Plant Heteropolysaccharides as Potential Anti-Diabetic Agents: A Review
Abstract
1. Introduction
2. The Intervention Mechanisms of Plant Heteropolysaccharides on Diabetes Mellitus
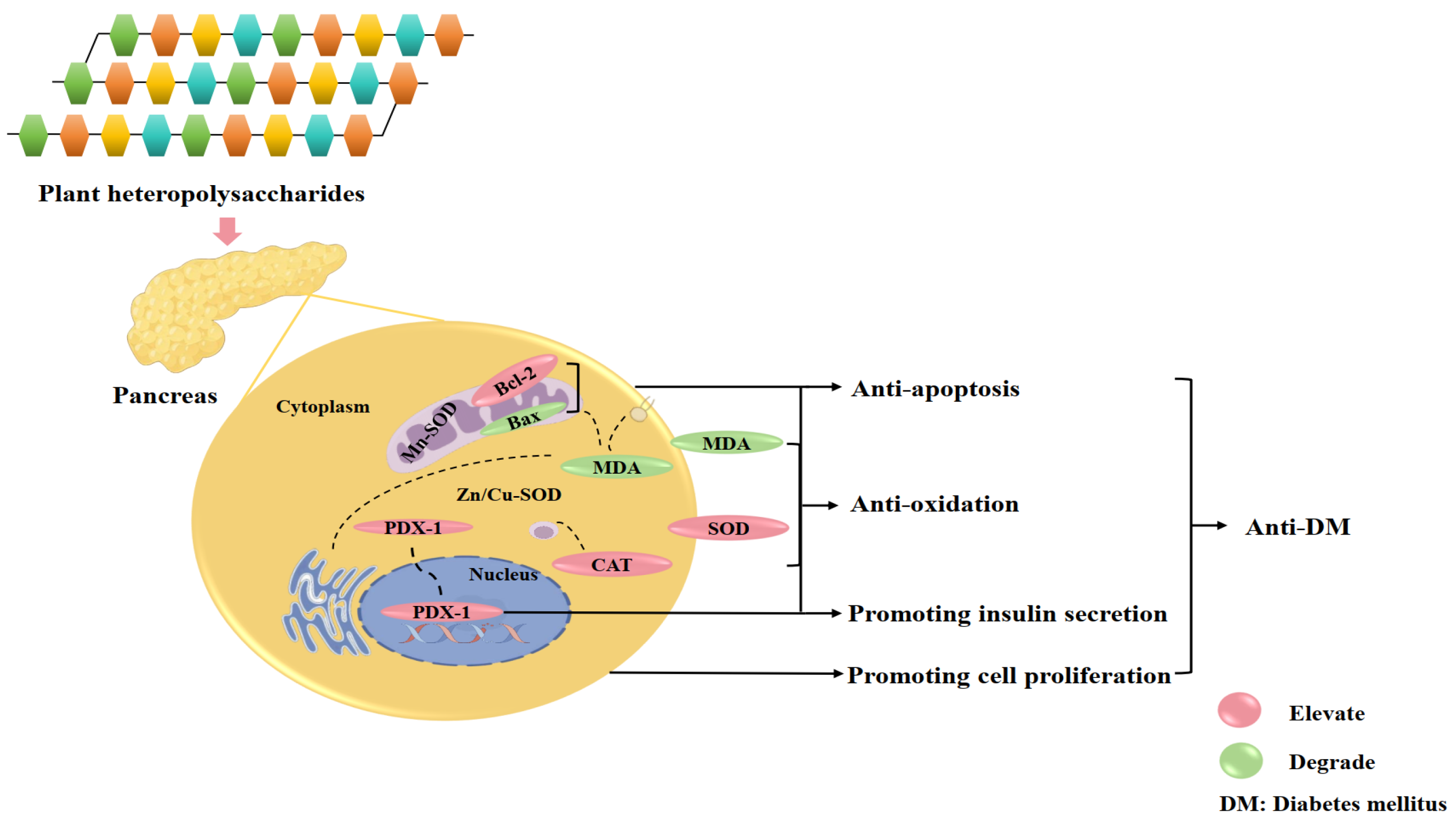
2.1. Regulation of Insulin Secretion and Pancreatic β-Cells Function
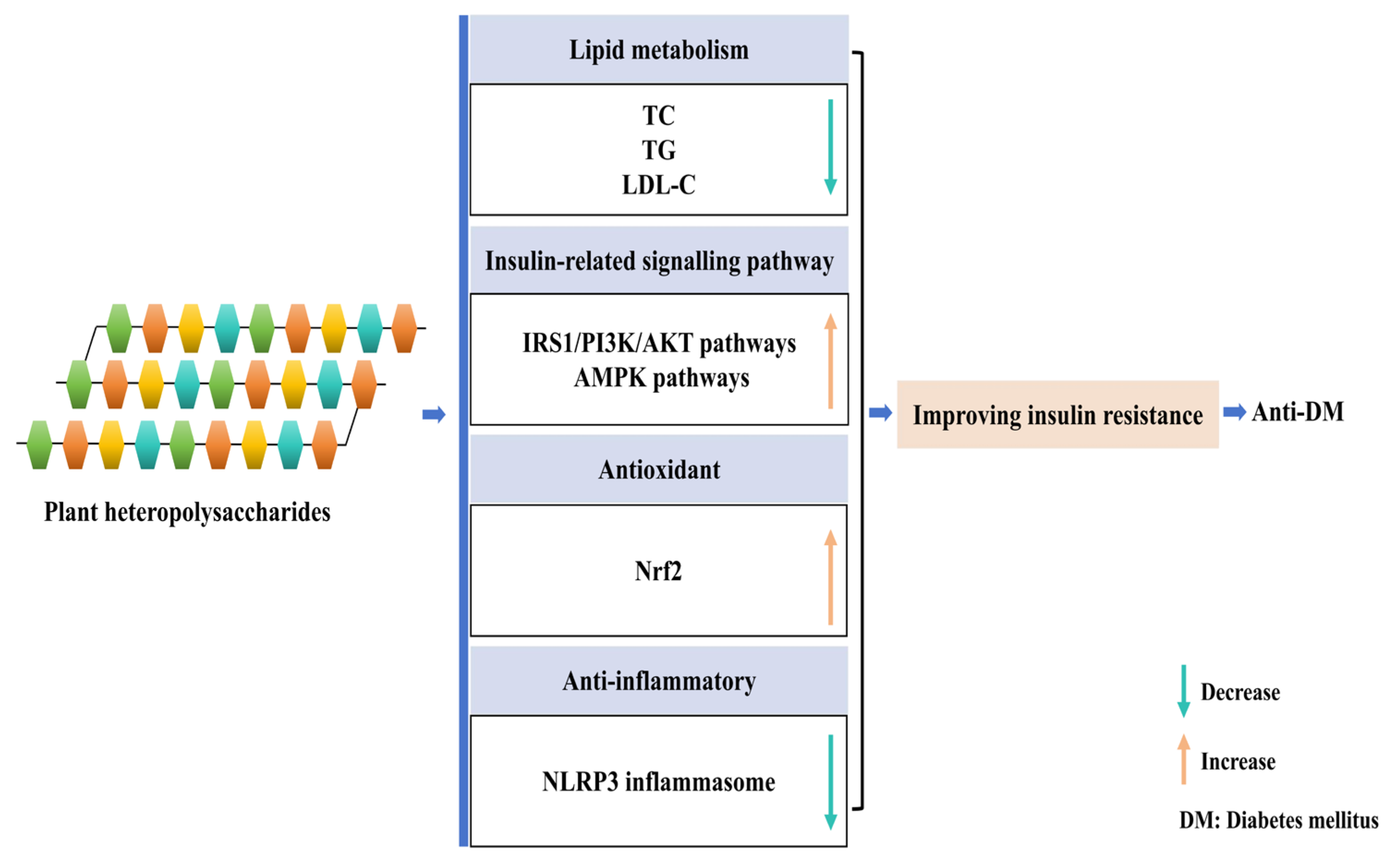
2.2. Improvement of Insulin Resistance
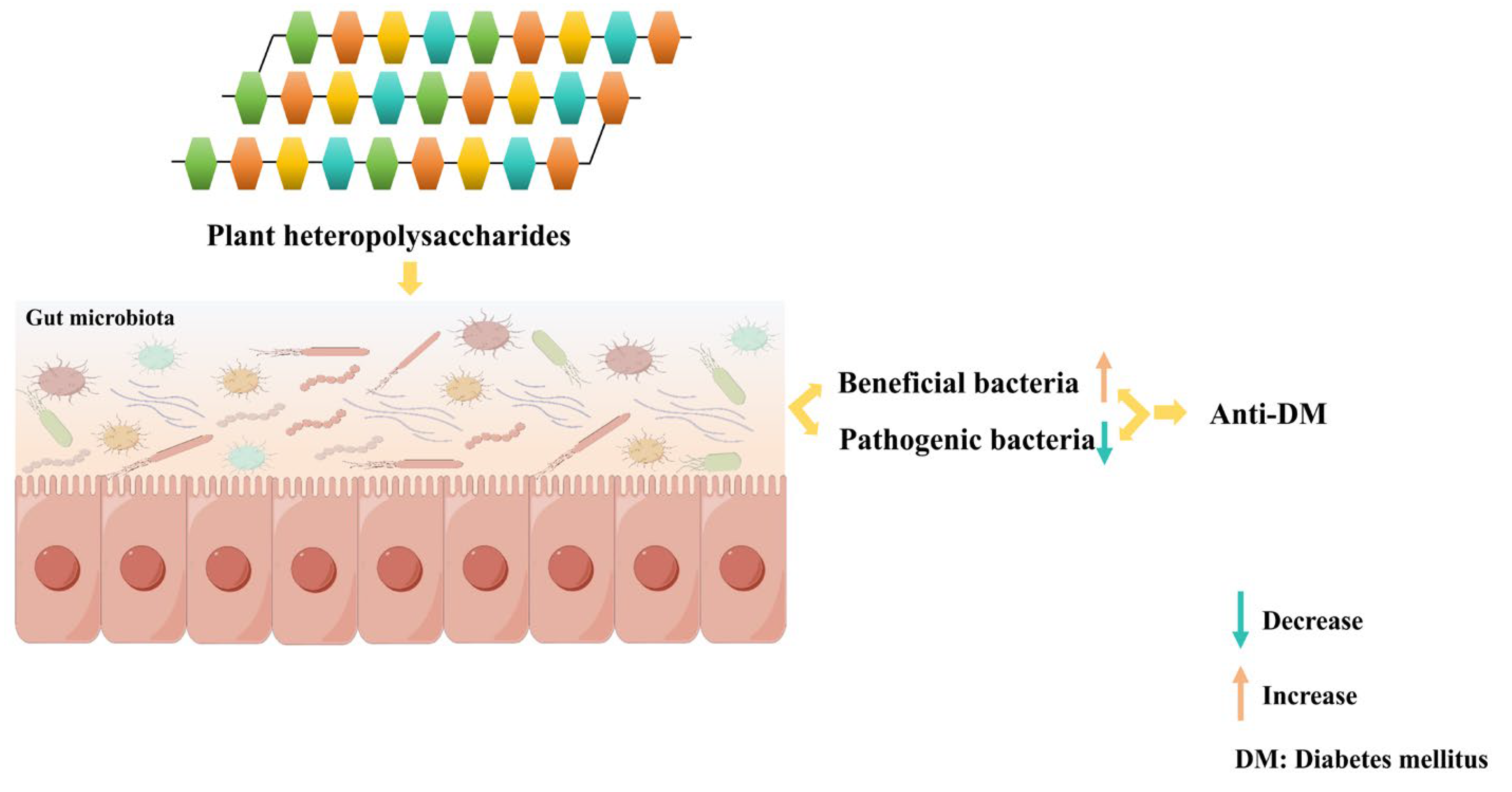
2.3. Modulation of the Gut Microbiota
3. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes mellitus: Classification, mediators, and complications; a gate to identify potential targets for the development of new effective treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna. J. Med. 2020, 10, 174–188. [Google Scholar] [PubMed]
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [PubMed]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar]
- Hivert, M.F.; White, F.; Allard, C.; James, K.; Majid, S.; Aguet, F.; Ardlie, K.G.; Florez, J.C.; Edlow, A.G.; Bouchard, L.; et al. Placental IGFBP1 levels during early pregnancy and the risk of insulin resistance and gestational diabetes. Nat. Med. 2024, 30, 1689–1695. [Google Scholar]
- Kaser, S.; Winhofer-Stöckl, Y.; Kazemi-Shirazi, L.; Hofer, S.E.; Brath, H.; Sourij, H.; Vila, G.; Abrahamian, H.; Riedl, M.; Weitgasser, R.; et al. Other specific types of diabetes and exocrine pancreatic insufficiency (Update 2019). Wien. Klin. Wochenschr. 2019, 131, 16–26. [Google Scholar]
- Susilawati, E.; Levita, J.; Susilawati, Y.; Sumiwi, S.A. Review of the case reports on metformin, sulfonylurea, and thiazolidinedione therapies in type 2 diabetes mellitus patients. Med. Sci. 2023, 11, 50. [Google Scholar]
- Weinberg Sibony, R.; Segev, O.; Dor, S.; Raz, I. Drug therapies for diabetes. Int. J. Mol. Sci. 2023, 24, 17147. [Google Scholar]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar]
- Tahrani, A.; Barnett, A.; Bailey, C. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 566–592. [Google Scholar] [PubMed]
- Pathak, V.; Pathak, N.M.; O’Neill, C.L.; Guduric-Fuchs, J.; Medina, R.J. Therapies for type 1 diabetes: Current scenario and future perspectives. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 1179551419844521. [Google Scholar] [PubMed]
- Hougen, I.; Whitlock, R.H.; Komenda, P.; Rigatto, C.; Clemens, K.K.; Tangri, N. Safety of add-on sulfonylurea therapy in patients with type 2 diabetes using metformin: A population-based real-world study. BMJ Open Diabetes Res. Care 2021, 9, e002352. [Google Scholar] [PubMed]
- Wang, E.; Wang, H.; Chakrabarti, S. Endothelial-to-mesenchymal transition: An underappreciated mediator of diabetic complications. Front. Endocrinol. 2023, 14, 1050540. [Google Scholar]
- Ma, X.; Mei, S.; Wuyun, Q.; Zhou, L.; Sun, D.; Yan, J. Epigenetics in diabetic cardiomyopathy. Clin. Epigenet. 2024, 16, 52. [Google Scholar]
- Zhang, Y.; Yang, B.; Sun, W.; Sun, X.; Zhao, J.; Li, Q. Structural characterization of squash polysaccharide and its effect on STZ-induced diabetes mellitus model in MIN6 cells. Int. J. Biol. Macromol. 2024, 270, 132226. [Google Scholar]
- Zhong, R.F.; Liu, C.J.; Hao, K.X.; Fan, X.D.; Jiang, J.G. Polysaccharides from Flos Sophorae immaturus ameliorates insulin resistance in IR-HepG2 cells by co-regulating signaling pathways of AMPK and IRS-1/PI3K/AKT. Int. J. Biol. Macromol. 2024, 280, 136088. [Google Scholar]
- Qu, Z.; Liu, H.; Yang, J.; Zheng, L.; Huang, J.; Wang, Z.; Xie, C.; Zuo, W.; Xia, X.; Sun, L.; et al. Selective utilization of medicinal polysaccharides by human gut Bacteroides and Parabacteroides species. Nat. Commun. 2025, 16, 638. [Google Scholar]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.G. Role of insulin in health and disease: An update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar]
- Zhou, Z.; Sun, B.; Yu, D.; Zhu, C. Gut microbiota: An important player in type 2 diabetes mellitus. Front. Cell Infect. Microbiol. 2022, 12, 834485. [Google Scholar]
- Campbell, J.E.; Newgard, C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 2021, 22, 142–158. [Google Scholar] [PubMed]
- Gheibi, S.; Kashfi, K.; Ghasemi, A. A practical guide for induction of type-2 diabetes in rat: Incorporating a high-fat diet and streptozotocin. Biomed. Pharmacother. 2017, 95, 605–613. [Google Scholar] [PubMed]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar]
- Qin, L.; Fan, B.; Zhou, Y.; Zheng, J.; Diao, R.; Wang, F.; Liu, J. Targeted gut microbiome therapy: Applications and prospects of probiotics, fecal microbiota transplantation and natural products in the management of type 2 diabetes. Pharmacol. Res. 2025, 213, 107625. [Google Scholar]
- Geraldo, R.; Castro, C.; Pinto, E.; Vasconcelos, M.W.; Neves, D. Effects of dietary polyphenols on vasculogenic erectile dysfunction: A systematic review of pre-clinical studies. Phytother. Res. 2025, 39, 2428–2444. [Google Scholar]
- Zhang, Y.; Ren, C.; Lu, G.; Mu, Z.; Cui, W.; Gao, H.; Wang, Y. Anti-diabetic effect of mulberry leaf polysaccharide by inhibiting pancreatic islet cell apoptosis and ameliorating insulin secretory capacity in diabetic rats. Int. Immunopharmacol. 2014, 22, 248–257. [Google Scholar]
- Li, Q.; Hu, J.; Nie, Q.; Chang, X.; Fang, Q.; Xie, J.; Li, H.; Nie, S. Hypoglycemic mechanism of polysaccharide from Cyclocarya paliurus leaves in type 2 diabetic rats by gut microbiota and host metabolism alteration. Sci. China Life Sci. 2021, 64, 117–132. [Google Scholar]
- Yang, B.; Luo, Y.; Wei, X.; Kan, J. Polysaccharide from Hovenia dulcis (Guaizao) improves pancreatic injury and regulates liver glycometabolism to alleviate STZ-induced type 1 diabetes mellitus in rats. Int. J. Biol. Macromol. 2022, 214, 655–663. [Google Scholar]
- Zhu, J.; Liu, W.; Yu, J.; Zou, S.; Wang, J.; Yao, W.; Gao, X. Characterization and hypoglycemic effect of a polysaccharide extracted from the fruit of Lycium barbarum L. Carbohydr. Polym. 2013, 98, 8–16. [Google Scholar]
- Jiang, S.; Du, P.; An, L.; Yuan, G.; Sun, Z. Anti-diabetic effect of Coptis Chinensis polysaccharide in high-fat diet with STZ-induced diabetic mice. Int. J. Biol. Macromol. 2013, 55, 118–122. [Google Scholar] [PubMed]
- Deng, Y.; Lei, J.; Luo, X.; Wang, S.P.; Tan, H.M.; Zhang, J.Y.; Wu, D.T. Prospects of Ganoderma polysaccharides: Structural features, structure-function relationships, and quality evaluation. Int. J. Biol. Macromol. 2025, 309, 142836. [Google Scholar]
- Zhang, X.; Cui, Y.; Zhang, X.; Zhang, Z.; Yu, Q.; Li, T.; Li, S. Preparation and structure-function relationships of homogalacturonan-rich and rhamnogalacturonan-I rich pectin: A review. Int. J. Biol. Macromol. 2025, 304, 140775. [Google Scholar]
- Zhang, Y.; Cao, Y.; Wang, F.; Wang, L.; Xiong, L.; Shen, X.; Song, H. Polysaccharide from Momordica charantia L. alleviates type 2 diabetes mellitus in mice by activating the IRS1/PI3K/Akt and AMPK signaling pathways and regulating the gut microbiota. J. Agric. Food Chem. 2025, 73, 7298–7309. [Google Scholar]
- Xie, S.Z.; Zhang, W.J.; Liu, W.; Bai, J.B.; Xie, S.L.; Wang, T.S.; Xu, G.B.; Wu, D.L. Physicochemical characterization and hypoglycemic potential of a novel polysaccharide from Polygonatum sibiricum red through PI3K/Akt mediated signaling pathway. J. Funct. Foods 2022, 93, 105080. [Google Scholar]
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Zarrabi, A.; et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother. 2022, 146, 112563. [Google Scholar]
- Taheri, R.; Mokhtari, Y.; Yousefi, A.M.; Bashash, D. The PI3K/Akt signaling axis and type 2 diabetes mellitus (T2DM): From mechanistic insights into possible therapeutic targets. Cell Biol. Int. 2024, 48, 1049–1068. [Google Scholar]
- Zhang, Y.; Wang, H.; Zhang, L.; Yuan, Y.; Yu, D. Codonopsis lanceolata polysaccharide CLPS alleviates high fat/high sucrose diet-induced insulin resistance via anti-oxidative stress. Int. J. Biol. Macromol. 2020, 145, 944–949. [Google Scholar]
- Zhao, Y.; Wang, Q.; Yan, S.; Zhou, J.; Huang, L.; Zhu, H.; Ye, F.; Zhang, Y.; Chen, L.; Chen, L.; et al. Bletilla striata polysaccharide promotes diabetic wound healing through inhibition of the NLRP3 inflammasome. Front. Pharmacol. 2021, 12, 659215. [Google Scholar]
- Luo, D.; Dong, X.; Huang, J.; Huang, C.; Fang, G.; Huang, Y. Pueraria lobata root polysaccharide alleviates glucose and lipid metabolic dysfunction in diabetic db/db mice. Pharm. Biol. 2021, 59, 382–390. [Google Scholar]
- Zhu, Y.; Wang, D.; Zhou, S.; Zhou, T. Hypoglycemic effects of Gynura divaricata (L.) DC polysaccharide and action mechanisms via modulation of gut microbiota in diabetic mice. J. Agric. Food Chem. 2024, 72, 9893–9905. [Google Scholar]
- Liu, W.; Jin, R.; Ma, F.; Zhao, P.; Su, Y.; Wang, J.; Zhang, Y.; Wang, R.; Zhu, J.; Liu, X. Effects of Dioscorea opposita polysaccharides on insulin resistance and gut microbiota in high-fat-diet induced type 2 diabetic rats. Int. J. Biol. Macromol. 2025, 304, 141004. [Google Scholar]
- Wang, G.; Zhang, Y.; Zhang, R.; Pan, J.; Qi, D.; Wang, J.; Yang, X. The protective effects of walnut green husk polysaccharide on liver injury, vascular endothelial dysfunction and disorder of gut microbiota in high fructose-induced mice. Int. J. Biol. Macromol. 2020, 162, 92–106. [Google Scholar] [PubMed]
- Zhao, Y.; Yang, X.; Ren, D.; Wang, D.; Xuan, Y. Preventive effects of jujube polysaccharides on fructose-induced insulin resistance and dyslipidemia in mice. Food Funct. 2014, 5, 1771–1778. [Google Scholar] [PubMed]
- Kavya, M.; Krishnan, R.; Suvachan, A.; Sathyan, S.; Tozuka, Y.; Kadota, K.; Nisha, P. The art and science of porous starch: Understanding the preparation method and structure-function relationship. Crit. Rev. Food Sci. Nutr. 2025, 65, 2880–2897. [Google Scholar]
- Rold, L.S.; Jensen, A.M.; Arenholt, L.; Leutscher, P.D.C.; Ovesen, P.G.; Hagstrøm, S.; Sørensen, S. Identifying microbiome-based changes and biomarkers prior to disease development in mother and child, with a focus on gestational diabetes mellitus: Protocol for the DANish maternal and offspring microbiome (DANMOM) cohort study. BMJ Open 2024, 14, e083358. [Google Scholar]
- Xu, C.; Huang, J.; Gao, Y.; Zhao, W.; Shen, Y.; Luo, F.; Yu, G.; Zhu, F.; Ni, Y. OBMeta: A comprehensive web server to analyze and validate gut microbial features and biomarkers for obesity-associated metabolic diseases. Bioinformatics 2023, 39, btad715. [Google Scholar]
- Yao, T.; Wang, H.; Lin, K.; Wang, R.; Guo, S.; Chen, P.; Wu, H.; Liu, T.; Wang, R. Exercise-induced microbial changes in preventing type 2 diabetes. Sci. China Life Sci. 2024, 67, 892–899. [Google Scholar]
- Zhao, X.; Zhang, Y.; Guo, R.; Yu, W.; Zhang, F.; Wu, F.; Shang, J. The alteration in composition and function of gut microbiome in patients with type 2 diabetes. J. Diabetes Res. 2020, 2020, 8842651. [Google Scholar]
- Craciun, C.I.; Neag, M.A.; Catinean, A.; Mitre, A.O.; Rusu, A.; Bala, C.; Roman, G.; Buzoianu, A.D.; Muntean, D.M.; Craciun, A.E. The relationships between gut microbiota and diabetes mellitus, and treatments for diabetes mellitus. Biomedicines 2022, 10, 308. [Google Scholar] [CrossRef]
- Li, H.; Fang, Q.; Nie, Q.; Hu, J.; Yang, C.; Huang, T.; Li, H.; Nie, S. Hypoglycemic and hypolipidemic mechanism of tea polysaccharides on type 2 diabetic rats via gut microbiota and metabolism alteration. J. Agric. Food Chem. 2020, 68, 10015–10028. [Google Scholar] [PubMed]
- Zhang, X.; Li, Q.; Han, N.; Song, C.; Lin, Y.; Zhang, L.; Ren, D.; Zhao, Y.; Yang, X.; Li, T. Effects of Fu brick tea polysaccharides on gut microbiota and fecal metabolites of HFD/STZ-induced type 2 diabetes rats. Food Funct. 2023, 14, 10910–10923. [Google Scholar] [PubMed]
- Wu, Z.; Zeng, W.; Zhang, X.; Yang, J. Characterization of acidic tea polysaccharides from yellow leaves of Wuyi rock tea and their hypoglycemic activity via intestinal flora regulation in rats. Foods 2022, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Huang, X.; Wu, G.; Ye, H.; Huang, W.; Nie, Q.; Chen, H.; Yin, J.; Chen, Y.; Nie, S. Polysaccharides from red kidney bean alleviating hyperglycemia and hyperlipidemia in type 2 diabetic rats via gut microbiota and lipid metabolic modulation. Food Chem. 2023, 404, 134598. [Google Scholar]
- Song, Q.; Cheng, S.W.; Li, D.; Cheng, H.; Lai, Y.S.; Han, Q.; Wu, H.Y.; Shaw, P.C.; Zuo, Z. Gut microbiota mediated hypoglycemic effect of Astragalus membranaceus polysaccharides in db/db mice. Front. Pharmacol. 2022, 13, 1043527. [Google Scholar]
- Ye, J.; Ma, J.; Rozi, P.; Kong, L.; Zhou, J.; Luo, Y.; Yang, H. The polysaccharides from seeds of Glycyrrhiza uralensis ameliorate metabolic disorders and restructure gut microbiota in type 2 diabetic mice. Int. J. Biol. Macromol. 2024, 264, 130622. [Google Scholar]
- Yuan, Y.; Zhou, J.; Zheng, Y.; Xu, Z.; Li, Y.; Zhou, S.; Zhang, C. Beneficial effects of polysaccharide-rich extracts from Apocynum venetum leaves on hypoglycemic and gut microbiota in type 2 diabetic mice. Biomed. Pharmacother. 2020, 127, 110182. [Google Scholar]
- Liu, G.; Liang, L.; Yu, G.; Li, Q. Pumpkin polysaccharide modifies the gut microbiota during alleviation of type 2 diabetes in rats. Int. J. Biol. Macromol. 2018, 115, 711–717. [Google Scholar]
- Xia, T.; He, W.; Luo, Z.; Wang, K.; Tan, X. Achyranthes bidentata polysaccharide ameliorates type 2 diabetes mellitus by gut microbiota-derived short-chain fatty acids-induced activation of the GLP-1/GLP-1R/cAMP/PKA/CREB/INS pathway. Int. J. Biol. Macromol. 2024, 270, 132256. [Google Scholar]
- Zang, Y.; Ge, Y.; Cao, Y.; Tang, H. Anti-diabetic effect of red quinoa polysaccharide on type 2 diabetic mellitus mice induced by streptozotocin and high-fat diet. Front. Microbiol. 2024, 15, 1308866. [Google Scholar]
- Xi, L.; Weibing, X.; Shuyong, F.; Sheng-Hua, L.; Xiong, F.; Chin-Ping, T.; Ping-Ping, W.; Zu-Man, D.; Chun, C. The effect of the molecular weight of blackberry polysaccharides on gut microbiota modulation and hypoglycemic effect in vivo. Food Funct. 2024, 15, 8586–8603. [Google Scholar] [PubMed]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa roxburghii Tratt fruit attenuates hyperglycemia and hyperlipidemia and regulates colon microbiota in diabetic db/db mice. J. Agric. Food Chem. 2020, 68, 147–159. [Google Scholar] [PubMed]
- Jia, R.B.; Li, Z.R.; Wu, J.; Ou, Z.R.; Liao, B.; Sun, B.; Lin, L.; Zhao, M. Mitigation mechanisms of Hizikia fusifarme polysaccharide consumption on type 2 diabetes in rats. Int. J. Biol. Macromol. 2020, 164, 2659–2670. [Google Scholar] [PubMed]
- Jia, R.B.; Li, Z.R.; Wu, J.; Ou, Z.R.; Sun, B.; Lin, L.; Zhao, M. Antidiabetic effects and underlying mechanisms of anti-digestive dietary polysaccharides from Sargassum fusiforme in rats. Food Funct. 2020, 11, 7023–7036. [Google Scholar]
- Ma, Q.; Zhai, R.; Xie, X.; Chen, T.; Zhang, Z.; Liu, H.; Nie, C.; Yuan, X.; Tu, A.; Tian, B.; et al. Hypoglycemic effects of Lycium barbarum polysaccharide in type 2 diabetes mellitus mice via modulating gut microbiota. Front. Nutr. 2022, 9, 916271. [Google Scholar]
- Zhou, W.; Liu, P.; Xu, W.; Ran, L.; Yan, Y.; Lu, L.; Zeng, X.; Cao, Y.; Mi, J. A purified fraction of polysaccharides from the fruits of Lycium barbarum L. improves glucose homeostasis and intestinal barrier function in high-fat diet-fed mice. Food Funct. 2023, 14, 5311–5325. [Google Scholar]
- Liu, H.; Xing, Y.; Wang, Y.; Ren, X.; Zhang, D.; Dai, J.; Xiu, Z.; Yu, S.; Dong, Y. Dendrobium officinale polysaccharide prevents diabetes via the regulation of gut microbiota in prediabetic mice. Foods 2023, 12, 2310. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Fu, X. Dendrobium officinale polysaccharide alleviates type 2 diabetes mellitus by restoring gut microbiota and repairing intestinal barrier via the LPS/TLR4/TRIF/NF-kB axis. J. Agric. Food Chem. 2023, 71, 11929–11940. [Google Scholar]
- Fang, J.; Lin, Y.; Xie, H.; Farag, M.A.; Feng, S.; Li, J.; Shao, P. Dendrobium officinale leaf polysaccharides ameliorated hyperglycemia and promoted gut bacterial associated SCFAs to alleviate type 2 diabetes in adult mice. Food Chem. X 2022, 13, 100207. [Google Scholar]
- Ruan, Q.; Chen, Y.; Wen, J.; Qiu, Y.; Huang, Y.; Zhang, Y.; Farag, M.A.; Zhao, C. Regulatory mechanisms of the edible alga Ulva lactuca polysaccharide via modulation of gut microbiota in diabetic mice. Food Chem. 2023, 409, 135287. [Google Scholar]
- Jia, R.B.; Li, Z.R.; Lin, L.; Luo, D.; Chen, C.; Zhao, M. The potential mechanisms of Macrocystis pyrifera polysaccharides mitigating type 2 diabetes in rats. Food Funct. 2022, 13, 7918–7929. [Google Scholar] [PubMed]
- Zheng, Q.; Zheng, Y.; Jia, R.B.; Luo, D.; Chen, C.; Zhao, M. Fucus vesiculosus polysaccharide alleviates type 2 diabetes in rats via remodeling gut microbiota and regulating glycolipid metabolism-related gene expression. Int. J. Biol. Macromol. 2023, 248, 126504. [Google Scholar]
- Zhou, W.; Han, L.J.; Raza, S.H.A.; Yue, Q.M.; Sun, S.N.; Zhao, Y.X.; Lv, L.F.; Deng, Y.R.; Yuan, Z.Z.; Alsharif, I.; et al. Polysaccharides in Berberis dasystachya improve intestinal flora depending on the molecular weight and ameliorate type 2 diabetes in rats. J. Funct. Foods 2023, 100, 105381. [Google Scholar]
- Xia, T.; Liu, C.S.; Hu, Y.N.; Luo, Z.Y.; Chen, F.L.; Yuan, L.X.; Tan, X.M. Coix seed polysaccharides alleviate type 2 diabetes mellitus via gut microbiota-derived short-chain fatty acids activation of IGF1/PI3K/AKT signaling. Food Res. Int. 2021, 150, 110717. [Google Scholar]
- Chen, C.; You, L.J.; Huang, Q.; Fu, X.; Zhang, B.; Liu, R.H.; Li, C. Modulation of gut microbiota by mulberry fruit polysaccharide treatment of obese diabetic db/db mice. Food Funct. 2018, 9, 3732–3742. [Google Scholar]
- Chen, X.; Wu, J.; Fu, X.; Wang, P.; Chen, C. Fructus mori polysaccharide alleviates diabetic symptoms by regulating intestinal microbiota and intestinal barrier against TLR4/NF-κB pathway. Int. J. Biol. Macromol. 2023, 249, 126038. [Google Scholar]
- Zhang, Z.; Lai, J.; Fan, X.; Wang, S.; Zhang, H.; Wang, L.; Wang, H. Extraction of polysaccharides from Polygonum cuspidatum with activity against type 2 diabetes via alterations in gut microbiota. Food Chem. 2025, 470, 140047. [Google Scholar]
- Zhang, B.; Wang, J.; Chen, X.; Xue, T.; Xin, J.; Liu, Y.; Wang, X.; Li, X. Laminaria japonica polysaccharide regulates fatty hepatosis through bile acids and gut microbiota in diabetes rat. Mar. Biotechnol. 2024, 26, 1165–1178. [Google Scholar]
- Yao, Y.; Yan, L.; Chen, H.; Wu, N.; Wang, W.; Wang, D. Cyclocarya paliurus polysaccharides alleviate type 2 diabetic symptoms by modulating gut microbiota and short-chain fatty acids. Phytomedicine 2020, 77, 153268. [Google Scholar]
- Ye, M.; Liu, Y.; Wang, F.; Yang, X.; Yang, X.; Gao, X.; Liu, W.; Yu, J. Polysaccharide extracted from Sarcandra glabra residue attenuate cognitive impairment by regulating gut microbiota in diabetic mice. Int. J. Biol. Macromol. 2024, 270, 132121. [Google Scholar]
- Ren, Y.; Mao, S.; Zeng, Y.; Chen, S.; Tian, J.; Ye, X. Pectin from Citrus unshiu Marc. alleviates glucose and lipid metabolism by regulating the gut microbiota and metabolites. Foods 2023, 12, 4094. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Luo, H.; He, F.; Du, Y.; Wang, J.; Zeng, H.; Xu, Z.; Sun, Y.; Li, M. Guava polysaccharides attenuate high fat and STZ-induced hyperglycemia by regulating gut microbiota and arachidonic acid metabolism. Int. J. Biol. Macromol. 2024, 276, 133725. [Google Scholar]
- Araújo-Rodrigues, H.; Sousa, A.S.; Relvas, J.B.; Tavaria, F.K.; Pintado, M. An Overview on mushroom polysaccharides: Health-promoting properties, prebiotic and gut microbiota modulation effects and structure-function correlation. Carbohydr. Polym. 2024, 333, 121978. [Google Scholar] [PubMed]
| Source | Experimental Models | Gut Microbiota | Function | References |
|---|---|---|---|---|
| Green tea | High fat diet combined with streptozotocin induced type 2 diabetic mellitus rats | Restored the relative abundance of Lachnospira, Victivallis, Roseburia, and Fluviicola | Hypoglycemic and hypolipidemic effects | [51] |
| Fu brick tea | High-fat diet/streptozotocin-induced type 2 diabetic mellitus rats | Increased the abundance of Ruminococcus, Lactobacillus, and Lachnospiraece_NK4A136_group; Reduced the abundance of Prevotella and Faecalibaculum | Hypoglycemic, hypolipidemic, and antioxidant effects | [52] |
| Yellow leaves of Wuyi rock tea | Streptozotocin-induced type 2 diabetic mellitus rats | Increased the abundance of Bifidobacterium, Blautia, Dorea, and Oscillospira; Decreased the abundance of Desulfovibrio and Lactobacillus | Hypoglycemic effect | [53] |
| Red kidney bean | Streptozotocin-induced type 2 diabetic rats | Enriched to Bacteroides, Phascolarctobacterium, Succinivibrio, and Blautia | Hypoglycemic and hypolipidemic effects | [54] |
| Astragalus membranaceus | Diabetic db/db mice | Increased the abundance of Akkermansia and Faecalibaculum | Hypoglycemic effect | [55] |
| Glycyrrhiza uralensis seeds | High-fat diet/streptozotocin-induced type 2 diabetic mellitus mice | Increased the abundances of Akkermansia, Lactobacillus, Romboutsia, and Faecalibaculum; Decreased the abundances of Escherichia-Shigella, and Clostridium sensu stricto 1 | Hypoglycemic, hypolipidemic, antioxidant, and anti-inflammatory effects | [56] |
| Apocynum venetum leaves | High-fat diet and streptozocin-induced type 2 diabetic mice | Increased the abundance of Odoribacter, Anaeroplasma, Parasutterella, and Muribaculum; Decreased the abundance of Enterococcus, Klebsiella, and Aerococcus | Hypoglycemic and hypolipidemic effects | [57] |
| Cucurbita pepo ‘lady godiva’ | High-fat diet induced type 2 diabetic rats | Enriched to Bacteroidetes, Prevotella, Deltaproteobacteria, Oscillospira, Veillonellaceae, Phascolarctobacterium, Sutterella, and Bilophila | Hypoglycemic and hypolipidemic effects | [58] |
| Achyranthes bidentata | High-sugar and high-fat diet/streptozotocin-induced type 2 diabetic mellitus mice | Increased the abundance of Alloprevotella, Bacteroides, Prevotellaceae_UCG_001, Prevotellaceae_NK3B31_group, and Akkermansia | Hypoglycemic effect | [59] |
| Chenopodium quinoa Willd. | High-fat diet and streptozocin-induced type 2 diabetic mice | Decreased the abundance of norank_f_Muribaculaceae and Lachnospiraceae_NK4A136_group; Increased the relative abundance of Akkermansia, unclassified_f_Lachnospiraceae, norank_f_Eubacterium_coprostanoligenes_group, unclassified_f_Atopobiaceae, and norank_f_Lachnospiraceae | Hypoglycemic, hypolipidemic, and antioxidant effects | [60] |
| Blackberry | High-fat diet and streptozocin-induced type 2 diabetic mice | Increased the abundance of Oscillospira, Bacteroidaceae, Bacteroides; Decreased the abundance of Allobaculum | Hypoglycemic effect | [61] |
| Rosa roxburghii tratt fruit | Type-2 diabetic db/db mice | Increased the abundances of Bacteroidaceae, Bacteroidaceae S24-7 group, and Lactobacillaceae | Hypoglycemic and hypolipidemic effects | [62] |
| Hizikia fusifarme | High-sugar and high-fat diet/streptozotocin-induced type 2 diabetic mellitus rats | Increased the abundance of Ruminococcaceae, Mollicutes_RF39-norank, Lachnospiraceae_NK4A136_group, Turicibacter, Faecalibaculum, and Lactobacillus; Decreased the abundance of Escherichia-Shigella | Hypoglycemic, hypolipidemic, antioxidant, and anti-inflammatory effects | [63] |
| Sargassum fusiforme | High-sugar and high-fat diet/streptozotocin-induced type 2 diabetic mellitus rats | Increased the abundance of Ruminococcaceae, Mollicutes_RF39-norank, and Prevotellaceae_NK3B31_group; Decreased the abundance of Escherichia-Shigella | Hypoglycemic, hypolipidemic, and antioxidant effects | [64] |
| Lycium barbarum | High fat diet combined with streptozotocin induced type 2 diabetic mellitus rats | Increased the abundance of Bacteroides, Ruminococcaceae_UCG-014, Intestinimonas, Mucispirillum, and Ruminococcaceae_UCG-009; Decreased the abundance of Allobaculum, Dubosiella, and Romboutsia | Hypoglycemic and hypolipidemic effects | [65] |
| Lycium barbarum L. | High-fat diet-induced diabetic mice | Increased the abundance of Allobaculum and Romboutsia | Hypoglycemic effect | [66] |
| Dendrobium officinale | High-sugar and high-fat diet/streptozotocin-induced prediabetic rice | Enriched to Roseburia, Bifidobacterium, Lactobacillus, Alloprevotella, and Bacteroides | Hypoglycemic effect | [67] |
| Dendrobium officinale | High-fat diet and streptozocin-induced type 2 diabetic mice | Inhibited the abundance of Helicobacter; Facilitated the proliferation of Allobaculum, Bifidobacterium, and Lactobacillus | Hypoglycemic, antioxidant, and anti-inflammatory effects | [68] |
| Dendrobium officinale leaf | High fat diet combined with streptozotocin induced type 2 diabetic mellitus mice | Increased the abundance of Lactobacillus, Bifidobacterium, and Akkermansia | Hypoglycemic and hypolipidemic effects | [69] |
| Ulva lactuca | High-sugar and high-fat diet/D-galactose and streptozotocin-induced aging type 2 diabetic mice | Increased the abundance of Alloprevotella and Pediococcus | Hypoglycemic effect | [70] |
| Macrocystis pyrifera | High-sugar and high-fat diet/streptozotocin-induced type 2 diabetic mellitus rats | Increased the abundance of Muribaculaceae_norank, Akkermansia, Bifidobacterium, Lactobacillus, Olsenella, Lachnospiraceae_NK4A136_group, Ruminococcaceae_UCG-014, Ruminococcus_1, Eubacterium_coprostanoligenes_group, and Ruminococcaceae_UCG-014; Decreased the abundance of Escherichia-Shigella | Hypoglycemic and hypolipidemic effects | [71] |
| Fucus vesiculosus | High-fat diet and streptozotocin-induced type 2 diabetic mellitus rats | Increased the abundance of Lactobacillus, Muribaculaceae_norank, Lachnospiraceae_Nk4A136_group, and Bacteroides; Reduced the abundance of Escherichia-Shigella, Herminiimonas, Citrobacter, and Pseudomonas | Hypoglycemic, hypolipidemic, and antioxidant effects | [72] |
| Berberis dasystachya | High-fat diet/streptozotocin-induced type 2 diabetic mellitus rats | Enriched to Ruminococcaceae NK4A214 group, Ruminococcus 2, Prevotellaceae NK3B31 group, Eubacterium coprostanoligenes group, Romboutsia, and Alloprevotella | Hypoglycemic and antioxidant effects | [73] |
| Coix seed | High-fat diet and streptozotocin-induced type 2 diabetic mellitus mice | Increased the abundance of Lactobacillus, Akkermansia, Bacteroides, and Bifidobacterium | Hypoglycemic effect | [74] |
| Fructus mori | Obese diabetic db/db mice | Enriched to Bacteroidales, Lactobacillus, Allobaculum, Bacteroides, and Akkermansia | Hypoglycemic, hypolipidemic, and antioxidant effects | [75] |
| Fructus mori | High-fat diet and streptozotocin-induced type 2 diabetic mellitus mice | The inhibition of Shigella and the restoration of Allobaculum and Bifidobacterium | Hypoglycemic, hypolipidemic, antioxidant, and anti-inflammatory effects | [76] |
| Polygonum cuspidatum | High-fat diet and streptozotocin-induced type 2 diabetic mellitus mice | Upregulated the population of Lactobacillus and Akkermansia | Hypoglycemic effect | [77] |
| Laminaria japonica | High-sugar and high-fat diet/streptozotocin-induced type 2 diabetic rats | Increased the abundance of Bacteroidia, Campylobacteria, Clostridia, Gammaproteobacteria, Negativicutes, and Verrucomicrobi | Hypoglycemic and hypolipidemic effects | [78] |
| Cyclocarya paliurus | High-fat diet and streptozotocin-induced type 2 diabetic rats | Increased the abundances of Ruminococcus bromii, Anaerotruncus colihominis, Clostridium methylpentosum, Rosebui ia intestinalis, Roseburia hominis, Clostridiumasparagiforme, Pseudoflavonifractorcapillosus, Intestinimonasbutyriciproducens, Intestinimonas_sp._GD2, Oscillibacter valericigenes, and Oscillibacter ruminantium | Hypoglycemic and hypolipidemic effects | [79] |
| Sarcandra glabra | Diabetes spontaneous mutation mice (Leptin receptor-deficient, Leprdb/db) | Enriched to Bacteroidales S24-7 | Hypoglycemic effect | [80] |
| Citrus unshiu Marc. | Diabetic C57BL/KsJ-db/db mice | Increased the abundance of Ligilactobacillus, Lactobacillus, and Limosilactobacillus | Hypoglycemic and hypolipidemic effects | [81] |
| Psidium guajava L. | High-fat diet and streptozotocin-induced type 2 diabetic mice | Inhibited Uncultured_f_Desulfovibrionaceae, Bilophila, and Desulfovibrio; Promoted the proliferation of Bifidobacterium and Bacteroides | Hypoglycemic, hypolipidemic, and anti-inflammatory effects | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, D.; Cui, C. Plant Heteropolysaccharides as Potential Anti-Diabetic Agents: A Review. Curr. Issues Mol. Biol. 2025, 47, 533. https://doi.org/10.3390/cimb47070533
He D, Cui C. Plant Heteropolysaccharides as Potential Anti-Diabetic Agents: A Review. Current Issues in Molecular Biology. 2025; 47(7):533. https://doi.org/10.3390/cimb47070533
Chicago/Turabian StyleHe, Dan, and Can Cui. 2025. "Plant Heteropolysaccharides as Potential Anti-Diabetic Agents: A Review" Current Issues in Molecular Biology 47, no. 7: 533. https://doi.org/10.3390/cimb47070533
APA StyleHe, D., & Cui, C. (2025). Plant Heteropolysaccharides as Potential Anti-Diabetic Agents: A Review. Current Issues in Molecular Biology, 47(7), 533. https://doi.org/10.3390/cimb47070533







