Phytochemicals in Breast Cancer Prevention and Therapy: Mechanisms, Efficacy, and Future Prospects
Abstract
1. Introduction
2. Classification of Phytochemicals Utilized in Breast Cancer Treatment
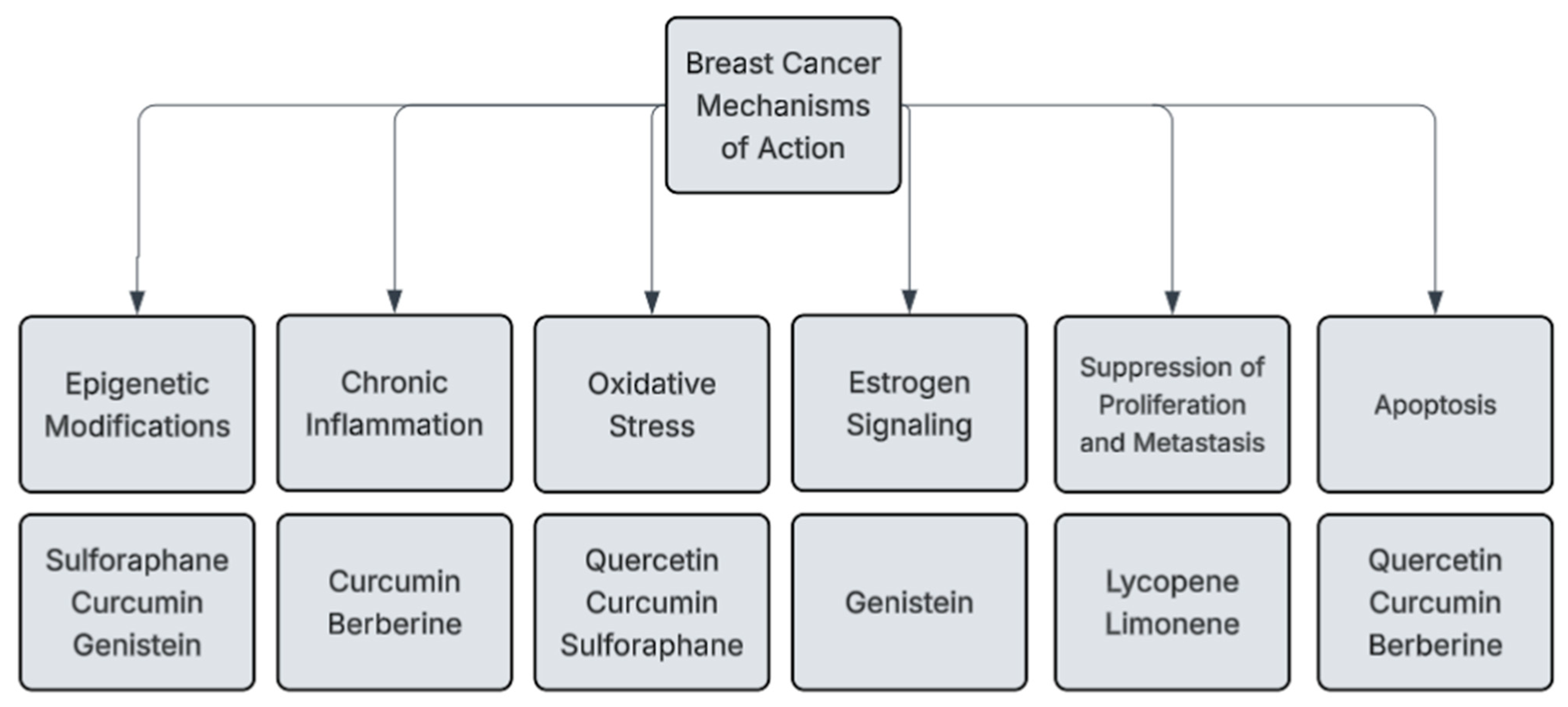
3. Mechanisms of Action for Phytochemicals in Breast Cancer
4. Phytochemicals and Breast Cancer Stem Cells
5. Phytochemicals and Immune Modulation in Breast Cancer
6. Clinical Trials and Human Studies on Phytochemicals in Breast Cancer
7. Challenges in Phytochemical Research Methodology
8. Potential for Integration into Breast Cancer Therapy
9. Safety, Toxicity, and Limitations
10. Future Prospects and Emerging Research
11. Ethical and Regulatory Perspectives on Phytochemical Use
12. Perspectives from Traditional Medicine
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Burguin, A.; Diorio, C.; Durocher, F. Breast Cancer Treatments: Updates and New Challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef]
- Johnson, I.T. Phytochemicals and cancer. Proc. Nutr. Soc. 2007, 66, 207–215. [Google Scholar] [CrossRef]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxidative Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef]
- Kapinova, A.; Kubatka, P.; Golubnitschaja, O.; Kello, M.; Zubor, P.; Solar, P.; Pec, M. Dietary phytochemicals in breast cancer research: Anticancer effects and potential utility for effective chemoprevention. Environ. Health Prev. Med. 2018, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Losada-Echeberría, M.; Herranz-López, M.; Micol, V.; Barrajón-Catalán, E. Polyphenols as Promising Drugs against Main Breast Cancer Signatures. Antioxidants 2017, 6, 88. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in Food and Their Health Benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Le Marchand, L. Cancer preventive effects of flavonoids—A review. Biomed. Pharmacother. 2002, 56, 296–301. [Google Scholar] [CrossRef]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A review on anti-cancer properties of Quercetin in breast cancer. Life Sci. 2020, 248, 117463. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.-P.; Wang, A.; Ye, J.-H.; Zheng, X.-Q.; Polito, C.A.; Lu, J.-L.; Li, Q.-S.; Liang, Y.-R. Suppressive Effects of Tea Catechins on Breast Cancer. Nutrients 2016, 8, 458. [Google Scholar] [CrossRef]
- Bhat, S.S.; Prasad, S.K.; Shivamallu, C.; Prasad, K.S.; Syed, A.; Reddy, P.; Cull, C.A.; Amachawadi, R.G. Genistein: A Potent Anti-Breast Cancer Agent. Curr. Issues Mol. Biol. 2021, 43, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Assumpção, J.H.M.; Takeda, A.A.S.; Sforcin, J.M.; Rainho, C.A. Effects of Propolis and Phenolic Acids on Triple-Negative Breast Cancer Cell Lines: Potential Involvement of Epigenetic Mechanisms. Molecules 2020, 25, 1289. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Zamanian, M.Y.; Jalal, S.M.; Noraldeen, S.A.M.; Ramírez-Coronel, A.A.; Oudaha, K.H.; Obaid, R.F.; Almulla, A.F.; Bazmandegan, G.; Kamiab, Z. A comprehensive review on Ellagic acid in breast cancer treatment: From cellular effects to molecular mechanisms of action. Food Sci. Nutr. 2023, 11, 7458–7468. [Google Scholar] [CrossRef]
- Das, U.; Manna, K.; Khan, A.; Sinha, M.; Biswas, S.N.; Sengupta, A.; Chakraborty, A.; Dey, S. Ferulic acid (FA) abrogates γ-radiation induced oxidative stress and DNA damage by up-regulating nuclear translocation of Nrf2 and activation of NHEJ pathway. Free Radic. Res. 2017, 51, 47–63. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Z. The Effect of Curcumin on Breast Cancer Cells. J. Breast Cancer 2013, 16, 133. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Smith, J.; Swanson, H.; Rubin, L. Carotenoid Lutein Selectively Inhibits Breast Cancer Cell Growth and Potentiates the Effect of Chemotherapeutic Agents through ROS-Mediated Mechanisms. Molecules 2018, 23, 905. [Google Scholar] [CrossRef]
- Sowmya Shree, G.; Yogendra Prasad, K.; Arpitha, H.S.; Deepika, U.R.; Nawneet Kumar, K.; Mondal, P.; Ganesan, P. β-carotene at physiologically attainable concentration induces apoptosis and down-regulates cell survival and antioxidant markers in human breast cancer (MCF-7) cells. Mol. Cell. Biochem. 2017, 436, 1–12. [Google Scholar] [CrossRef]
- Trejo-Solís, C.; Pedraza-Chaverrí, J.; Torres-Ramos, M.; Jiménez-Farfán, D.; Cruz Salgado, A.; Serrano-García, N.; Osorio-Rico, L.; Sotelo, J. Multiple Molecular and Cellular Mechanisms of Action of Lycopene in Cancer Inhibition. Evid.-Based Complement. Altern. Med. 2013, 2013, 705121. [Google Scholar] [CrossRef]
- Rampogu, S.; Balasubramaniyam, T.; Lee, J.-H. Phytotherapeutic applications of alkaloids in treating breast cancer. Biomed. Pharmacother. 2022, 155, 113760. [Google Scholar] [CrossRef]
- Rabi, T.; Bishayee, A. Terpenoids and breast cancer chemoprevention. Breast Cancer Res. Treat. 2008, 115, 223–239. [Google Scholar] [CrossRef]
- Miller, J.A.; Lang, J.E.; Ley, M.; Nagle, R.; Hsu, C.-H.; Thompson, P.A.; Cordova, C.; Waer, A.; Chow, H.-H.S. Human breast tissue disposition and bioactivity of limonene in women with early stage breast cancer. Cancer Prev. Res. 2013, 6, 577–584. [Google Scholar] [CrossRef]
- Shoaib, S.; Ansari, M.A.; Ghazwani, M.; Hani, U.; Jamous, Y.F.; Alali, Z.; Wahab, S.; Ahmad, W.; Weir, S.A.; Alomary, M.N.; et al. Prospective Epigenetic Actions of Organo-Sulfur Compounds against Cancer: Perspectives and Molecular Mechanisms. Cancers 2023, 15, 697. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; Pinti, M.; Nasi, M.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cossarizza, A. Interfering with ROS Metabolism in Cancer Cells: The Potential Role of Quercetin. Cancers 2010, 2, 1288–1311. [Google Scholar] [CrossRef]
- Li, X.; Zhou, N.; Wang, J.; Liu, Z.; Wang, X.; Zhang, Q.; Liu, Q.; Gao, L.; Wang, R. Quercetin suppresses breast cancer stem cells (CD44+/CD24−) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018, 196, 56–62. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin Induces Apoptosis via Caspase Activation, Regulation of Bcl-2, and Inhibition of PI-3-Kinase/Akt and ERK Pathways in a Human Hepatoma Cell Line (HepG2). J. Nutr. 2006, 136, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- de la Parra, C.; Castillo-Pichardo, L.; Cruz-Collazo, A.; Cubano, L.; Redis, R.; Calin, G.A.; Dharmawardhane, S. Soy Isoflavone Genistein-Mediated Downregulation of miR-155 Contributes to the Anticancer Effects of Genistein. Nutr. Cancer 2016, 68, 154–164. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules 2021, 27, 167. [Google Scholar] [CrossRef]
- Manica, D.; da Silva, G.B.; da Silva, A.P.; Marafon, F.; de Maciel, S.F.V.O.; Bagatini, M.D.; Moreno, M. Curcumin promotes apoptosis of human melanoma cells by caspase 3. Cell Biochem. Funct. 2023, 41, 1295–1304. [Google Scholar] [CrossRef]
- Bardak, H.; Uğuz, A.C.; Bardak, Y. Curcumin regulates intracellular calcium release and inhibits oxidative stress parameters, VEGF, and caspase-3/-9 levels in human retinal pigment epithelium cells. Physiol. Int. 2017, 104, 301–315. [Google Scholar] [CrossRef]
- Wang, S.-L.; Li, Y.; Wen, Y.; Chen, Y.-F.; Na, L.-X.; Li, S.-T.; Sun, C.-H. Curcumin, a Potential Inhibitor of Up-regulation of TNF-alpha and IL-6 Induced by Palmitate in 3T3-L1 Adipocytes through NF-kappaB and JNK Pathway. Biomed. Environ. Sci. 2009, 22, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Al-Yousef, N.; Shinwari, Z.; Al Shahrani, B.; Al Showimi, M.; Al Moghrabi, N. Curcumin induces re-expression of BRCA1 and suppression of γ synuclein by modulating DNA promoter methylation in breast cancer cell lines. Oncol. Rep. 2020, 43, 827–838. [Google Scholar] [CrossRef]
- Lycopene Inhibition of Cell Cycle Progression in Breast and Endometrial Cancer Cells is Associated with Reduction in Cyclin D Levels and Retention of p27Kip1 in the Cyclin E–cdk2 Complexes. Portal de Periódicos Da CAPES; Portal.periodicos. CAPES. 2024. Available online: https://www.periodicos.capes.gov.br/index.php/acervo/buscador.html?task=detalhes&id=W1964084914 (accessed on 30 June 2025).
- Park, G.B.; Park, S.H.; Kim, D.; Kim, Y.S.; Yoon, S.H.; Hur, D.Y. Berberine induces mitochondrial apoptosis of EBV-transformed B cells through p53-mediated regulation of XAF1 and GADD45α. Int. J. Oncol. 2016, 49, 411–421. [Google Scholar] [CrossRef]
- Yao, M.; Zheng, J.; Ai, Y.; Wang, Y.; Wang, J. Berberine inhibits NLRP3 inflammasome pathway in human triple-negative breast cancer MDA-MB-231 cell. BMC Complement. Altern. Med. 2019, 19, 216. [Google Scholar] [CrossRef]
- Miller, J.A.; Thompson, P.A.; Hakim, I.A.; Chow, H.-H.S.; Thomson, C.A. d-Limonene: A bioactive food component from citrus and evidence for a potential role in breast cancer prevention and treatment. Oncol. Rev. 2010, 5, 31–42. [Google Scholar] [CrossRef]
- Morimitsu, Y.; Nakagawa, Y.; Hayashi, K.; Fujii, H.; Kumagai, T.; Nakamura, Y.; Osawa, T.; Horio, F.; Itoh, K.; Iida, K.; et al. A Sulforaphane Analogue That Potently Activates the Nrf2-dependent Detoxification Pathway. J. Biol. Chem. 2001, 277, 3456–3463. [Google Scholar] [CrossRef]
- Sinha, S.; Sharma, S.; Sharma, A.; Vora, J.; Shrivastava, N. Sulforaphane-cisplatin combination inhibits the stemness and metastatic potential of TNBCs via down regulation of sirtuins-mediated EMT signaling axis. Phytomedicine 2021, 84, 153492. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, S.M.; Royce, S.G.; Licciardi, P.V.; Karagiannis, T.C. Dietary Sulforaphane in Cancer Chemoprevention: The Role of Epigenetic Regulation and HDAC Inhibition. Antioxid. Redox Signal. 2015, 22, 1382–1424. [Google Scholar] [CrossRef]
- Preiser, J.-C. Oxidative Stress. J. Parenter. Enter. Nutr. 2012, 36, 147–154. [Google Scholar] [CrossRef]
- Hecht, F.; Pessoa, C.F.; Gentile, L.B.; Rosenthal, D.; Carvalho, D.P.; Fortunato, R.S. The role of oxidative stress on breast cancer development and therapy. Tumor Biol. 2016, 37, 4281–4291. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and their effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar] [CrossRef]
- Younas, M.; Hano, C.; Giglioli-Guivarc’h, N.; Abbasi, B.H. Mechanistic evaluation of phytochemicals in breast cancer remedy: Current understanding and future perspectives. RSC Adv. 2018, 8, 29714–29744. [Google Scholar] [CrossRef] [PubMed]
- Danforth, D.N. The Role of Chronic Inflammation in the Development of Breast Cancer. Cancers 2021, 13, 3918. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Si, H. Synergistic anti-inflammatory effects and mechanisms of the combination of resveratrol and curcumin in human vascular endothelial cells and rodent aorta. J. Nutr. Biochem. 2022, 108, 109083. [Google Scholar] [CrossRef]
- Szczepanek, J.; Skorupa, M.; Jarkiewicz-Tretyn, J.; Cybulski, C.; Tretyn, A. Harnessing Epigenetics for Breast Cancer Therapy: The Role of DNA Methylation, Histone Modifications, and MicroRNA. Int. J. Mol. Sci. 2023, 24, 7235. [Google Scholar] [CrossRef]
- Laxmi, A.; Garg, S.S.; Singh, A.; Prabhakar, P.K.; Gupta, J. Histone Modifying Potential of Dietary Phytochemicals: Implications in Treating Breast Cancer. Curr. Pharmacol. Rep. 2023, 9, 489–510. [Google Scholar] [CrossRef]
- Prajapati, K.S.; Gupta, S.; Kumar, S. Targeting Breast Cancer-Derived Stem Cells by Dietary Phytochemicals: A Strategy for Cancer Prevention and Treatment. Cancers 2022, 14, 2864. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Xie, C.; Zhu, J.; Meng, Y.; Chen, Y.; Li, Y.; Jiang, Y.; Yang, X.; Wang, S.; et al. Sonic hedgehog and Wnt/β-catenin pathways mediate curcumin inhibition of breast cancer stem cells. Anti-Cancer Drugs 2018, 29, 208–215. [Google Scholar] [CrossRef]
- Naujokat, C.; McKee, D.L. The “Big Five” Phytochemicals Targeting Cancer Stem Cells: Curcumin, EGCG, Sulforaphane, Resveratrol and Genistein. Curr. Med. Chem. 2020, 27, 28. [Google Scholar] [CrossRef]
- Castro, N.P.; Rangel, M.C.; Merchant, A.S.; MacKinnon, G.; Cuttitta, F.; Salomon, D.S.; Kim, Y.S. Sulforaphane Suppresses the Growth of Triple-negative Breast Cancer Stem-like Cells In vitro and In vivo. Cancer Prev. Res. 2019, 12, 147–158. [Google Scholar] [CrossRef]
- Javed, Z.; Khan, K.; Herrera-Bravo, J.; Naeem, S.; Iqbal, M.J.; Sadia, H.; Qadri, Q.R.; Raza, S.; Irshad, A.; Akbar, A.; et al. Genistein as a regulator of signaling pathways and microRNAs in different types of cancers. Cancer Cell Int. 2021, 21, 388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, H.; Zheng, F.; Liu, H.; Qiu, F.; Chen, Y.; Liang, C.-L.; Dai, Z. Resveratrol exerts antitumor effects by downregulating CD8+ CD122+ Tregs in murine hepatocellular carcinoma. OncoImmunology 2020, 9, 1829346. [Google Scholar] [CrossRef]
- Pan, J.; Shen, J.; Si, W.; Du, C.; Chen, D.; Xu, L.; Yao, M.; Fu, P.; Fan, W. Resveratrol promotes MICA/B expression and natural killer cell lysis of breast cancer cells by suppressing c-Myc/miR-17 pathway. Oncotarget 2017, 8, 65743–65758. [Google Scholar] [CrossRef]
- Lee-Chang, C.; Bodogai, M.; Martin-Montalvo, A.; Wejksza, K.; Sanghvi, M.; Moaddel, R.; de Cabo, R.; Biragyn, A. Inhibition of Breast Cancer Metastasis by Resveratrol-Mediated Inactivation of Tumor-Evoked Regulatory B Cells. J. Immunol. 2013, 191, 4141–4151. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Cicero, A.F.G.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2019, 59, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Sun, Q.; Liu, Q.; Li, H.; Zhang, W.; Sun, C. Focus on immune checkpoint PD-1/PD-L1 pathway: New advances of polyphenol phytochemicals in tumor immunotherapy. Biomed. Pharmacother. 2022, 154, 113618. [Google Scholar] [CrossRef]
- Fernandez-Prades, L.; Brasal-Prieto, M.; Alba, G.; Martin, V.; Montserrat-de, S.; Cejudo-Guillen, M.; Santa-Maria, C.; Hala Dakhaoui Granados, B.; Sobrino, F.; Palomares, F.; et al. Sulforaphane Reduces the Chronic Inflammatory Immune Response of Human Dendritic Cells. Nutrients 2023, 15, 3405. [Google Scholar] [CrossRef]
- Santhiravel, S.; Bekhit, A.E.-D.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The Impact of Plant Phytochemicals on the Gut Microbiota of Humans for a Balanced Life. Int. J. Mol. Sci. 2022, 23, 8124. [Google Scholar] [CrossRef]
- Cao, X.; Wang, X.; Ren, Y.; Sun, Y.; Yang, Z.; Ge, J.; Ping, W. Lonicera caerulea L. polyphenols improve short-chain fatty acid levels by reshaping the microbial structure of fermented feces in vitro. Front. Microbiol. 2023, 14, 1228700. [Google Scholar] [CrossRef]
- Nakkarach, A.; Foo, H.L.; Song, A.A.L.; Mutalib, N.E.A.; Nitisinprasert, S.; Withayagiat, U. Anti-cancer and anti-inflammatory effects elicited by short chain fatty acids produced by Escherichia coli isolated from healthy human gut microbiota. Microb. Cell Factories 2021, 20, 36. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Calderón-Montaño, J.M.; Salvador, J.; Robles, A.; López-Lázaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef]
- Farhood, B.; Mortezaee, K.; Goradel, N.H.; Khanlarkhani, N.; Salehi, E.; Nashtaei, M.S.; Najafi, M.; Sahebkar, A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell. Physiol. 2018, 234, 5728–5740. [Google Scholar] [CrossRef]
- Alqahtani, A.M.; Chidambaram, K.; Pino-Figueroa, A.; Chandrasekaran, B.; Dhanaraj, P.; Venkatesan, K. Curcumin-Celecoxib: A synergistic and rationale combination chemotherapy for breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1916–1927. [Google Scholar] [CrossRef]
- Crew, K.D.; Brown, P.; Greenlee, H.; Bevers, T.B.; Arun, B.; Hudis, C.; McArthur, H.L.; Chang, J.; Rimawi, M.; Vornik, L.; et al. Phase IB randomized, double-blinded, placebo-controlled, dose escalation study of polyphenon E in women with hormone receptor-negative breast cancer. Cancer Prev. Res. 2012, 5, 1144–1154. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S242S. [Google Scholar] [CrossRef]
- Del Rio, D.; Costa, L.G.; Lean, M.E.J.; Crozier, A. Polyphenols and health: What compounds are involved? Nutr. Metab. Cardiovasc. Dis. 2010, 20, 1–6. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-Dimensional Cell Culture: A Breakthrough in Vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, srep19103. [Google Scholar] [CrossRef]
- Hatamipour, M.; Johnston, T.P.; Sahebkar, A. One Molecule, Many Targets and Numerous Effects: The Pleiotropy of Curcumin Lies in its Chemical Structure. Curr. Pharm. Des. 2018, 24, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lin, Q.; Zhao, H.; Li, X.; Sang, S.; McClements, D.J.; Long, J.; Jin, Z.; Wang, J.; Qiu, C. Bioaccessibility and bioavailability of phytochemicals: Influencing factors, improvements, and evaluations. Food Hydrocoll. 2023, 135, 108165. [Google Scholar] [CrossRef]
- Rajani, M.; Kanaki, N.S. Phytochemical Standardization of Herbal Drugs and Polyherbal Formulations; Springer: Berlin/Heidelberg, Germany, 2008; pp. 349–369. [Google Scholar] [CrossRef]
- Rodríguez-Fragoso, L.; Martínez-Arismendi, J.L.; Orozco-Bustos, D.; Reyes-Esparza, J.; Torres, E.; Burchiel, S.W. Potential Risks Resulting from Fruit/Vegetable-Drug Interactions: Effects on Drug-Metabolizing Enzymes and Drug Transporters. J. Food Sci. 2011, 76, R112–R124. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Peeters, M.; Bogaerts, A.; Smits, E.; Deben, C. Oxidative Stress-Inducing Anticancer Therapies: Taking a Closer Look at Their Immunomodulating Effects. Antioxidants 2020, 9, 1188. [Google Scholar] [CrossRef]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunol. 2021, 71, 507–526. [Google Scholar] [CrossRef]
- Forcados, G.E.; James, D.B.; Sallau, A.B.; Muhammad, A.; Mabeta, P. Oxidative Stress and Carcinogenesis: Potential of Phytochemicals in Breast Cancer Therapy. Nutr. Cancer 2017, 69, 365–374. [Google Scholar] [CrossRef]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem.-Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex Interactions between Phytochemicals. The Multi-Target Therapeutic Concept of Phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Mahanta, S.K.; Challa, S.R. Phytochemicals as pro-oxidants in Cancer: Therapeutic implications. In Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Springer: Singapore, 2022; pp. 1–9. [Google Scholar] [CrossRef]
- Lin, J.K.-S.; Tujios, S.R. Hidden Dangers: Herbal and Dietary Supplement Induced Hepatotoxicity. Livers 2023, 3, 618–636. [Google Scholar] [CrossRef]
- Gómez-Garduño, J.; León-Rodríguez, R.; Alemón-Medina, R.; Pérez-Guillé, B.E.; Soriano-Rosales, R.E.; González-Ortiz, A.; Chávez-Pacheco, J.L.; Solorio-López, E.; Fernandez-Pérez, P.; Rivera-Espinosa, L. Phytochemicals That Interfere With Drug Metabolism and Transport, Modifying Plasma Concentration in Humans and Animals. Dose-Response A Publ. Int. Hormesis Soc. 2022, 20, 15593258221120485. [Google Scholar] [CrossRef]
- Buriani, A.; Fortinguerra, S.; Carrara, M. Clinical Perspectives in Diagnostic-Omics and Personalized Medicine Approach to Monitor Effectiveness and Toxicity of Phytocomplexes; Springer EBooks: Berlin/Heidelberg, Germany, 2017; pp. 385–476. [Google Scholar] [CrossRef]
- Özenver, N.; Efferth, T. Integration of phytochemicals and phytotherapy into cancer precision medicine. In Approaching Complex Diseases, Network-Based Pharmacology and Systems Approach in Bio-Medicine; Bizzarri, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 355–393. Available online: www.researchgate.net/publication/340727805_Integration_of_Phytochemicals_and_Phytotherapy_into_Cancer_Precision_Medicine (accessed on 30 June 2025).
- Varghese, R.; Shringi, H.; Efferth, T.; Ramamoorthy, S. Artificial intelligence driven approaches in phytochemical research: Trends and prospects. Phytochem. Rev. 2025, 24, 1–16. [Google Scholar] [CrossRef]
- Feng, C.; Cheng, J.; Sun, M.; Qiao, C.; Feng, Q.; Fang, N.; Ge, Y.; Rui, M. Artificial intelligence-driven identification and mechanistic exploration of synergistic anti-breast cancer compound combinations from Prunella vulgaris L.-Taraxacum mongolicum Hand.-Mazz. herb pair. Front. Pharmacol. 2025, 15, 1522787. [Google Scholar] [CrossRef]
- Sarkar, T.; Bharadwaj, K.K.; Salauddin, M.; Pati, S.; Chakraborty, R. Phytochemical Characterization, Antioxidant, Anti-inflammatory, Anti-diabetic properties, Molecular Docking, Pharmacokinetic Profiling, and Network Pharmacology Analysis of the Major Phytoconstituents of Raw and Differently Dried Mangifera indica (Himsagar cultivar): An In Vitro and In Silico Investigations. Appl. Biochem. Biotechnol. 2021, 194, 950–987. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, N.D.; Šavikin, K.; Zdunić, G.; Menković, N. Potential of encapsulated phytochemicals in hydrogel particles. In Encapsulation of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 305–342. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Dietary Supplements. 2023. Available online: https://www.fda.gov/food/dietary-supplements (accessed on 30 June 2025).
- Muela-Molina, C.; Perelló-Oliver, S.; García-Arranz, A. False and misleading health-related claims in food supplements on Spanish radio: An analysis from a European Regulatory Framework. Public Health Nutr. 2021, 24, 5156. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration, Human Foods Program. Structure/Function Claims. 2024. Available online: https://www.fda.gov/food/nutrition-food-labeling-and-critical-foods/structurefunction-claims (accessed on 30 June 2025).
- European Medicines Agency. Homepage. 2024. Available online: https://www.ema.europa.eu/en/homepage (accessed on 30 June 2025).
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Jiang, H.; Li, M.; Du, K.; Ma, C.; Cheng, Y.; Wang, S.; Nie, X.; Fu, C.; He, Y. Traditional Chinese Medicine for adjuvant treatment of breast cancer: Taohong Siwu Decoction. Chin. Med. 2021, 16, 129. [Google Scholar] [CrossRef]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.S.; Nesari, T.M.; Dedge, A.P.; Dhumal, V.R.; Shengule, S.A.; Gadgil, M.S.; Salvi, S.; Valiathan, M.V.S. Dosha phenotype specific Ayurveda intervention ameliorates asthma symptoms through cytokine modulations: Results of whole system clinical trial. J. Ethnopharmacol. 2017, 197, 110–117. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Arado, G.M.; Amatto, P.D.P.G.; Marins, M.; Rizzi, E.S.; França, S.D.C.; Coppede, J.D.S.; Carmona, F.; Pereira, A.M.S. Anti-inflammatory and/or immunomodulatory activities of Uncaria tomentosa (cat’s claw) extracts: A systematic review and meta-analysis of in vivo studies. Front. Pharmacol. 2024, 15, 1378408. [Google Scholar] [CrossRef]

| Phytochemical | Natural Source | Observed Effects | References |
|---|---|---|---|
| Quercetin | Apples, onions, berries | Increases antioxidant enzyme activity to neutralize ROS, protects DNA from oxidative damage, inhibits PI3K/Akt signaling | [25,26,27] |
| Genistein | Soybeans | Binds to estrogen receptors ERα and Erβ, affects miRNA expression | [13,28] |
| Curcumin | Turmeric | Suppresses lipid peroxidation and activates the Nrf2 pathway, activates caspase-3 and caspase-9, Inhibits NF-κB for anti-inflammatory effects, modifies DNA methylation patterns to restore normal gene expression | [29,30,31,32,33] |
| Lycopene | Tomatoes, watermelon | Decreases cyclin and CDK production and activity to prevent uncontrollable growth | [34] |
| Berberine | Barberries | Induces apoptosis through mitochondrial dysfunction and activation of the p53 tumor suppressor pathway, Reduces macrophage-induced inflammation in the tumor microenvironment | [35,36] |
| Limonene | Citrus fruits | Disrupts Ras signaling, which is necessary for tumor proliferation | [37] |
| Sulforaphane | Broccoli sprouts | Triggers phase II detoxification enzymes, Suppress epithelial–mesenchymal transition to prevent metastasis, Inhibits histone deacetylases for reactivation of tumor-suppressor genes | [38,39,40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodali, N.; Madu, C.O.; Lu, Y. Phytochemicals in Breast Cancer Prevention and Therapy: Mechanisms, Efficacy, and Future Prospects. Curr. Issues Mol. Biol. 2025, 47, 527. https://doi.org/10.3390/cimb47070527
Kodali N, Madu CO, Lu Y. Phytochemicals in Breast Cancer Prevention and Therapy: Mechanisms, Efficacy, and Future Prospects. Current Issues in Molecular Biology. 2025; 47(7):527. https://doi.org/10.3390/cimb47070527
Chicago/Turabian StyleKodali, Neha, Chikezie O. Madu, and Yi Lu. 2025. "Phytochemicals in Breast Cancer Prevention and Therapy: Mechanisms, Efficacy, and Future Prospects" Current Issues in Molecular Biology 47, no. 7: 527. https://doi.org/10.3390/cimb47070527
APA StyleKodali, N., Madu, C. O., & Lu, Y. (2025). Phytochemicals in Breast Cancer Prevention and Therapy: Mechanisms, Efficacy, and Future Prospects. Current Issues in Molecular Biology, 47(7), 527. https://doi.org/10.3390/cimb47070527




