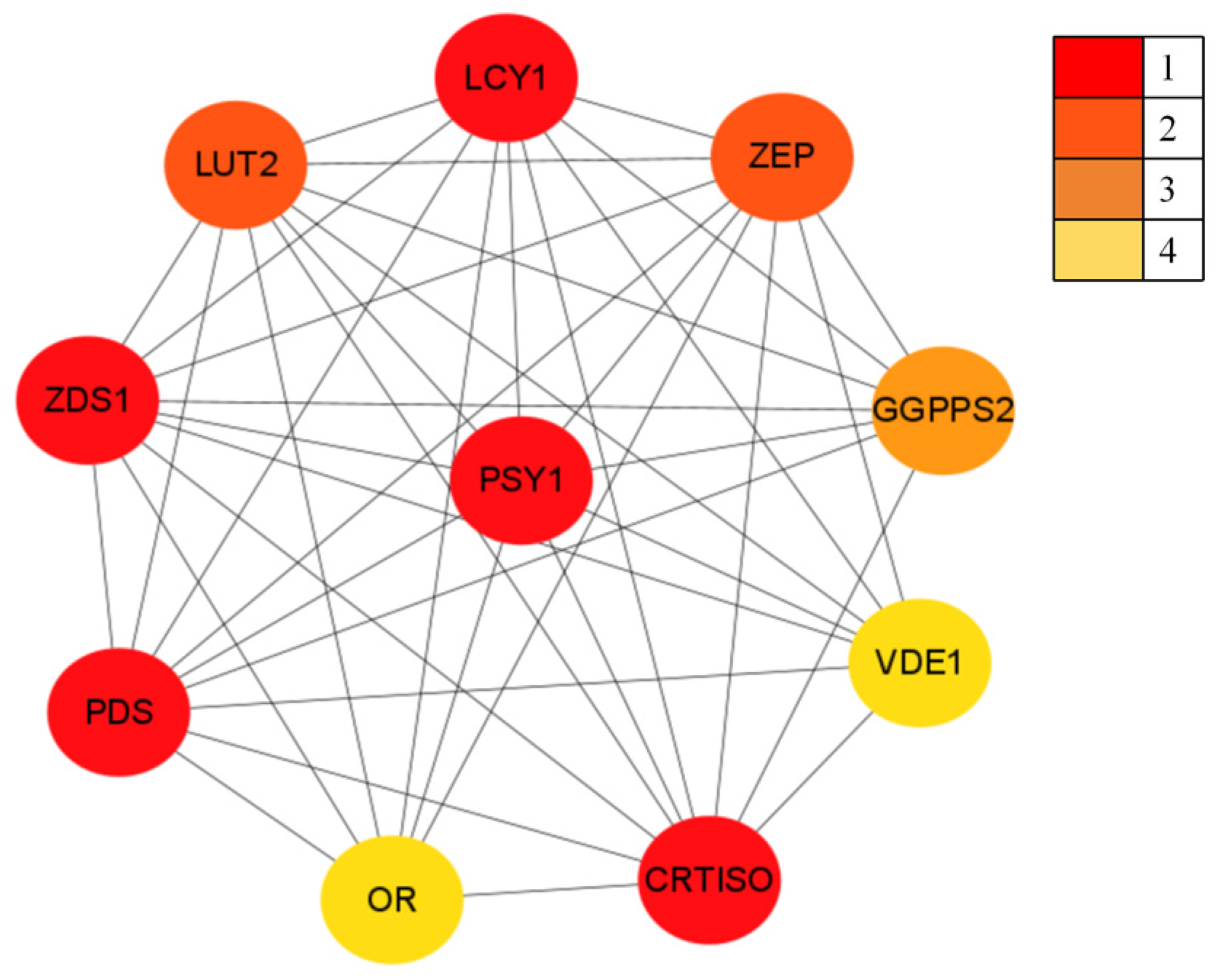
3.2.5. PjPSY1 Interacting Proteins
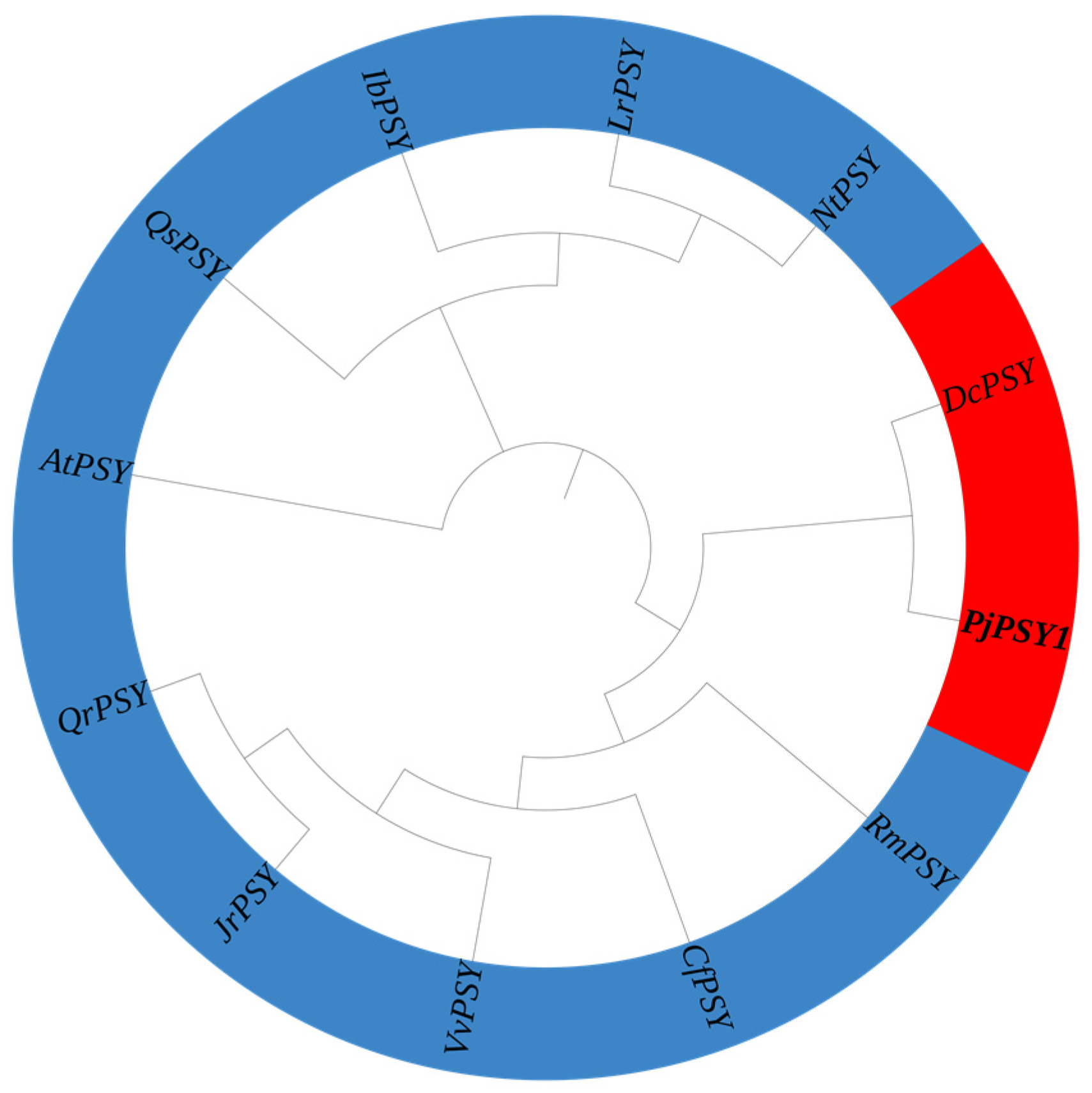
3.2.6. Evolutionary Tree Analyses
3.3. PjPSY1 Gene Function
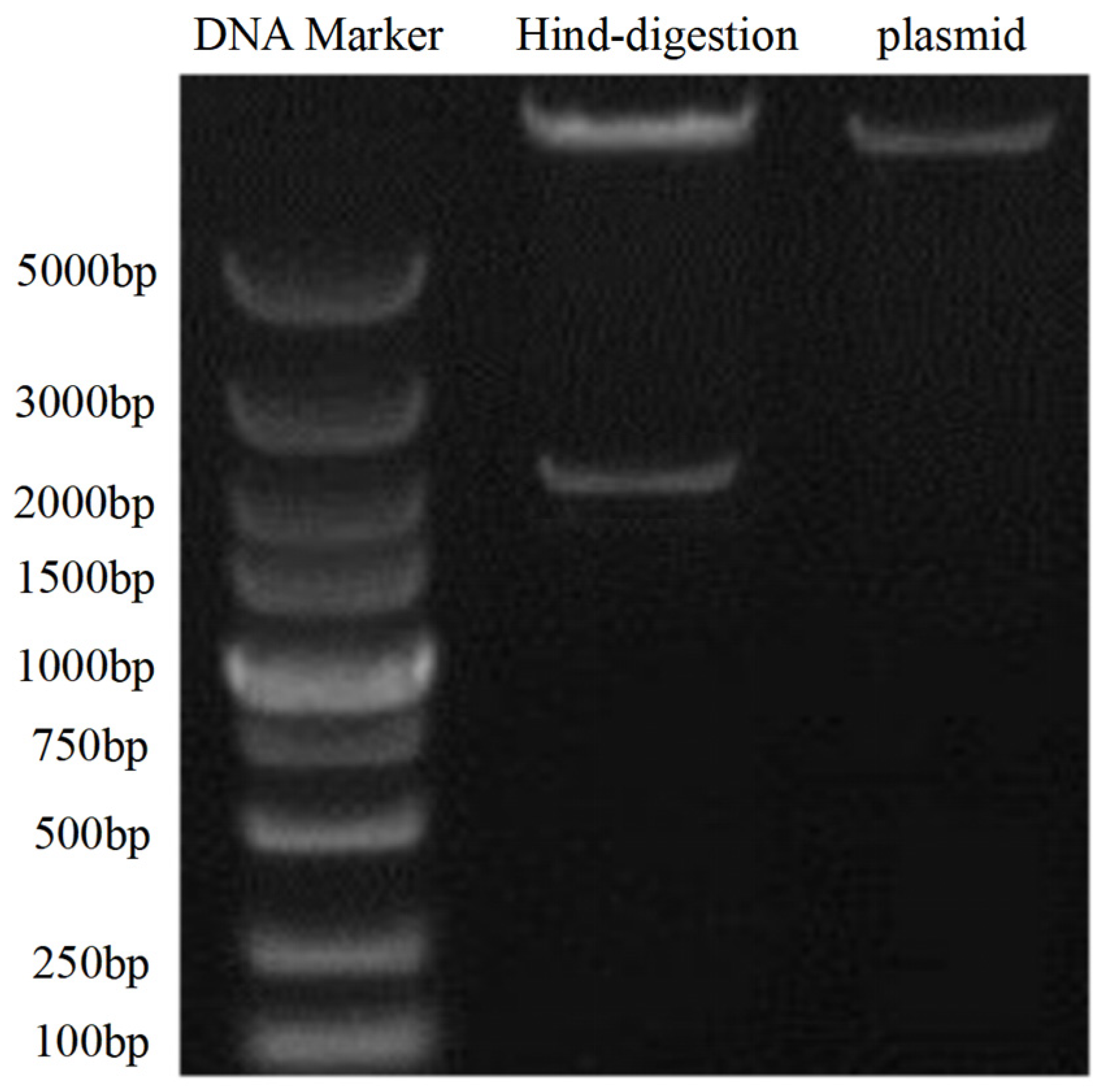
3.3.1. Successful Construction of the pBI121-PjPSY1 Recombinant Vector
3.3.2. Successful Acquisition of Trans-PjPSY1 Arabidopsis thaliana
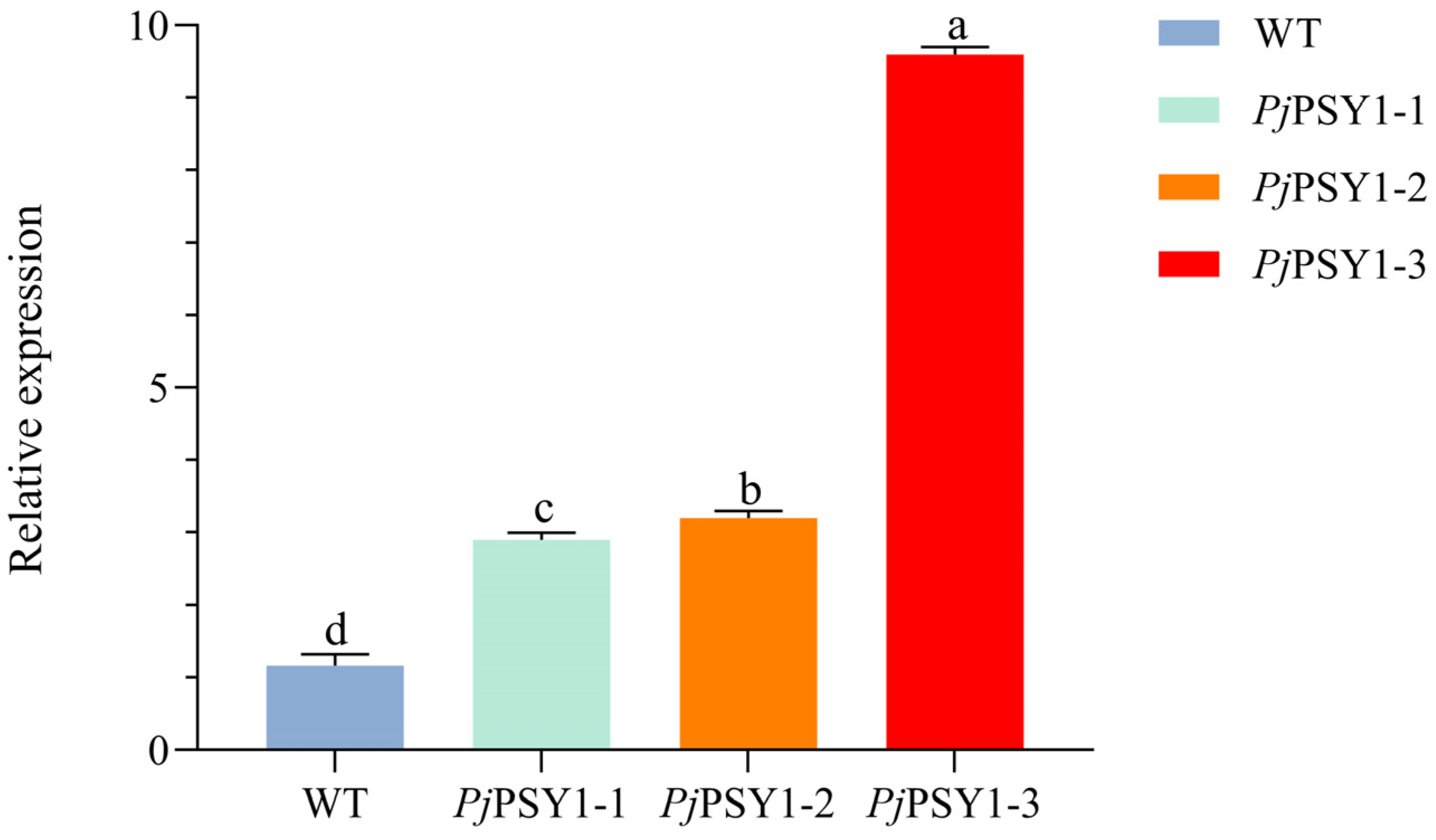
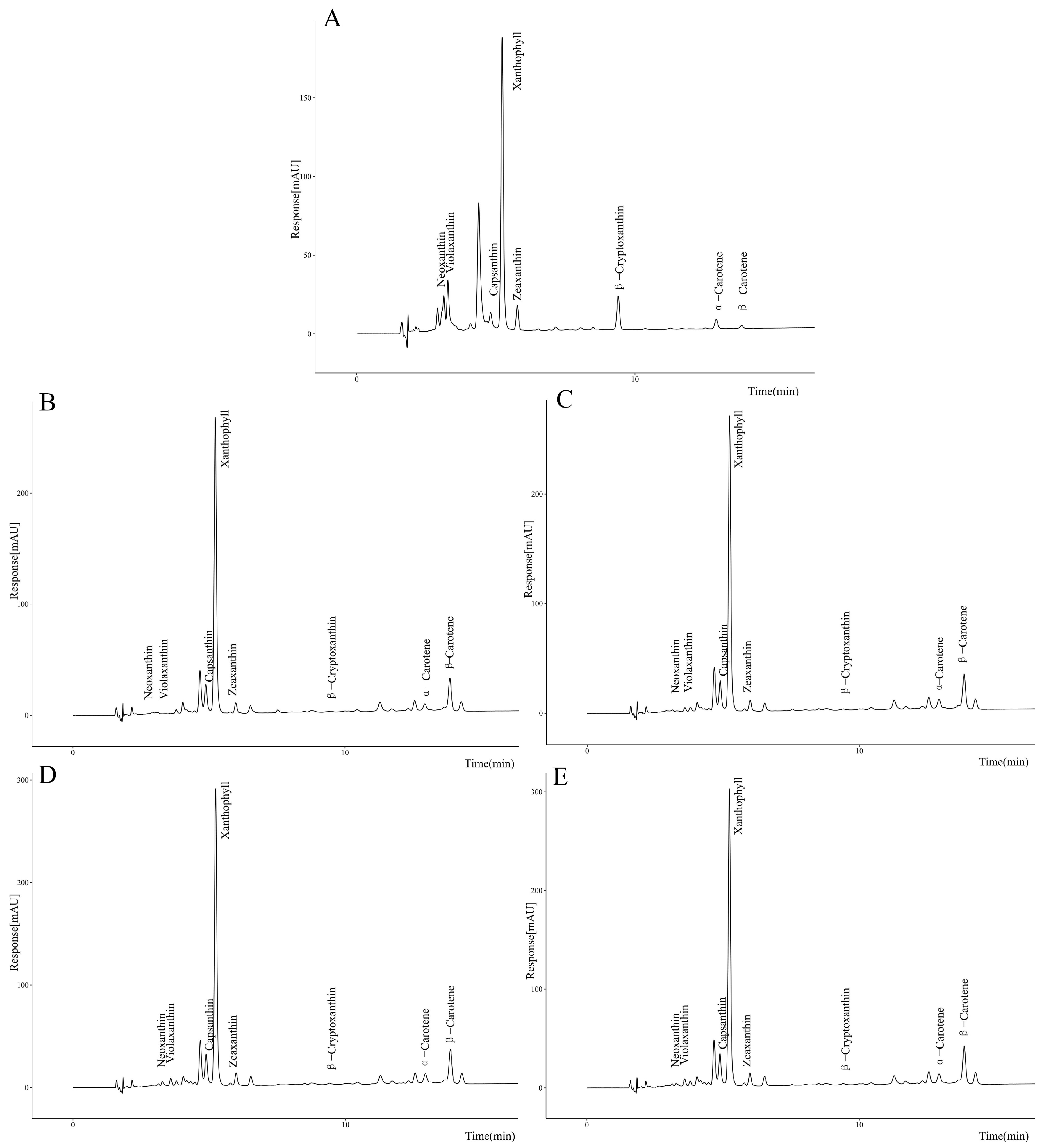
3.3.3. Correlation Between PjPSY1 Expression and Carotenoid Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, X.; Mu, Y.; Wu, F.; Fu, N.; Shi, F.; Zhang, Y. Analysis of codon bias in Panax japonicus transcriptome. Jiangsu Agric. Sci. 2019, 47, 59–63. [Google Scholar]
- Chen, Y.; Rao, B.; Shen, Y.; Cheng, Q.; Zhao, Q. Analysis and Identification of Characteristic Components of Amino Acids and Pmanxsaponins in Panax japonicus C. A. Mey. Chin. J. Chromatogr. 2003, 21, 248–250. [Google Scholar]
- Xu, B.; Chen, P.; Chen, X. Study on extraction process of polysaccharide constituents from Panax japonicus by enzyme method. China J. Chin. Mater. Medica 2008, 33, 1549–1551. [Google Scholar]
- Zhang, Y.T. Study on Extraction and Measurement on Flavonoid Glycosides from Panax japonicus Production in Guizhou. J. Anhui Agric. Sci. 2009, 37, 16371–16372. [Google Scholar]
- Zhang, L.; Yang, B.M. Nutrition Ingredients of Different Organs of Panax japonicus and Their Correlations in Guizhou. Guizhou Agric. Sci. 2006, 34, 34–35. [Google Scholar]
- Xi-lun, H.U.N.; Ting-ting, T.A.G.; Rui, J.I.; Liang, E.; Jing, L.I.; Lai, Z.H.N. Study on the Physiological Mechanism of Fruit Coloration of Panax japonicus. North. Hortic. 2024, 6, 100–107. [Google Scholar]
- Xavier, A.A.O.; Pérez-Gálvez, A. Carotenoids as a Source of Antioxidants in the Diet. Subcell. Biochem. 2016, 79, 359–375. [Google Scholar] [CrossRef]
- Johnson, J.D. Do Carotenoids Serve as Transmembrane Radical Channels? Free Radic. Biol. Med. 2009, 47, 321–323. [Google Scholar] [CrossRef]
- Domonkos, I.; Kis, M.; Gombos, Z.; Ughy, B. Carotenoids, Versatile Components of Oxygenic Photosynthesis. Prog. Lipid. Res. 2013, 52, 539–561. [Google Scholar] [CrossRef]
- Scolnik, P.A.; Bartley, G.E. Nucleotide Sequence of an Arabidopsis cDNA for Phytoene Synthase. Plant Physiol. 1994, 104, 1471–1472. [Google Scholar] [CrossRef]
- Welsch, R.; Wüst, F.; Bär, C.; Al-Babili, S.; Beyer, P. A Third Phytoene Synthase Is Devoted to Abiotic Stress-Induced Abscisic Acid Formation in Rice and Defines Functional Diversification of Phytoene Synthase Genes. Plant Physiol. 2008, 147, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Vallabhaneni, R.; Yu, J.; Rocheford, T.; Wurtzel, E.T. The Maize Phytoene Synthase Gene Family: Overlapping Roles for Carotenogenesis in Endosperm, Photomorphogenesis, and Thermal Stress Tolerance. Plant Physiol. 2008, 147, 1334–1346. [Google Scholar] [CrossRef]
- Bartley, G.E.; Viitanen, P.V.; Bacot, K.O.; Scolnik, P.A. A Tomato Gene Expressed during Fruit Ripening Encodes an Enzyme of the Carotenoid Biosynthesis Pathway. J. Biol. Chem. 1992, 267, 5036–5039. [Google Scholar] [CrossRef] [PubMed]
- Bartley, G.E.; Scolnik, P.A. cDNA Cloning, Expression during Development, and Genome Mapping of PSY2, a Second Tomato Gene Encoding Phytoene Synthase. J. Biol. Chem. 1993, 268, 25718–25721. [Google Scholar] [CrossRef]
- Stauder, R.; Welsch, R.; Camagna, M.; Kohlen, W.; Balcke, G.U.; Tissier, A.; Walter, M.H. Strigolactone Levels in Dicot Roots Are Determined by an Ancestral Symbiosis-Regulated Clade of the PHYTOENE SYNTHASE Gene Family. Front. Plant Sci. 2018, 9, 255. [Google Scholar] [CrossRef]
- Wang, B.; Lin, L.; Chen, M.H.; Liu, J.T.; Ye, X.R.; Zhu, H.S. Analysis of Carotenoids Contents in Pumpkin by Ultra Performance Liquid Chromatography. J. Agric. 2017, 7, 22–27. [Google Scholar]
- Niyogi, K.K. PHOTOPROTECTION REVISITED: Genetic and Molecular Approaches. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1999, 50, 333–359. [Google Scholar] [CrossRef]
- Ampomah-Dwamena, C.; Driedonks, N.; Lewis, D.; Shumskaya, M.; Chen, X.; Wurtzel, E.T.; Espley, R.V.; Allan, A.C. The Phytoene Synthase Gene Family of Apple (Malus × Domestica) and Its Role in Controlling Fruit Carotenoid Content. BMC Plant Biol. 2015, 15, 185. [Google Scholar] [CrossRef]
- Giorio, G.; Stigliani, A.L.; D’Ambrosio, C. Phytoene Synthase Genes in Tomato (Solanumlycopersicum L.)—New Data on the Structures, the Deduced Amino Acid Sequences and the Expression Patterns. FEBS J. 2008, 275, 527–535. [Google Scholar] [CrossRef]
- Salvini, M.; Bernini, A.; Fambrini, M.; Pugliesi, C. Cloning and expression analysis of phytoene synthase gene in Tagetes erecta L. J. Hefei Univ. Technol. 2024, 47, 1547–1552. [Google Scholar]
- Tetali, S.D. Terpenes and Isoprenoids: A Wealth of Compounds for Global Use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Zhao, Y.; Teng, K.; Tan, P.; Liu, Z.; Yang, Z.; Han, L.; Chao, Y. Expression of ZjPSY, a Phytoene Synthase Gene from Zoysia Japonica Affects Plant Height and Photosynthetic Pigment Contents. Plants 2022, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. The Dual Role of Phytoene Synthase Genes in. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2015. [Google Scholar]
- Zheng, B. Molecular Cloning and Functional Analysis of Violaxanthin Deepoxidase Gene from Phyllostachys. Master’s Thesis, China Academy of Forestry, Beijing, China, 2011. [Google Scholar]
- Zhou, X.; Rao, S.; Wrightstone, E.; Sun, T.; Lui, A.C.W.; Welsch, R.; Li, L. Phytoene Synthase: The Key Rate-Limiting Enzyme of Carotenoid Biosynthesis in Plants. Front. Plant Sci. 2022, 13, 884720. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R. Abscisic Acid Synthesis and Response. Arabidopsis. Book 2013, 11, e0166. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, T.; Jin, R.; Huang, X.; Liang, E.; Zhang, L. Bioinformatics Analysis and Functional Verification of Phytoene Synthase Gene PjPSY1 of Panax japonicus C. A. Meyer. Curr. Issues Mol. Biol. 2025, 47, 551. https://doi.org/10.3390/cimb47070551
Tang T, Jin R, Huang X, Liang E, Zhang L. Bioinformatics Analysis and Functional Verification of Phytoene Synthase Gene PjPSY1 of Panax japonicus C. A. Meyer. Current Issues in Molecular Biology. 2025; 47(7):551. https://doi.org/10.3390/cimb47070551
Chicago/Turabian StyleTang, Tingting, Rui Jin, Xilun Huang, E Liang, and Lai Zhang. 2025. "Bioinformatics Analysis and Functional Verification of Phytoene Synthase Gene PjPSY1 of Panax japonicus C. A. Meyer" Current Issues in Molecular Biology 47, no. 7: 551. https://doi.org/10.3390/cimb47070551
APA StyleTang, T., Jin, R., Huang, X., Liang, E., & Zhang, L. (2025). Bioinformatics Analysis and Functional Verification of Phytoene Synthase Gene PjPSY1 of Panax japonicus C. A. Meyer. Current Issues in Molecular Biology, 47(7), 551. https://doi.org/10.3390/cimb47070551




