Antioxidant Activity of Radix Cyathula officinalis Kuan Polysaccharides and Their Modulatory Effects on the Gut Microbiota of Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Preparation of Polysaccharides
2.4. Component Content Analysis
2.5. In Vitro Antioxidant Assay
2.6. Cultivation and Synchronization of C. elegans
2.7. In Vivo Antioxidant Assay in C. elegans
2.7.1. Lifespan and Thermal Stress Assay
2.7.2. Reproductive Assay
2.7.3. ROS Detection
2.7.4. Antioxidant Enzyme Activity Measurement
2.7.5. Gut Microbiota Analysis
2.8. Statistical Analysis
3. Results
3.1. Component Composition and In Vitro Antioxidant Activity of Crude RCP
3.1.1. Component Composition of Crude RCP
3.1.2. In Vitro Antioxidant Activity of Crude RCP
3.1.3. Principal Component Analysis (PCA)
3.1.4. Component Composition and Fractionation of RCP
3.2. In Vivo Antioxidant Activities of RCP in C. elegans
3.2.1. Effects on Lifespan and Reproduction
3.2.2. Effects of rRCP and wRCP on Antioxidant Enzyme Activities in C. elegans
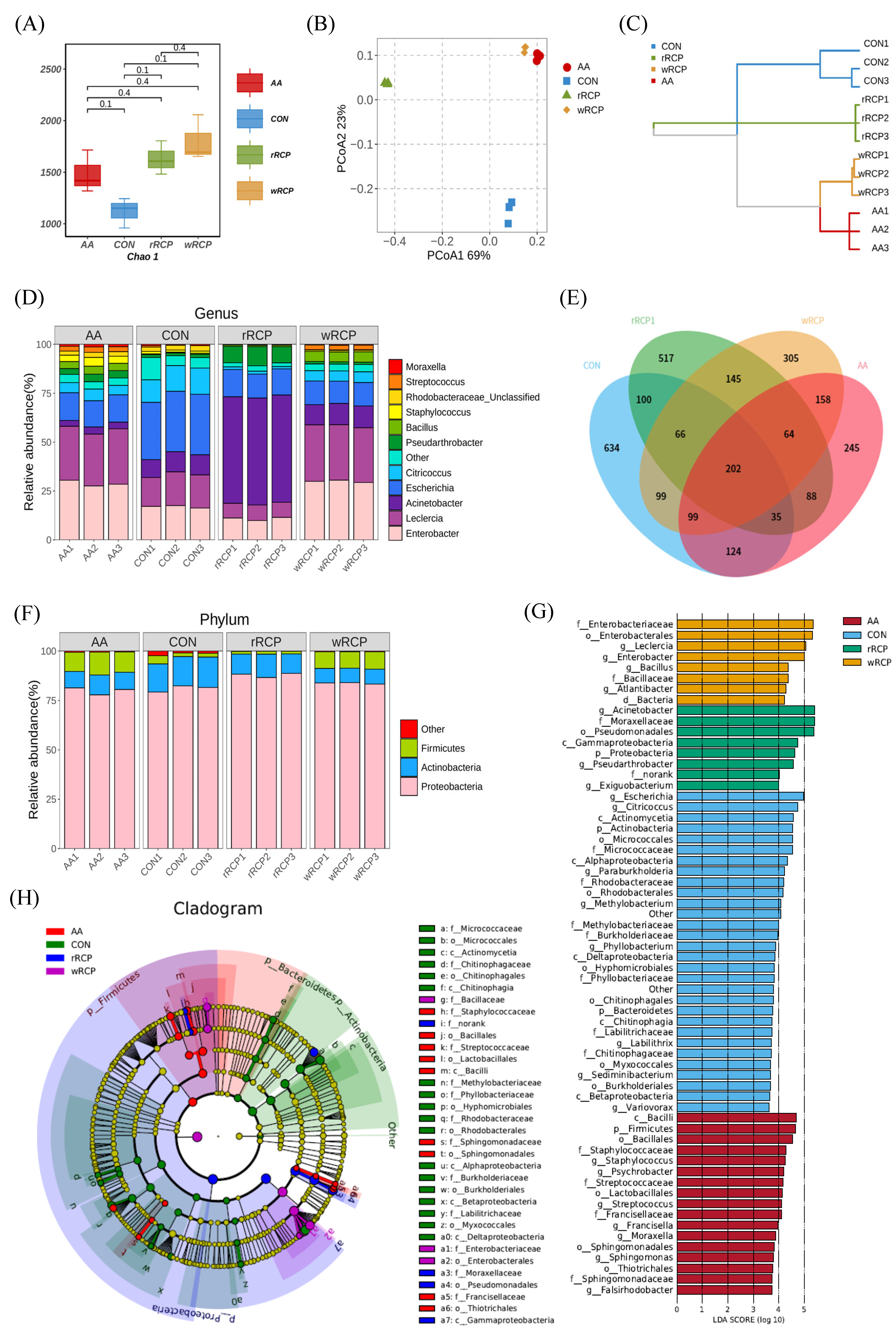
3.3. Effect of RCP on Gut Microbiota of C. elegans
3.3.1. Effect of RCP on Microbial Diversity
3.3.2. Effect of RCP on Gut Microbiota Composition and Structure
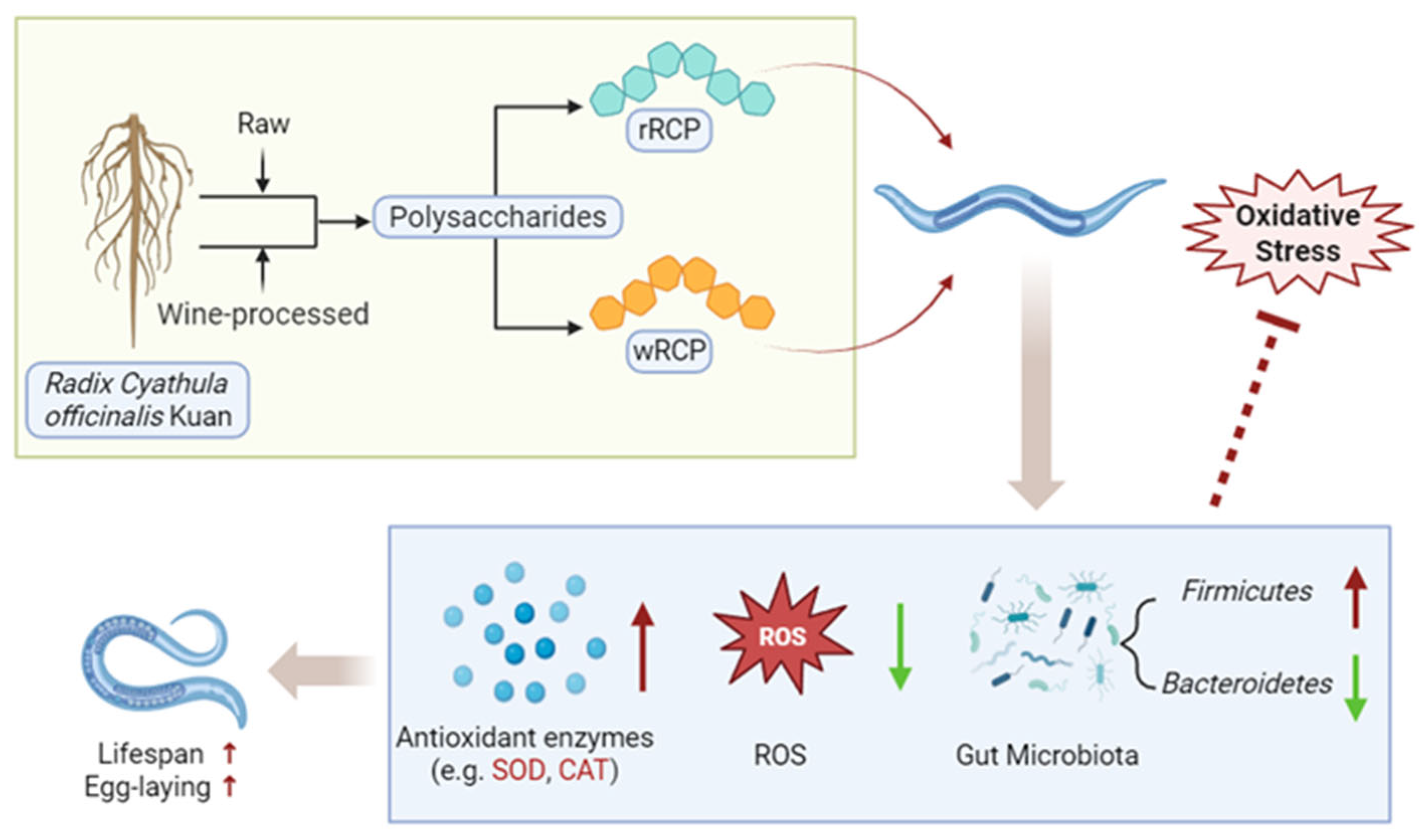
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ratan, R.R. Oxidative Stress and Huntington’s Disease: The Good, The Bad, and The Ugly. J. Huntingt. Dis. 2016, 5, 217–237. [Google Scholar] [CrossRef]
- Shetty, A.K.; Kodali, M.; Upadhya, R.; Madhu, L.N. Emerging Anti-Aging Strategies—Scientific Basis and Efficacy. Aging Dis. 2018, 9, 1165–1184. [Google Scholar] [CrossRef]
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential applications of antioxidants—A review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Zhou, H.; Dai, C.; Cui, X.; Zhang, T.; Che, Y.; Duan, K.; Yi, L.; Nguyen, A.D.; Li, N.; De Souza, C.; et al. Immunomodulatory and antioxidant effects of Glycyrrhiza uralensis polysaccharide in Lohmann Brown chickens. Front. Vet. Sci. 2022, 9, 959449. [Google Scholar] [CrossRef]
- Guan, T.; Wei, X.; Xu, P.; Chen, K.; Zou, Y.; Chen, M.; Zhu, Z. Comparison of structural and antioxidant activity of polysaccharide extracted from truffles. J. Food Sci. 2022, 87, 2999–3012. [Google Scholar] [CrossRef]
- Imre, B.; García, L.; Puglia, D.; Vilaplana, F. Reactive compatibilization of plant polysaccharides and biobased polymers: Review on current strategies, expectations and reality. Carbohydr. Polym. 2019, 209, 20–37. [Google Scholar] [CrossRef]
- Li, H.; Ma, F.; Hu, M.; Ma, C.W.; Xiao, L.; Zhang, J.; Xiang, Y.; Huang, Z. Polysaccharides from medicinal herbs as potential therapeutics for aging and age-related neurodegeneration. Rejuvenation Res. 2014, 17, 201–204. [Google Scholar] [CrossRef]
- Luan, F.; Ji, Y.; Peng, L.; Liu, Q.; Cao, H.; Yang, Y.; He, X.; Zeng, N. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Codonopsis pilosula: A review. Carbohydr. Polym. 2021, 261, 117863. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Yang, W.; Huang, G. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.K.; Shen, Y.; Fan, L.; Zhang, Z.R.; Zhao, Y.Q.; You, L.C.; Zhang, Y.; Zhang, Q.; Su, Z.T.; Cao, S.Z. Effects of Pueraria Polysaccharides on Serum Antioxidant and Immune Indexes and Gut Microbiota of Calves. Chin. J. Anim. Nutr. 2023, 35, 1696–1704. [Google Scholar]
- Chen, Q.Y.; Wang, Y.; Yin, N.; Wang, R.F.; Zheng, Y.; Yang, Y.P.; An, X.P.; Qi, J.W. Polysaccharides from fermented wheat bran enhanced the growth performance of zebrafish (Danio rerio) through improving gut microflora and antioxidant status. Aquac. Rep. 2022, 25, 101188. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, X.; Wu, D.; Ouyang, Y.; Gao, L.; Chen, Z.; El-Seedi, H.R.; Wang, M.F.; Chen, X.; Zhao, C. Characterization of the structure and analysis of the anti-oxidant effect of microalga Spirulina platensis polysaccharide on Caenorhabditis elegans mediated by modulating microRNAs and gut microbiota. Int. J. Biol. Macromol. 2020, 163, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- Li, R.P.; Liu, C.G.; Zhang, S.; Dong, L.Y.; Wang, Q.X.; Xu, S.X.; Li, G.Q.; Tian, Y.; Chai, Y.Q.; Liu, S.Z. Effects of Cordyceps cicadae polysaccharide on growth performance, antioxidant capacity and intestinal microbial diversity of broiler chickens. Chin. J. Vet. Sci. 2021, 41, 1118–1126. [Google Scholar] [CrossRef]
- Cui, J.; Fu, C.N.; li, L.A.; Ma, J.F.; Qing, S.Y. Effects of brown algae polysaccharide on growth, antioxidantcapacity, intestinal flora and cytokines in piglets. Anim. Husb. Vet. Med. 2019, 51, 66–69. [Google Scholar]
- National Pharmacopoeia Commission. Cyathulae Radix. In Pharmacopoeia of The People’s Republic of China, 2020 ed.; Kuang, L.J., Li, C.X., Cao, S.L., Li, H.R., Wang, Z., Wu, S.S., Fan, Y., Eds.; Chinese Medicine Science Press: Beijing, China, 2020; Volume 1, pp. 39–40. [Google Scholar]
- Feng, H.; Du, X.; Liu, J.; Han, X.; Cao, X.; Zeng, X. Novel polysaccharide from Radix Cyathulae officinalis Kuan can improve immune response to ovalbumin in mice. Int. J. Biol. Macromol. 2014, 65, 121–128. [Google Scholar] [CrossRef]
- Han, X.; Shen, S.; Liu, T.; Du, X.; Cao, X.; Feng, H.; Zeng, X. Characterization and antioxidant activities of the polysaccharides from Radix Cyathulae officinalis Kuan. Int. J. Biol. Macromol. 2015, 72, 544–552. [Google Scholar] [CrossRef]
- Yao, Z.; Gan, F.; Zeng, Y.; Ren, L.; Zeng, Y. Elucidating Cyathula Officinals’ mechanism in osteoarthritis treatment: Network pharmacology and empirical evidence on anti-inflammatory actions. Heliyon 2024, 10, e27999. [Google Scholar] [CrossRef]
- Chen, X.M.; Tian, G.Y. Structural elucidation and antitumor activity of a fructan from Cyathula officinalis Kuan. Carbohydr. Res. 2003, 338, 1235–1241. [Google Scholar] [CrossRef]
- Li, J.L.; Han, X.F.; Liu, T.Q.; Zeng, X.Y. Effects of polysaccharides from the roots of Radix Cyathulae officinalis Kuan on in vivo antioxidant capacities of D-galactose-induced aging mouse model. Chin. J. Antibiot. 2014, 39, 553–559. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Zheng, J.; Park, Y. Caenorhabditis elegans: A Convenient In Vivo Model for Assessing the Impact of Food Bioactive Compounds on Obesity, Aging, and Alzheimer’s Disease. Annu. Rev. Food Sci. Technol. 2018, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.R. Study on Quality Evaluation and Anti-Inflammatory and Antioxidantactivity of Saposhnikovia divaricata from Different Origins. Master’s Thesis, Jilin Agricultural University, Changchun, 2024. [Google Scholar]
- Tong, K.; Li, Z.L.; Yan, S.; Deng, M.S.; Tang, Y.B.; Jiang, M.J.; Yang, J.H.; Tian, M.L. HPLC fingerprint of crude Cyathula officinalis and its processed products stir-fried with wine and salt and variation of their index components. Chin. Tradit. Herbal. Drugs 2016, 47, 580–584. [Google Scholar]
- National Pharmacopoeia Commission. General rules for the processing. In Pharmacopoeia of The People’s Republic of China, 2020 ed.; Cai, H., Yu, H.P., Wang, L.F., Liu, X.Q., Eds.; Chinese Medicine Science Press: Beijing, China, 2015; Volume 4, p. 31. [Google Scholar]
- Qiu, X.; Wang, Q.; Wang, R.; Xian, B.; Wu, Q.H.; Pei, J. Extraction of polysaccharide from Chuanniuxi and analysis of monosaccharide. Pharm. Clin. Chin. Mater. Medica 2020, 11, 6–9+13. [Google Scholar]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Feng, X. Preparation of Polysaccharides from Polygonatirhizoma and its Effects on Intestine and Lung Inaged Mice. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2020. [Google Scholar]
- Wu, Q.W.; Li, D.H.; Song, Q.J.; Li, G.F.; Li, X.W.; Yang, X.R.; Li, Y.F. Comparative study on chemical composition and in vitro anti-oxidant activity ofAstragali Radix fresh-cut pieces and traditional pieces. Chin. Tradit. Herbal. Drugs 2022, 53, 7039–7047. [Google Scholar]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef]
- Panahi, Y.; Rajaee, S.M.; Johnston, T.P.; Sahebkar, A. Neuroprotective effects of antioxidants in the management of neurodegenerative disorders: A literature review. J. Cell Biochem. 2019, 120, 2742–2748. [Google Scholar] [CrossRef]
- Essa, M.M.; Moghadas, M.; Ba-Omar, T.; Walid Qoronfleh, M.; Guillemin, G.J.; Manivasagam, T.; Justin-Thenmozhi, A.; Ray, B.; Bhat, A.; Chidambaram, S.B.; et al. Protective Effects of Antioxidants in Huntington’s Disease: An Extensive Review. Neurotox. Res. 2019, 35, 739–774. [Google Scholar] [CrossRef]
- Zou, Y.F.; JiZe, X.P.; Li, C.Y.; Zhang, C.W.; Fu, Y.P.; Yin, Z.Q.; Li, Y.P.; Song, X.; Li, L.X.; Zhao, X.H.; et al. Polysaccharide from aerial part of Chuanminshen violaceum alleviates oxidative stress and inflammatory response in aging mice through modulating intestinal microbiota. Front. Immunol. 2023, 14, 1159291. [Google Scholar] [CrossRef]
- Zeng, B.; Yan, Y.; Zhang, Y.; Wang, C.; Huang, W.; Zhong, X.; Chen, Z.; Xie, M.; Yang, Z. Dendrobium officinale Polysaccharide (DOP) inhibits cell hyperproliferation, inflammation and oxidative stress to improve keratinocyte psoriasis-like state. Adv. Med. Sci. 2024, 69, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Pu, Q.; Qiu, H.; Di, D.; Cao, Y. A polysaccharide from Lycium barbarum L.: Structure and protective effects against oxidative stress and high-glucose-induced apoptosis in ARPE-19 cells. Int. J. Biol. Macromol. 2022, 201, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Q.; Hao, J.W.; Wang, J.Z. Investigating the Extraction Process of Radix cyathulae Polysaccharides by the Single Factor Method. For. By-Product. Spec. China 2019, 4, 17–20. [Google Scholar] [CrossRef]
- Ye, X.J.; Huang, Y.X.; Li, S.F. Study on Extraction of Cyathulae Radix Polysaccharides by Compound Enzymes Method. China J. Pharm. Econ. 2018, 13, 24–26. [Google Scholar]
- Wang, Y.Y.; Zhang, M.F.; Zha, L.L.; Guo, Y. Research on the Extraction Process of Polysaccharides in Cyathulae Radix and Achyranthis Bidentatae Radix. J. Chang. Norm. Univ. 2016, 35, 77–81. [Google Scholar]
- Lai, X. Preliminary Screening of Potential Quality Markers (Q-Marker) of Cyathula officinalis Kuan. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2019. [Google Scholar]
- Chen, A.M.; Pan, J.; Bo, H.U.; Chen, J.W.; Wei, W.U. Determination of antioxidant activities of polysaccharides from Cyathulae Radix and optimization of microwave extraction. Chin. Tradit. Pat. Med. 2017, 39, 80–84. [Google Scholar]
- Hromádková, Z.; Paulsen, B.S.; Polovka, M.; Košťálová, Z.; Ebringerová, A. Structural features of two heteroxylan polysaccharide fractions from wheat bran with anti-complementary and antioxidant activities. Carbohydr. Polym. 2013, 93, 22–30. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Wang, M.; Ma, L.; Ye, H.; Zeng, X. A comparative study of the neutral and acidic polysaccharides from Allium macrostemon Bunge. Carbohydr. Polym. 2015, 117, 980–987. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, S.; Shen, S.; Wang, H.; Yuan, M.; Liu, J.; Huang, Y.; Ding, C. The antioxidant activities effect of neutral and acidic polysaccharides from Epimedium acuminatum Franch. on Caenorhabditis elegans. Carbohydr. Polym. 2016, 144, 122–130. [Google Scholar] [CrossRef]
- Fan, J.; Wang, Y.; Yang, J.; Gu, D.; Kang, S.; Liu, Y.; Jin, H.; Wei, F.; Ma, S. Anti-aging activities of neutral and acidic polysaccharides from Polygonum multiflorum Thunb in Caenorhabditis elegans. Int. J. Biol. Macromol. 2024, 257, 128724. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Wang, P.; Yin, C. Pharmacodynamic evaluation and mechanism study of Corni Fructus polysaccharide on anti-aging of Caenorhabditis elegans. J. Food Saf. Qual. 2022, 13, 6996–7003. [Google Scholar] [CrossRef]
- Zheng, J.; Gao, C.; Wang, M.; Tran, P.; Mai, N.; Finley, J.W.; Heymsfield, S.B.; Greenway, F.L.; Li, Z.; Heber, D.; et al. Lower Doses of Fructose Extend Lifespan in Caenorhabditis elegans. J. Diet. Suppl. 2017, 14, 264–277. [Google Scholar] [CrossRef][Green Version]
- Rathor, L.; Akhoon, B.A.; Pandey, S.; Srivastava, S.; Pandey, R. Folic acid supplementation at lower doses increases oxidative stress resistance and longevity in Caenorhabditis elegans. Age 2015, 37, 113. [Google Scholar] [CrossRef]
- Peng, Y.; Dai, S.; Lu, Y.; Xiong, L.; Huang, J.; Liu, Z.; Gong, Y. Theanine Improves High-Dose Epigallocatechin-3-Gallate-Induced Lifespan Reduction in Caenorhabditis elegans. Foods 2021, 10, 1404. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Tan, M.; Chang, S.; Zhu, Y.; Rong, G.; Wei, G.; Zhang, J.; Zhao, B.; Zhao, Q.S. Effects of different processing (Paozhi) on structural characterization and antioxidant activities of polysaccharides from Cistanche deserticola. Int. J. Biol. Macromol. 2023, 245, 125507. [Google Scholar] [CrossRef]
- Fang, Q.; Hu, J.; Nie, Q.; Nie, S. Effects of polysaccharides on glycometabolism based on gut microbiota alteration. Trends Food Sci. Technol. 2019, 92, 65–70. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, Q.M.; Zha, X.Q.; Luo, J.P. Dendrobium fimbriatum Hook polysaccharide ameliorates dextran-sodium-sulfate-induced colitis in mice via improving intestinal barrier function, modulating intestinal microbiota, and reducing oxidative stress and inflammatory responses. Food Funct. 2022, 13, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Li, X.; Liu, D.; Gao, X.; Chen, Y.; Chen, Z.; Fu, C.; Lin, L.; Liu, B.; Zhao, C. Physicochemical characterization and antioxidant effects of green microalga Chlorella pyrenoidosa polysaccharide by regulation of microRNAs and gut microbiota in Caenorhabditis elegans. Int. J. Biol. Macromol. 2021, 168, 152–162. [Google Scholar] [CrossRef]
- Song, W.; Wang, Y.; Li, G.; Xue, S.; Zhang, G.; Dang, Y.; Wang, H. Modulating the gut microbiota is involved in the effect of low-molecular-weight Glycyrrhiza polysaccharide on immune function. Gut Microbes 2023, 15, 2276814. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, X.; Yuan, S.; Wang, Y.; Liao, X.; Zhong, M.; He, Q.; Shen, H.; Liao, W.; Shen, J. Flammulina velutipes polysaccharide improves C57BL/6 mice gut health through regulation of intestine microbial metabolic activity. Int. J. Biol. Macromol. 2021, 167, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Fábrega, M.J.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Algieri, F.; Badía, J.; Giménez, R.; Gálvez, J.; Baldomà, L. Intestinal Anti-inflammatory Effects of Outer Membrane Vesicles from Escherichia coli Nissle 1917 in DSS-Experimental Colitis in Mice. Front. Microbiol. 2017, 8, 1274. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Nicholson, S.E.; Watts, L.T.; Burmeister, D.M.; Merrill, D.; Scroggins, S.; Zou, Y.; Lai, Z.; Grandhi, R.; Lewis, A.M.; Newton, L.M.; et al. Moderate Traumatic Brain Injury Alters the Gastrointestinal Microbiome in a Time-Dependent Manner. Shock 2019, 52, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Maynard, C.; Weinkove, D. The Gut Microbiota and Ageing. In Biochemistry and Cell Biology of Ageing: Part I Biomedical Science; Harris, J.R., Korolchuk, V.I., Eds.; Springer: Singapore, 2018; pp. 351–371. [Google Scholar]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4586–4591. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Zhou, Q.; Gao, J.; Sun, X.; Liang, Y.; Ye, M.; Liang, D.; Ling, C.; Fang, B. In Vitro Characterization of Polysaccharides from Fresh Tea Leaves in Simulated Gastrointestinal Digestion and Gut Microbiome Fermentation. Foods 2024, 13, 1561. [Google Scholar] [CrossRef]
- Zhao, J.; He, R.; Zhong, H.; Liu, S.; Liu, X.; Hussain, M.; Sun, P. A cold-water extracted polysaccharide-protein complex from Grifola frondosa exhibited anti-tumor activity via TLR4-NF-κB signaling activation and gut microbiota modification in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2023, 239, 124291. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Ding, J.; Shen, T.; Liang, Y.; Wei, F.; Wu, Y.; Iqbal, M.; Kulyar, M.F.; Li, K.; Wei, K. Radix paeoniae alba polysaccharide attenuates lipopolysaccharide-induced intestinal injury by regulating gut microbiota. Front. Microbiol. 2022, 13, 1064657. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, M.; Li, X.; Han, Y.; Wu, F.; Liu, Y. The effects of feeding Lactobacillus pentosus on growth, immunity, and disease resistance in Haliotis discus hannai Ino. Fish. Shellfish. Immunol. 2018, 78, 42–51. [Google Scholar] [CrossRef]
- Veisi, R.S.; Taghdir, M.; Abbaszadeh, S.; Hedayati, A. Dietary Effects of Probiotic Lactobacillus casei on Some Immunity Indices of Common Carp (Cyprinus carpio) Exposed to Cadmium. Biol. Trace Elem. Res. 2023, 201, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Ye, J.Z.; Lv, L.X.; Xu, H.; Yang, L.Y.; Jiang, X.W.; Wu, W.R.; Shi, D.; Fang, D.Q.; Bian, X.Y.; et al. Pretreatment With Bacillus cereus Preserves Against D-Galactosamine-Induced Liver Injury in a Rat Model. Front. Microbiol. 2019, 10, 1751. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Xu, S.; Yang, J.; Wang, K.; Zhan, X. Bacillus subtilis DSM29784 Alleviates Negative Effects on Growth Performance in Broilers by Improving the Intestinal Health Under Necrotic Enteritis Challenge. Front. Microbiol. 2021, 12, 723187. [Google Scholar] [CrossRef]
- Palkovicsné Pézsa, N.; Kovács, D.; Rácz, B.; Farkas, O. Effects of Bacillus licheniformis and Bacillus subtilis on Gut Barrier Function, Proinflammatory Response, ROS Production and Pathogen Inhibition Properties in IPEC-J2-Escherichia coli/Salmonella Typhimurium Co-Culture. Microorganisms 2022, 10, 936. [Google Scholar] [CrossRef]
- Mols, M.; Abee, T. Primary and secondary oxidative stress in Bacillus. Environ. Microbiol. 2011, 13, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Chen, Y.; Yang, J.; Wen, X. Characterization and comparison of polysaccharides from Achyranthes bidentata, Cyathula officinalis and Achyranthes aspera by saccharides mapping. J. Pharm. Biomed. Anal. 2023, 227, 115272. [Google Scholar] [CrossRef]
- Wang, C.; Hua, D.; Yan, C. Structural characterization and antioxidant activities of a novel fructan from Achyranthes bidentata Blume, a famous medicinal plant in China. Ind. Crops Prod. 2015, 70, 427–434. [Google Scholar] [CrossRef]
- Chen, X.-M.; Tian, G.-Y. Structural Features of Fructans from the Root of Cyathula officinalis Kuan. Chin. J. Chem. 2003, 21, 858–863. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, C.; Hou, X.; Yan, C. Structural characterization and osteoprotective effects of a polysaccharide purified from Achyranthes bidentata. Int. J. Biol. Macromol. 2019, 139, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Chen, Y.; Hu, D.; Yao, S.; Yang, J.; Wen, X. A graminan type fructan from Achyranthes bidentata prevents the kidney injury in diabetic mice by regulating gut microbiota. Carbohydr. Polym. 2024, 339, 122275. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Li, T.; Wang, B.; Jin, C.; Li, S.; Li, Y.; Li, M.; Ding, K. A pectic polysaccharide isolated from Achyranthes bidentata is metabolized by human gut Bacteroides spp. Int. J. Biol. Macromol. 2023, 248, 125785. [Google Scholar] [CrossRef]
- Chen, X.; Bahramimehr, F.; Shahhamzehei, N.; Fu, H.; Lin, S.; Wang, H.; Li, C.; Efferth, T.; Hong, C. Anti-aging effects of medicinal plants and their rapid screening using the nematode Caenorhabditis elegans. Phytomedicine 2024, 129, 155665. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, C.; Cao, Y.; Chen, Y. Caenorhabditis elegans as an in vivo model for the identification of natural antioxidants with anti-aging actions. Biomed. Pharmacother. 2023, 167, 115594. [Google Scholar] [CrossRef]
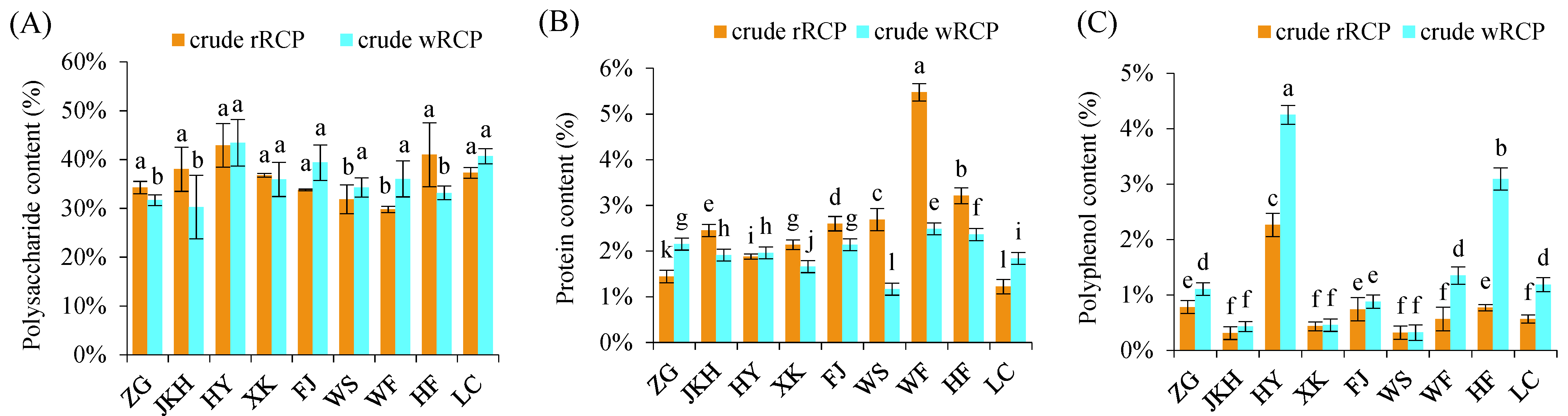
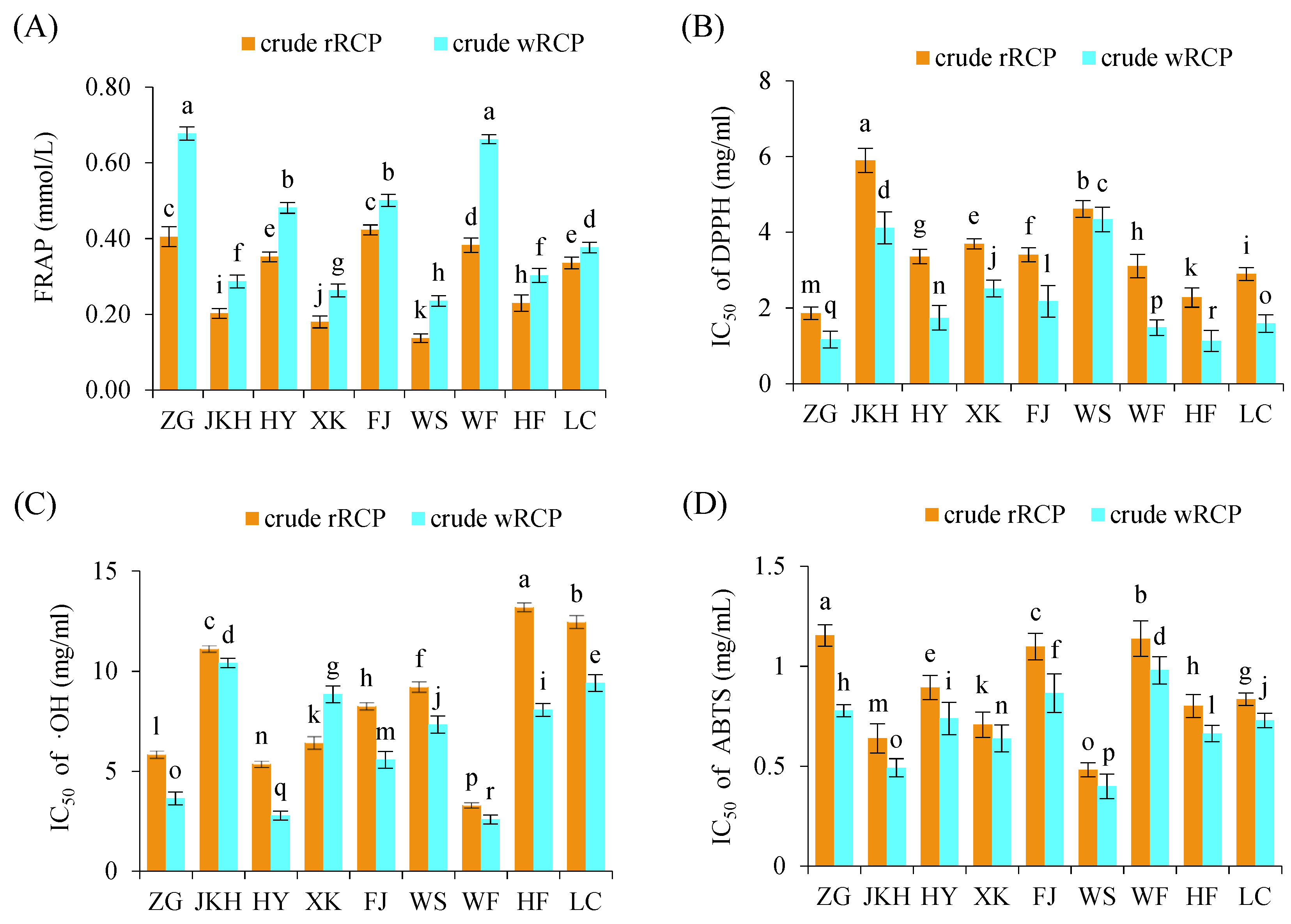
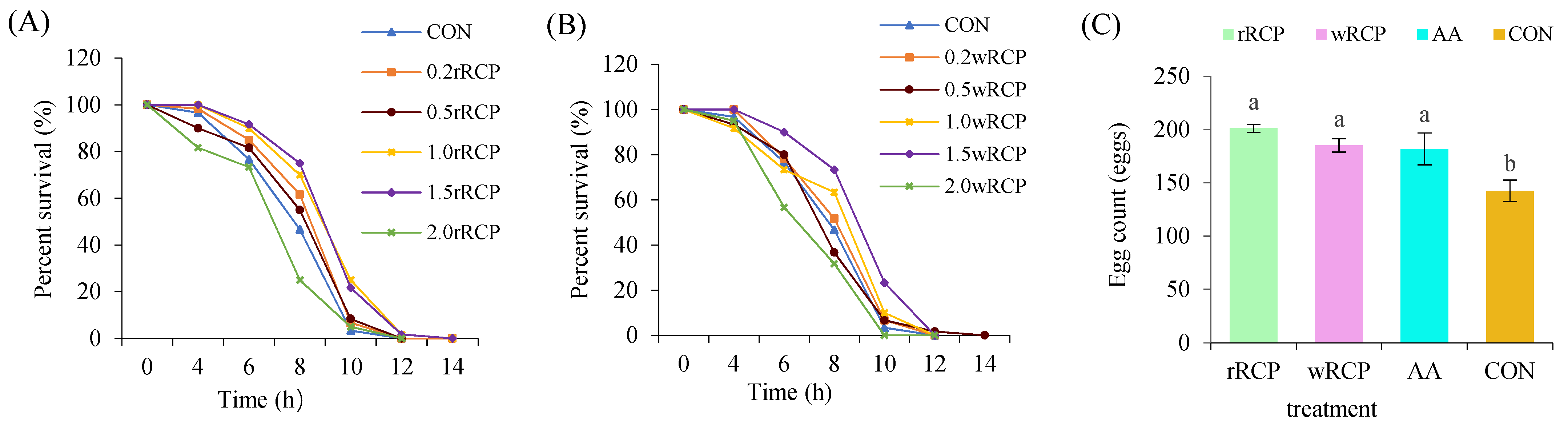
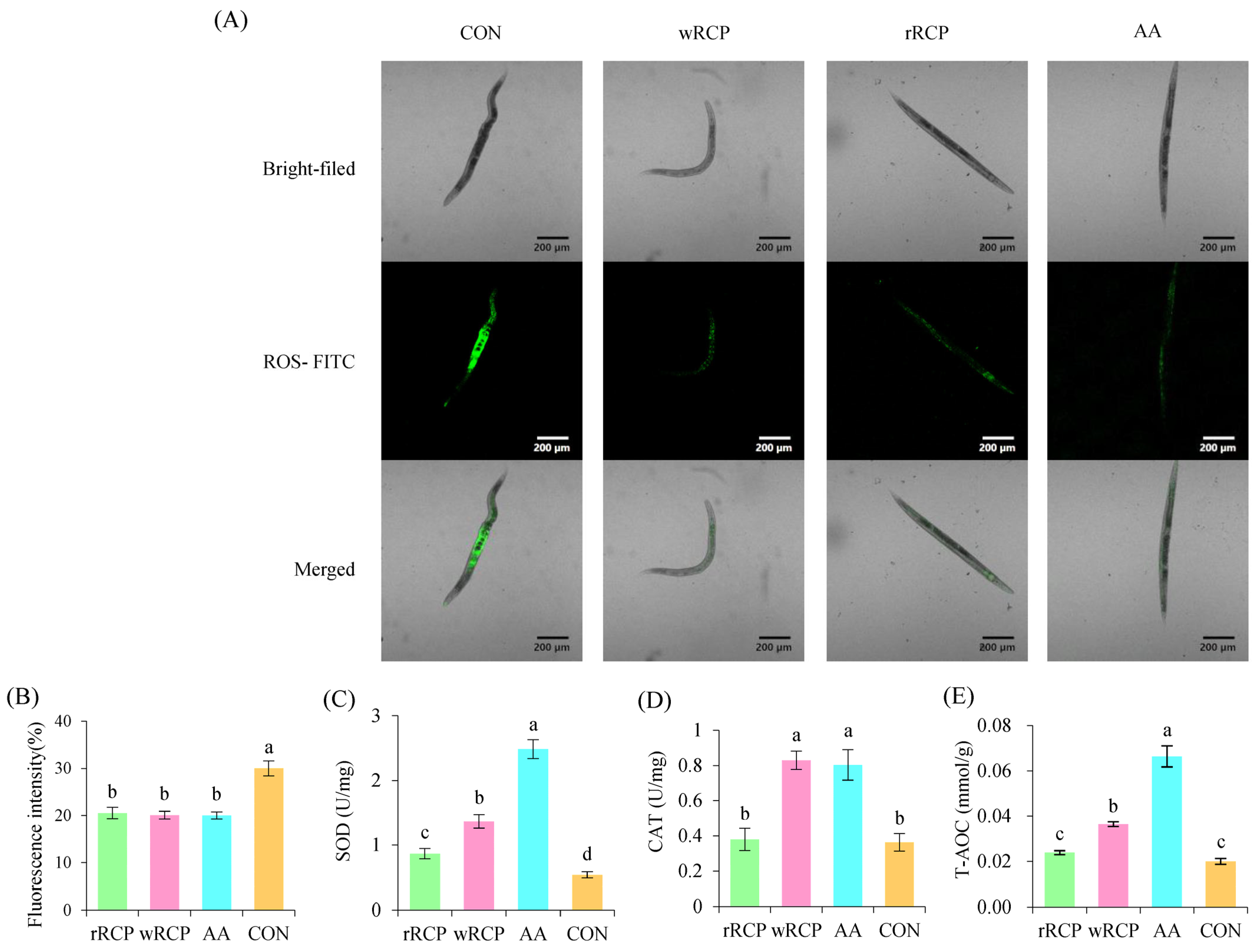
| Component | Initial Eigenvalues | Extraction Sums of Squared Loadings | ||||
|---|---|---|---|---|---|---|
| Total | % of variance | Cumulative % | Total | % of variance | Cumulative % | |
| 1 | 2.550 | 51.001 | 51.001 | 2.550 | 51.001 | 51.001 |
| 2 | 1.049 | 20.974 | 71.975 | 1.049 | 20.974 | 71.975 |
| 3 | 0.626 | 12.527 | 84.502 | |||
| 4 | 0.547 | 10.931 | 95.433 | |||
| 5 | 0.228 | 4.567 | 100.000 | |||
| Constituent | ||
|---|---|---|
| 1 | 2 | |
| FRAP | 0.910 | −0.061 |
| DPPH | −0.786 | −0.217 |
| •OH | −0.772 | 0.250 |
| ABTS | 0.708 | −0.012 |
| Polysaccharide content | 0.090 | 0.967 |
| Regions | Comprehensive Score (Y) | |
|---|---|---|
| Crude rRCP | ZG | 1.209 |
| HY | 1.191 | |
| WF | 0.512 | |
| FJ | 0.242 | |
| HF | −0.159 | |
| LC | −0.458 | |
| XK | −0.543 | |
| JKH | −1.86 | |
| WS | −2.431 | |
| Crude wRCP | ZG | 2.103 |
| HY | 2.088 | |
| WF | 2.037 | |
| FJ | 1.344 | |
| HF | 0.657 | |
| LC | −0.26 | |
| XK | −0.597 | |
| JKH | −1.767 | |
| WS | −2.309 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Chen, X.; Wu, L.; Xie, L.; Chen, M.; Qiu, Y.; Liu, F.; Chen, J.; Tian, M. Antioxidant Activity of Radix Cyathula officinalis Kuan Polysaccharides and Their Modulatory Effects on the Gut Microbiota of Caenorhabditis elegans. Curr. Issues Mol. Biol. 2025, 47, 538. https://doi.org/10.3390/cimb47070538
Li R, Chen X, Wu L, Xie L, Chen M, Qiu Y, Liu F, Chen J, Tian M. Antioxidant Activity of Radix Cyathula officinalis Kuan Polysaccharides and Their Modulatory Effects on the Gut Microbiota of Caenorhabditis elegans. Current Issues in Molecular Biology. 2025; 47(7):538. https://doi.org/10.3390/cimb47070538
Chicago/Turabian StyleLi, Rui, Xinyue Chen, Lijuan Wu, Lei Xie, Mengqiu Chen, Yujie Qiu, Fan Liu, Ji Chen, and Mengliang Tian. 2025. "Antioxidant Activity of Radix Cyathula officinalis Kuan Polysaccharides and Their Modulatory Effects on the Gut Microbiota of Caenorhabditis elegans" Current Issues in Molecular Biology 47, no. 7: 538. https://doi.org/10.3390/cimb47070538
APA StyleLi, R., Chen, X., Wu, L., Xie, L., Chen, M., Qiu, Y., Liu, F., Chen, J., & Tian, M. (2025). Antioxidant Activity of Radix Cyathula officinalis Kuan Polysaccharides and Their Modulatory Effects on the Gut Microbiota of Caenorhabditis elegans. Current Issues in Molecular Biology, 47(7), 538. https://doi.org/10.3390/cimb47070538


_Kim.png)



