Transcriptomic Analysis Reveals C-C Motif Chemokine Receptor 1 as a Critical Pathogenic Hub Linking Sjögren’s Syndrome and Periodontitis
Abstract
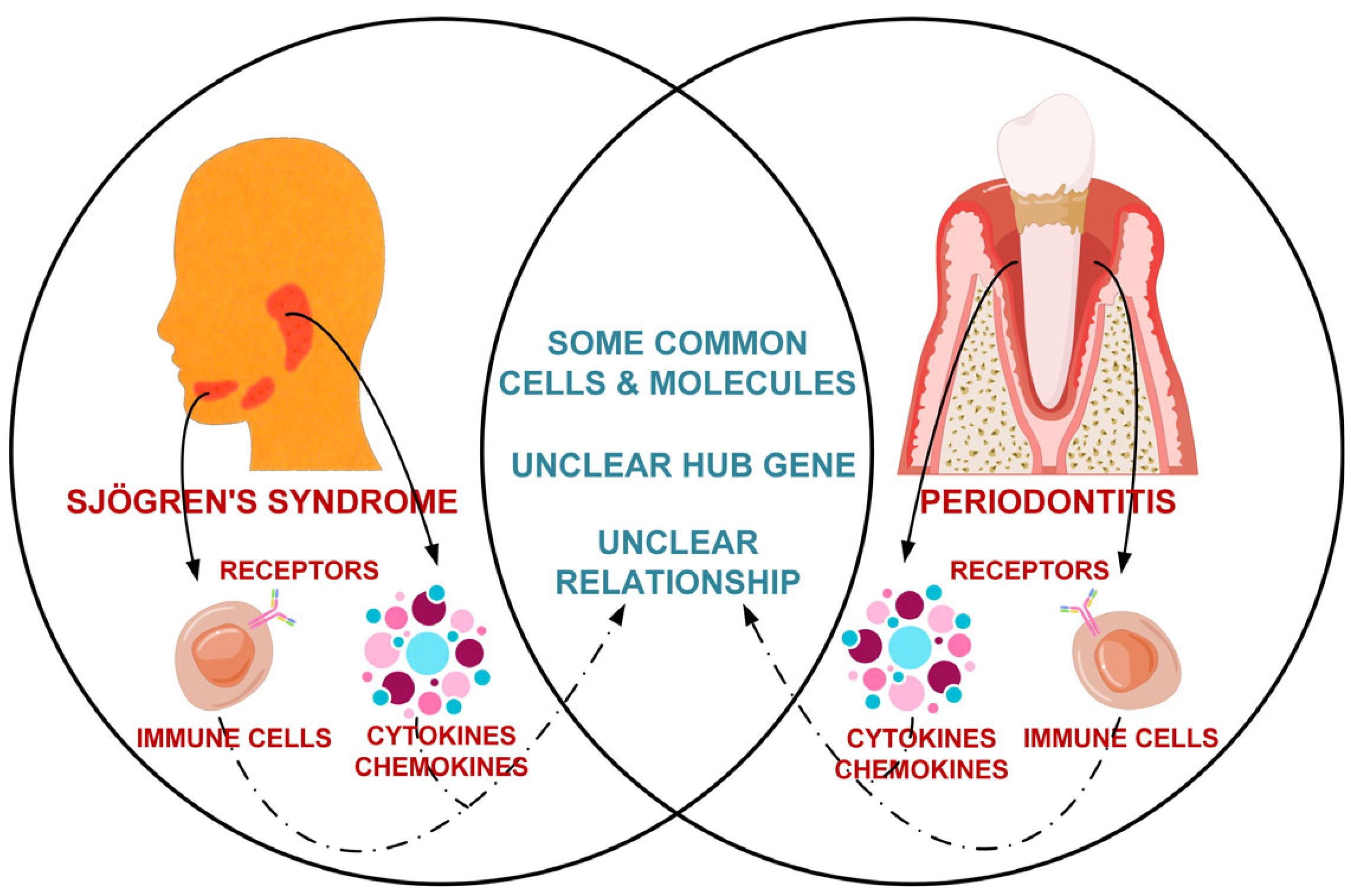
1. Introduction
2. Materials and Methods
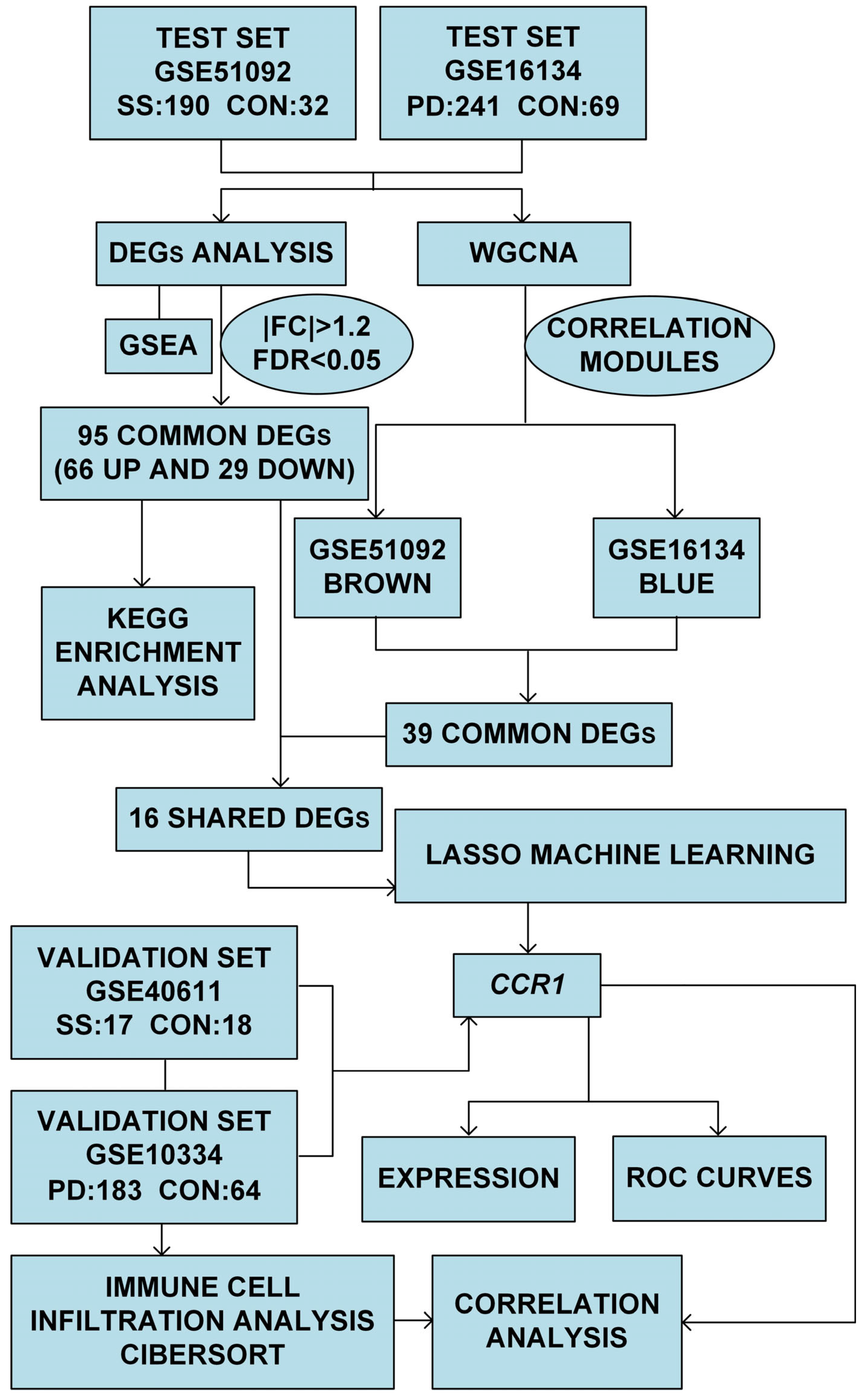
2.1. Study Design and Methodology
2.2. Data Collection
2.3. Differentially Expressed Genes (DEGs) Analysis and Functional Enrichment Analysis
2.4. Weighted Gene Co-Expression Network Analysis (WGCNA)
2.5. Least Absolute Shrinkage and Selection Operator (LASSO) Machine Learning
2.6. Evaluation of Expressions and Receiver Operating Characteristic (ROC) Curves
2.7. Immune Infiltration Analysis
2.8. Statistical Analysis
3. Results
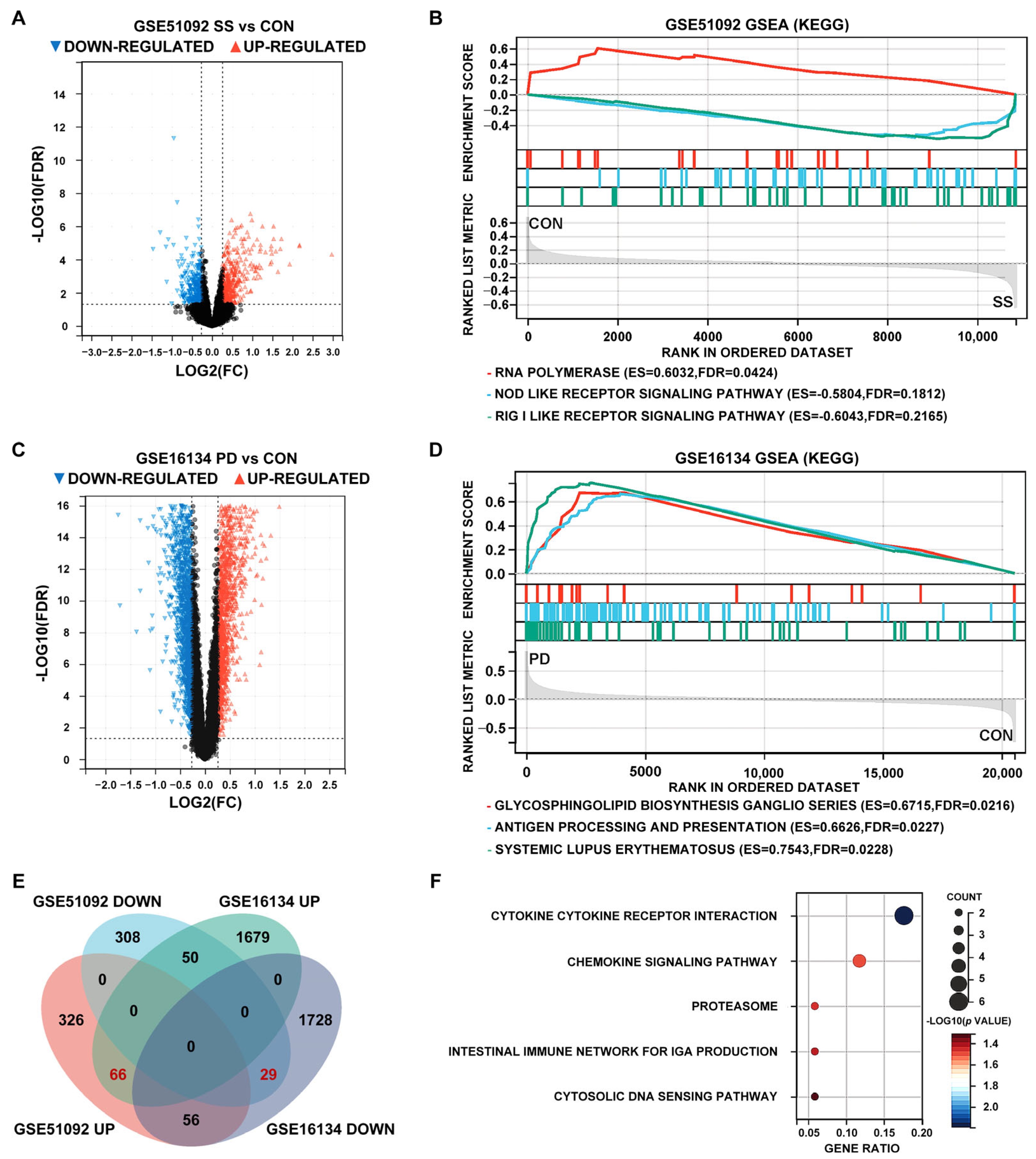
3.1. Identification of DEGs and GSEA Pathways of SS and PD in the Test Sets
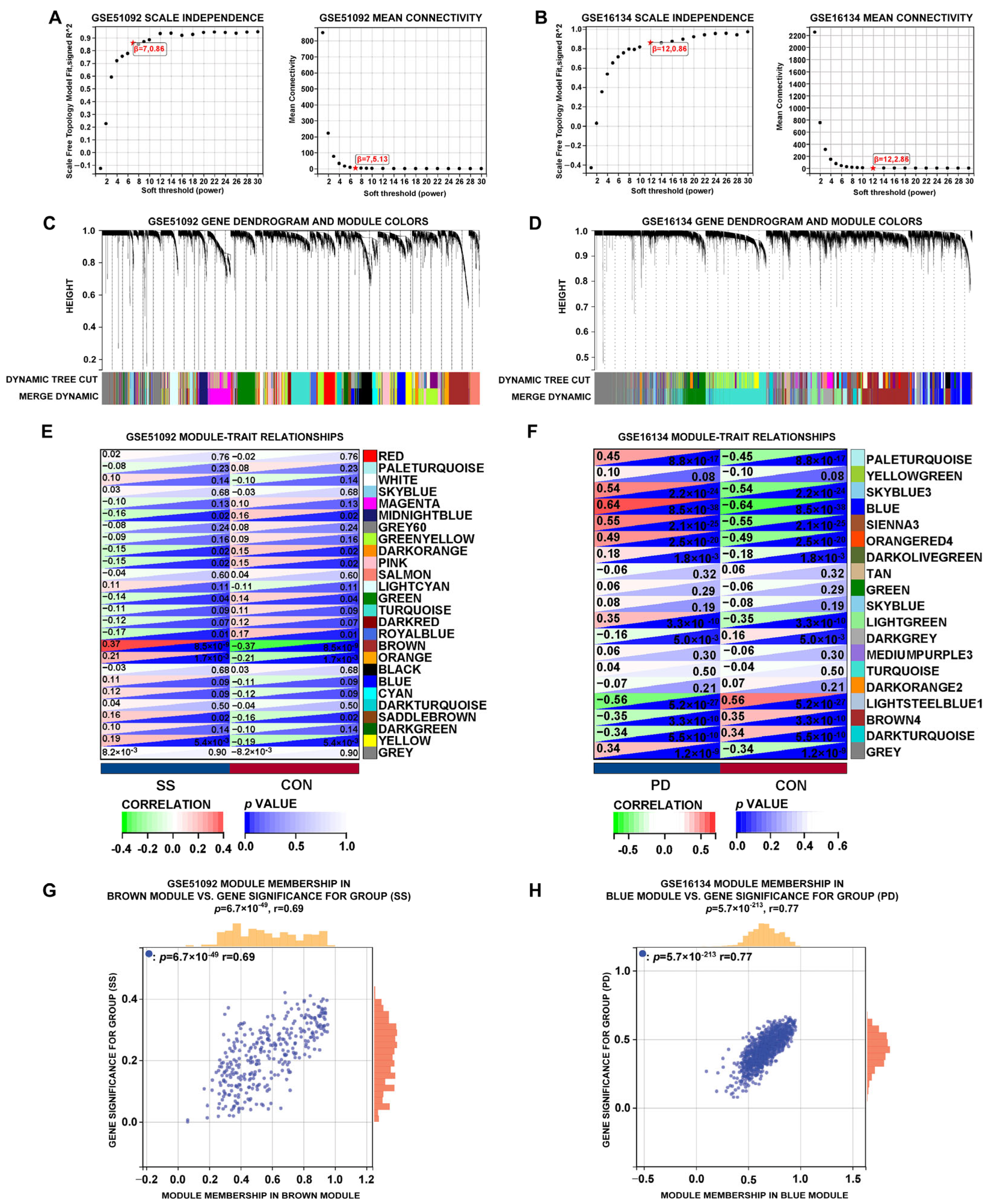
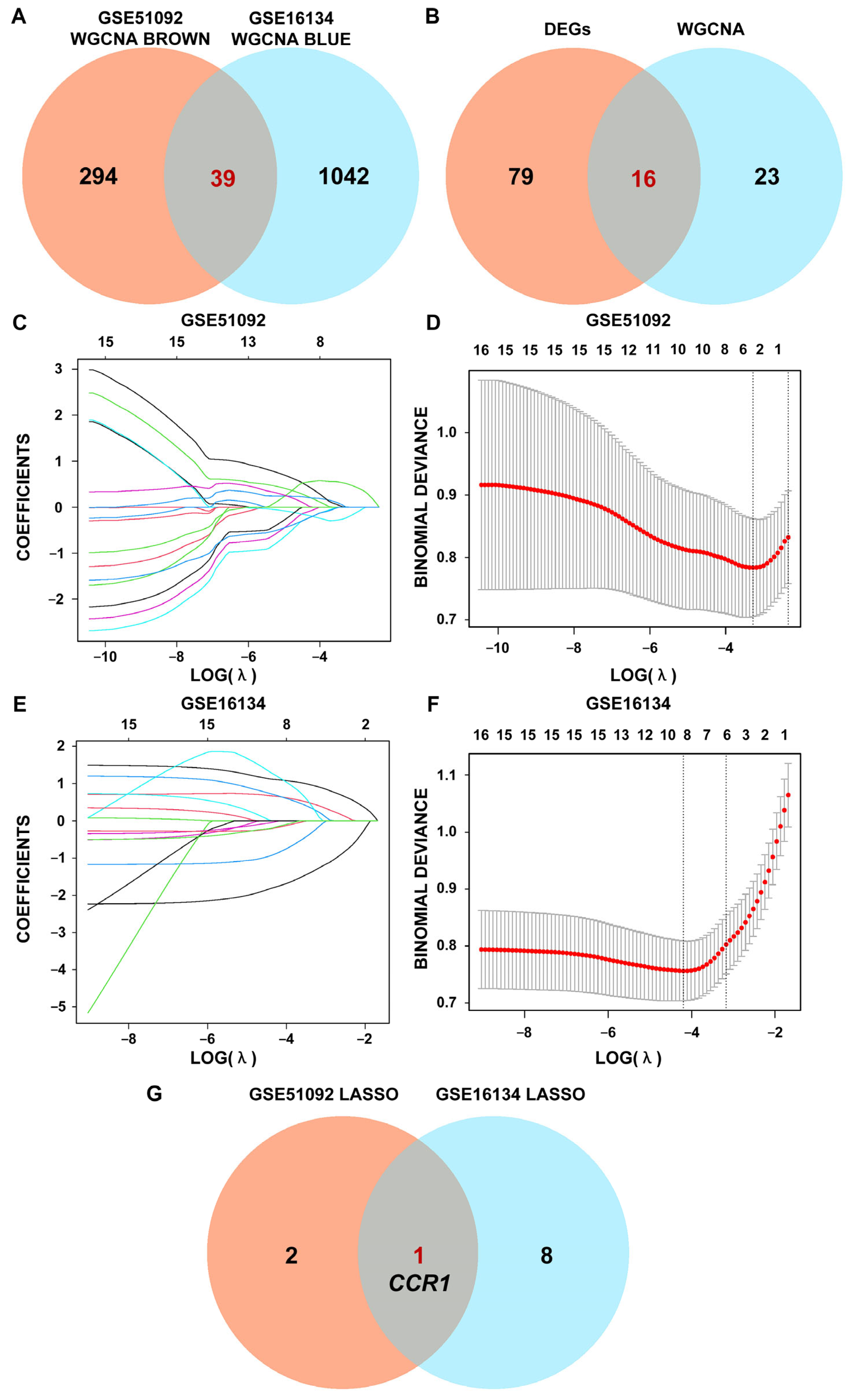
3.2. WGCNA of SS and PD in the Test Sets
3.3. Interacted DEG of SS and PD Using LASSO Regression
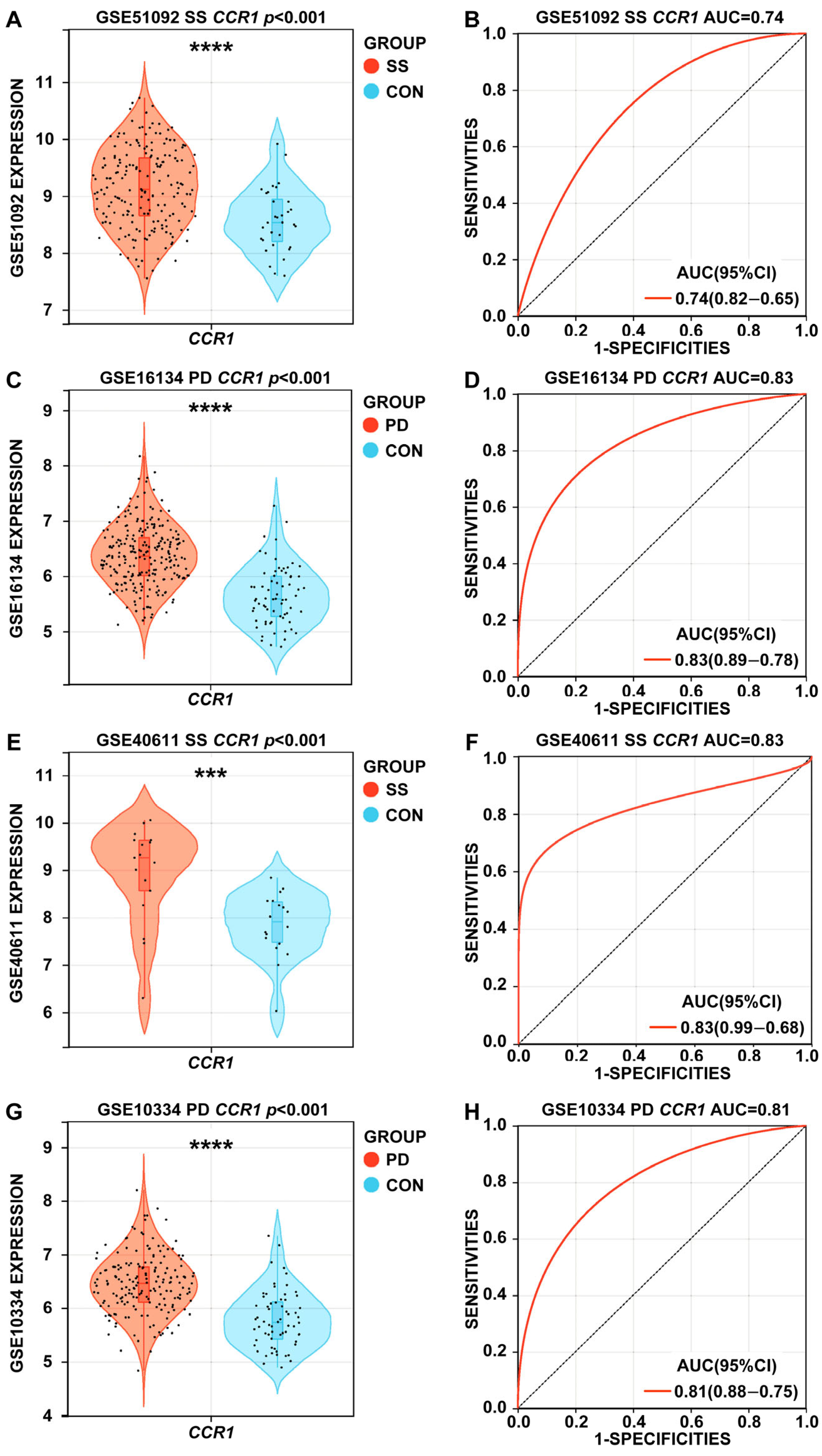
3.4. Expression Levels and ROC Curves of CCR1 in SS and PD
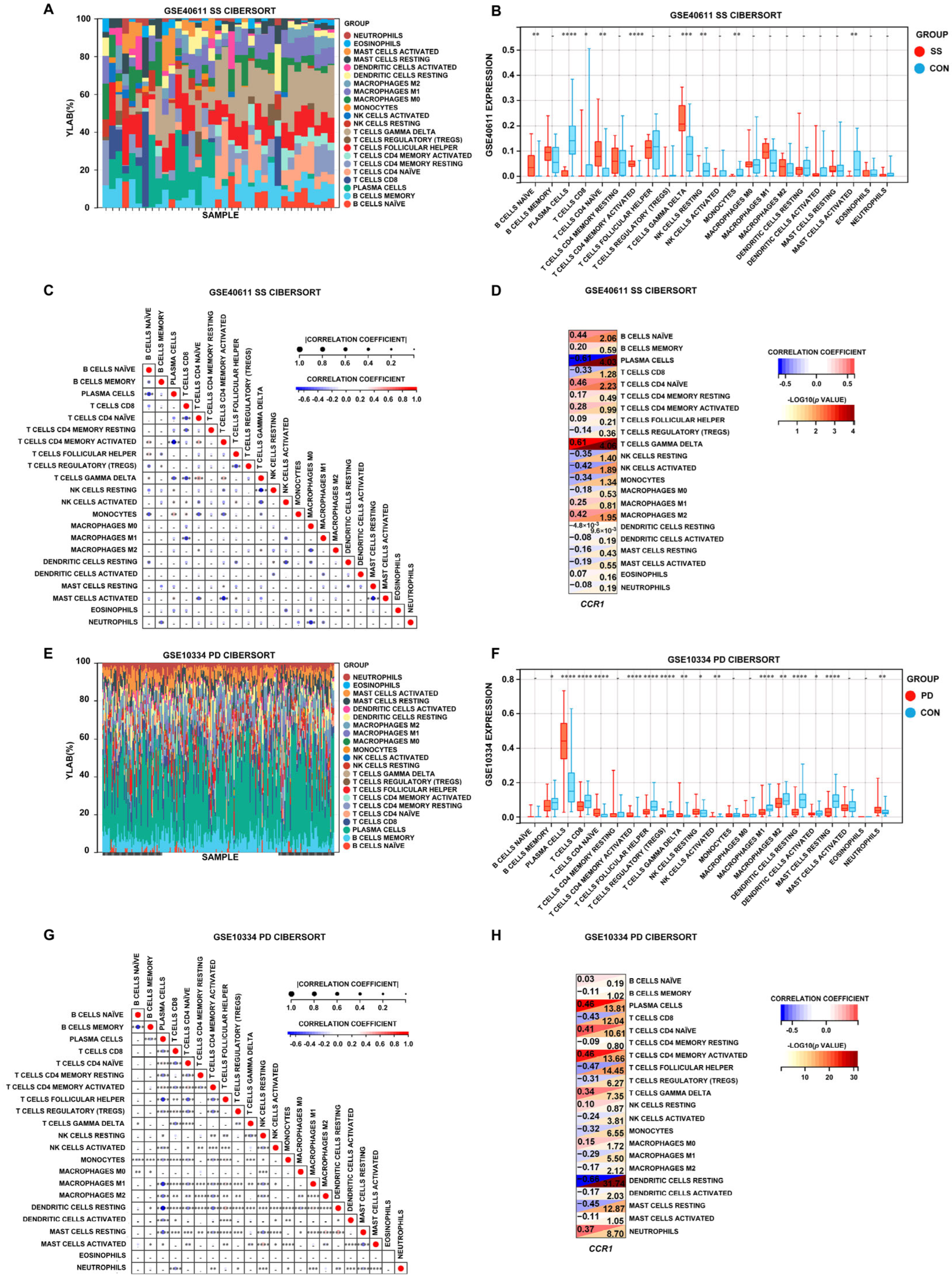
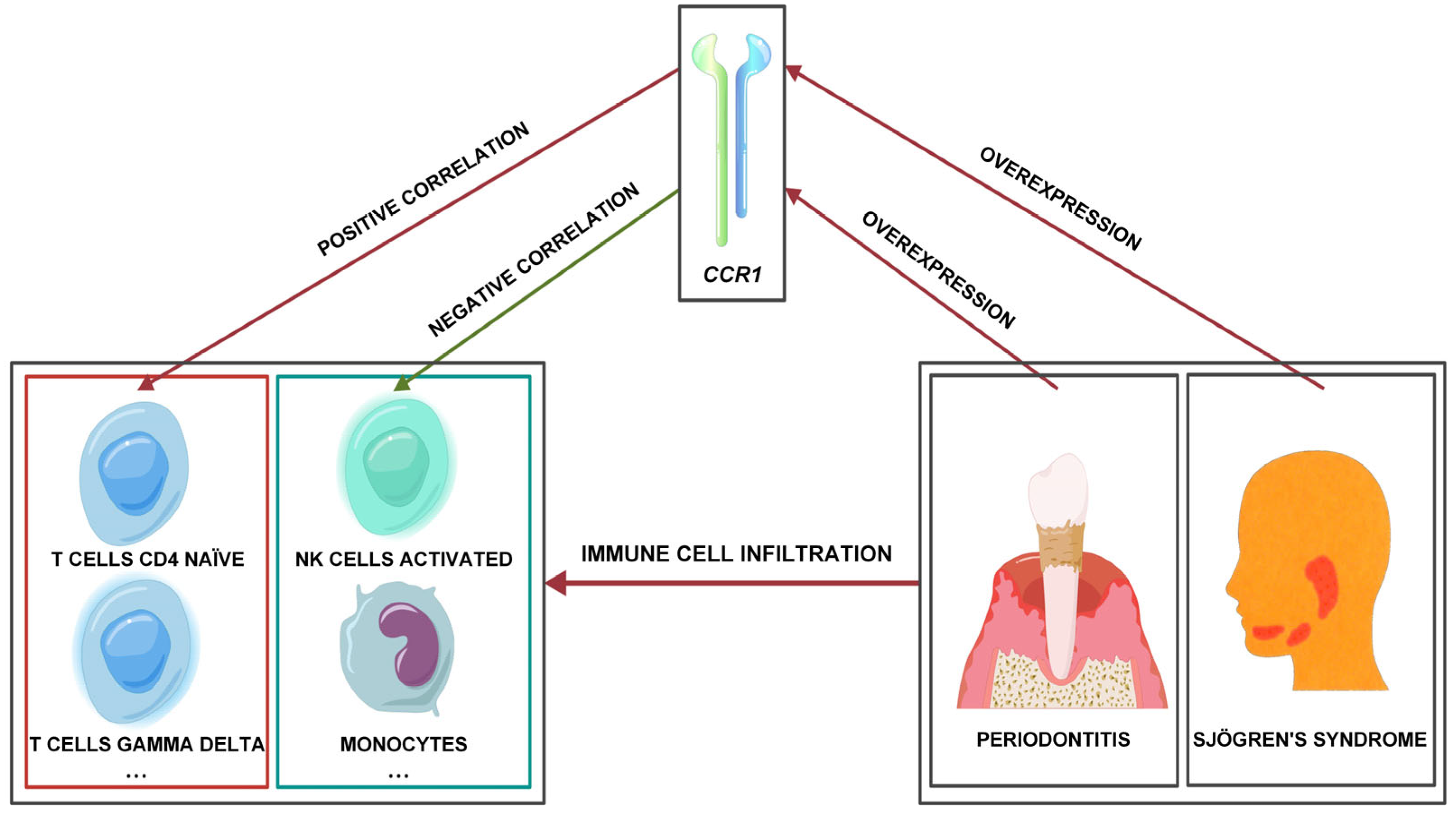
3.5. Immune Cell Infiltration in SS and PD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SS | Sjögren’s syndrome |
| PD | Periodontitis |
| ComBat | Co-variance batch adjustment |
| RMA | Robust multi-array average |
| DEGs | Differentially expressed genes |
| CCR1 | C-C motif chemokine receptor 1 |
| GSEA | Gene set enrichment analysis |
| KEGG | Kyoto encyclopedia of genes and genomes |
| WGCNA | Weighted gene co-expression network analysis |
| LASSO | Least absolute shrinkage and selection operator |
| ROC | Receiver operating characteristic |
| NES | Normalized enrichment score |
| MAD | Median absolute deviation |
| TOM | Topological overlap matrix |
| AUC | Area under the curve |
| CIBERSORT | cell-type identification by estimating relative subsets of RNA transcripts |
References
- Balint, G.; Watson, B.W.; Kean, C.A.; Kean, W.; Rainsford, K.D. Sjögren’s syndrome. Inflammopharmacology 2024, 32, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Gómez-Autet, M.; Morales, P.; Rebassa, J.B.; Llinas Del Torrent, C.; Jagerovic, N.; Pardo, L.; Franco, R. Multimodal Single-Cell Sequencing of B Cells in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2024, 76, 255–267. [Google Scholar] [CrossRef]
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of primary Sjögren’s syndrome: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989. [Google Scholar] [CrossRef]
- Khatri, B.; Tessneer, K.L.; Rasmussen, A.; Aghakhanian, F.; Reksten, T.R.; Adler, A.; Alevizos, I.; Anaya, J.M.; Aqrawi, L.A.; Baecklund, E.; et al. Genome-wide association study identifies Sjögren’s risk loci with functional implications in immune and glandular cells. Nat. Commun. 2022, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; Seror, R.; Bootsma, H.; Bowman, S.J.; Dörner, T.; Gottenberg, J.E.; Mariette, X.; Theander, E.; Bombardieri, S. Characterization of systemic disease in primary Sjögren’s syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology 2015, 54, 2230–2238. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Diaz, P.I.; van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontology 2020 2020, 83, 14–25. [Google Scholar] [CrossRef]
- Darby, I. Risk factors for periodontitis & peri-implantitis. Periodontology 2000 2022, 90, 9–12. [Google Scholar] [CrossRef]
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of periodontitis in dentate people between 2011 and 2020: A systematic review and meta-analysis of epidemiological studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016, 8, 369ra176. [Google Scholar] [CrossRef]
- Bolstad, A.I.; Sehjpal, P.; Lie, S.A.; Fevang, B.S. Periodontitis in patients with systemic lupus erythematosus: A nationwide study of 1,990 patients. J. Periodontol. 2022, 93, 364–372. [Google Scholar] [CrossRef]
- Lugonja, B.; Yeo, L.; Milward, M.R.; Smith, D.; Dietrich, T.; Chapple, I.L.; Rauz, S.; Williams, G.P.; Barone, F.; de Pablo, P.; et al. Periodontitis prevalence and serum antibody reactivity to periodontal bacteria in primary Sjögren’s syndrome: A pilot study. J. Clin. Periodontol. 2016, 43, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Scardina, G.A.; Ruggieri, A.; Messina, P. Periodontal disease and Sjögren syndrome: A possible correlation? Angiology 2010, 61, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Zoellner, H.; Chapple, C.C.; Hunter, N. Microvasculature in gingivitis and chronic periodontitis: Disruption of vascular networks with protracted inflammation. Microsc. Res. Tech. 2002, 56, 15–31. [Google Scholar] [CrossRef]
- Nayar, G.; Gauna, A.; Chukkapalli, S.; Velsko, I.; Kesavalu, L.; Cha, S. Polymicrobial infection alters inflammatory microRNA in rat salivary glands during periodontal disease. Anaerobe 2016, 38, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Tseng, C.F.; Liu, J.M.; Chuang, H.C.; Lei, W.T.; Liu, L.Y.; Yu, Y.C.; Hsu, R.J. Association between Periodontal Disease and Subsequent Sjögren’s Syndrome: A Nationwide Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 771. [Google Scholar] [CrossRef]
- Song, S.; Yang, Y.; Ren, T.; Shen, Y.; Ding, S.; Chang, X.; Liu, C. Enhanced co-expression of TIGIT and PD-1 on γδ T cells correlates with clinical features and laboratory parameters in patients with primary Sjögren’s syndrome. Clin. Rheumatol. 2025, 44, 1245–1257. [Google Scholar] [CrossRef]
- Barel, O.; Aizenbud, Y.; Tabib, Y.; Jaber, Y.; Leibovich, A.; Horev, Y.; Zubeidat, K.; Saba, Y.; Eli-Berchoer, L.; Heyman, O.; et al. γδ T Cells Differentially Regulate Bone Loss in Periodontitis Models. J. Dent. Res. 2022, 101, 428–436. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Zhao, K.; Zhang, S.; Chen, Z. Exploration of the shared diagnostic genes and mechanisms between periodontitis and primary Sjögren’s syndrome by integrated comprehensive bioinformatics analysis and machine learning. Int. Immunopharmacol. 2024, 141, 112899. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wu, C.Y.; Chen, M.H.; Huang, H.Y.; Chen, Y.H.; Tsao, Y.P.; Lai, Y.L.; Lee, S.Y. Periodontal conditions in patients with Sjögren’s syndrome: A meta-analysis. J. Dent. Sci. 2021, 16, 1222–1232. [Google Scholar] [CrossRef]
- Maarse, F.; Jager, D.H.J.; Alterch, S.; Korfage, A.; Forouzanfar, T.; Vissink, A.; Brand, H.S. Sjögren’s syndrome is not a risk factor for periodontal disease: A systematic review. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 118), 225–233. [Google Scholar] [PubMed]
- de Goés Soares, L.; Rocha, R.L.; Bagordakis, E.; Galvão, E.L.; Douglas-De-Oliveira, D.W.; Falci, S.G.M. Relationship between sjögren syndrome and periodontal status: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 223–231. [Google Scholar] [CrossRef]
- Lu, M.C.; Jheng, C.H.; Tsai, T.Y.; Koo, M.; Lai, N.S. Increased dental visits in patients prior to diagnosis of primary Sjögren’s syndrome: A population-based study in Taiwan. Rheumatol. Int. 2014, 34, 1555–1561. [Google Scholar] [CrossRef]
- Reckelkamm, S.L.; Alayash, Z.; Holtfreter, B.; Nolde, M.; Baumeister, S.E. Sjögren’s Disease and Oral Health: A Genetic Instrumental Variable Analysis. J. Dent. Res. 2024, 103, 263–268. [Google Scholar] [CrossRef]
- Lin, T.C.; Tseng, C.F.; Wang, Y.H.; Yu, H.C.; Chang, Y.C. Patients with chronic periodontitis present increased risk for primary Sjögren syndrome: A nationwide population-based cohort study. PeerJ 2018, 6, e5109. [Google Scholar] [CrossRef] [PubMed]
- Antoniazzi, R.P.; Miranda, L.A.; Zanatta, F.B.; Islabão, A.G.; Gustafsson, A.; Chiapinotto, G.A.; Oppermann, R.V. Periodontal conditions of individuals with Sjögren’s syndrome. J. Periodontol. 2009, 80, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Pers, J.O.; D’arbonneau, F.; Devauchelle-Pensec, V.; Saraux, A.; Pennec, Y.L.; Youinou, P. Is periodontal disease mediated by salivary BAFF in Sjögren’s syndrome? Arthritis Rheum. 2005, 52, 2411–2414. [Google Scholar] [CrossRef]
- Ambrósio, L.M.; Rovai, E.S.; França, B.N.; Balzarini, D.A.; Abreu, I.S.; Lopes, S.B.; Nunes, T.B.; Lourenço, S.V.; Pasoto, S.G.; Saraiva, L.; et al. Effects of periodontal treatment on primary sjȫgren’s syndrome symptoms. Braz. Oral Res. 2017, 31, e8. [Google Scholar] [CrossRef]
- Willis, I.M.; Moir, R.D. Signaling to and from the RNA Polymerase III Transcription and Processing Machinery. Annu. Rev. Biochem. 2018, 87, 75–100. [Google Scholar] [CrossRef]
- Kong, X.; Yuan, Z.; Cheng, J. The function of NOD-like receptors in central nervous system diseases. J. Neurosci. Res. 2017, 95, 1565–1573. [Google Scholar] [CrossRef]
- Kato, H.; Fujita, T. RIG-I-like receptors and autoimmune diseases. Curr. Opin. Immunol. 2015, 37, 40–45. [Google Scholar] [CrossRef]
- Leng, H.; Simon, A.K.; Horwood, N.J. Blocking glycosphingolipid production alters autophagy in osteoclasts and improves myeloma bone disease. Autophagy 2024, 20, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Pishesha, N.; Harmand, T.J.; Ploegh, H.L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 2022, 22, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Teruya, H.; Shoda, H.; Itamiya, T.; Tsuchida, Y.; Okamura, T.; Fujio, K. Body weight in systemic lupus erythematosus is associated with disease activity and the adaptive immune system, independent of type I IFN. Front. Immunol. 2025, 16, 1503559. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, R.; Lv, H.; Song, X.; Xu, X.; Chai, L.; Lv, W.; Shang, Z.; Jiang, Y.; Zhang, R. Prioritization of candidate genes for periodontitis using multiple computational tools. J. Periodontol. 2014, 85, 1059–1069. [Google Scholar] [CrossRef]
- Repeke, C.E.; Ferreira, S.B., Jr.; Claudino, M.; Silveira, E.M.; de Assis, G.F.; Avila-Campos, M.J.; Silva, J.S.; Garlet, G.P. Evidences of the cooperative role of the chemokines CCL3, CCL4 and CCL5 and its receptors CCR1+ and CCR5+ in RANKL+ cell migration throughout experimental periodontitis in mice. Bone 2010, 46, 1122–1130. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Xia, H.; Xia, G.; Xu, J.; Lin, S.; Guo, L.; Liu, Y. The effect of the Litcubanine A on the treatment of murine experimental periodontitis by inhibiting monocyte-macrophage chemotaxis and osteoclast differentiation. J. Periodontal Res. 2023, 58, 948–958. [Google Scholar] [CrossRef]
- Chen, L.; Lu, D.; Yu, K.; He, S.; Liu, L.; Zhang, X.; Feng, B.; Wang, X. Bioinformatics Analysis for Identification of Key Genes in Salivary Gland and the Potential of a Combination of Biomarkers for the Diagnosis of SS. J. Inflamm. Res. 2021, 14, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhou, P.; Hang, Y.; Wu, D.; Zhao, N.; Yao, G.; Tang, X.; Sun, L. CFL1 restores the migratory capacity of bone marrow mesenchymal stem cells in primary Sjögren’s syndrome by regulating CCR1 expression. Int. Immunopharmacol. 2024, 128, 111485. [Google Scholar] [CrossRef]
- Imgenberg-Kreuz, J.; Sandling, J.K.; Björk, A.; Nordlund, J.; Kvarnström, M.; Eloranta, M.L.; Rönnblom, L.; Wahren-Herlenius, M.; Syvänen, A.C.; Nordmark, G. Transcription profiling of peripheral B cells in antibody-positive primary Sjögren’s syndrome reveals upregulated expression of CX3CR1 and a type I and type II interferon signature. Scand. J. Immunol. 2018, 87, e12662. [Google Scholar] [CrossRef]
- Ray, S.; Agarwal, P.; Arora, R.; Kapoor, S.; Tyagi, A.K. Expression analysis of calcium-dependent protein kinase gene family during reproductive development and abiotic stress conditions in rice (Oryza sativa L. ssp. indica). Mol. Genet. Genom. 2007, 278, 493–505. [Google Scholar] [CrossRef]
- Zhao, X.; Gu, M.; Xu, X.; Wen, X.; Yang, G.; Li, L.; Sheng, P.; Meng, F. CCL3/CCR1 mediates CD14+CD16− circulating monocyte recruitment in knee osteoarthritis progression. Osteoarthr. Cartil. 2020, 28, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, F.; Zhang, C.; Li, N.; Huang, H.; Shao, Z.; Zhang, M.; Zhan, X.; He, Y.; Ju, Z.; et al. Eosinophil-derived chemokine (hCCL15/23, mCCL6) interacts with CCR1 to promote eosinophilic airway inflammation. Signal Transduct. Target. Ther. 2021, 6, 91. [Google Scholar] [CrossRef]
- Chang, H.W.; Kanegasaki, S.; Jin, F.; Deng, Y.; You, Z.; Chang, J.H.; Kim, D.Y.; Timilshina, M.; Kim, J.R.; Lee, Y.J.; et al. A common signaling pathway leading to degranulation in mast cells and its regulation by CCR1-ligand. Allergy 2020, 75, 1371–1381. [Google Scholar] [CrossRef]
- Gladue, R.P.; Cole, S.H.; Roach, M.L.; Tylaska, L.A.; Nelson, R.T.; Shepard, R.M.; McNeish, J.D.; Ogborne, K.T.; Neote, K.S. The human specific CCR1 antagonist CP-481,715 inhibits cell infiltration and inflammatory responses in human CCR1 transgenic mice. J. Immunol. 2006, 176, 3141–3148. [Google Scholar] [CrossRef]
- Gong, H.; Qiu, X.; Li, P.; Zhao, R.; Wang, B.; Zhu, L.; Huo, X. Immune infiltration analysis reveals immune cell signatures in salivary gland tissue of primary Sjögren’s syndrome. Front. Med. 2023, 10, 1033232. [Google Scholar] [CrossRef] [PubMed]
- Fessler, J.; Fasching, P.; Raicht, A.; Hammerl, S.; Weber, J.; Lackner, A.; Hermann, J.; Dejaco, C.; Graninger, W.B.; Schwinger, W.; et al. Lymphopenia in primary Sjögren’s syndrome is associated with premature aging of naïve CD4+ T cells. Rheumatology 2021, 60, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Sequí-Sabater, J.M.; Beretta, L. Defining the Role of Monocytes in Sjögren’s Syndrome. Int. J. Mol. Sci. 2022, 23, 12765. [Google Scholar] [CrossRef]
- Kaieda, S.; Fujimoto, K.; Todoroki, K.; Abe, Y.; Kusukawa, J.; Hoshino, T.; Ida, H. Mast cells can produce transforming growth factor β1 and promote tissue fibrosis during the development of Sjögren’s syndrome-related sialadenitis. Mod. Rheumatol. 2022, 32, 761–769. [Google Scholar] [CrossRef]
- Jiang, Q.; Huang, X.; Yu, W.; Huang, R.; Zhao, X.; Chen, C. mTOR Signaling in the Regulation of CD4+ T Cell Subsets in Periodontal Diseases. Front. Immunol. 2022, 13, 827461. [Google Scholar] [CrossRef]
- Seidel, A.; Seidel, C.L.; Weider, M.; Junker, R.; Gölz, L.; Schmetzer, H. Influence of Natural Killer Cells and Natural Killer T Cells on Periodontal Disease: A Systematic Review of the Current Literature. Int. J. Mol. Sci. 2020, 21, 9766. [Google Scholar] [CrossRef]
- Almubarak, A.; Tanagala, K.K.K.; Papapanou, P.N.; Lalla, E.; Momen-Heravi, F. Disruption of Monocyte and Macrophage Homeostasis in Periodontitis. Front. Immunol. 2020, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.S.F.; Dos Santos, J.N.; Rocha, C.A.G.; Cury, P.R. Association Between Mast Cells and Collagen Maturation in Chronic Periodontitis in Humans. J. Histochem. Cytochem. 2018, 66, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, S.; Meng, C.; He, Y.; Huang, X.J.; You, H.B. Inhibition of CC chemokine receptor 1 ameliorates osteoarthritis in mouse by activating PPAR-γ. Mol. Med. 2024, 30, 74. [Google Scholar] [CrossRef]
- Futamatsu, H.; Suzuki, J.; Koga, N.; Adachi, S.; Kosuge, H.; Maejima, Y.; Haga, T.; Hirao, K.; Horuk, R.; Isobe, M. CCR1 antagonist prevents the development of experimental autoimmune myocarditis in association with T cell inactivation. J. Mol. Cell. Cardiol. 2006, 40, 853–861. [Google Scholar] [CrossRef] [PubMed]
| Accession | Type | Disease | Control | Case | Source | Platform |
|---|---|---|---|---|---|---|
| GSE51092 | Test | SS | 32 | 190 | Blood | GPL6884 |
| GSE16134 | Test | PD | 69 | 241 | Gingiva | GPL570 |
| GSE40611 | Validation | SS | 18 | 17 | Parotid | GPL10558 |
| GSE10334 | Validation | PD | 64 | 183 | Gingiva | GPL570 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Su, J.; Tang, S.; Jiang, J.; Wei, W.; Chen, J.; Wu, D. Transcriptomic Analysis Reveals C-C Motif Chemokine Receptor 1 as a Critical Pathogenic Hub Linking Sjögren’s Syndrome and Periodontitis. Curr. Issues Mol. Biol. 2025, 47, 523. https://doi.org/10.3390/cimb47070523
Lin Y, Su J, Tang S, Jiang J, Wei W, Chen J, Wu D. Transcriptomic Analysis Reveals C-C Motif Chemokine Receptor 1 as a Critical Pathogenic Hub Linking Sjögren’s Syndrome and Periodontitis. Current Issues in Molecular Biology. 2025; 47(7):523. https://doi.org/10.3390/cimb47070523
Chicago/Turabian StyleLin, Yanjun, Jingjing Su, Shupin Tang, Jun Jiang, Wenwei Wei, Jiang Chen, and Dong Wu. 2025. "Transcriptomic Analysis Reveals C-C Motif Chemokine Receptor 1 as a Critical Pathogenic Hub Linking Sjögren’s Syndrome and Periodontitis" Current Issues in Molecular Biology 47, no. 7: 523. https://doi.org/10.3390/cimb47070523
APA StyleLin, Y., Su, J., Tang, S., Jiang, J., Wei, W., Chen, J., & Wu, D. (2025). Transcriptomic Analysis Reveals C-C Motif Chemokine Receptor 1 as a Critical Pathogenic Hub Linking Sjögren’s Syndrome and Periodontitis. Current Issues in Molecular Biology, 47(7), 523. https://doi.org/10.3390/cimb47070523








