Salivary Biomarkers as a Predictive Factor in Anxiety, Depression, and Stress
Abstract
1. Introduction
2. Literature Review
- ✓ Study Selection and Data Extraction.
- ✓ Data Synthesis.
- ✓ Inclusion criteria were original articles and reviews published in English; studies involving human participants; articles reporting salivary biomarkers in relation to anxiety, depression, or psychological stress; and peer-reviewed journal publications.
- ✓ Exclusion criteria were animal studies, studies without access to the full text, case reports, editorials, and conference abstracts without supporting data.
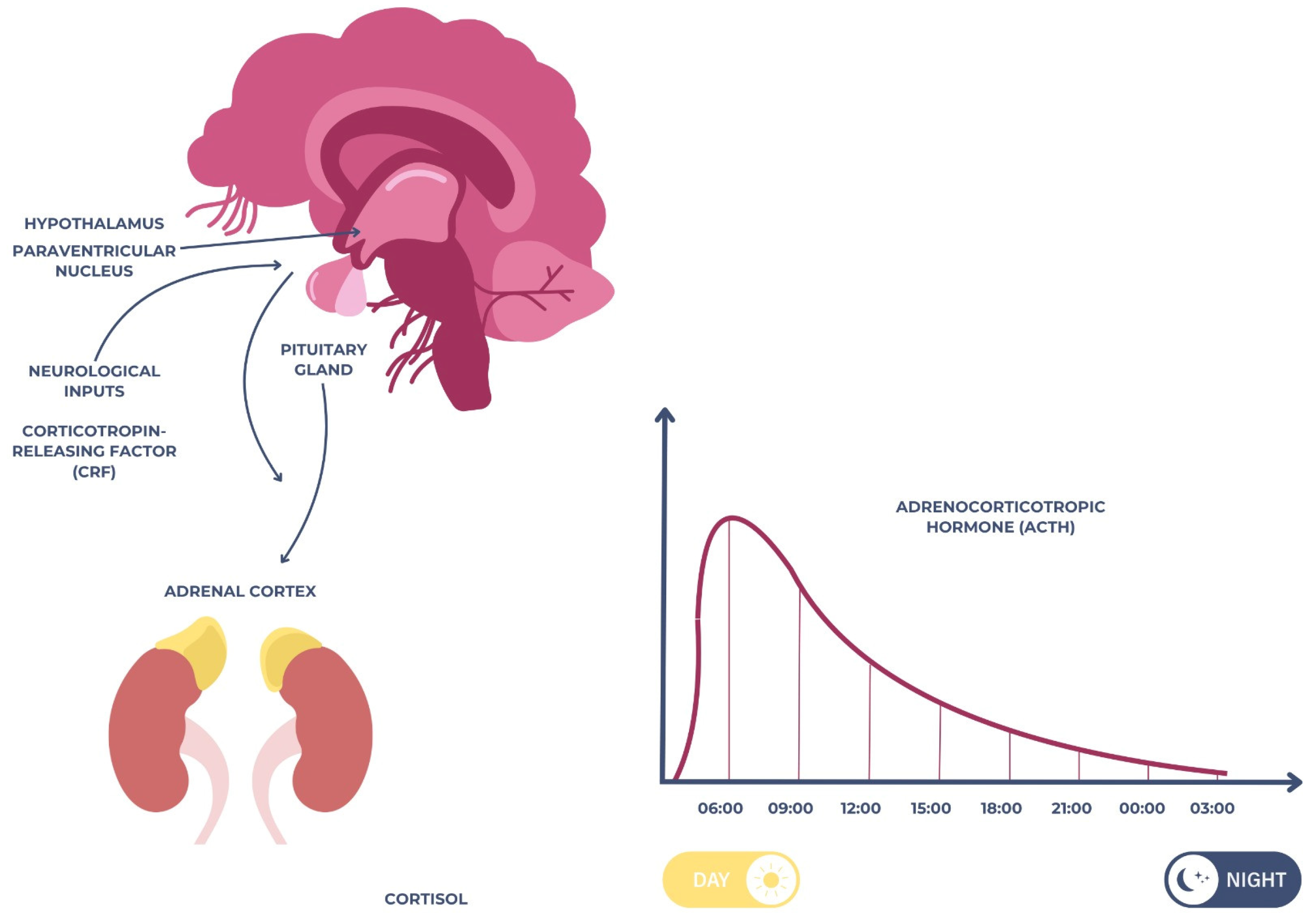
2.1. Cortisol
2.2. Salivary Alpha-Amylase (sAA)
2.3. Salivary Immunoglobulin A (sIgA)
2.4. Chromogranin A (CgA)
2.5. Interleukin-6 (IL-6)
2.6. Tumor Necrosis Factor-Alpha (TNF-α)
2.7. C-Reactive Protein (CRP)
2.8. Brain-Derived Neurotrophic Factor (BDNF)
2.9. Salivary MicroRNAs (miRNAs)
2.10. S100 Proteins
2.11. Additional Biomarkers of Potential Relevance
3. Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| HPA | hypothalamic–pituitary–adrenal |
| SAM | sympathetic–adrenal–medullary |
| sAA | alpha-amylase |
| CgA | chromogranin A |
| sIgA | secretory immunoglobulin A |
| miRNAs | salivary microRNAs |
| IL-6 | interleukin-6 |
| CRP | C-reactive protein |
| TNF-α | tumor necrosis factor-alpha |
| BDNF | brain-derived neurotrophic factor |
| MDD | major depressive disorder |
| GAD | generalized anxiety disorder |
| WHO | World Health Organization |
| PVN | paraventricular nucleus |
| CAR | cortisol awakening response |
| CRF | corticotropin-releasing factor |
| ACTH | adrenocorticotropic hormone |
| SNS | sympathetic nervous system |
| CAMP | cyclic adenosine monophosphate |
| pIgR | polymeric immunoglobulin receptor |
| PKA | protein kinase A |
| PTSD | post-traumatic stress disorder |
| IDO | indoleamine 2,3-dioxygenase |
| ACEs | Adverse Childhood Experiences |
| qRT-PCR | quantitative reverse transcription polymerase chain reaction |
References
- Goodwin, G.M.; Stein, D.J. Generalised Anxiety Disorder and Depression: Contemporary Treatment Approaches. Adv. Ther. 2021, 38, 45–51. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Pranjic, N.; Karabasic, A. Disability Weights and Years Lived with Disability of Depression with and Without Suicidality. Mater Sociomed 2023, 35, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.B.; Ock, M.; Jung, Y.S.; Kim, K.B.; Kim, Y.E.; Kim, K.A.; Yoon, S.J. Estimation of Years Lived with Disability Using a Prevalence-Based Approach: Application to Major Psychiatric Disease in Korea. Int. J. Environ. Res. Public Health 2021, 18, 9056. [Google Scholar] [CrossRef] [PubMed]
- Go, D.S.; Kim, Y.E.; Paik, J.W.; Roh, S.; Yoon, S.J. A comparison of disease burden and the government budget for mental health in Korea. Ment. Health 2020, 31, 471–478. [Google Scholar] [CrossRef]
- Sic, A.; Bogicevic, M.; Brezic, N.; Nemr, C.; Knezevic, N.N. Chronic Stress and Headaches: The Role of the HPA Axis and Autonomic Nervous System. Biomedicines 2025, 13, 463. [Google Scholar] [CrossRef]
- DeMorrow, S. Role of the Hypothalamic-Pituitary-Adrenal Axis in Health and Disease. Int. J. Mol. Sci. 2018, 19, 986. [Google Scholar] [CrossRef]
- Surdu, A.; Foia, L.G.; Luchian, I.; Trifan, D.; Tatarciuc, M.S.; Scutariu, M.M.; Ciupilan, C.; Budala, D.G. Saliva as a Diagnostic Tool for Systemic Diseases—A Narrative Review. Medicina 2025, 61, 243. [Google Scholar] [CrossRef]
- Boroumand, M.; Olianas, A.; Cabras, T.; Manconi, B.; Fanni, D.; Faa, G.; Desiderio, C.; Messana, I.; Castagnola, M. Saliva, a bodily fluid with recognized and potential diagnostic applications. Sep. Sci. 2021, 44, 3677–3690. [Google Scholar] [CrossRef]
- Chojnowska, S.; Ptaszyńska-Sarosiek, I.; Kępka, A.; Knaś, M.; Waszkiewicz, N. Salivary Biomarkers of Stress, Anxiety and Depression. Clin. Med. 2021, 10, 517. [Google Scholar] [CrossRef]
- Tammayan, M.; Jantaratnotai, N.; Pachimsawat, P. Differential responses of salivary cortisol, amylase, and chromogranin A to academic stress. PLoS ONE 2021, 16, e0256172. [Google Scholar] [CrossRef] [PubMed]
- Gholami, N.; Hosseini Sabzvari, B.; Razzaghi, A.; Salah, S. Effect of stress, anxiety and depression on unstimulated salivary flow rate and xerostomia. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 247–252. [Google Scholar]
- Zhang, C.Z.; Cheng, X.Q.; Li, J.Y.; Zhang, P.; Yi, P.; Xu, X.; Zhou, X.D. Saliva in the diagnosis of diseases. Int. Oral Sci. 2016, 8, 133–137. [Google Scholar] [CrossRef]
- Nunes, L.A.; Mussavira, S.; Bindhu, O.S. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: A systematic review. Biochem. Med. 2015, 25, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Ohe, S.; Asano, K.; Ishida, M. Effects of a One-Day Experiential Sheep-Rearing Experience on Motivation, Anxiety, and Frontal Lobe Brain Activity in Patients with Chronic Psychiatric Disorders: A Crossover Pilot Study. Psychiatry Int. 2024, 5, 134–153. [Google Scholar] [CrossRef]
- Shahsavarani, A.M.; Azad Marz Abadi, E.; Hakimi Kalkhoran, M. Stress: Facts and theories through literature review. Int. Med Rev. 2015, 2, 230–241. [Google Scholar]
- Slavich, G.M. Life stress and health. Teach. Psychol. 2016, 43, 346–355. [Google Scholar] [CrossRef]
- Vlenterie, R.; Geuijen, P.M.; van Gelder, M.M.H.J.; Roeleveld, N. Questionnaires and salivary cortisol to measure stress and depression in mid-pregnancy. PLoS ONE 2021, 16, e0250459. [Google Scholar] [CrossRef]
- Sacchini, S.; Bombardi, C.; Arbelo, M.; Herráez, P. The Hypothalamus of the Beaked Whales: The Paraventricular, Supraoptic, and Suprachiasmatic Nuclei. Biology 2023, 12, 1319. [Google Scholar] [CrossRef]
- Kageyama, K.; Iwasaki, Y.; Daimon, M. Hypothalamic Regulation of Corticotropin-Releasing Factor under Stress and Stress Resilience. Int. J. Mol. Sci. 2021, 22, 12242. [Google Scholar] [CrossRef]
- O’Byrne, N.A.; Yuen, F.; Butt, W.Z.; Liu, P.Y. Sleep and Circadian Regulation of Cortisol: A Short Review. Curr. Opin. Endocr. Metab. Res. 2021, 18, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Andreadi, A.; Andreadi, S.; Todaro, F.; Ippoliti, L.; Bellia, A.; Magrini, A.; Chrousos, G.P.; Lauro, D. Modified Cortisol Circadian Rhythm: The Hidden Toll of Night-Shift Work. Int. J. Mol. Sci. 2025, 26, 2090. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Nandam, L.S.; Brazel, M.; Zhou, M.; Jhaveri, D.J. Cortisol and Major Depressive Disorder-Translating Findings From Humans to Animal Models and Back. Front. Psychiatry 2020, 10, 974. [Google Scholar] [CrossRef]
- Ram, D.; Shapira, J.; Holan, G.; Magora, F.; Cohen, S.; Davidovich, E. Audiovisual video eyeglass distraction during dental treatment in children. Quintessence Int. 2010, 41, 673–679. [Google Scholar] [PubMed]
- Mathu-Muju, K.; Wright, J.T. Diagnosis and treatment of molar incisor hypomineralization. Contemp. Dent. Pract. 2006, 27, 604–610. [Google Scholar]
- Adam, E.K.; Quinn, M.E.; Tavernier, R.; McQuillan, M.T.; Dahlke, K.A.; Gilbert, K.E. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 83, 25–41. [Google Scholar] [CrossRef]
- Perini, G.; Cotta Ramusino, M.; Sinforiani, E.; Bernini, S.; Petrachi, R.; Costa, A. Cognitive impairment in depression: Recent advances and novel treatments. Neuropsychiatr. Dis. Treat. 2019, 15, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- James, K.A.; Stromin, J.I.; Steenkamp, N.; Combrinck, M.I. Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front. Endocrinol. 2023, 14, 1085950. [Google Scholar] [CrossRef]
- Vargas, I.; Lopez-Duran, N. The cortisol awakening response after sleep deprivation: Is the cortisol awakening response a “response” to awakening or a circadian process? Health Psychol. 2020, 25, 900–912. [Google Scholar] [CrossRef]
- Dedovic, K.; Ngiam, J. The cortisol awakening response and major depression: Examining the evidence. Neuropsychiatr. Dis. Treat. 2015, 11, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Devine, J.K.; Wolf, J.M. Determinants of cortisol awakening responses to naps and nighttime sleep. Psychoneuroendocrinology 2016, 63, 128–134. [Google Scholar] [CrossRef]
- Stalder, T.; Kirschbaum, C.; Kudielka, B.M.; Adam, E.K.; Pruessner, J.C.; Wust, S.; Dockray, S.; Smyth, N.; Evans, P.; Hellhammer, D.H.; et al. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 2016, 63, 414–432. [Google Scholar] [CrossRef] [PubMed]
- Keevil, B.G. Improving the Dexamethasone Suppression Test. Clin. Chem. 2021, 67, 929–931. [Google Scholar] [CrossRef]
- Foreman, D.M.; Goodyer, I.M. Salivary cortisol hypersecretion in juvenile depression. Child Psychol. Psychiatry 1988, 29, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Burke, H.M.; Fernald, L.C.; Gertler, P.J.; Adler, N.E. Depressive symptoms are associated with blunted cortisol stress responses in very low-income women. Psychosom. Med. 2005, 67, 211–216. [Google Scholar] [CrossRef]
- Qiu, Q.; Yang, L.; He, M.; Gao, W.; Mar, H.; Li, J.; Wang, G. The Effects of Forest Therapy on the Blood Pressure and Salivary Cortisol Levels of Urban Residents: A Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 458. [Google Scholar] [CrossRef] [PubMed]
- Nasser, A.; Ozenne, B.; Høgsted, E.S.; Jensen, P.S.; Frokjaer, V.G. Reliability of three versus five saliva sampling times for assessing the cortisol awakening response. Psychoneuroendocrinology 2023, 147, 105950. [Google Scholar] [CrossRef]
- Constantin, V.; Luchian, I.; Goriuc, A.; Budala, D.G.; Bida, F.C.; Cojocaru, C.; Butnaru, O.M.; Virvescu, D.I. Salivary Biomarkers Identification: Advances in Standard and Emerging Technologies. Oral 2025, 5, 26. [Google Scholar] [CrossRef]
- Salahuddin, M.F.; Bugingo, R.; Mahdi, F.; Spencer, D.; Manzar, M.D.; Paris, J.J. Physiological and Psychological Impacts of Shift Work Among Student Pharmacists: Sex Differences in Stress and Health Outcomes. Psychiatry Int. 2025, 6, 47. [Google Scholar] [CrossRef]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakova, O.G.; Hurt, C.M.; Xiang, Y.; Dell’Acqua, M.L.; Zhang, Q.; Tsien, R.W.; Kobilka, B.K. Organization of beta-adrenoceptor signaling compartments by sympathetic innervation of cardiac myocytes. Cell Biol. 2007, 176, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y.; Pandey, G.N. Adenylyl cyclase-cyclicAMP signaling in mood disorders: Role of the crucial phosphorylating enzyme protein kinase A. Neuropsychiatr. Dis. Treat. 2008, 4, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Zanassi, P.; Paolillo, M.; Feliciello, A.; Avvedimento, E.V.; Gallo, V.; Schinelli, S. cAMP-dependent protein kinase induces cAMP-response element-binding protein phosphorylation via an intracellular calcium release/ERK-dependent pathway in striatal neurons. Biol. Chem. 2001, 276, 11487–11495. [Google Scholar] [CrossRef]
- Ali, N.; Nater, U.M. Salivary Alpha-Amylase as a Biomarker of Stress in Behavioral Medicine. Int. Behav. Med. 2020, 27, 337–342. [Google Scholar] [CrossRef]
- Vineetha, R.; Pai, K.M.; Vengal, M.; Gopalakrishna, K.; Narayanakurup, D. Usefulness of salivary alpha amylase as a biomarker of chronic stress and stress related oral mucosal changes—A pilot study. Clin. Exp. Dent. 2014, 6, e132–e137. [Google Scholar] [CrossRef]
- Vors, O.; Marqueste, T.; Mascret, N. The Trier Social Stress Test and the Trier Social Stress Test for groups: Qualitative investigations. PLoS ONE 2018, 13, e0195722. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Navarrete, F.; Sala, F.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Biomarkers in Psychiatry: Concept, Definition, Types and Relevance to the Clinical Reality. Front. Psychiatry 2020, 11, 432. [Google Scholar] [CrossRef]
- Christidis, N.; Baghernejad, P.; Deyhim, A.; Jasim, H. Salivary Alpha-Amylase in Experimentally-Induced Muscle Pain. Diagnostics 2020, 10, 722. [Google Scholar] [CrossRef]
- Dixit, S.; Kumar, A.; Srinivasan, K. A Current Review of Machine Learning and Deep Learning Models in Oral Cancer Diagnosis: Recent Technologies, Open Challenges, and Future Research Directions. Diagnostics 2023, 13, 1353. [Google Scholar] [CrossRef]
- Takai, N.; Yamaguchi, M.; Aragaki, T.; Eto, K.; Uchihashi, K.; Nishikawa, Y. Effect of physiological stress on salivary cortisol and amylase levels in healthy young adults. Arch. Oral Biol. 2004, 49, 963–968. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Kammerer, M.; O’Reilly, R.; Taylor, A.; Glover, V. Salivary α-amylase stability, diurnal profile and lack of response to the cold hand test in young women. Stress 2009, 12, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Strahler, J.; Mueller, A.; Rosenloecher, F.; Kirschbaum, C.; Rohleder, N. Salivary alpha-amylase stress reactivity across different age groups. Psychophysiology 2010, 47, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Watanabe, K.; Sugimura, N.; Shishido, I.; Konya, I.; Fujita, T.; Yoshimitsu, Y.; Kato, S.; Ito, Y.M.; Yano, R. Salivary Biomarker Profiles and Chronic Fatigue among Nurses Working Rotation Shifts: An Exploratory Pilot Study. Healthcare 2022, 10, 1416. [Google Scholar] [CrossRef]
- Martínez-Borrás, R.; Navarrete, J.; Bellosta-Batalla, M.; Martínez-Brotóns, C.; Martínez-Rubio, D. Changes in Salivary Immunoglobulin A, Stress, and Burnout in a Workplace Mindfulness Intervention: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 6226. [Google Scholar] [CrossRef]
- Engeland, C.G.; Hugo, F.N.; Hilgert, J.B.; Nascimento, G.G.; Celeste, R.K.; Lim, H.J.; Marucha, P.T.; Bosch, J.A. Psychological distress and salivary secretory immunity. Brain Behav. Immun. 2016, 52, 11–17. [Google Scholar] [CrossRef]
- Yang, Y.; Koh, D.; Ng, V.; Lee, C.Y.; Chan, G.; Dong, F.; Goh, S.H.; Anantharaman, V.; Chia, S.E. Self perceived work related stress and the relation with salivary IgA and lysozyme among emergency department nurses. Occup. Environ. Med. 2002, 59, 836–841. [Google Scholar] [CrossRef]
- Koh, D.; Ng, V.; Naing, L. Alpha Amylase as a Salivary Biomarker of Acute Stress of Venepuncture from Periodic Medical Examinations. Front. Public Health 2014, 2, 121. [Google Scholar] [CrossRef]
- Dia, M.M.; Bocanegra, O.L.; Teixeira, R.R.; Soares, S.S.; Espindola, F.S. Response of salivary markers of autonomic activity to elite competition. Int. Sports Med. 2012, 33, 763–768. [Google Scholar]
- Ma, D.; Serbin, L.A.; Stack, D.M. Children’s anxiety symptoms and salivary immunoglobulin A: A mutual regulatory system? Dev. Psychobiol. 2018, 60, 202–215. [Google Scholar] [CrossRef]
- Irshad, L.; Faustini, S.; Evans, L.; Drayson, M.T.; Campbell, J.P.; Heaney, J.L.J. Salivary free light chains as a new biomarker to measure psychological stress: The impact of a university exam period on salivary immunoglobulins, cortisol, DHEA and symptoms of infection. Psychoneuroendocrinology 2020, 122, 104912. [Google Scholar] [CrossRef] [PubMed]
- Racine, N.; McArthur, B.A.; Cooke, J.E.; Eirich, R.; Zhu, J.; Madigan, S. Global Prevalence of Depressive and Anxiety Symptoms in Children and Adolescents During COVID-19: A Meta-analysis. JAMA Pediatr. 2021, 175, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Fuentes, D.A.; Gutiérrez-Chablé, L.E.; Méndez-Martínez, S.; García-Flores, M.A.; Ayón-Aguilar, J. Confinamiento y distanciamiento social: Estrés, ansiedad, depresión en niños y adolescentes [Confinement and social distancing: Stress, anxiety, depression in children and adolescents]. Rev. Med. Inst. Mex. Seguro Soc. 2022, 60, 338–344. [Google Scholar] [PubMed]
- Nakane, H.; Asami, O.; Yamada, Y.; Harada, T.; Matsui, N.; Kanno, T.; Yanaihara, N. Salivary chromogranin a as an index of psychosomatic stress response. Biomed. Res. 1998, 19, 401–406. [Google Scholar] [CrossRef]
- Kanamura, Y.; Kikukawa, A.; Shimamura, K. Salivary chromogranin-A as a marker of psychological stress during a cognitive test battery in humans. Stress 2006, 9, 127–131. [Google Scholar] [CrossRef]
- Obara, S.; Iwama, H. Assessment of psychological tension after premedication by measurement of salivary chromogranin A. Clin. Anesthesiol. 2005, 17, 554–557. [Google Scholar] [CrossRef]
- Lee, T.; Shimizu, T.; Iijima, M.; Obinata, K.; Yamashiro, Y.; Nagasawa, S. Evaluation of psychosomatic stress in children by measuring salivary chromogranin A. Acta Paediatr. 2006, 95, 935–939. [Google Scholar] [CrossRef]
- Miyakawa, M.; Matsui, T.; Kishikawa, H.; Murayama, R.; Uchiyama, I.; Itoh, T.; Yoshida, T. Salivary chromogranin A as a measure of stress response to noise. Noise Health 2006, 8, 108. [Google Scholar] [CrossRef]
- Duits, P.; Cath, D.C.; Lissek, S.; Hox, J.J.; Hamm, A.O.; Engelhard, I.M.; Van Den Hout, M.A.; Baas, J.M.P. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress. Anxiety 2015, 32, 239–253. [Google Scholar] [CrossRef]
- Takatsuji, K.; Sugimoto, Y.; Ishizaki, S.; Ozaki, Y.; Matsuyama, E.; Yamaguchi, Y. The effects of examination stress on salivary cortisol, immunoglobulin A, and chromogranin A in nursing students. Biomed. Res. 2008, 29, 221–224. [Google Scholar] [CrossRef]
- Qing, H.; Desrouleaux, R.; Israni-Winger, K.; Mineur, Y.S.; Fogelman, N.; Zhang, C.; Rashed, S.; Palm, N.W.; Sinha, R.; Picciotto, M.R.; et al. Origin and Function of Stress-Induced IL-6 in Murine Models. Cell 2020, 182, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.; Srinivasan, M.; Shanmugam, A.; Ward, A.; Ganapathy, V.; Bloom, J.; Sharma, A.; Sharma, S. Interleukin-6 trans-signaling inhibition prevents oxidative stress in a mouse model of early diabetic retinopathy. Redox Biol. 2020, 34, 101574. [Google Scholar] [CrossRef]
- Rodney, T.; Taylor, P.; Dunbar, K.; Perrin, N.; Lai, C.; Roy, M.; Gill, J. High IL-6 in military personnel relates to multiple traumatic brain injuries and post-traumatic stress disorder. Behav. Brain Res. 2020, 392, 112715. [Google Scholar] [CrossRef] [PubMed]
- Voges, J.F.; Müller-Pinzler, L.; Neis, M.; Luebber, F.; Lange, T.; Hundt, J.E.; Kasten, M.; Krämer, U.M.; Krach, S.; Rademacher, L. Association of stress-related neural activity and baseline interleukin-6 plasma levels in healthy adults. Stress 2022, 25, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wagnon, I.M.; Jabur, L.J.; Niedermayer, G.; Münch, G.; Karl, T.; Chesworth, R.; Gyengesi, E. Chronic interleukin-6 mediated neuroinflammation decreases anxiety, and impaires spatial memory in aged female mice. Front. Neurosci. 2023, 17, 1267818. [Google Scholar] [CrossRef]
- Shen, S.Y.; Liang, L.F.; Shi, T.L.; Shen, Z.Q.; Yin, S.Y.; Zhang, J.R.; Li, W.; Mi, W.L.; Wang, Y.Q.; Zhang, Y.Q.; et al. Microglia-Derived Interleukin-6 Triggers Astrocyte Apoptosis in the Hippocampus and Mediates Depression-Like Behavior. Adv. Sci. 2025, 12, e2412556. [Google Scholar] [CrossRef]
- Zannas, A.S.; Gordon, J.L.; Hinderliter, A.L.; Girdler, S.S.; Rubinow, D.R. IL-6 Response to Psychosocial Stress Predicts 12-month Changes in Cardiometabolic Biomarkers in Perimenopausal Women. Clin. Endocrinol. Metab. 2020, 105, e3757–3765. [Google Scholar] [CrossRef]
- Bekkevold, O.J.; Damås, J.K.; Brumpton, B.M.; Åsvold, B.O. The causal role of C-reactive protein and interleukin-6 on anxiety and depression symptoms and life satisfaction: Mendelian randomisation analyses in the HUNT study. Psychol. Med. 2023, 53, 7561–7568. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Dolovich, C.; Bernstein, C.N.; Singh, H.; Nugent, Z.; Tennakoon, A.; Shafer, L.A.; Marrie, R.A.; Sareen, J.; Targownik, L.E. Anxiety and Depression Leads to Anti-Tumor Necrosis Factor Discontinuation in Inflammatory Bowel Disease. Clin. Gastroenterol Hepatol. 2021, 19, 1200–1208. [Google Scholar] [CrossRef]
- Yao, L.; Pan, L.; Qian, M.; Sun, W.; Gu, C.; Chen, L.; Tang, X.; Hu, Y.; Xu, L.; Wei, Y.; et al. Tumor Necrosis Factor-α Variations in Patients with Major Depressive Disorder Before and After Antidepressant Treatment. Front. Psychiatry 2020, 11, 518837. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhang, D.; Sang, Y.; Zheng, N.; Chen, J.; Qiu, X.; Liu, X. Relationship between Tumor Necrosis Factor-Alpha and Neuropeptide Y Expression and Neurological Function Score in Epileptic Children. Iran. Public Health 2021, 50, 1056–1064. [Google Scholar] [CrossRef]
- Pondeljak, N.; Lugović-Mihić, L.; Davidović, B.L.; Karlović, D.; Hanžek, M.; Neuberg, M. Serum Levels of IL-6 and TNF-α, Salivary Morning Cortisol and Intensity of Psychological Stress in Patients with Allergic Contact Hand Dermatitis and Healthy Subjects. Life 2025, 15, 351. [Google Scholar] [CrossRef] [PubMed]
- Limaye, A.; Hall, B.E.; Zhang, L.; Cho, A.; Prochazkova, M.; Zheng, C.; Walker, M.; Adewusi, F.; Burbelo, P.D.; Sun, Z.J.; et al. Targeted TNF-α Overexpression Drives Salivary Gland Inflammation. Dent. Res. 2019, 98, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Slavish, D.C.; Graham-Engeland, J.E.; Smyth, J.M.; Engeland, C.G. Salivary markers of inflammation in response to acute stress. Brain Behav. Immun. 2015, 44, 253–269. [Google Scholar] [CrossRef]
- Kibune, R.; Muraoka, K.; Morishita, M.; Ariyoshi, W.; Awano, S. Relationship between Dynamics of TNF-α and Its Soluble Receptors in Saliva and Periodontal Health State. Dent. J. 2022, 10, 25. [Google Scholar] [CrossRef]
- Friend, S.F.; Nachnani, R.; Powell, S.B.; Risbrough, V.B. C-Reactive protein: Marker of risk for post-traumatic stress disorder and its potential for a mechanistic role in trauma response and recovery. Eur. J. Neurosci. 2022, 55, 2297–2310. [Google Scholar] [CrossRef]
- Shah, K.; Kumari, R.; Jain, M. Unveiling stress markers: A systematic review investigating psychological stress biomarkers. Dev. Psychobiol. 2024, 66, e22490. [Google Scholar] [CrossRef]
- Kennedy, E.; Niedzwiedz, C.L. The association of anxiety and stress-related disorders with C-reactive protein (CRP) within UK Biobank. Brain Behav. Immun. Health 2022, 19, 100410. [Google Scholar] [CrossRef]
- Akhter, N.; Wilson, A.; Arefanian, H.; Thomas, R.; Kochumon, S.; Al-Rashed, F.; Abu-Farha, M.; Al-Madhoun, A.; Al-Mulla, F.; Ahmad, R.; et al. Endoplasmic Reticulum Stress Promotes the Expression of TNF-α in THP-1 Cells by Mechanisms Involving ROS/CHOP/HIF-1α and MAPK/NF-κB Pathways. Int. J. Mol. Sci. 2023, 24, 15186. [Google Scholar] [CrossRef]
- Mehta, N.D.; Haroon, E.; Xu, X.; Woolwine, B.J.; Li, Z.; Felger, J.C. Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: Preliminary results. Brain Behav. Immun. 2018, 73, 725–730. [Google Scholar] [CrossRef]
- Murawska-Ciałowicz, E.; Wiatr, M.; Ciałowicz, M.; Gomes de Assis, G.; Borowicz, W.; Rocha-Rodrigues, S.; Paprocka-Borowicz, M.; Marques, A. BDNF Impact on Biological Markers of Depression—Role of Physical Exercise and Training. Int. J. Environ. Res. Public Health 2021, 18, 7553. [Google Scholar] [CrossRef]
- Song, M.; Martinowich, K.; Lee, F.S. BDNF at the synapse: Why location matters. Mol. Psychiatry 2017, 22, 1370–1375. [Google Scholar] [CrossRef]
- de Assis, G.G.; de Almondes, K.M. Exercise-dependent BDNF as a modulatory factor for the executive processing of individuals in course of cognitive decline. A systematic review. Front. Psychol. 2017, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Kayser, S.; Engelmann, J.; Schlicht, K.F.; Dreimüller, N.; Tüscher, O.; Müller-Dahlhaus, F.; Braus, D.F.; Tadić, A.; Neyazi, A.; et al. Plasma brain-derived neurotrophic factor (pBDNF) and executive dysfunctions in patients with major depressive disorder. World Biol. Psychiatry 2019, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.H.; Heng, B.C.; Lim, L.W. New insights on brain-derived neurotrophic factor epigenetics: From depression to memory extinction. Ann. N. Y. Acad. Sci. 2021, 1484, 9–31. [Google Scholar] [CrossRef] [PubMed]
- Laske, C.; Banschbach, S.; Stransky, E.; Bosch, S.; Straten, G.; Machann, J.; Fritsche, A.; Hipp, A.; Niess, A.; Eschweiler, G.W. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. Int. Neuropsychopharmacol. 2010, 13, 595–602. [Google Scholar] [CrossRef]
- Anderson, G.; Berk, M.; Dean, O.; Moylan, S.; Maes, M. Role of immune-inflammatory and oxidative and nitrosative stress pathways in the etiology of depression: Therapeutic implications. CNS Drugs 2014, 28, 1–10. [Google Scholar] [CrossRef]
- Bus, B.A.; Tendolkar, I.; Franke, B.; de Graaf, J.; den Heijer, M.; Buitelaar, J.K.; Oude Voshaar, R.C. Serum brain-derived neurotrophic factor: Determinants and relationship with depressive symptoms in a community population of middle-aged and elderly people. World Biol. Psychiatry 2012, 13, 39–47. [Google Scholar] [CrossRef]
- Schmidt, A.T.; Hicks, S.D.; Bergquist, B.K.; Maloney, K.A.; Dennis, V.E.; Bammel, A.C. Preliminary Evidence for Neuronal Dysfunction Following Adverse Childhood Experiences: An Investigation of Salivary MicroRNA Within a High-Risk Youth Sample. Genes 2024, 15, 1433. [Google Scholar] [CrossRef]
- Thounaojam, M.C.; Kaushik, D.K.; Kundu, K.; Basu, A. MicroRNA-29b modulates Japanese encephalitis virus-induced microglia activation by targeting tumor necrosis factor α-induced protein 3. J. Neurochem. 2014, 129, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, R.; Liu, Y.; Liu, D.; Jiang, H.; Pan, F. FKBP5 and specific microRNAs via glucocorticoid receptor in the basolateral amygdala involved in the susceptibility to depressive disorder in early adolescent stressed rats. J. Psychiatr. Res. 2017, 95, 102–113. [Google Scholar] [CrossRef]
- Sillivan, S.E.; Jamieson, S.; de Nijs, L.; Jones, M.; Snijders, C.; Klengel, T.; Joseph, N.F.; Krauskopf, J.; Kleinjans, J.; Vinkers, C.H.; et al. MicroRNA regulation of persistent stress-enhanced memory. Mol. Psychiatry 2020, 25, 965–976. [Google Scholar]
- Chen, R.J.; Kelly, G.; Sengupta, A.; Heydendael, W.; Nicholas, B.; Beltrami, S.; Luz, S.; Peixoto, L.; Able, T.; Bhatnagar, S. MicroRNAs as biomarkers of resilience or vulnerability to stress. Neuroscience 2015, 305, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Volk, N.; Pape, J.C.; Engel, M.; Zannas, A.S.; Cattane, N.; Cattaneo, A.; Binder, E.B.; Chen, A. Amygdalar microRNA-15a is essential for coping with chronic stress. Cell Rep. 2016, 17, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T.; Bargiel, W.; Grabarczyk, M.; Skibinska, M. Peripheral S100B Protein Levels in Five Major Psychiatric Disorders: A Systematic Review. Brain Sci. 2023, 13, 1334. [Google Scholar] [CrossRef]
- Michetti, F.; D’Ambrosi, N.; Toesca, A.; Puglisi, M.A.; Serrano, A.; Marchese, E.; Corvino, V.; Geloso, M.C. The S100B story: From biomarker to active factor in neural injury. J. Neurochem. 2019, 148, 168–187. [Google Scholar] [CrossRef]
- Chen, S.; Tian, L.; Chen, N.; Xiu, M.; Wang, Z.; Yang, G.; Wang, C.; Yang, F.; Tan, Y. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017, 247, 6–11. [Google Scholar] [CrossRef]
- Milleit, B.; Smesny, S.; Rothermundt, M.; Preul, C.; Schroeter, M.L.; Von Eiff, C.; Ponath, G.; Milleit, C.; Sauer, H.; Gaser, C. Serum S100B protein is specifically related to white matter changes in Schizophrenia. Front. Cell. Neurosci. 2016, 10, 33. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Kang, M.M.; Li, G.Y.; Xiong, P.; Chen, H.X.; Kang, L.; Li, S.; Lu, C.L.; Li, Q.Q.; Bai, M.Y. Predictive effect of Bayes discrimination in the level of serum protein factors and cognitive dysfunction in schizophrenia. Psychiatr. Res. 2022, 151, 539–545. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2021, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Scalise, E.; Ielapi, N.; Bracale, U.M.; Andreucci, M.; Serra, R. Metalloproteinases as Biomarkers and Sociomarkers in Human Health and Disease. Biomolecules 2024, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Overall, C.M.; Dufour, A. Matrix metalloproteinases in the CNS: Interferons get nervous. Cell. Mol. Life Sci. 2019, 76, 3083–3095. [Google Scholar] [CrossRef]
- Costa, D.; Ielapi, N.; Minici, R.; Bevacqua, E.; Ciranni, S.; Cristodoro, L.; Torcia, G.; Di Taranto, M.D.; Bracale, U.M.; Andreucci, M.; et al. Metalloproteinases between History, Health, Disease, and the Complex Dimension of Social Determinants of Health. Vasc. Dis. 2023, 2, 282–298. [Google Scholar] [CrossRef]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Zhang, H.; Liu, X.; Pan, S.; Li, C. The Role of Extracellular Matrix Metalloproteinase Inducer Glycosylation in Regulating Matrix Metalloproteinases in Periodontitis. Periodontal Res. 2018, 53, 391–402. [Google Scholar] [CrossRef]


| Biomarker | System Represented | Relevance in Mental Health | Detection Method | Limitations | Temporal Applicability | Saliva Type |
|---|---|---|---|---|---|---|
| Cortisol | HPA axis | Elevated in stress, depression; reflects HPA dysregulation | ELISA, immunoassay | High circadian variability, influenced by medications | Acute | Unstimulated |
| Alpha-amylase (sAA) | SAM system | Rapidly increases under acute stress; reflects sympathetic activity | Enzymatic assay | Affected by salivary flow rate, transient changes | Acute | Stimulated or resting (often both) |
| Secretory IgA (sIgA) | Mucosal immunity | Reduced in chronic stress and depression; indicates immune suppression | Immunoassay (ELISA) | Sensitive to oral health and local inflammation | Acute/Long-Term | Stimulated |
| Chromogranin A (CgA) | SAM system | Correlates with sympathetic arousal; rises during acute stress | Immunoassay | Requires timing; overlaps with other markers | Acute | Stimulated |
| Interleukin-6 (IL-6) | Immune–inflammatory | Elevated in chronic stress, anxiety, and depression; promotes inflammation | ELISA, multiplex cytokine panels | Low specificity, varies with comorbid conditions | Acute/Long-Term | Unstimulated |
| Tumor Necrosis Factor-alpha (TNF-α) | Immune–inflammatory | Linked to neuroinflammation and neurotransmitter imbalance in depression | ELISA, multiplex cytokine panels | Overlap with other inflammatory markers; systemic effects | Acute/Long-Term | Unstimulated |
| C-Reactive Protein (CRP) | Systemic inflammation | Correlates with treatment resistance and fatigue in depression | ELISA, high-sensitivity immunoassay | Limited saliva standardization; influenced by infections | Long-Term | Stimulated |
| Brain-Derived Neurotrophic Factor (BDNF) | Neuroplasticity | Reduced in MDD and anxiety; linked to synaptic and cognitive dysfunction | ELISA, immunoassay | Low salivary concentrations; assay variability | Long-Term | Unstimulated |
| microRNAs (miRNAs) | Gene regulation | Differential profiles associated with depression, PTSD, stress | qRT-PCR, sequencing | Lack of standardization, normalization challenges | Long-Term | Unstimulated (standardized protocols recommended) |
| First Author/Year | Study Design | Sample Size | Population | Biomarkers Investigated | Key Findings | Evidence Strength/Limitations |
|---|---|---|---|---|---|---|
| Chojnowska et al., 2021 [10] | Review | N/A | General population—multiple studies | Cortisol, sAA, CgA, sIgA | Salivary biomarkers reflect HPA/SAM axis activation and mucosal immunity in stress and affective disorders | Narrative synthesis; lacks unified methodology |
| Tammayan et al., 2021 [11] | Cross-sectional | n = 90 students | Dental students under exam stress | Cortisol, sAA, CgA | Elevated salivary cortisol and sAA correlated with subjective stress scores | Good sample; limited to academic context |
| Gholami et al., 2017 [12] | Clinical comparative | n = 60 (30 depressed, 30 controls) | Depressed vs. healthy adults | Salivary flow rate | Depressed patients had significantly reduced salivary flow rate | Small sample; focused only on xerostomia |
| Shimizu et al., 2024 [15] | Pilot crossover study | n = 18 psychiatric patients | Patients with depression/anxiety | Salivary biomarkers + physiological monitoring | Combined biometric and salivary analysis identified distinct stress response profiles | Pilot data; needs replication |
| Strahler et al., 2010 [53] | Experimental–age comparison | n = 60 | Young, middle-aged, and older adults | Salivary alpha-amylase (sAA) | Stress-induced sAA reactivity varied by age; highest in younger adults | Age-diverse sample; limited to acute stressor design |
| Irshad et al., 2020 [61] | Observational (exam period) | n = unspecified | University students during exam stress | Cortisol, DHEA, salivary immunoglobulins, free light chains | Salivary biomarkers varied significantly during stress and were associated with infection symptoms | Multimodal biomarker approach; small sample size |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budala, D.G.; Luchian, I.; Virvescu, D.I.; Tudorici, T.; Constantin, V.; Surlari, Z.; Butnaru, O.; Bosinceanu, D.N.; Bida, C.; Hancianu, M. Salivary Biomarkers as a Predictive Factor in Anxiety, Depression, and Stress. Curr. Issues Mol. Biol. 2025, 47, 488. https://doi.org/10.3390/cimb47070488
Budala DG, Luchian I, Virvescu DI, Tudorici T, Constantin V, Surlari Z, Butnaru O, Bosinceanu DN, Bida C, Hancianu M. Salivary Biomarkers as a Predictive Factor in Anxiety, Depression, and Stress. Current Issues in Molecular Biology. 2025; 47(7):488. https://doi.org/10.3390/cimb47070488
Chicago/Turabian StyleBudala, Dana Gabriela, Ionut Luchian, Dragos Ioan Virvescu, Teona Tudorici, Vlad Constantin, Zinovia Surlari, Oana Butnaru, Dan Nicolae Bosinceanu, Cosmin Bida, and Monica Hancianu. 2025. "Salivary Biomarkers as a Predictive Factor in Anxiety, Depression, and Stress" Current Issues in Molecular Biology 47, no. 7: 488. https://doi.org/10.3390/cimb47070488
APA StyleBudala, D. G., Luchian, I., Virvescu, D. I., Tudorici, T., Constantin, V., Surlari, Z., Butnaru, O., Bosinceanu, D. N., Bida, C., & Hancianu, M. (2025). Salivary Biomarkers as a Predictive Factor in Anxiety, Depression, and Stress. Current Issues in Molecular Biology, 47(7), 488. https://doi.org/10.3390/cimb47070488










