Mechanistic Insights into the Bornyl Diphosphate Synthase from Lavandula angustifolia
Abstract
1. Introduction
2. Materials and Methods
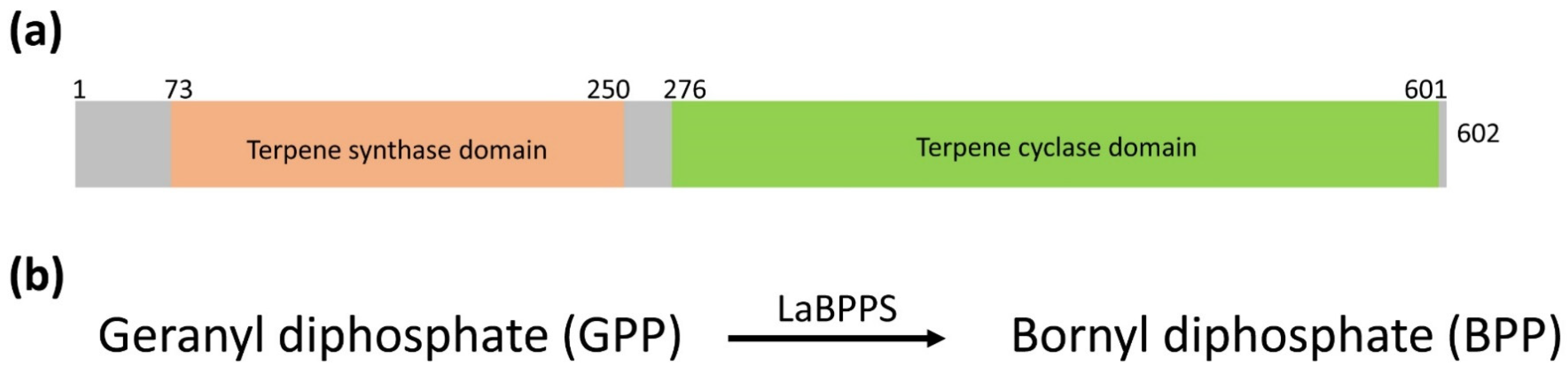
2.1. Bioinformatics Analysis
2.2. Protein Constructs, Expression, and Purification
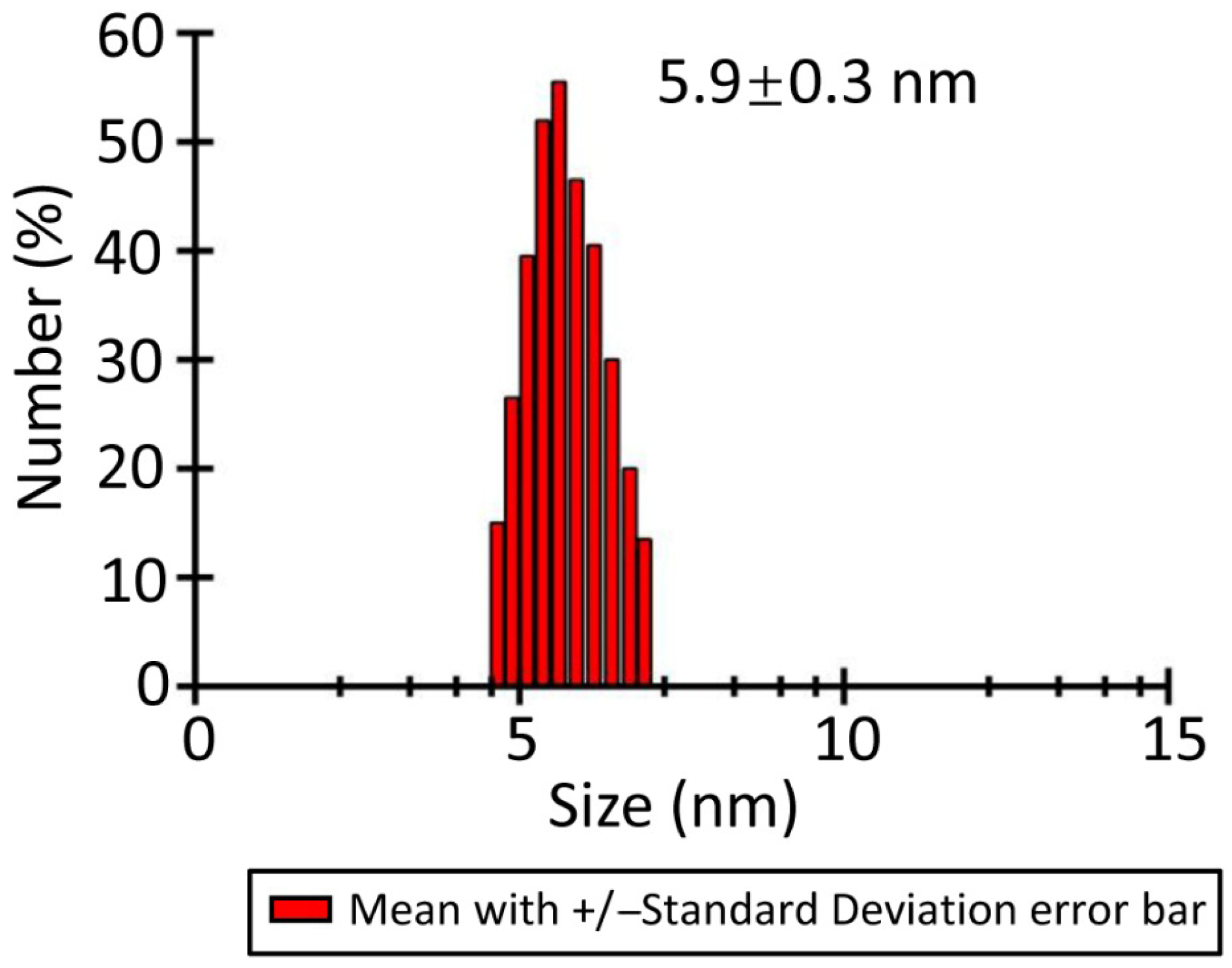
2.3. Dynamic Light Scattering (DLS) Experiments
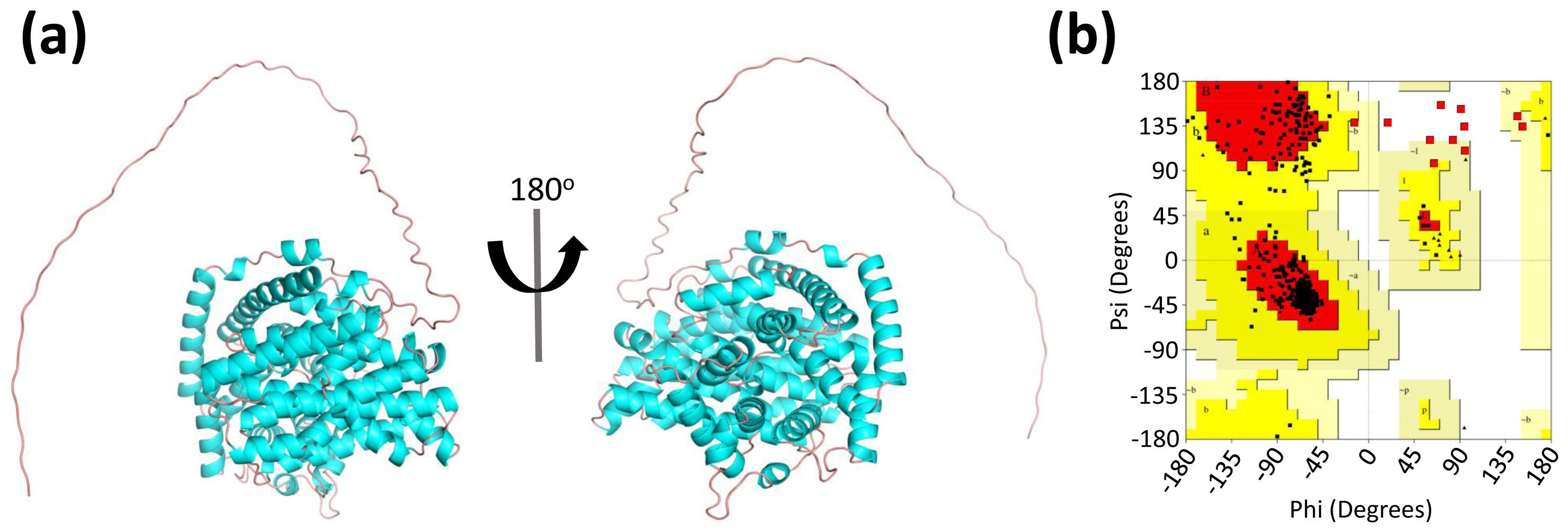
2.4. Structure Prediction and Quality Assessment of LaBPPS
2.5. Enzymatic Activity Assays
2.6. GC-MS Analysis
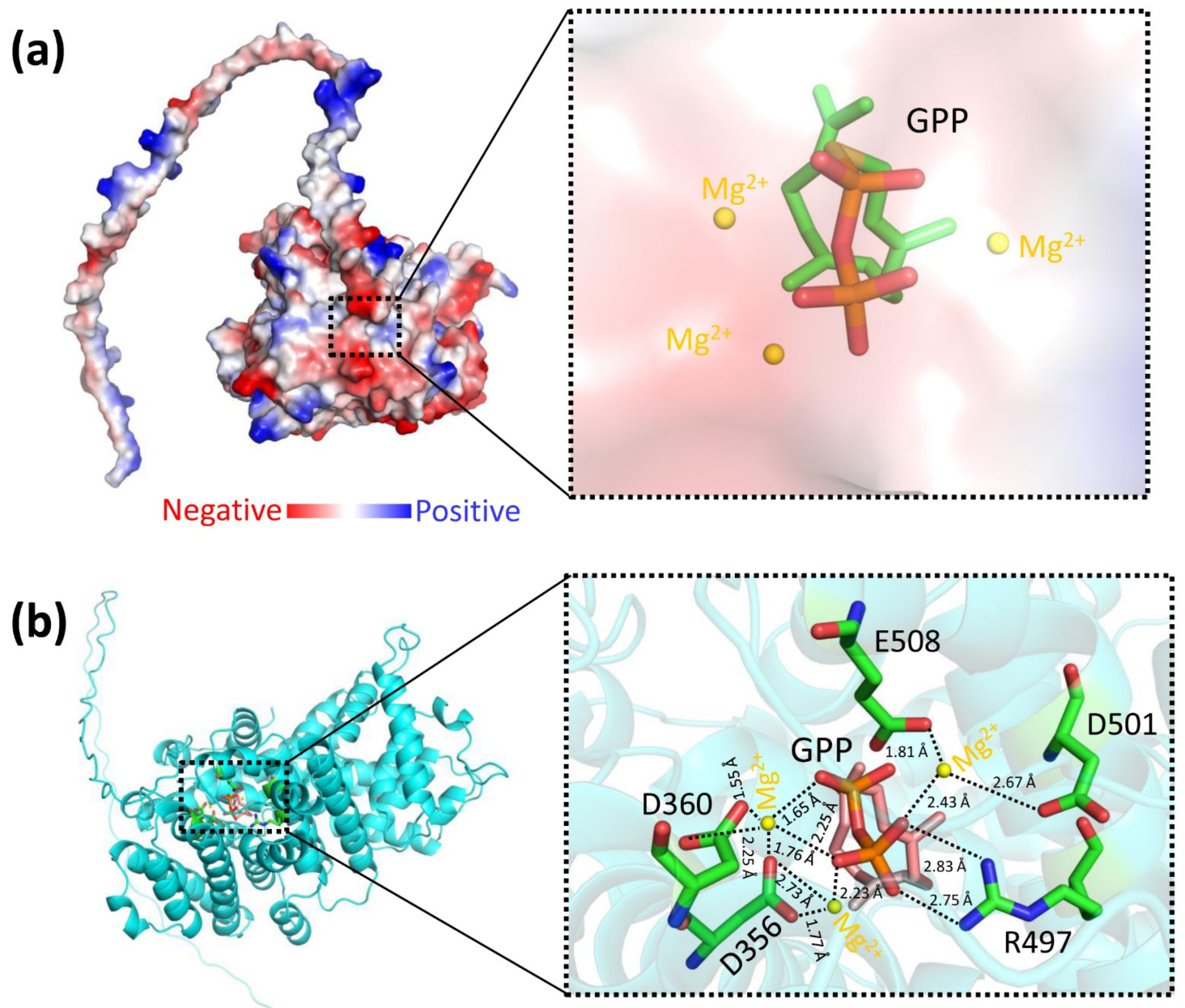
2.7. Molecular Docking
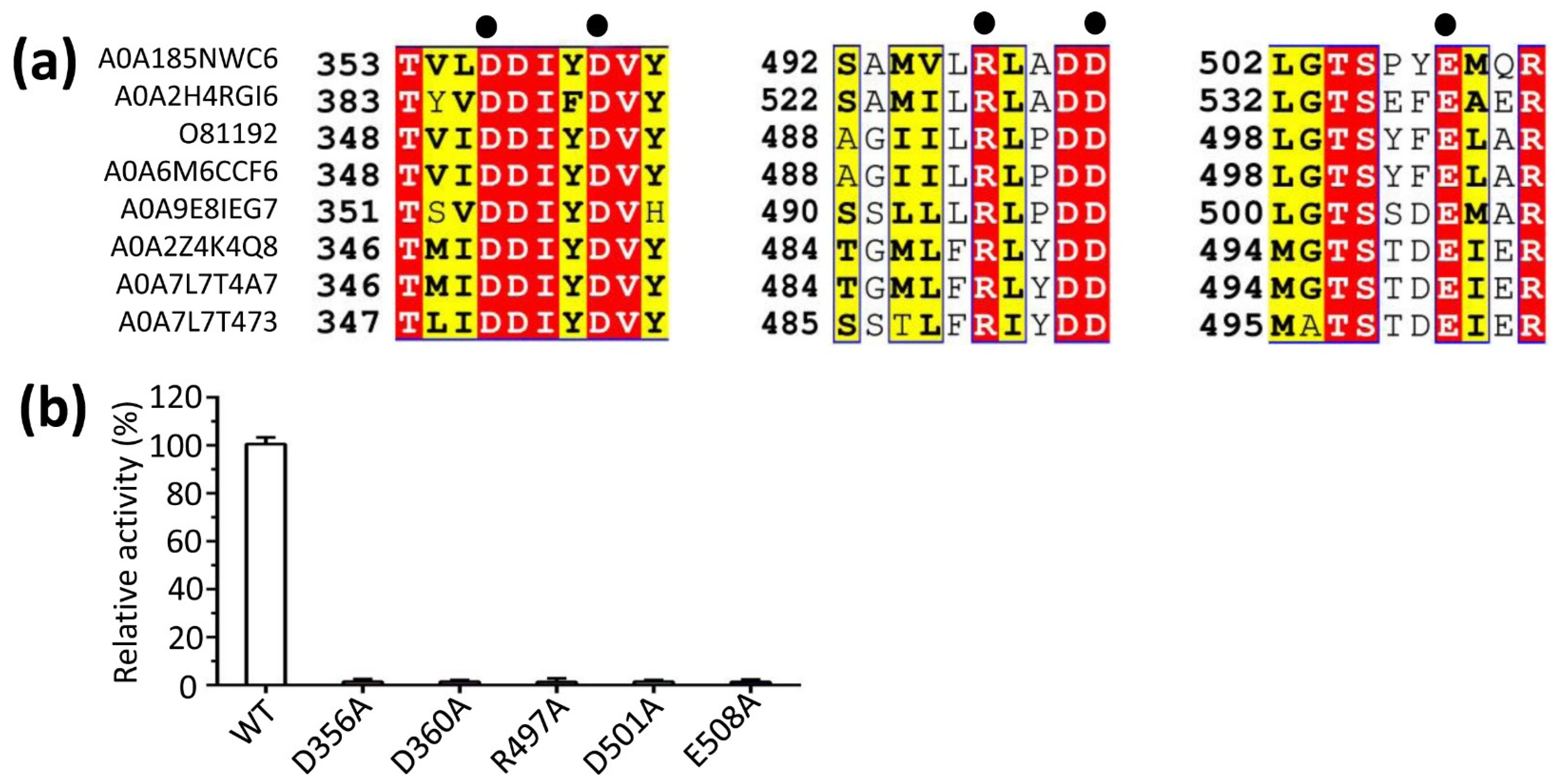
2.8. Enzymatic Activity Assay for Site-Directed Mutagenesis Proteins
2.9. LaBPPS Expression Profiles Analyzed Using RT-qPCR
2.10. Statistical Analysis
3. Results
3.1. Bioinformatics Analysis
3.2. Prediction and Quality Assessment of LaBPPS Structure
3.3. Characterization of LaBPPS by Dynamic Light Scattering
3.4. The Predicted Ligand-Binding Sites of LaBPPS-GPP Complex
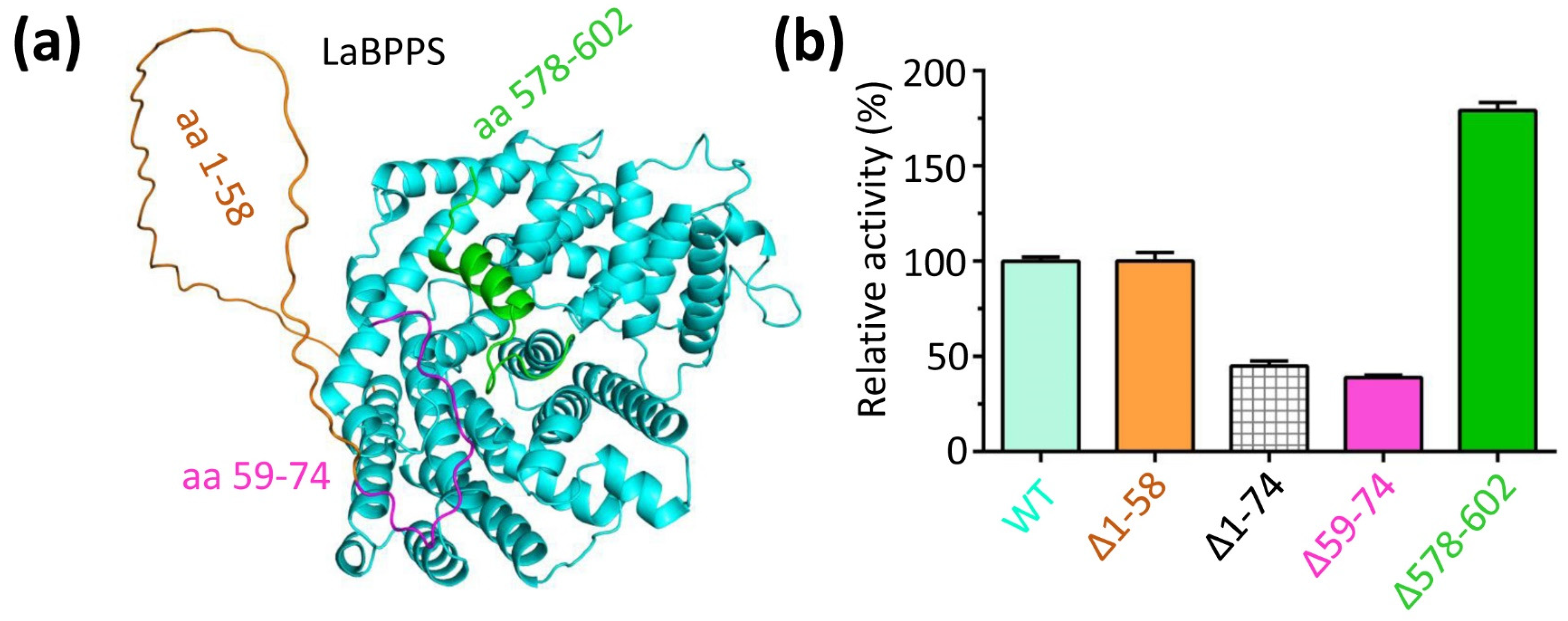
3.5. The N- and C-Terminus Regulated the Catalytic Activity of LaBPPS
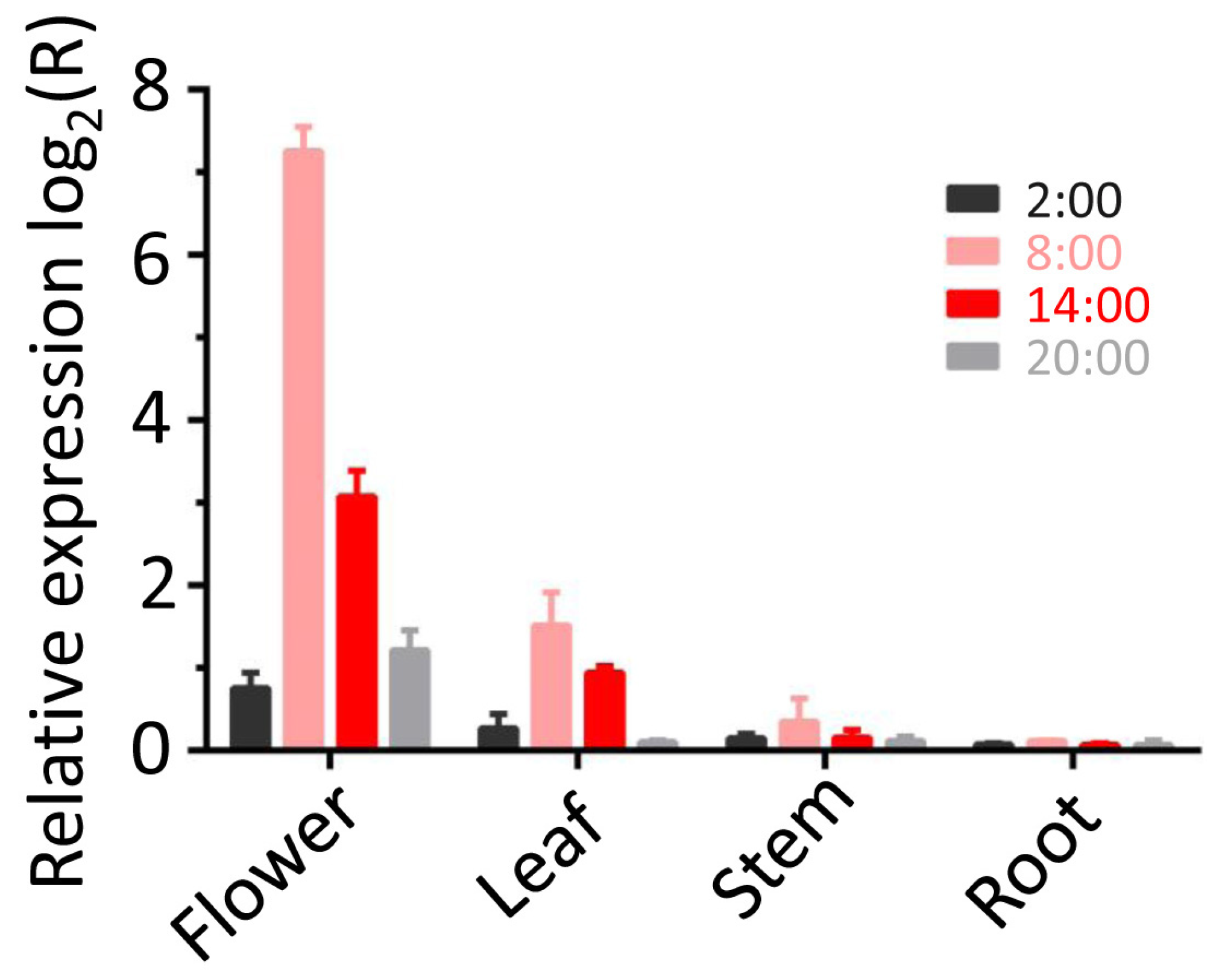
3.6. Spatial and Temporal Regulation Patterns of LaBPPS Gene
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crișan, I.; Ona, A.; Vârban, D.; Muntean, L.; Vârban, R.; Stoie, A.; Mihăiescu, T.; Morea, A. Current Trends for Lavender (Lavandula angustifolia Mill.) Crops and Products with Emphasis on Essential Oil Quality. Plants 2023, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- De Melo Alves Silva, L.C.; de Oliveira Mendes, F.d.C.; de Castro Teixeira, F.; de Lima Fernandes, T.E.; Barros Ribeiro, K.R.; da Silva Leal, K.C.; Dantas, D.V.; Neves Dantas, R.A. Use of Lavandula angustifolia essential oil as a complementary therapy in adult health care: A scoping review. Heliyon 2023, 9, e15446. [Google Scholar] [CrossRef]
- Wilson, T.M.; Poulson, A.; Packer, C.; Carlson, R.E.; Buch, R.M. Essential Oil Profile and Yield of Corolla, Calyx, Leaf, and Whole Flowering Top of Cultivated Lavandula angustifolia Mill. (Lamiaceae) from Utah. Molecules 2021, 26, 2343. [Google Scholar] [CrossRef] [PubMed]
- Landmann, C.; Fink, B.; Festner, M.; Dregus, M.; Engel, K.-H.; Schwab, W. Cloning and functional characterization of three terpene synthases from lavender (Lavandula angustifolia). Arch. Biochem. Biophys. 2007, 465, 417–429. [Google Scholar] [CrossRef]
- Hedayati, S.; Tarahi, M.; Iraji, A.; Hashempur, M.H. Recent developments in the encapsulation of lavender essential oil. Adv. Colloid Interface Sci. 2024, 331, 103229. [Google Scholar] [CrossRef]
- Khan, S.U.; Hamza, B.; Mir, R.H.; Fatima, K.; Malik, F. Lavender Plant: Farming and Health Benefits. Curr. Mol. Med. 2024, 24, 702–711. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Luan, F.; Duan, J.; Zou, J.; Sun, J.; Shi, Y.; Guo, D.; Wang, C.; Wang, X. Therapeutic Potential of Essential Oils Against Ulcerative Colitis: A Review. J. Inflamm. Res. 2024, 17, 3527–3549. [Google Scholar] [CrossRef]
- Liu, D.; Deng, H.; Song, H. Insights into the functional mechanisms of the sesquiterpene synthase GEAS and GERDS in lavender. Int. J. Biol. Macromol. 2025, 299, 140195. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, P. Aroma Characteristics of Lavender Extract and Essential Oil from Lavandula angustifolia Mill. Molecules 2020, 25, 5541. [Google Scholar] [CrossRef]
- Malloggi, E.; Menicucci, D.; Cesari, V.; Frumento, S.; Gemignani, A.; Bertoli, A. Lavender aromatherapy: A systematic review from essential oil quality and administration methods to cognitive enhancing effects. Appl. Psychol. Health Well-Being 2021, 14, 663–690. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Teibo, J.O.; Wasef, L.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al-kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; Papadakis, M. A review of the bioactive components and pharmacological properties of Lavandula species. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 877–900. [Google Scholar] [CrossRef]
- Braunstein, G.D.; Braunstein, E.W. Are Prepubertal Gynaecomastia and Premature Thelarche Linked to Topical Lavender and Tea Tree Oil Use? Touchreviews Endocrinol. 2023, 19, 9. [Google Scholar] [CrossRef]
- Liu, D.; Song, H.; Deng, H.; Abdiriyim, A.; Zhang, L.; Jiao, Z.; Li, X.; Liu, L.; Bai, S. Insights into the functional mechanisms of three terpene synthases from Lavandula angustifolia (Lavender). Front. Plant Sci. 2024, 15, 877–900. [Google Scholar] [CrossRef] [PubMed]
- Bungau, A.F.; Radu, A.-F.; Bungau, S.G.; Vesa, C.M.; Tit, D.M.; Purza, A.L.; Endres, L.M. Emerging Insights into the Applicability of Essential Oils in the Management of Acne Vulgaris. Molecules 2023, 28, 6395. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Sohel, M.; Hossain, M.S.; Ansari, F.; Islam, M.T.; Sultana, F.; Al Mamun, A.; Islam, M.M.; Amin, M.N. A comprehensive review on clinically proven natural products in the management of nerve pain, with mechanistic insights. Heliyon 2023, 9, e15346. [Google Scholar] [CrossRef]
- Prosche, S.; Stappen, I. Flower Power: An Overview on Chemistry and Biological Impact of Selected Essential Oils from Blossoms. Planta Medica 2024, 90, 595–626. [Google Scholar] [CrossRef]
- Vairinhos, J.; Miguel, M.G. Essential oils of spontaneous species of the genus Lavandula from Portugal: A brief review. Z. Naturforschung C 2020, 75, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Aarshageetha, P.; Janci, P.R.R.; Tharani, N.D. Role of Alternate Therapies to Improve the Quality of Life in Menopausal Women: A Systematic Review. J. Mid-Life Health 2023, 14, 153–158. [Google Scholar] [CrossRef]
- Liu, D.; Du, Y.; Abdiriyim, A.; Zhang, L.; Song, D.; Deng, H.; Wen, X.; Zhang, Y.; Sun, B. Molecular functional mechanisms of two alcohol acetyltransferases in Lavandula x intermedia (lavandin). Front. Chem. 2025, 13, 1627286. [Google Scholar] [CrossRef]
- Adal, A.M.; Sarker, L.S.; Malli, R.P.N.; Liang, P.; Mahmoud, S.S. RNA-Seq in the discovery of a sparsely expressed scent-determining monoterpene synthase in lavender (Lavandula). Planta 2018, 249, 271–290. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kuzuyama, T. Structural and Mechanistic Insight into Terpene Synthases that Catalyze the Irregular Non-Head-to-Tail Coupling of Prenyl Substrates. ChemBioChem 2018, 20, 29–33. [Google Scholar] [CrossRef]
- Benabdelkader, T.; Guitton, Y.; Pasquier, B.; Magnard, J.L.; Jullien, F.; Kameli, A.; Legendre, L. Functional characterization of terpene synthases and chemotypic variation in three lavender species of section Stoechas. Physiol. Plant. 2014, 153, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Guitton, Y.; Nicolè, F.; Moja, S.; Valot, N.; Legrand, S.; Jullien, F.; Legendre, L. Differential accumulation of volatile terpene and terpene synthase mRNAs during lavender (Lavandula angustifolia and L. x intermedia) inflorescence development. Physiol. Plant. 2010, 138, 150–163. [Google Scholar] [CrossRef]
- Gonzalez, V.; Touchet, S.; Grundy, D.J.; Faraldos, J.A.; Allemann, R.K. Evolutionary and Mechanistic Insights from the Reconstruction of α-Humulene Synthases from a Modern (+)-Germacrene A Synthase. J. Am. Chem. Soc. 2014, 136, 14505–14512. [Google Scholar] [CrossRef] [PubMed]
- Jullien, F.d.r.; Petit, C.c.; Moja, S.; Bony, A.l.; Guitton, Y.; Nicolè, F.; Baudino, S.; Legrand, S.; Benabdelkader, T.; Poirot, K.; et al. Isolation and functional characterization of a s-cadinol synthase, a new sesquiterpene synthase from Lavandula angustifolia. Plant Mol. Biol. 2014, 84, 227–241. [Google Scholar] [CrossRef]
- Despinasse, Y.; Fiorucci, S.; Antonczak, S.; Moja, S.; Bony, A.; Nicolè, F.; Baudino, S.; Magnard, J.-L.; Jullien, F. Bornyl-diphosphate synthase from Lavandula angustifolia: A major monoterpene synthase involved in essential oil quality. Phytochemistry 2017, 137, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Adal, A.M.; Najafianashrafi, E.; Sarker, L.S.; Mahmoud, S.S. Cloning, functional characterization and evaluating potential in metabolic engineering for lavender ( +)-bornyl diphosphate synthase. Plant Mol. Biol. 2022, 111, 117–130. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Gasteiger, E. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Liu, D.; Dou, W.; Song, H.; Deng, H.; Tian, Z.; Chen, R.; Liu, Z.; Jiao, Z.; Akhberdi, O. Insights into the functional mechanism of the non-specific lipid transfer protein nsLTP in Kalanchoe fedtschenkoi (Lavender scallops). Protein Expr. Purif. 2025, 226, 106607. [Google Scholar] [CrossRef]
- Koblan, L.W.; Doman, J.L.; Wilson, C.; Levy, J.M.; Tay, T.; Newby, G.A.; Maianti, J.P.; Raguram, A.; Liu, D.R. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 2018, 36, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Papamichail, D.; Liu, H.; Machado, V.; Gould, N.; Coleman, J.R.; Papamichail, G. Codon Context Optimization in Synthetic Gene Design. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018, 15, 452–459. [Google Scholar] [CrossRef]
- Ranaghan, M.J.; Li, J.J.; Laprise, D.M.; Garvie, C.W. Assessing optimal: Inequalities in codon optimization algorithms. BMC Biol. 2021, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tian, Z.; Tusong, K.; Mamat, H.; Luo, Y. Expression, purification and characterization of CTP synthase PyrG in Staphylococcus aureus. Protein Expr. Purif. 2024, 221, 106520. [Google Scholar] [CrossRef]
- Wayment-Steele, H.K.; Ojoawo, A.; Otten, R.; Apitz, J.M.; Pitsawong, W.; Hömberger, M.; Ovchinnikov, S.; Colwell, L.; Kern, D. Predicting multiple conformations via sequence clustering and AlphaFold2. Nature 2023, 625, 832–839. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- De Beer, T.A.P.; Berka, K.; Thornton, J.M.; Laskowski, R.A. PDBsum additions. Nucleic Acids Res. 2014, 42, D292–D296. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Jabłońska, J.; Pravda, L.; Vařeková, R.S.; Thornton, J.M. PDBsum: Structural summaries of PDB entries. Protein Sci. 2017, 27, 129–134. [Google Scholar] [CrossRef]
- Laskowski, R.A. PDBsum1: A standalone program for generating PDBsum analyses. Protein Sci. 2022, 31, e4473. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.F.C.; Guest, P.C. Multiplex Analyses Using Real-Time Quantitative PCR. In Multiplex Biomarker Techniques: Methods and Applications; Humana: New York, NY, USA, 2017; Volume 1546, pp. 125–133. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Analysis and Normalization of Real-Time Polymerase Chain Reaction (PCR) Experimental Data. Cold Spring Harb. Protoc. 2018, 2018, pdb.top095000. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, D.; Abdiriyim, A.; Zhang, L.; Ruzitohti, B. Functional and mechanistic insights into the stealth protein full-length CpsY is conducive to understanding immune evasion mechanisms by Mycobacterium tuberculosis. Tuberculosis 2025, 152, 102616. [Google Scholar] [CrossRef]
- Liu, D.; Abdiriyim, A.; Zhang, L.; Yu, F. Functional and Mechanistic Insights into the Fatty-Acid CoA Ligase FadK in Escherichia coli. Front. Biosci.-Landmark 2025, 30, 36701. [Google Scholar] [CrossRef]
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Žídek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A.; et al. Highly accurate protein structure prediction for the human proteome. Nature 2021, 596, 590–596. [Google Scholar] [CrossRef]
- Bertoni, M.; Kiefer, F.; Biasini, M.; Bordoli, L.; Schwede, T. Modeling protein quaternary structure of homo- and hetero-oligomers beyond binary interactions by homology. Sci. Rep. 2017, 7, 10480. [Google Scholar] [CrossRef]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository—New features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Studer, G.; Rempfer, C.; Waterhouse, A.M.; Gumienny, R.; Haas, J.; Schwede, T.; Elofsson, A. QMEANDisCo—Distance constraints applied on model quality estimation. Bioinformatics 2020, 36, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Babbar, R.; Sharma, P.; Arora, R.; Sharma, T.; Garg, M.; Singh, S.; Kumar, S.; Sindhu, R.K. Unveiling the phyto-restorative potential of ethereal distillates for atopic dermatitis: An advanced therapeutic approach. J. Complement. Integr. Med. 2024, 22, 213–227. [Google Scholar] [CrossRef]
- Pokajewicz, K.; Czarniecka-Wiera, M.; Krajewska, A.; Maciejczyk, E.; Wieczorek, P.P. Lavandula x intermedia—A Bastard Lavender or a Plant of Many Values? Part II. Biological Activities and Applications of Lavandin. Molecules 2023, 28, 2986. [Google Scholar] [CrossRef] [PubMed]
- Sharmeen, J.; Mahomoodally, F.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Dagli, N.; Dagli, R.; Mahmoud, R.; Baroudi, K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 335. [Google Scholar] [CrossRef]
- Matera, R.; Lucchi, E.; Valgimigli, L. Plant Essential Oils as Healthy Functional Ingredients of Nutraceuticals and Diet Supplements: A Review. Molecules 2023, 28, 901. [Google Scholar] [CrossRef]
| Primers | Primer Sequence (5′-3′) |
|---|---|
| D356A (F) | GTCCTGgcaGACATCTACGATG |
| D356A (R) | GTAGATGTCtgcCAGGACCGTGATC |
| D360A (F) | CATCTACgcaGTATACGGCACCC |
| D360A (R) | CGTATACtgcGTAGATGTCGTCCAGG |
| R497A (F) | GGTTCTGgcaCTGGCAGATGAC |
| R497A (R) | CTGCCAGtgcCAGAACCATG |
| D501A (F) | CTGGCAGATgcaCTGGGCACCTCCC |
| D501A (R) | GGTGCCCAGtgcATCTGCCAGACGC |
| E508A (F) | CCCGTACgcaATGCAACGTGGTG |
| E508A (R) | GTTGCATtgcGTACGGGGAGGTG |
| Codon Optimization | Codon Adaptation Index (CAI) Value | GC Content Value |
|---|---|---|
| Before codon optimization | 46.2% | 43.0% |
| After codon optimization | 81.6% | 51.9% |
| Residues | Residues in Most Favored Regions | Residues in Additional Allowed Regions | Residues in Generously Allowed Regions | Residues in Disallowed Regions | ||||
|---|---|---|---|---|---|---|---|---|
| Residual Properties | Number of residues | Total % of residues | Number of residues | Total % of residues | Number of residues | Total % of residues | Number of residues | Total % of residues |
| LaBPPS | 495 | 89.0 | 50 | 9.0 | 6 | 1.1 | 5 | 0.9 |
| Constructs | Km (μM) | Kcat (min−1) |
|---|---|---|
| WT (wild-type) | 29.37 ± 1.21 | 6.23 ± 0.47 |
| ∆1–58 | 28.12 ± 1.75 | 6.78 ± 0.35 |
| ∆1–74 | 41.72 ± 2.15 | 3.19 ± 0.12 |
| ∆59–74 | 38.49 ± 2.58 | 3.37 ± 0.42 |
| ∆578–602 | 14.83 ± 1.09 | 11.71 ± 0.86 |
| Tissues | Time Points | Metabolite (Quantity μg/g Dry Tissue) |
|---|---|---|
| Flower | 2:00 | 1.29 ± 0.56 |
| 8:00 | 239.53 ± 6.47 | |
| 14:00 | 13.27 ± 1.14 | |
| 20:00 | 3.47 ± 0.83 | |
| Leaf | 2:00 | 0.38 ± 0.02 |
| 8:00 | 8.49 ± 0.92 | |
| 14:00 | 1.95 ± 0.73 | |
| 20:00 | 0.09 ± 0.01 | |
| Stem | 2:00 | 0.11 ± 0.06 |
| 8:00 | 0.95 ± 0.06 | |
| 14:00 | 0.25 ± 0.03 | |
| 20:00 | 0.13 ± 0.09 | |
| Root | 2:00 | 0.08 ± 0.02 |
| 8:00 | 0.38 ± 0.01 | |
| 14:00 | 0.06 ± 0.01 | |
| 20:00 | 0.05 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Li, N.; Yu, F.; Du, Y.; Song, H.; Yao, W. Mechanistic Insights into the Bornyl Diphosphate Synthase from Lavandula angustifolia. Curr. Issues Mol. Biol. 2025, 47, 517. https://doi.org/10.3390/cimb47070517
Liu D, Li N, Yu F, Du Y, Song H, Yao W. Mechanistic Insights into the Bornyl Diphosphate Synthase from Lavandula angustifolia. Current Issues in Molecular Biology. 2025; 47(7):517. https://doi.org/10.3390/cimb47070517
Chicago/Turabian StyleLiu, Dafeng, Na Li, Feng Yu, Yanyan Du, Hongjun Song, and Wenshuang Yao. 2025. "Mechanistic Insights into the Bornyl Diphosphate Synthase from Lavandula angustifolia" Current Issues in Molecular Biology 47, no. 7: 517. https://doi.org/10.3390/cimb47070517
APA StyleLiu, D., Li, N., Yu, F., Du, Y., Song, H., & Yao, W. (2025). Mechanistic Insights into the Bornyl Diphosphate Synthase from Lavandula angustifolia. Current Issues in Molecular Biology, 47(7), 517. https://doi.org/10.3390/cimb47070517






