Plateau Environment, Gut Microbiota, and Depression: A Possible Concealed Connection?
Abstract
1. Introduction
- Immune Regulation: This process results in mucosal immunity and interacts with Toll-like receptors (TLRs), thereby modulating immune responses [10].
- Gut-brain axis communication: This process regulates the synthesis of neurotransmitters, including serotonin and gamma-aminobutyric acid (GABA), and influences the function of the central nervous system via neural, endocrine, and immune pathways [10].
| Years | Region/Country | Number of Persons/Survey Methods | Incidence of Depression |
|---|---|---|---|
| 2019 [11] | Yushu Prefecture, Qinghai/China | Central Epidemiological Research Depression Scale (24,141 Tibetans; Average age 34.33 years) | Participants with depressive symptoms (score ≥ 8) accounted for 52.3% of the total sample, and participants with depression (score ≥ 14) accounted for 28.6%. |
| 2021 [14] | Yushu Prefecture, Qinghai/China | Central Epidemiological Research Depression Scale; Connor-Davidson elasticity Scale; Strengths and difficulties questionnaire (11,160 participants; Average age =14.34 years) | The prevalence of depression was 29.2%. Higher levels of prosocial behavior were significantly associated with lower levels of depression. |
| 2022 [20] | Peru | Dependent variable depressive symptoms using patient health questionnaire (34,971 residents aged 15+) | Among those living between 1500 and 2499 m and ≥2500 m, 7.23% and 7.12%, respectively, had depressive symptoms. Compared with the reference group (<1500 m), the prevalence of screening depression was 41% higher in those living above 2500 m and 38% higher in those living between 1500 and 2499 m. |
| 2022 [21] | Residence elevation ≥ 900 m | 9 patient health questionnaires (PHQ-9); 7-item Generalized Anxiety Disorder Questionnaire (GAD-7) (3731 medical students) | High-altitude residence (>900 m) was significantly associated with total PHQ-9 score (OR = 1.32, 95% CI = 1.001–1.75, p < 0.05) and PHQ-9 suicidal ideation (OR = 1.79, 95% CI = 1.08–0.02, p = 0.02). Moving from low-altitude to high-altitude was associated with PHQ-9 total score (OR = 1.47, 95% CI = 1.087–1.98, p = 0.01), GAD-7 total score (OR = 1.40, 95% CI = 1.0040–1.95, p < 0.05) and PHQ-9 suicidal ideation (OR = 1.10,95% CI =1.01–1.19, p = 0.02). |
| 2022 [22] | Tibet/China | Self-rating Anxiety Scale and self-rating Depression Scale (84 participants; The mean age was 35.67 ± 7.69 years) | The incidence of anxiety and depression increased significantly during the first 7 days of rapid ascent to 4500 m above sea level. |
2. Characteristics of the Plateau Environment and Its Impact on the Human Body and Gut
2.1. Uniqueness of the Plateau Environment
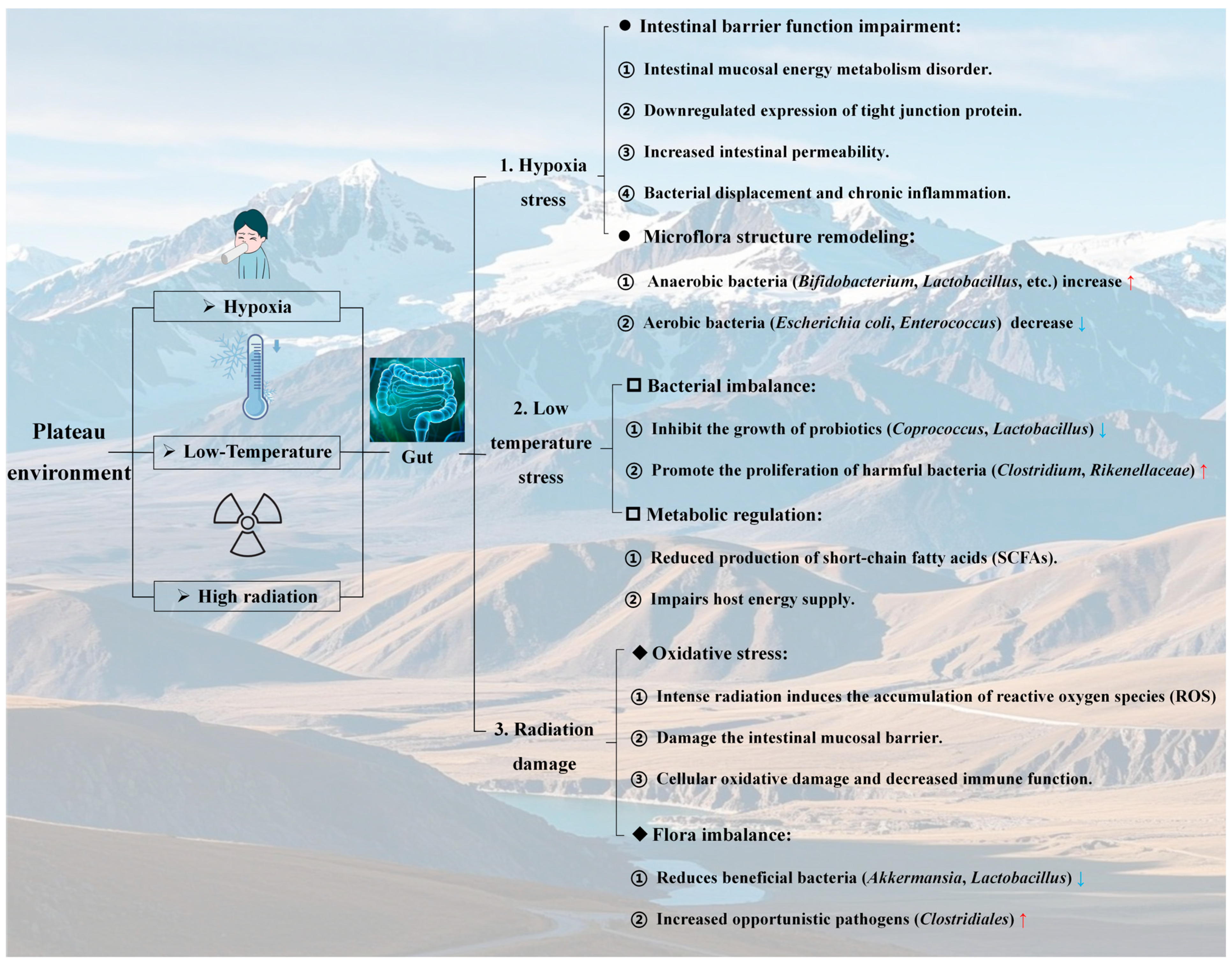
- The unique triad of hypoxia, low-temperature, and high UV radiation in plateau environments creates a landscape that profoundly challenges human physiological homeostasis. The drastic reduction in oxygen availability, extreme temperature fluctuations, intense solar radiation, and arid conditions collectively impose cumulative stress on thermoregulation, oxidative defense, and fluid balance. These environmental pressures not only directly impact organ systems but also prime the gut microbiota for dysregulation, setting the stage for subsequent disruptions in the gut-brain axis discussed in subsequent sections. Understanding these climatic stressors is critical for deciphering the mechanistic links between plateau habitats and depression via microbial remodeling.
2.2. Influence of the Altitude Hypoxic Environment on the Gut
- Altitude hypoxia imposes dual stresses on the gut: Remodeling the microbial composition toward anaerobic dominance while disrupting mucosal barrier function via oxidative stress and energy metabolism deficits. The observed shifts in the microbiota (e.g., increased Bacteroides and decreased Bifidobacteria) and compromised tight junctions highlight a vicious cycle of dysbiosis and inflammation. These findings underscore hypoxia as a key driver of gut-brain axis disruption in plateau environments, linking microbial and barrier dysfunction to downstream mental health risks such as depression.
2.3. Effects of the Low-Temperature Environment on the Gut
- Low-temperature stress at high altitudes affects gut microbiota homeostasis, and SCFAs provide protection: low-temperature stress in plateau environments disrupts gut microbiota homeostasis by suppressing probiotics (e.g., Coprococcus and Lactobacillaceae) and promoting pathogenic bacteria (e.g., Clostridium and Rikenellaceae) while reducing SCFA production. The protective role of SCFAs (e.g., butyrate) in restoring microbial balance and enhancing cold tolerance highlights their potential as therapeutic targets for mitigating gut dysfunction and associated mental health risks in cold-exposed populations.
2.4. Effects of a High-Altitude Radiation Environment on the Gut Tract
- High-altitude intense radiation exacerbates the imbalance in the gut microbiota and the “radiation-microbiota-inflammation” axis effect by inducing oxidative stress and causing mucosal damage. The high-altitude environment, characterized by intense radiation, exacerbates the ecological imbalance of gut microorganisms via two mechanisms: the induction of oxidative stress and damage to the gut mucosa. This manifests as a reduction in the abundance of beneficial bacteria (e.g., Achaemenia and Lactobacillus) and an overproliferation of opportunistic pathogenic bacteria (e.g., Clostridium and Helicobacter). Such microbial dysbiosis not only compromises the local immune defense of the gut tract but also amplifies radiation-induced damage through systemic inflammatory pathways. These findings underscore the threat posed by the “radiation-microbiota-inflammation” axis to gut homeostasis and overall health in high-altitude environments, offering microbiological evidence for investigating the link between radiation exposure and mental disorders, such as depression.
3. The Function of the Gut Microbiota and Its Relationship with Depression
3.1. The Role of the Gut Microbiota in Human Health
3.1.1. The Role of the Gut Microbiota in Nutrient Metabolism
3.1.2. Effects of the Gut Microbiota on the Gut Barrier
3.1.3. Gut Microbiota Homeostasis and Dysregulation: Immune Regulation and Health Implications
3.1.4. In Summary
- Vitamin synthesis: Beneficial bacteria, such as Bifidobacterium and Lactobacillus, synthesize essential vitamins, including B vitamins (e.g., B1 and B12) and vitamin K. This compensates for the insufficiency of endogenous synthesis in the human body and supports energy metabolism and nervous system function [65,66].
- Dietary fiber degradation: Through glycolysis, indigestible carbohydrates are metabolized into SCFAs, such as butyric acid and propionic acid, which contribute to the energy supply, inflammation regulation, and proliferation of intestinal epithelial cells [67].
- Core conclusion: The gut microbiota, which functions as a “metabolic organ,” enhances the efficiency of nutrient utilization via multiple pathways. Dysregulation of these microbiota may contribute to diseases associated with nutritional imbalances.
- Core conclusion: The gut microbiota establishes the primary defense mechanism against pathogens through a dual-action process involving “protection mediated by beneficial bacteria and suppression of harmful bacteria.” Disruption of their homeostasis serves as a critical trigger for intestinal permeability alterations and systemic inflammatory responses.
- Maintenance of an anti-inflammatory microenvironment: SCFAs (e.g., butyric acid) activate the PPAR-γ pathway, promote mitochondrial oxidative phosphorylation in colonic cells, inhibit the proliferation of aerobic pathogenic bacteria, and reduce the expression of inflammatory factors (e.g., iNOS) [87,88].
- Immune-related risks: Disruptions in microbiota composition (e.g., decreased Firmicutes and increased Proteobacteria) disrupt immune tolerance, induce excessive activation of Th17 cells, and are closely linked to immune-mediated diseases, such as IBD, diabetes and depression [97,98,99,100,101,102,103,104,105,106,107,108,109,110].
- Core Conclusion: The gut microbiota plays a crucial role in maintaining mucosal immune homeostasis through metabolism-immune interactions. Dysfunction of these microbiota can initiate a systemic inflammatory cascade, serving as a common pathological basis for various chronic diseases.
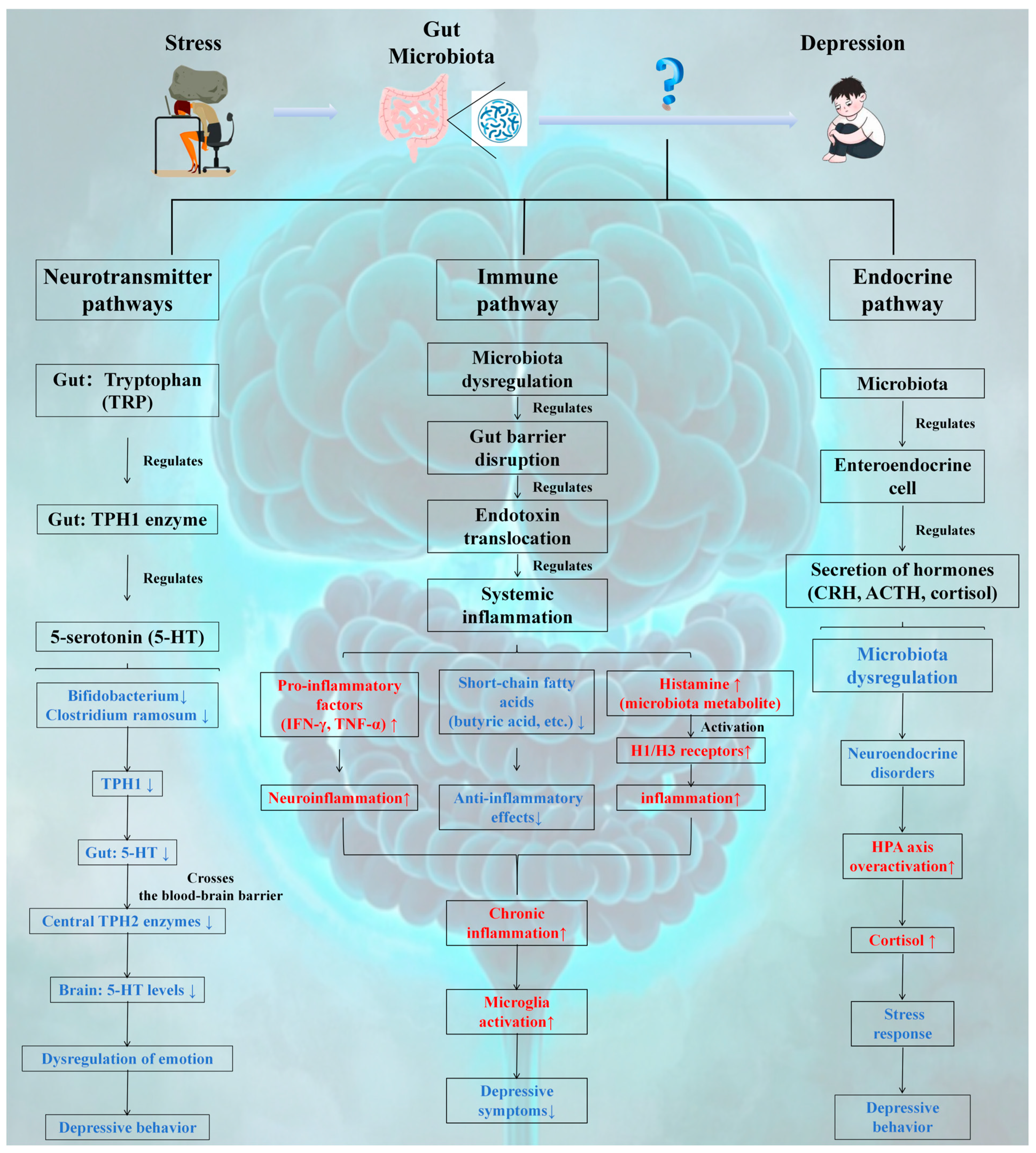
3.2. Potential Mechanisms by Which the Gut Microbiota Affects Depression
3.2.1. Neurotransmitter Pathway
- The gut microbiota plays a pivotal role in emotion regulation by modulating the 5-HT synthesis pathway:
- (1)
- Peripheral Dominant Synthesis: The intestine accounts for approximately 90% of 5-HT synthesis, which is catalyzed by the TPH1 enzyme in ECs through tryptophan metabolism. The activity of this enzyme is bidirectionally regulated by microbial metabolites (e.g., SCFAs and butyric acid) via the GPR41/43 signaling pathway and epigenetic modifications (such as DNA demethylation).
- (2)
- Gut-Brain Axis Linkage: Peripheral 5-HT influences the activity of the central TPH2 enzyme across the blood-brain barrier, establishing a microbiota-metabolism-neural regulatory axis. For example, probiotic intervention can increase 5-HT levels by upregulating TPH1 expression and inhibiting the IDO pathway, thereby alleviating depressive-like behaviors (as observed in CUMS model rats).
- (3)
- Pathology Association: Harmful bacteria, such as Clostridium ramosum, suppress 5-HT synthesis by competitively inhibiting tryptophan binding or inducing inflammation, thus elucidating the causal relationship between dysbiosis of the gut microbiota and depression.
- (4)
- Core points: The gut microbiota has emerged as a critical molecular hub in the gut–brain axis, mediating depression through the precise regulation of the 5-HT metabolic pathway. Intervention strategies targeting the microbiota-5-HT axis, such as probiotic administration and SCFA supplementation, exhibit significant antidepressant potential.
3.2.2. Immune Pathways
- The gut microbiota serves as a pivotal regulatory link in the pathogenesis of depression via immune pathways:
- (1)
- Barrier-Maintenance of Immune Homeostasis: Beneficial bacteria, such as Bifidobacterium and Lactobacillus, preserve mucosal immune equilibrium by reinforcing intestinal epithelial tight junctions and secreting SCFAs, such as butyric acid, which inhibit inflammatory signaling cascades.
- (2)
- Imbalance Triggers the Inflammatory Cascade: Dysbiosis of the gut microbiota facilitates the overgrowth of opportunistic pathogenic bacteria, such as Escherichia coli, leading to intestinal barrier disruption, endotoxin release, and activation of the TLR pathway. This induces systemic inflammation, characterized by elevated levels of IFN-γ and TNF-α, subsequently triggering neuroinflammation through blood–brain barrier permeability.
- (3)
- Metabolism-mediated inflammation: Histamine, a bacterial metabolite, promotes immune cell activation via H1/H3 receptor stimulation. Bacteria that produce histamine, such as Klebsiella pneumoniae, are associated with depressive-like behaviors. For example, the use of an H3 antagonist such as JNJ10181457 to target histamine receptors can mitigate inflammation and alleviate depressive symptoms.
- (4)
- Core points: Intestinal microbes maintain immune homeostasis through a tripartite mechanism encompassing “barrier protection, metabolic regulation, anti-inflammatory effects, and immune modulation.” Dysregulation of this system drives depression via the “microbiota-inflammation-brain” axis, highlighting the antidepressant potential of anti-inflammatory strategies that target microbial metabolites, such as SCFAs and histamine.
3.2.3. Endocrine Pathway
- The gut microbiota serves as a pivotal link in the regulation of depression through endocrine pathways.
- (1)
- Modulation of the gut-brain axis hormone network: Metabolites produced by the gut microbiota, such as propionic acid and butyric acid, regulate the secretion of hormones (e.g., CRH, ACTH, and cortisol) from GECs. Dysregulation of the microbiota activates the GPR43 receptor, inducing transcription of the CRH gene in the hypothalamus. This leads to excessive activation of the HPA axis, characterized by elevated cortisol levels, which subsequently triggers stress responses and depressive-like behaviors.
- (2)
- Epigenetic and inflammatory interactions: SCFA deficiency promotes NF-κB p65 acetylation and sustains inflammatory signaling by reducing histone deacetylase 2 (HDAC2) activity. Conversely, supplementation with butyric acid restores GR sensitivity and balances HPA axis feedback by reversing histone modifications, such as H3K9 deacetylation.
- ✓
- Epigenetic Switch function of SCFAs
- ✓
- Plateau-Specific Evidence
- ✓
- Interaction with Neurotransmitters
- (1)
- Probiotic Intervention Potential: Specific probiotics, including Bifidobacterium and Lactobacillus, have been shown to improve depressive behaviors by modulating baseline corticosterone levels and stress responses within the HPA axis (e.g., Bifidobacterium CECT 7765). However, further human studies are needed to validate their clinical efficacy.
- (2)
- Core Points: Gut microbes regulate HPA axis homeostasis via the “microbiome-metabolism-neuroendocrine” axis. Dysregulation of this axis contributes to depression through histone modification and inflammatory pathways. Targeted probiotic interventions offer a promising therapeutic direction for stress-related depression.
3.2.4. Interactions of Neuro-Immune-Metabolic Pathways
- (1)
- Neuroimmune interactions:
- 5-HT deficiency impairs microglial polarization toward the anti-inflammatory M2 phenotype.
- Neuroinflammatory cytokines (e.g., IL-6) inhibit TPH1/2 activity, reducing 5-HT synthesis [149].
- (2)
- Immune-metabolic interactions
- Proinflammatory factors (e.g., TNF-α) suppress SCFA-producing bacteria (e.g., Roseburia).
- SCFA deficiency weakens the gut barrier, promoting LPS translocation and systemic inflammation [150].
- (3)
- Metabolic–neuro interactions
- SCFAs regulate the HPA axis via the vagus nerve; deficiency increases cortisol, inhibiting brain-derived neurotrophic factor (BDNF) expression.
- HPA hyperactivity impairs enterochromaffin cell function, reducing 5-HT synthesis [151].
3.3. Findings in Clinical Studies
4. Correlations Between the Plateau Environment, the Gut Microbiota, and Depression
4.1. Influence of the Plateau Environment on Depression via the Gut Microbiota
4.1.1. Neurotransmitter Conduction
4.1.2. Immune Regulation
4.1.3. Metabolic Pathways
4.1.4. Interaction Mechanism of Multiple Environmental Factors
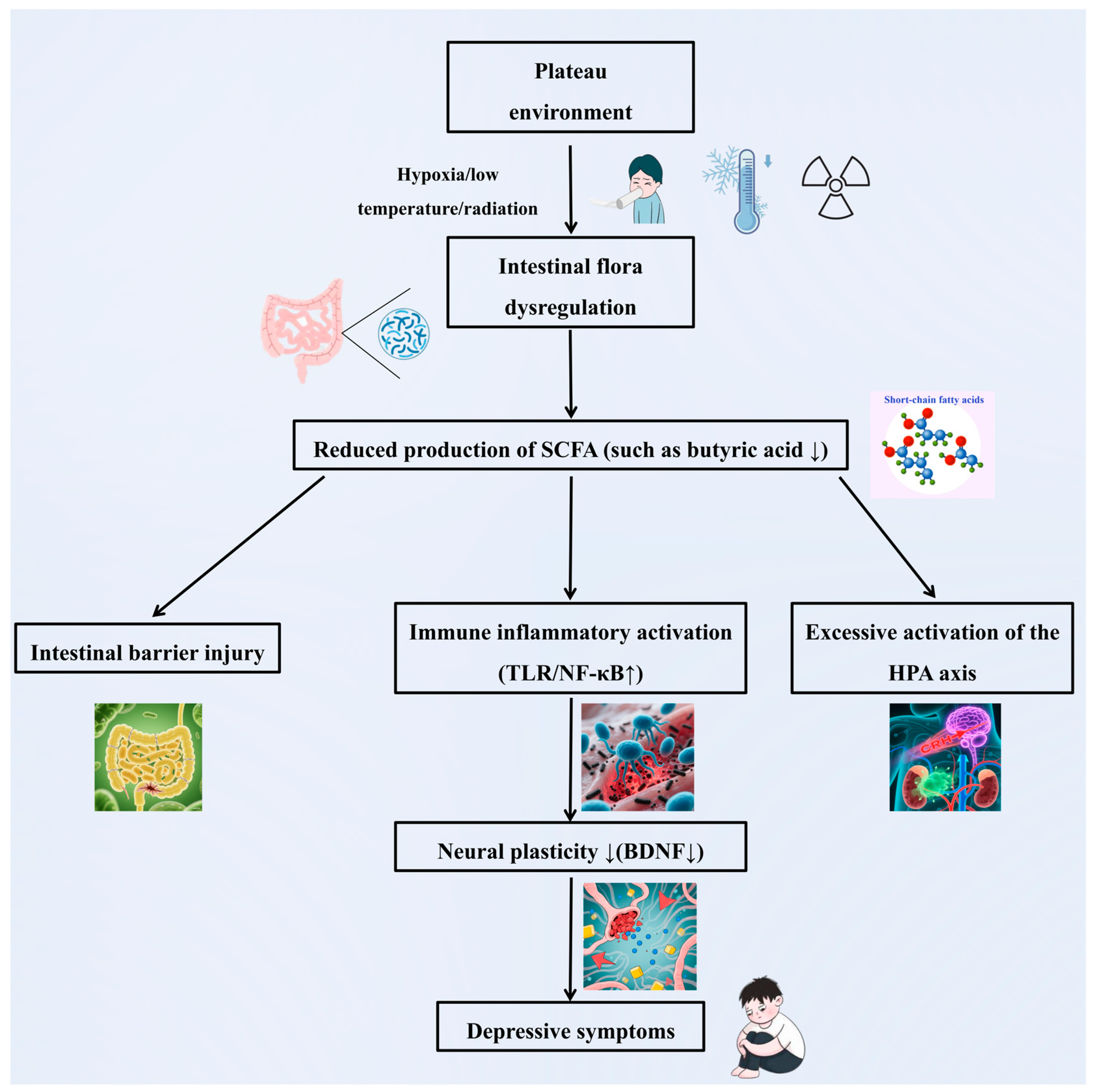
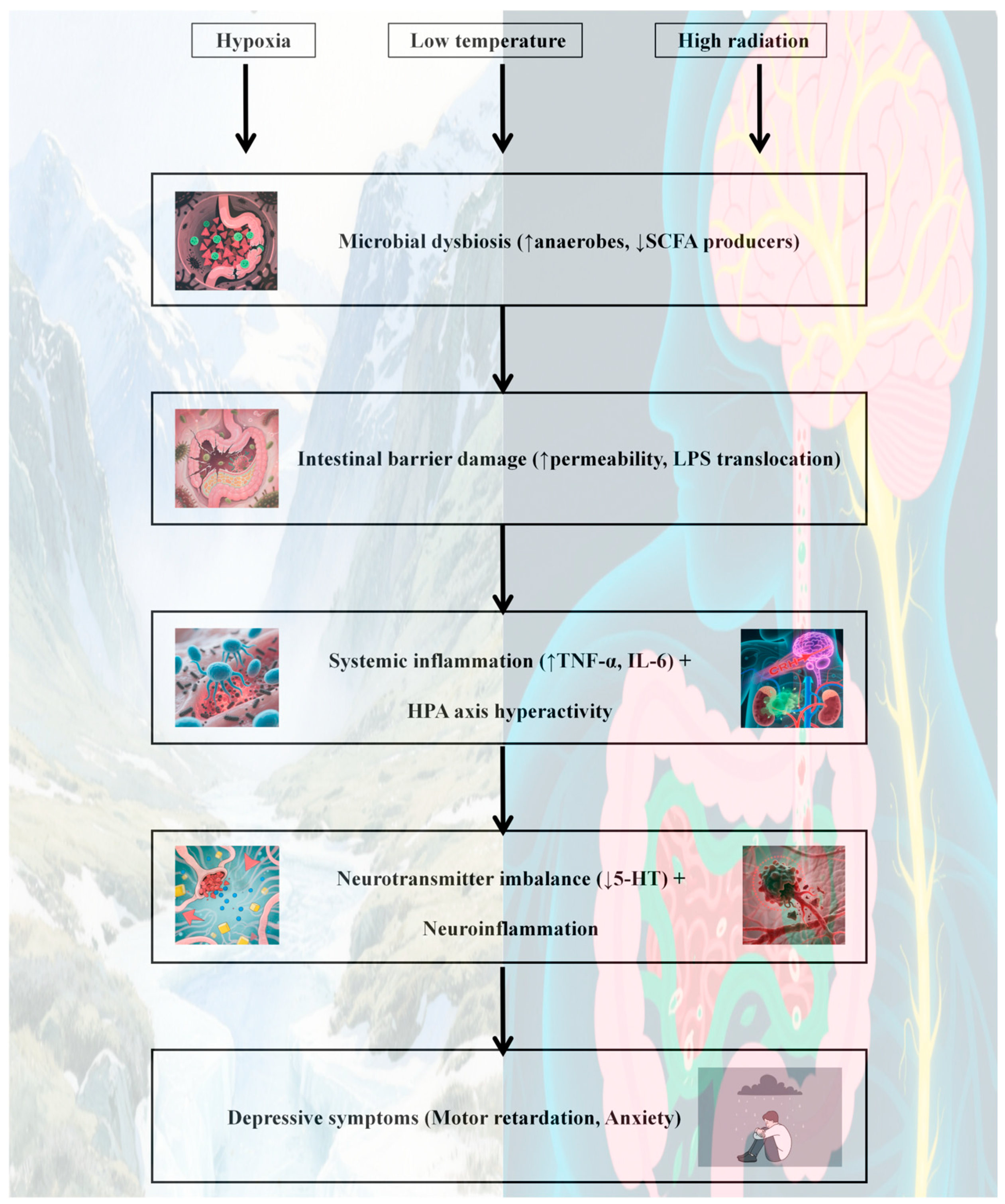
- A potential core mechanism by which the high-altitude environment may induce depression via the gut microbiota could involve multipathway synergistic dysregulation, though this remains speculative and requires validation (Figure 4):
- (1)
- Impaired nerve conduction: Hypoxia, low-temperature, and radiation result in a reduced abundance of beneficial bacteria such as Bifidobacterium and Lactobacillus. This inhibits tryptophan metabolism and 5-HT synthesis, thereby disrupting mood regulation pathways.
- (2)
- Immune-inflammatory activation: Dysbiosis compromises intestinal barrier function, leading to endotoxin translocation. This activates systemic inflammation (e.g., elevated IL-6 and TNF-α) and induces neuroinflammation and neuroplasticity damage through the blood–brain barrier.
- (3)
- Metabolic homeostasis imbalance: Reduced production of SCFAs, particularly butyric acid, weakens intestinal barrier protection and anti-inflammatory capacity. Concurrently, excessive activation of the HPA axis (e.g., elevated cortisol levels) results in a vicious cycle of “stress–inflammation.”
- (4)
- Multifactorial synergistic toxicity: Hypoxia promotes an anaerobic/aerobic imbalance in the microbiota, low-temperature suppresses probiotic metabolism, and radiation induces oxidative stress. The cumulative effects of these factors exacerbate microbial dysregulation and amplify depression risk through the “microbiota-inflammation-brain” axis.
- (5)
- Core points: Multiple stressors in high-altitude environments trigger the key pathological chain of depression via the “neuro-immune-metabolite” three-dimensional regulatory network of the gut microbiota. Interventions targeting bacterial metabolites (e.g., butyric acid) or probiotics hold promise as precise prevention and control strategies for high-altitude depression.
4.1.5. Limitations
4.2. Population-Specific Differences in the Gut Microbiota-Immune-Neuroendocrine Axis in High-Altitude Environments
4.2.1. Populations Exposed Acutely to High Altitudes
4.2.2. Chronic Plateau Adaptation Population
4.2.3. Compound Risk in Special Populations
4.2.4. Limitations
- The observed associations must be interpreted alongside potential confounders. For example, sleep disorders—prevalent in high-altitude environments owing to hypoxia-induced breathing disruptions [193]—are independently linked to both gut dysbiosis and depression. Additionally, genetic factors (e.g., hypoxia-inducible factor polymorphisms in Tibetan populations), dietary patterns (high fat/low fiber intake), and social stressors (isolation, limited mental health access) may confound the proposed microbiota–brain axis. Future studies should employ multivariate regression to control for these variables and use twin designs to disentangle genetic and environmental effects.
- Comorbidity and socioeconomic factors: This review also acknowledges limitations in addressing comorbidities (e.g., chronic mountain sickness, pulmonary hypertension) and socioeconomic factors (e.g., limited mental health access, cultural stigma), which may confound the microbiota-depression relationship in plateau populations. Future studies should adopt multidisciplinary approaches to disentangle these interacting variables.
- Genetic adaptation in high-altitude populations, such as Tibetans, may influence the gut microbiota composition and depression risk. For example, Tibetans exhibit unique genetic variants in the HIF-1α and EPAS1 pathways, which regulate hypoxia tolerance and may interact with gut bacteria to modulate SCFA production [194]. Additionally, dietary patterns in plateau regions—characterized by high intake of ghee, red meat, and low fiber—promote the growth of Collinsella and reduce SCFA-producing bacteria such as Ruminococcaceae. These dietary habits may exacerbate gut dysbiosis and inflammation, independent of altitude stress.
5. Intervention Strategies: From Theory to Practice
5.1. Potential Methods for Improving Depression in Plateau Environments Through the Regulation of the Gut microbiota
- (1)
- Dietary Fiber
- (2)
- Probiotics
- (3)
- Fecal Microbiota Transplantation
5.2. Other Supporting Interventions
- (1)
- Psychological Intervention
- (2)
- Exercise
- The depression intervention strategy targeting the intestinal microbiota in the plateau environment can be summarized as a comprehensive plan with “microbiota regulation as the primary approach and multimodal support as an auxiliary component”.
- (1)
- Dietary Fiber Intervention: Consuming foods rich in dietary fiber, such as oats and barley, selectively promotes the proliferation of Bifidobacteria and Lactobacillus. This increases the levels of SCFAs (e.g., inulin supplementation for three weeks can increase Bifidobacteria abundance), strengthens the intestinal barrier, suppresses inflammation, and improves mood regulation function.
- (2)
- Probiotic application: Supplementing probiotics, such as Lactobacillus and Bifidobacterium, repairs intestinal mucosal barrier damage caused by hypoxia at high altitudes (e.g., Bifidobacterium longifolium JBLC-141 upregulates tight junction protein expression). It reduces proinflammatory factor levels, and the daily intake of probiotic-rich yogurt by the Tibetan population may correlate with their adaptability to high-altitude conditions.
- (3)
- FMT: As an emerging therapeutic approach, FMT rapidly restores intestinal diversity in patients with severe microbiota imbalance and alleviates symptoms of refractory depression. However, overcoming technical challenges, such as donor screening and dose standardization, is necessary. Currently, FMT remains in the stages of animal experimentation and clinical exploration.
- (4)
- Supportive interventions: CBT reshaped negative cognition to alleviate high-intensity adaptation stress. Regular exercise (e.g., 1–2 aerobic sessions per week) increases serum serotonin levels and enhances hypoxia tolerance. The integration of CBT and exercise with microbiota regulation further mitigates the risk of depression through neuroimmune-metabolic pathways.
- (5)
- Core Points: Flora regulation based on dietary fiber and probiotics constitutes the core strategy for addressing high-altitude depression. The FMT demonstrates potential breakthrough value. Psychological and exercise interventions play synergistic roles via neuro-immune-metabolic pathways, collectively constructing a multilevel prevention and control system.
6. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.H.; Xin, Z.B.; Huang, Y.Z.; Yu, J. Climate suitability assessment on the Qinghai-Tibet Plateau. Sci. Total Environ. 2022, 816, 151653. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Qi, P.; Bai, L.H.; Yan, X.D.; Zhang, L. Review of the relationship and underlying mechanisms between the Qinghai-Tibet plateau and host intestinal flora. Front. Microbiol. 2022, 13, 1055632. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Wang, K.; Wang, X.M.; Pang, Y.L.; Jiang, C.T. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell. 2021, 12, 360–373. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.L.; Zhao, X.J.; Liu, X.S.; Zhao, L.; Jia, Q.; Shi, J.L.; Xu, X.; Hao, L.J.; Xu, Z.G.; Zhong, Q.; et al. Impacts of the Plateau Environment on the Gut Microbiota and Blood Clinical Indexes in Han and Tibetan Individuals. mSystems 2020, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.X.; Wang, J.Z.; Liu, P.H.; Tu, H.W.; Zhang, R.Y.; Zhang, Y.Y.; Sun, N.; Zhang, K.R. Gut microbiota composition in depressive disorder: A systematic review, meta-analysis, and meta-regression. Transl. Psychiatry 2023, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Yuan, W.Q.; Wu, S.M.; Yang, Y.H.; Cui, D.J. Novel mechanisms of intestinal flora regulation in high-altitude hypoxia. Heliyon 2024, 10, 38220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.L.; Yuan, Y.T.; Zhang, S.S.; Guo, C.; Li, X.L.; Li, G.Y.; Xiong, W.; Zeng, Z.Y. Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract. Front. Immunol. 2020, 11, 575. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zhou, Y.Y.; Liang, Y.M.; Liu, Z.K. A Large Sample Survey of Tibetan People on the Qinghai-Tibet Plateau: Current Situation of Depression and Risk Factors. Int. J. Environ. Res. Public. Health 2019, 17, 289. [Google Scholar] [CrossRef]
- Lei, X.Y.; Xiao, L.M.; Liu, Y.N.; Li, Y.M. Prevalence of Depression among Chinese University Students: A Meta-Analysis. PLoS ONE 2016, 11, 0153454. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.J.; Chang, K.; Chen, W.; Sou, K.L.; Latkin, C.; Yeung, A. Exploring the association between depression and shenjing shuairuo in a population representative epidemiological study of Chinese adults in Guangzhou; China. Transcult. Psychiatry 2018, 55, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Eli, B.; Zhou, Y.; Liang, Y.; Cheng, J.; Wang, J.; Huang, C.; Xuan, X.; Liu, Z. Depression in Children and Adolescents on the Qinghai-Tibet Plateau: Associations with Resilience and Prosocial Behavior. Int. J. Environ. Res. Public. Health 2021, 18, 440. [Google Scholar] [CrossRef]
- Rao, W.W.; Xu, D.D.; Cao, X.L.; Wen, S.Y.; Che, W.I.; Ng, C.H.; Ungvari, G.S.; He, F.; Xiang, Y.T. Prevalence of depressive symptoms in children and adolescents in China: A meta-analysis of observational studies. Psychiatry Res. 2019, 272, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.F.; Tang, S.Q.; Ren, Z.H.; Wong, D.F.K. Prevalence of depressive symptoms among adolescents in secondary school in mainland China: A systematic review and meta-analysis. J. Affect. Disord. 2019, 245, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gao, X.Y.; Huang, X.R.; Fan, Y.Q.; Wang, Y.E.; Zhang, Y.L.; Chen, X.F.; Wen, J.L.; He, H.W.; Hong, Y.M.; et al. Moderate altitude exposure impacts host fasting blood glucose and serum metabolome by regulation of the intestinal flora. Sci. Total Environ. 2023, 905, 167016. [Google Scholar] [CrossRef]
- Qi, P.; Lv, J.; Bai, L.H.; Yan, X.D.; Zhang, L. Effects of Hypoxemia by Acute High-Altitude Exposure on Human Intestinal Flora and Metabolism. Microorganisms 2023, 11, 2284. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 5, 372. [Google Scholar] [CrossRef]
- Zegarra-Rodríguez, C.A.; Plasencia-Dueñas, N.R.; Failoc-Rojas, V.E. Disparities in the prevalence of screened depression at different altitudes in Peru: A retrospective analysis of the ENDES 2019. PLoS ONE 2022, 17, 0278947. [Google Scholar] [CrossRef]
- Kious, B.M.; Bakian, A.; Zhao, J.; Mickey, B.; Guille, C.; Renshaw, P.; Sen, S. Altitude and risk of depression and anxiety: Findings from the intern health study. Int. Rev. Psychiatry 2019, 31, 637–645. [Google Scholar] [CrossRef]
- Kan, H.; Zhang, X. Change in sleep, gastrointestinal symptoms; and mood states at high altitude (4500 m) for 6 months. Sleep Breath. 2025, 29, 72. [Google Scholar] [CrossRef]
- West, J.B. Recent Advances in High Altitude Medicine and Biology. High. Alt. Med. Biol. 2015, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. High altitude medicine and biology in China. High. Alt. Med. Biol. 2015, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.C. High Altitude Dermatology. Indian J. Dermatol. 2017, 62, 59–65. [Google Scholar] [CrossRef]
- Duan, G.N.; Song, C.; Liu, Y.F.; Fu, Z.G.; Zhang, C.; Han, X.; Li, Y.; Zhou, Y. Study on the dynamic effects of plateau hypoxic and cold environment on the thermal adaptation of short-term sojourners in Xizang. J. Therm. Biol. 2024, 119, 103774. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.T. High-altitude illnesses: Physiology, risk factors, prevention, and treatment. Rambam Maimonides Med. J. 2011, 2, 22. [Google Scholar] [CrossRef]
- Brighenti, S.; Tolotti, M.; Bruno, M.C.; Wharton, G.; Pusch, M.T.; Bertoldi, W. Ecosystem shifts in Alpine streams under glacier retreat and rock glacier thaw: A review. Sci. Total Environ. 2019, 675, 542–559. [Google Scholar] [CrossRef]
- Flanagan, P.X.; Basara, J.B.; Xiao, X.M. Long-term analysis of the asynchronicity between temperature and precipitation maxima in the United States Great Plains. Int. J. Climatol. 2017, 37, 3919–3933. [Google Scholar] [CrossRef]
- Rangwala, I.; Miller, J.R. Climate change in mountains: A review of elevation-dependent warming and its possible causes. Clim. Change 2012, 114, 527–547. [Google Scholar] [CrossRef]
- Yang, Z.H.; Bai, C.J.; Pu, Y.W.; Kong, Q.H.; Guo, Y.B.; Ouzhuluobu; Gengdeng; Liu, X.Y.; Zhao, Q.; Qiu, Z.C.; et al. Genetic adaptation of skin pigmentation in highland Tibetans. Proc. Natl. Acad. Sci. USA 2022, 119, e2200421119. [Google Scholar] [CrossRef] [PubMed]
- Engelke, M.; Jensen, J.M.; Ekanayake-Mudiyanselage, S.; Proksch, E. Effects of xerosis and aging on epidermal proliferation and differentiation. Br. J. Dermatol. 1997, 137, 219–225. [Google Scholar] [CrossRef]
- Beretta, E.; Lanfranconi, F.; Grasso, G.S.; Bartesaghi, M.; Alemayehu, H.K.; Pratali, L.; Catuzzo, B.; Giardini, G.; Miserocchi, G. Air blood barrier phenotype correlates with alveolo-capillary O equilibration in hypobaric hypoxia. Respir. Physiol. Neurobiol. 2017, 246, 53–58. [Google Scholar] [CrossRef]
- Okada, Y.; Paton, J.F.R.; López-Barneo, J.; Wilson, R.J.A.; Marina, N.; Pokorski, M. Editorial: Hypoxia and Cardiorespiratory Control. Front. Physiol. 2021, 12, 820815. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Peng, Y.J.; Nanduri, J. Adaptive cardiorespiratory changes to chronic continuous and intermittent hypoxia. Handb. Clin. Neurol. 2022, 188, 103–123. [Google Scholar]
- Nakamura, H.; Shibata, M.; Watanabe, N. Intravital Observation of Microvascular Remodeling During Chronic Exposure to Hypoxia in Mice. Adv. Exp. Med. Biol. 2018, 1072, 245–249. [Google Scholar] [PubMed]
- Han, N.; Pan, Z.; Liu, G.; Yang, R.; Yujing, B. Hypoxia: The “Invisible Pusher” of Gut Microbiota. Front. Microbiol. 2021, 12, 690600. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Maity, C.; Ghosh, K.; Pati, B.R.; Mondal, K.C. Dynamics of predominant microbiota in the human gastrointestinal tract and change in luminal enzymes and immunoglobulin profile during high-altitude adaptation. Folia Microbiol. 2013, 58, 523–528. [Google Scholar] [CrossRef]
- Adak, A.; Maity, C.; Ghosh, K.; Mondal, K.C. Alteration of predominant gastrointestinal flora and oxidative damage of large intestine under simulated hypobaric hypoxia. Z. Gastroenterol. 2014, 52, 180–186. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Colgan, S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol. 2015, 309, 350–360. [Google Scholar] [CrossRef]
- Zhang, F.X.; Wu, W.M.; Deng, Z.Y.; Zheng, X.F.; Zhang, J.C.; Deng, S.X.; Chen, J.Y.; Ma, Q.; Wang, Y.; Yu, X.H.; et al. High altitude increases the expression of hypoxia-inducible factor 1α and inducible nitric oxide synthase with intest-inal mucosal barrier failure in rats. Int. J. Clin. Exp. Pathol. 2015, 8, 5189–5195. [Google Scholar] [PubMed]
- Yang, Y.H.; Yan, F.; Yuan, W.; Shi, P.S.; Wu, S.M.; Cui, D.J. High-altitude hypoxia promotes BRD4-mediated activation of the Wnt/β-catenin pathway and disruption of intestinal barrier. Cell. Signal. 2024, 120, 111187. [Google Scholar] [CrossRef] [PubMed]
- McInnis, K.; Haman, F.; Doucet, É. Humans in the cold: Regulating energy balance. Obes. Rev. 2020, 21, e12978. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; van Marken Lichtenbelt, W.D.; Strobbe, H.; Schrauwen, P. Energy metabolism in humans at a lowered ambient temperature. Eur. J. Clin. Nutr. 2002, 56, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, J.; Moeller, A.H. The Effects of Temperature on Animal Gut Microbiomes. Front. Microbiol. 2020, 11, 384. [Google Scholar] [CrossRef]
- Liu, J.; Peng, F.; Cheng, H.; Zhang, D.D.; Zhang, Y.X.; Wang, L.X.; Tang, F.; Wang, J.; Wan, Y.; Wu, J.; et al. Chronic cold environment regulates rheumatoid arthritis through modulation of gut microbiota-derived bile acids. Sci. Total Environ. 2023, 903, 166837. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.Q.; Zhao, M.X.; Xu, Y.; Fu, B.; Zhang, L.; Wu, S.; Yang, D.F.; Jia, C.X. Cold Exposure-induced Alterations in the Brain Peptidome and Gut Microbiome Are Linked to Energy Homeostasis in Mice. Mol. Cell Proteomics. 2023, 22, 100525. [Google Scholar] [CrossRef]
- Li, Y.L.; Zhou, E.K.; Yu, Y.H.; Wang, B.; Zhang, L.; Lei, R.Y.; Xue, B.D.; Tian, X.Y.; Niu, J.P.; Liu, J.T.; et al. Butyrate attenuates cold-induced hypertension via gut microbiota and activation of brown adipose tissue. Sci. Total Environ. 2024, 943, 173835. [Google Scholar] [CrossRef]
- Worthmann, A.; John, C.; Rühlemann, M.C.; Baguhl, M.; Heinsen, F.A.; Schaltenberg, N.; Heine, M.; Schlein, C.; Evangelakos, I.; Mineo, C.; et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat. Med. 2017, 23, 839–849. [Google Scholar] [CrossRef]
- Li, B.G.; Li, L.; Li, M.; Lam, S.M.; Wang, G.L.; Wu, Y.G.; Zhang, H.L.; Niu, C.Q.; Zhang, X.Y.; Liu, X.; et al. Microbiota Depletion Impairs Thermogenesis of Brown Adipose Tissue and Browning of White Adipose Tissue. Cell Rep. 2019, 26, 2720–2737. [Google Scholar] [CrossRef]
- Khakisahneh, S.; Zhang, X.Y.; Nouri, Z.; Wang, D.H. Gut Microbiota and Host Thermoregulation in Response to Ambient Temperature Fluctuations. mSystems 2020, 5, e00514-20. [Google Scholar] [CrossRef]
- Burke, G.; Faithfull, S.; Probst, H. Radiation induced skin reactions during and following radiotherapy: A systematic review of interventions. Radiography 2022, 28, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Szumiel, I. Ionizing radiation-induced oxidative stress; epigenetic changes and genomic instability: The pivotal role of mitochondria. Int. J. Radiat. Biol. 2015, 91, 1–12. [Google Scholar] [CrossRef]
- Santivasi, W.L.; Xia, F. Ionizing radiation-induced DNA damage; response; and repair. Antioxid. Redox Signal. 2014, 21, 251–259. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Prantil-Baun, R.; Jiang, A.; Potla, R.; Mammoto, T.; Weaver, J.C.; Ferrante, T.C.; Kim, H.J.; Cabral, J.M.S.; Levy, O.; et al. Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip. Cell Death Dis. 2018, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.Y.; Liang, Y.F.; Tian, S.J.; Jin, J.; Zhao, Y.M.; Fan, H.J. Radiation-Induced Intestinal Injury: Injury Mechanism and Potential Treatment Strategies. Toxics 2023, 11, 1011. [Google Scholar] [CrossRef] [PubMed]
- Hauer-Jensen, M.; Denham, J.W.; Andreyev, H.J. Radiation enteropathy--pathogenesis; treatment and prevention. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 470–479. [Google Scholar] [CrossRef]
- Liu, X.D.; Zhou, Y.; Wang, S.Z.; Guan, H.; Hu, S.; Huang, R.X.; Zhou, P.K. Impact of Low-Dose Ionizing Radiation on the Composition of the Gut Microbiota of Mice. Toxicol. Sci. 2019, 171, 258–268. [Google Scholar] [CrossRef]
- Zhao, T.S.; Xie, L.W.; Cai, S.; Xu, J.Y.; Zhou, H.; Tang, L.F.; Yang, C.; Fang, S.; Li, M.; Tian, Y. Dysbiosis of Gut Microbiota Is Associated With the Progression of Radiation-Induced Intestinal Injury and Is Alleviated by Oral Compound Probiotics in Mouse Model. Front. Cell Infect. Microbiol. 2021, 11, 717636. [Google Scholar] [CrossRef]
- He, K.Y.; Lei, X.Y.; Wu, D.H.; Zhang, L.; Li, J.Q.; Li, Q.T.; Yin, W.T.; Zhao, Z.L.; Liu, H.; Xiang, X.Y.; et al. Akkermansia muciniphila protects the intestine from irradiation-induced injury by secretion of propionic acid. Gut Microbes 2023, 15, 2293312. [Google Scholar] [CrossRef]
- Jameus, A.; Dougherty, J.; Narendrula, R.; Levert, D.; Valiquette, M.; Pirkkanen, J.; Lalonde, C.; Bonin, P.; Gagnon, J.D.; Appanna, V.D.; et al. Acute Impacts of Ionizing Radiation Exposure on the Gastrointestinal Tract and Gut Microbiome in Mice. Int. J. Mol. Sci. 2024, 25, 3339. [Google Scholar] [CrossRef]
- Kachrimanidou, M.; Tsintarakis, E. Insights into the Role of Human Gut Microbiota in Clostridioides difficile Infection. Microorganisms 2020, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.E.; Slusher, N.A.; Cabana, M.D.; Lynch, S.V. Role of the gut microbiota in defining human health. Expert. Rev. Anti Infect. Ther. 2010, 8, 435–454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ju, Z.J.; Zuo, T. Time for food: The impact of diet on gut microbiota and human health. Nutrition 2018, 51–52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Cummings, J.H.; Allison, C. Protein degradation by human intestinal bacteria. J. Gen. Microbiol. 1986, 132, 1647–1656. [Google Scholar] [CrossRef]
- Chassard, C.; Lacroix, C. Carbohydrates and the human gut microbiota. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 453–460. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.Y.; Fei, W.D.; Ye, Y.Y.; Zhao, M.D.; Zheng, C.H. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef]
- Skrypnik, K.; Suliburska, J. Association between the gut microbiota and mineral metabolism. J. Sci. Food Agric. 2018, 98, 2449–2460. [Google Scholar] [CrossRef]
- Montazeri-Najafabady, N.; Ghasemi, Y.; Dabbaghmanesh, M.H.; Talezadeh, P.; Koohpeyma, F.; Gholami, A. Supportive Role of Probiotic Strains in Protecting Rats from Ovariectomy-Induced Cortical Bone Loss. Probiotics Antimicrob. Proteins 2019, 11, 1145–1154. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Djafarian, K.; Fazeli, M.R.; Yekaninejad, M.S.; Rostamian, A.; Keshavarz, S.A. Effects of a Multispecies Probiotic Supplement on Bone Health in Osteopenic Postmenopausal Women: A Randomized, Double-blind, Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.F.; Lyu, J.; Wang, S.S. The role of intestinal microbes on intestinal barrier function and host immunity from a metabolite perspective. Front. Immunol. 2023, 14, 1277102. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Piao, X.S.; Mahfuz, S.; Long, S.F.; Wang, J. The interaction among gut microbes; the intestinal barrier and short-chain fatty acidshort chain fatty acids. Anim. Nutr. 2021, 9, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Zuo, L.; Dong, J.; Zhu, W.; Li, Y.; Gu, L.; Gong, J.; Li, Q.; Li, N.; et al. Intestinal dysbacteriosis contributes to decreased intestinal mucosal barrier function and increased bacterial translocation. Lett. Appl. Microbiol. 2014, 58, 384–392. [Google Scholar] [CrossRef]
- Cinova, J.; De Palma, G.; Stepankova, R.; Kofronova, O.; Kverka, M.; Sanz, Y.; Tuckova, L. Role of intestinal bacteria in gliadin-induced changes in intestinal mucosa: Study in germ-free rats. PLoS ONE 2011, 6, e16169. [Google Scholar] [CrossRef]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Souza, H.S.; Tortori, C.J.; Castelo-Branco, M.T.; Carvalho, A.T.; Margallo, V.S.; Delgado, C.F.; Dines, I.; Elia, C.C. Apoptosis in the intestinal mucosa of patients with inflammatory bowel disease: Evidence of altered expression of FasL and perforin cytotoxic pathways. Int. J. Color. Dis. 2005, 20, 277–286. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef]
- Ijssennagger, N.; Belzer, C.; Hooiveld, G.J.; Dekker, J.; van Mil, S.W.; Müller, M.; Kleerebezem, M.; van der Meer, R. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc. Natl. Acad. Sci. USA 2015, 112, 10038–10043. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.J.; Wu, S.; Wu, X.; Huso, D.L.; Karim, B.; Franco, A.A.; Rabizadeh, S.; Golub, J.E.; Mathews, L.E.; Shin, J.; et al. Induction of persistent colitis by a human commensal; enterotoxigenic Bacteroides fragilis; in wild-type C57BL/6 mice. Infect. Immun. 2009, 77, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Katahira, J.; Sugiyama, H.; Inoue, N.; Horiguchi, Y.; Matsuda, M.; Sugimoto, N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J. Biol. Chem. 1997, 272, 26652–26658. [Google Scholar] [CrossRef] [PubMed]
- Veshnyakova, A.; Piontek, J.; Protze, J.; Waziri, N.; Heise, I.; Krause, G. Mechanism of Clostridium perfringens enterotoxin interaction with claudin-3/-4 protein suggests structural modifications of the toxin to target specific claudins. J. Biol. Chem. 2012, 287, 1698–1708. [Google Scholar] [CrossRef]
- Gu, M.; Samuelson, D.R.; de la Rua, N.M.; Charles, T.P.; Taylor, C.M.; Luo, M.; Siggins, R.W.; Shellito, J.E.; Welsh, D.A. Host innate and adaptive immunity shapes the gut microbiota biogeography. Microbiol. Immunol. 2022, 66, 330–341. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Béguet-Crespel, F.; Marinelli, L.; Jamet, A.; Ledue, F.; Blottière, H.M.; Lapaque, N. Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Sci. Rep. 2018, 8, 9742. [Google Scholar] [CrossRef]
- Byndloss, M.X.; Olsan, E.E.; Rivera-Chávez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 2017, 357, 570–575. [Google Scholar] [CrossRef]
- den Besten, G.; Gerding, A.; van Dijk, T.H.; Ciapaite, J.; Bleeker, A.; van Eunen, K.; Havinga, R.; Groen, A.K.; Reijngoud, D.J.; Bakker, B.M. Protection against the Metabolic Syndrome by Guar Gum-Derived Short-Chain Fatty Acids Depends on Peroxisome Proliferator-Activated Receptor γ and Glucagon-Like Peptide-1. PLoS ONE 2015, 10, e0136364. [Google Scholar] [CrossRef]
- Athman, R.; Philpott, D. Innate immunity via Toll-like receptors and Nod proteins. Curr. Opin. Microbiol. 2004, 7, 25–32. [Google Scholar] [CrossRef]
- Oviedo-Boyso, J.; Bravo-Patiño, A.; Baizabal-Aguirre, V.M. Collaborative action of Toll-like and NOD-like receptors as modulators of the inflammatory response to pathogenic bacteria. Mediat. Inflamm. 2014, 2014, 432785. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Alcon-Giner, C.; Dalby, M.J.; Caim, S.; Ketskemety, J.; Shaw, A.; Sim, K.; Lawson, M.A.E.; Kiu, R.; Leclaire, C.; Chalklen, L.; et al. Microbiota Supplementation with Bifidobacterium and Lactobacillus Modifies the Preterm Infant Gut Microbiota and Metabolome: An Observational Study. Cell Rep. Med. 2020, 1, 100077. [Google Scholar] [CrossRef] [PubMed]
- Tinevez, J.Y.; Arena, E.T.; Anderson, M.; Nigro, G.; Injarabian, L.; André, A.; Ferrari, M.; Campbell-Valois, F.X.; Devin, A.; Shorte, S.L.; et al. Shigella-mediated oxygen depletion is essential for intestinal mucosa colonization. Nat. Microbiol. 2019, 4, 2001–2009. [Google Scholar] [CrossRef]
- Martinson, J.N.V.; Walk, S.T. Escherichia coli Residency in the Gut of Healthy Human Adults. EcoSal Plus 2020, 9, 10. [Google Scholar] [CrossRef]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Rigottier-Gois, L. Dysbiosis in inflammatory bowel diseases: The oxygen hypothesis. ISME J. 2013, 7, 1256–1261. [Google Scholar] [CrossRef]
- Knoop, K.A.; McDonald, K.G.; Kulkarni, D.H.; Newberry, R.D. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 2016, 65, 1100–1109. [Google Scholar] [CrossRef]
- Turner, N.A.; Anderson, D.J. Hospital Infection Control: Clostridioides difficile. Clin. Colon Rectal Surg. 2020, 33, 98–108. [Google Scholar] [CrossRef]
- Denève, C.; Janoir, C.; Poilane, I.; Fantinato, C.; Collignon, A. New trends in Clostridium difficile virulence and pathogenesis. Int. J. Antimicrob. Agents 2009, 33, S24–S28. [Google Scholar] [CrossRef]
- Rodriguez, S.; Hernandez, M.B.; Tarchini, G.; Zaleski, M.; Vatanchi, M.; Cardona, L.; Castro-Pavia, F.; Schneider, A. Risk of Clostridium difficile infection in hospitalized patients receiving metronidazole for a non-C difficile infection. Clin. Gastroenterol. Hepatol. 2014, 12, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Rafey, A.; Jahan, S.; Farooq, U.; Akhtar, F.; Irshad, M.; Nizamuddin, S.; Parveen, A. Antibiotics Associated With Clostridium difficile Infection. Cureus 2023, 15, e39029. [Google Scholar] [CrossRef]
- Schubert, A.M.; Sinani, H.; Schloss, P.D. Antibiotic-Induced Alterations of the Murine Gut Microbiota and Subsequent Effects on Colonization Resistance against Clostridium difficile. mBio 2015, 6, e00974. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Gassler, N.; Rohr, C.; Schneider, A.; Kartenbeck, J.; Bach, A.; Obermüller, N.; Otto, H.F.; Autschbach, F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, 216–228. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Lai, Y.J.; Huang, X.F.; Sun, H.W.; Hui, Q.; Hu, S.S. Research Progress in the Relationship between Intestinal Flora and Diabetes Mellitus. Endocr. Metab. Immune Disord. Drug Targets 2025, 25, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Kunugi, H. Intestinal Flora and Mental Disorders: A Focus on Mood Disorders and Autism Spectrum Disorder. Brain Nerve. 2021, 73, 871–877. [Google Scholar]
- Wu, W.H.; Zegarra-Ruiz, D.F.; Diehl, G.E. Intestinal Microbes in Autoimmune and Inflammatory Disease. Front. Immunol. 2020, 11, 597966. [Google Scholar] [CrossRef]
- Zou, Y.F.; Song, X.J.; Liu, N.; Sun, W.; Liu, B. Intestinal Flora: A Potential New Regulator of Cardiovascular Disease. Aging Dis. 2022, 13, 753–772. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.Y.; Yu, Z.J.; Zhou, Y.X.; Wang, S.Y.; Jia, M.Q.; Chen, T.; Zhang, X.J. Gut microbiota modulates neurotransmitter and gut-brain signaling. Microbiol. Res. 2024, 287, 127858. [Google Scholar] [CrossRef]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef]
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hörtnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Mandić, A.D.; Woting, A.; Jaenicke, T.; Sander, A.; Sabrowski, W.; Rolle-Kampcyk, U.; von Bergen, M.; Blaut, M. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Sci. Rep. 2019, 9, 1177. [Google Scholar] [CrossRef]
- Tian, P.J.; Wang, G.; Zhao, J.X.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef]
- Li, H.W.; Wang, P.; Huang, L.Q.; Li, P.; Zhang, D.L. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol. Motil. 2019, 31, e13677. [Google Scholar] [CrossRef]
- Peterson, D.A.; Cardona, R.A. Specificity of the adaptive immune response to the gut microbiota. Adv. Immunol. 2010, 107, 71–107. [Google Scholar]
- Purchiaroni, F.; Tortora, A.; Gabrielli, M.; Bertucci, F.; Gigante, G.; Ianiro, G.; Ojetti, V.; Scarpellini, E.; Gasbarrini, A. The role of intestinal microbiota and the immune system. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 323–333. [Google Scholar] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Kopschina Feltes, P.; Doorduin, J.; Klein, H.C.; Juárez-Orozco, L.E.; Dierckx, R.A.; Moriguchi-Jeckel, C.M.; de Vries, E.F. Anti-inflammatory treatment for major depressive disorder: Implications for patients with an elevated immune profile and nonresponders to standard antidepressant therapy. J. Psychopharmacol. 2017, 31, 1149–1165. [Google Scholar] [CrossRef]
- Gao, J.W.; Cao, B.; Zhao, R.Y.; Li, H.H.; Xu, Q.X.; Wei, B. Critical Signal Transduction Pathways and Intestinal Barrier: Implications for Pathophysiology and Therapeutics. Pharmaceuticals 2023, 16, 1216. [Google Scholar] [CrossRef]
- Galley, J.D.; Yu, Z.; Kumar, P.; Dowd, S.E.; Lyte, M.; Bailey, M.T. The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor. Gut Microbes 2014, 5, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Galley, J.D.; Nelson, M.C.; Yu, Z.; Dowd, S.E.; Walter, J.; Kumar, P.S.; Lyte, M.; Bailey, M.T. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.C.; Berk, M. Increased IgA and IgM responses against gut commensals in chronic depression: Further evidence for increased bacterial translocation or leaky gut. J. Affect. Disord. 2012, 141, 55–62. [Google Scholar] [CrossRef]
- Li, N.N.; Wang, Q.; Wang, Y.; Sun, A.J.; Lin, Y.W.; Jin, Y.; Li, X.B. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress 2019, 22, 592–602. [Google Scholar] [CrossRef]
- De Palma, G.; Shimbori, C.; Reed, D.E.; Yu, Y.; Rabbia, V.; Lu, J.; Jimenez-Vargas, N.; Sessenwein, J.; Lopez-Lopez, C.; Pigrau, M.; et al. Histamine production by the gut microbiota induces visceral hyperalgesia through histamine 4 receptor signaling in mice. Sci. Transl. Med. 2022, 14, 1895. [Google Scholar] [CrossRef]
- Iida, T.; Yoshikawa, T.; Kárpáti, A.; Matsuzawa, T.; Kitano, H.; Mogi, A.; Harada, R.; Naganuma, F.; Nakamura, T.; Yanai, K. JNJ10181457, a histamine H3 receptor inverse agonist, regulates in vivo microglial functions and improves depression-like behaviours in mice. Biochem. Biophys. Res. Commun. 2017, 488, 534–540. [Google Scholar] [CrossRef]
- Su, W.J.; Zhang, T.; Jiang, C.L.; Wang, W. Clemastine Alleviates Depressive-Like Behavior Through Reversing the Imbalance of Microglia-Related Pro-inflammatory State in Mouse Hippocampus. Front. Cell. Neurosci. 2018, 12, 412. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Shu, C.; Xiao, L.; Wang, G.H. Histamine and histamine receptors: Roles in major depressive disorder. Front. Psychiatry 2022, 13, 825591. [Google Scholar] [CrossRef]
- Ahlman, H.; Nilsson, O. The gut as the largest endocrine organ in the body. Ann. Oncol. 2001, 12 (Suppl. S2), S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Fothergill, L.J.; Callaghan, B.; Hunne, B.; Bravo, D.M.; Furness, J.B. Costorage of Enteroendocrine Hormones Evaluated at the Cell and Subcellular Levels in Male Mice. Endocrinology 2017, 158, 2113–2123. [Google Scholar] [CrossRef]
- Ge, T.T.; Yao, X.X.; Zhao, H.S.; Yang, W.; Zou, X.H.; Peng, F.Z.; Li, B.J.; Cui, R.J. Gut microbiota and neuropsychiatric disorders: Implications for neuroendocrine-immune regulation. Pharmacol. Res. 2021, 173, 105909. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Yang, W.J.; Li, Y.Q.; Cong, Y.Z. Enteroendocrine Cells: Sensing Gut Microbiota and Regulating Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2020, 26, 11–20. [Google Scholar] [CrossRef]
- Frankiensztajn, L.M.; Elliott, E.; Koren, O. The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis; implications for anxiety and stress disorders. Curr. Opin. Neurobiol. 2020, 62, 76–82. [Google Scholar] [CrossRef]
- Hueston, C.M.; Barnum, C.J.; Eberle, J.A.; Ferraioli, F.J.; Buck, H.M.; Deak, T. Stress-dependent changes in neuroinflammatory markers observed after common laboratory stressors are not seen following acute social defeat of the Sprague Dawley rat. Physiol. Behav. 2011, 104, 187–198. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Moya-Pérez, A.; Perez-Villalba, A.; Benítez-Páez, A.; Campillo, I.; Sanz, Y. Bifidobacterium CECT 7765 modulates early stress-induced immune; neuroendocrine and behavioral alterations in mice. Brain Behav. Immun. 2017, 65, 43–56. [Google Scholar] [CrossRef]
- Fukui, H.; Oshima, T.; Tanaka, Y.; Oikawa, Y.; Makizaki, Y.; Ohno, H.; Tomita, T.; Watari, J.; Miwa, H. Effect of probiotic Bifidobacterium bifidum G9-1 on the relationship between gut microbiota profile and stress sensitivity in maternally separated rats. Sci. Rep. 2018, 8, 12384. [Google Scholar] [CrossRef]
- Tian, P.; O’Riordan, K.J.; Lee, Y.K.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress 2020, 12, 100216. [Google Scholar] [CrossRef]
- Stenman, L.K.; Patterson, E.; Meunier, J.; Roman, F.J.; Lehtinen, M.J. Strain specific stress-modulating effects of candidate probiotics: A systematic screening in a mouse model of chronic restraint stress. Behav. Brain Res. 2020, 379, 112376. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.D.; Niu, H.M.; Zhao, Q.; Shi, J.; An, C.H.; Wang, S.Y.; Zhou, C.H.; Chen, S.Y.; Fu, Y.X.; Zhang, Y.Q.; et al. Impact of high-altitude acclimatization and de-acclimatization on the intestinal microbiota of rats in a natural high-altitude environment. Front. Microbiol. 2024, 15, 1371247. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, X. Comparative analyses of fecal microbiota in Tibetan and Chinese Han living at low or high altitude by barcoded 454 pyrosequencing. Sci. Rep. 2015, 5, 14682. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Chu, C.; Artis, D.; Chiu, I.M. Neuro-immune Interactions in the Tissues. Immunity 2020, 52, 464–474. [Google Scholar] [CrossRef]
- Li, M.; Van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short-chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Chung, Y.E.; Chen, H.C.; Chou, H.L.; Chen, I.M.; Lee, M.S.; Chuang, L.C.; Liu, Y.W.; Lu, M.L.; Chen, C.H.; Wu, C.S.; et al. Exploration of microbiota targets for major depressive disorder and mood related traits. J. Psychiatr. Res. 2019, 111, 74–82. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhang, L.; Wang, X.Q.; Wang, Z.; Zhang, J.J.; Jiang, R.H.; Wang, X.Q.; Wang, K.; Liu, Z.J.; Xia, Z.W.; et al. Similar Fecal Microbiota Signatures in Patients With Diarrhea-Predominant Irritable Bowel Syndrome and Patients With Depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Sheu, J.C.; Venkatachalam, A.; Runge, J.K.; Luna, R.A.; Calarge, C.A. Gut microbiome in adolescent depression. J. Affect. Disord. 2021, 292, 500–507. [Google Scholar] [CrossRef]
- Bai, S.; Xie, J.; Bai, H.; Tian, T.; Zou, T.; Chen, J.J. Gut Microbiota-Derived Inflammation-Related Serum Metabolites as Potential Biomarkers for Major Depressive Disorder. J. Inflamm. Res. 2021, 14, 3755–3766. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Ling, Z.X.; Zhang, Y.H.; Mao, H.J.; Ma, Z.P.; Yin, Y.; Wang, W.H.; Tang, W.X.; Tan, Z.L.; Shi, J.F.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Caso, J.R.; MacDowell, K.S.; González-Pinto, A.; García, S.; de Diego-Adeliño, J.; Carceller-Sindreu, M.; Sarramea, F.; Caballero-Villarraso, J.; Gracia-García, P.; De la Cámara, C.; et al. Gut microbiota; innate immune pathways; and inflammatory control mechanisms in patients with major depressive disorder. Transl. Psychiatry 2021, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The Next-Generation Probiotics for the Brain. Curr. Microbiol. 2021, 78, 449–463. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Nazari, S.; Etesam, F.; Nourimajd, S.; Ahmadpanah, M.; Jahromi, S.R. The Effect of Synbiotic as an Adjuvant Therapy to Fluoxetine in Moderate Depression: A Randomized Multicenter Trial. Arch. Neurosci. 2018, 5, e60507. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.J.K.; Milev, R.V. The Efficacy, Safety, and Tolerability of Probiotics on Depression: Clinical Results From an Open-Label Pilot Study. Front. Psychiatry 2021, 12, 618279. [Google Scholar] [CrossRef]
- Noori, M.; Shateri, Z.; Babajafari, S.; Eskandari, M.H.; Parastouei, K.; Ghasemi, M.; Afshari, H.; Samadi, M. The effect of probiotic-fortified kefir on depression, appetite, oxidative stress, and inflammatory parameters in Iranian overweight and obese elderly: A randomized, double-blind, placebo-controlled clinical trial. J. Health Popul. Nutr. 2025, 44, 30. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Galea, I. The blood-brain barrier in systemic infection and inflammation. Cell Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef]
- Xie, Z.Q.; Huang, J.J.; Sun, G.Q.; He, S.; Luo, Z.Y.; Zhang, L.N.; Li, L.; Yao, M.; Du, C.; Yu, W.J.; et al. Integrated multi-omics analysis reveals gut microbiota dysbiosis and systemic disturbance in major depressive disorder. Psychiatry Res. 2024, 334, 115804. [Google Scholar] [CrossRef]
- Hu, Y.C.; Pan, Z.Y.; Huang, Z.Y.; Li, Y.; Han, N.; Zhuang, X.M.; Peng, H.; Gao, Q.S.; Wang, Q.; Yang Lee, B.J.; et al. Gut Microbiome-Targeted Modulations Regulate Metabolic Profiles and Alleviate Altitude-Related Cardiac Hypertrophy in Rats. Microbiol. Spectr. 2022, 10, e0105321. [Google Scholar] [CrossRef]
- Luo, H.; Zhou, D.J.; Chen, Z.; Zhou, Q.Q.; Wu, K.; Tian, K.; Li, Z.W.; Xiao, Z.L. Establishment and evaluation of an experimental rat model for high-altitude intestinal barrier injury. Exp. Ther. Med. 2017, 13, 475–482. [Google Scholar] [CrossRef]
- McKenna, Z.J.; Gorini Pereira, F.; Gillum, T.L.; Amorim, F.T.; Deyhle, M.R.; Mermier, C.M. High-altitude exposures and intestinal barrier dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R192–R203. [Google Scholar] [CrossRef]
- Al-Hashem, F.H.; Assiri, A.S.; Shatoor, A.S.; Elrefaey, H.M.; Alessa, R.M.; Alkhateeb, M.A. Increased systemic low-grade inflammation in high altitude native rats mediated by adrenergic receptors. Saudi Med. J. 2014, 35, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Chien, W.C.; Chung, C.H.; Her, Y.N.; Yao, C.Y.; Lee, B.L.; Li, F.L.; Wan, F.J.; Tzeng, N.S. Acute Mountain Sickness and the Risk of Subsequent Psychiatric Disorders-A Nationwide Cohort Study in Taiwan. Int. J. Environ. Res. Public. Health 2023, 20, 2868. [Google Scholar] [CrossRef]
- Woolcott, O.O. The Lake Louise Acute Mountain Sickness Score: Still a Headache. High. Alt. Med. Biol. 2021, 22, 351–352. [Google Scholar] [CrossRef]
- Das, B.; Ghosh, T.S.; Kedia, S.; Rampal, R.; Saxena, S.; Bag, S.; Mitra, R.; Dayal, M.; Mehta, O.; Surendranath, A.; et al. Analysis of the Gut Microbiome of Rural and Urban Healthy Indians Living in Sea Level and High Altitude Areas. Sci. Rep. 2018, 8, 10104. [Google Scholar] [CrossRef] [PubMed]
- Lahti, L.; Salonen, A.; Kekkonen, R.A.; Salojärvi, J.; Jalanka-Tuovinen, J.; Palva, A.; Orešič, M.; de Vos, W.M. Associations between the human intestinal microbiota; Lactobacillus rhamnosus GG and serum lipids indicated by integrated analysis of high-throughput profiling data. PeerJ 2013, 1, e32. [Google Scholar] [CrossRef]
- Pham, K.; Parikh, K.; Heinrich, E.C. Hypoxia and Inflammation: Insights From High-Altitude Physiology. Front. Physiol. 2021, 12, 676782. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.S.; Fan, Q.L.; Hu, Q.Z.; Hou, Q. Research progress on the mechanism of cerebral blood flow regulation in hypoxia environment at plateau. Bioengineered 2022, 13, 6353–6358. [Google Scholar] [CrossRef]
- Merelli, A.; Repetto, M.; Lazarowski, A.; Auzmendi, J. Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J. Alzheimers Dis. 2021, 82 (Suppl. S1), S109–S126. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.X.; Gozal, D. Reactive oxygen species and the brain in sleep apnea. Respir. Physiol. Neurobiol. 2010, 174, 307–316. [Google Scholar] [CrossRef]
- Sohal, R.S.; Allen, R.G. Oxidative stress as a causal factor in differentiation and aging: A unifying hypothesis. Exp. Gerontol. 1990, 25, 499–522. [Google Scholar] [CrossRef]
- Maiti, P.; Singh, S.B.; Mallick, B.; Muthuraju, S.; Ilavazhagan, G. High altitude memory impairment is due to neuronal apoptosis in hippocampus; cortex and striatum. J. Chem. Neuroanat. 2008, 36, 227–238. [Google Scholar] [CrossRef]
- Bailey, D.M.; Brugniaux, J.V.; Filipponi, T.; Marley, C.J.; Stacey, B.; Soria, R.; Rimoldi, S.F.; Cerny, D.; Rexhaj, E.; Pratali, L.; et al. Exaggerated systemic oxidative-inflammatory-nitrosative stress in chronic mountain sickness is associated with cognitive decline and depression. J. Physiol. 2019, 597, 611–629. [Google Scholar] [CrossRef]
- Hwang, J.; DeLisi, L.E.; Öngür, D.; Riley, C.; Zuo, C.; Shi, X.; Sung, Y.H.; Kondo, D.; Kim, T.S.; Villafuerte, R.; et al. Cerebral bioenergetic differences measured by phosphorus-31 magnetic resonance spectroscopy between bipolar disorder and healthy subjects living in two different regions suggesting possible effects of altitude. Psychiatry Clin. Neurosci. 2019, 73, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Kious, B.M.; Kondo, D.G.; Renshaw, P.F. Living High and Feeling Low: Altitude, Suicide, and Depression. Harv. Rev. Psychiatry 2018, 26, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Qu, C.Y.; Zhao, L.N.; Zhang, J.H.; Huang, P.; Gao, D.R.; Wei, Q.M.; Qin, F.; Zhao, J.X. Effects of high-/low-temperature and high-altitude hypoxic environments on gut microbiota of sports people: A retrospective analysis. Sports Med. Health Sci. 2023, 5, 83–90. [Google Scholar] [CrossRef]
- Wang, X.B.; Wu, X.Y.; Shang, Y.Q.; Gao, Y.; Li, Y.; Wei, Q.G.; Dong, Y.H.; Mei, X.S.; Zhou, S.Y.; Sun, G.L.; et al. High-Altitude Drives the Convergent Evolution of Alpha Diversity and Indicator Microbiota in the Gut Microbiomes of Ungulates. Front. Microbiol. 2022, 13, 953234. [Google Scholar] [CrossRef]
- Zhao, J.S.; Yao, Y.F.; Dong, M.M.; Xiao, H.T.; Xiong, Y.; Yang, S.Z.; Li, D.Y.; Xie, M.; Ni, Q.Y.; Zhang, M.W.; et al. Diet and high altitude strongly drive convergent adaptation of gut microbiota in wild macaques, humans, and dogs to high altitude environments. Front. Microbiol. 2023, 14, 1067240. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.Y.; Chen, Z.S.; Xie, Z.J.; Liu, J.X.; Xiao, X.M. Mechanisms underlying changes in intestinal permeability during pregnancy and their implications for maternal and infant health. J. Reprod. Immunol. 2025, 168, 104423. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Zhang, X.J.; Yao, G.X.; Jin, J.J.; Zhang, T.X.; Sun, C.H.; Wang, Z.; Zhang, Q.Y. Intestinal flora and pregnancy complications: Current insights and future prospects. Imeta 2024, 3, e167. [Google Scholar] [CrossRef]
- Clements, S.J.; R Carding, S. Diet, the intestinal microbiota; and immune health in aging. Crit. Rev. Food Sci. Nutr. 2018, 58, 651–661. [Google Scholar] [CrossRef]
- Chen, L.A.; Boyle, K. The Role of the Gut Microbiome in Health and Disease in the Elderly. Curr. Gastroenterol. Rep. 2024, 26, 217–230. [Google Scholar] [CrossRef]
- Devita, M.; De Salvo, R.; Ravelli, A.; De Rui, M.; Coin, A.; Sergi, G.; Mapelli, D. Recognizing Depression in the Elderly: Practical Guidance and Challenges for Clinical Management. Neuropsychiatr. Dis. Treat. 2022, 18, 2867–2880. [Google Scholar] [CrossRef]
- San, T.; Polat, S.; Cingi, C.; Eskiizmir, G.; Oghan, F.; Cakir, B. Effects of high altitude on sleep and respiratory system and theirs adaptations. Sci. World J. 2013, 4, 241569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sun, L.; Liu, J.; Shi, B.; Zhang, Y.; Qu-Zong, C.R.; Dorji, T.; Wang, T.; Yuan, H.; Yang, J. Meta-analysis identifying gut microbial biomarkers of Qinghai-Tibet Plateau populations and the functionality of microbiota-derived butyrate in high-altitude adaptation. Gut Microbes 2024, 16, 2350151. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.F.; Zhou, S.J.; Burger, H.; Bockting, C.L.H.; Williams, A.D. Psychological interventions for depression in Chinese university students: A systematic review and meta-analysis. J. Affect. Disord. 2020, 262, 440–450. [Google Scholar] [CrossRef]
- Gautam, M.; Tripathi, A.; Deshmukh, D.; Gaur, M. Cognitive Behavioral Therapy for Depression. Indian J. Psychiatry 2020, 62 (Suppl. S2), S223–S229. [Google Scholar] [CrossRef] [PubMed]
- Cooney, G.M.; Dwan, K.; Greig, C.A.; Lawlor, D.A.; Rimer, J.; Waugh, F.R.; McMurdo, M.; Mead, G.E. Exercise for depression. Cochrane Database Syst. Rev. 2013, 2013, CD004366. [Google Scholar]
- Xie, Y.M.; Wu, Z.T.; Sun, L.M.; Zhou, L.; Wang, G.H.; Xiao, L.; Wang, H.L. The Effects and Mechanisms of Exercise on the Treatment of Depression. Front. Psychiatry 2021, 12, 705559. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe. 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Fu, J.X.; Zheng, Y.; Gao, Y.; Xu, W.H. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Healey, G.; Murphy, R.; Butts, C.; Brough, L.; Whelan, K.; Coad, J. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: A randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br. J. Nutr. 2018, 119, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.I.; Kambashi, B.; Rodriguez, J.; Cani, P.D.; et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695. [Google Scholar] [CrossRef]
- Costabile, A.; Kolida, S.; Klinder, A.; Gietl, E.; Bäuerlein, M.; Frohberg, C.; Landschütze, V.; Gibson, G.R. A double-blind; placebo-controlled; cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe artichoke (Cynara scolymus) in healthy human subjects. Br. J. Nutr. 2010, 104, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Guo, X.S.; Long, R.J.; Kreuzer, M.; Ding, L.M.; Shang, Z.H.; Zhang, Y.; Yang, Y.; Cui, G.X. Importance of functional ingredients in yak milk-derived food on health of Tibetan nomads living under high-altitude stress: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 292–302. [Google Scholar] [CrossRef]
- Zhao, Q.L.; Yang, Q.F.; Li, X.M. Study on Nutritional Interventions for Gastrointestinal Stress Response in Military Personnel with Acute Exposure to High Altitude. Clin. J. Med. Off. 2011, 39, 3. [Google Scholar]
- Doll, J.P.K.; Vázquez-Castellanos, J.F.; Schaub, A.C.; Schweinfurth, N.; Kettelhack, C.; Schneider, E.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Fecal Microbiota Transplantation (FMT) as an Adjunctive Therapy for Depression-Case Report. Front. Psychiatry 2022, 13, 815422. [Google Scholar] [CrossRef]
- Kurokawa, S.; Kishimoto, T.; Mizuno, S.; Masaoka, T.; Naganuma, M.; Liang, K.C.; Kitazawa, M.; Nakashima, M.; Shindo, C.; Suda, W.; et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome; functional diarrhea and functional constipation: An open-label observational study. J. Affect. Disord. 2018, 235, 506–512. [Google Scholar] [CrossRef]
- Lin, H.; Guo, Q.Q.; Wen, Z.Y.; Tan, S.L.; Chen, J.; Lin, L.J.; Chen, P.C.; He, J.Q.; Wen, J.B.; Chen, Y. The multiple effects of fecal microbiota transplantation on diarrhea-predominant irritable bowel syndrome (IBS-D) patients with anxiety and depression behaviors. Microb. Cell Fact. 2021, 20, 233. [Google Scholar] [CrossRef]
- Rao, J.J.; Qiao, Y.; Xie, R.N.; Lin, L.; Jiang, J.; Wang, C.M.; Li, G.Y. Fecal microbiota transplantation ameliorates stress-induced depression-like behaviors associated with the inhibition of glial and NLRP3 inflammasome in rat brain. J. Psychiatr. Res. 2021, 137, 147–157. [Google Scholar] [CrossRef]
- Cooke, N.C.A.; Bala, A.; Allard, J.P.; Hota, S.; Poutanen, S.; Taylor, V.H. The safety and efficacy of fecal microbiota transplantation in a population with bipolar disorder during depressive episodes: Study protocol for a pilot randomized controlled trial. Pilot Feasibility Stud. 2021, 7, 142. [Google Scholar] [CrossRef]
- Kim, K.O.; Gluck, M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin. Endosc. 2019, 52, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Schmitter, M.; Spijker, J.; Smit, F.; Tendolkar, I.; Derksen, A.M.; Oostelbos, P.; Wijnen, B.F.M.; van Doesum, T.J.; Smits, J.A.J.; Vrijsen, J.N. Exercise enhances: Study protocol of a randomized controlled trial on aerobic exercise as depression treatment augmentation. BMC Psychiatry 2020, 20, 585. [Google Scholar] [CrossRef] [PubMed]
- Streeter, C.C.; Gerbarg, P.L.; Brown, R.P.; Scott, T.M.; Nielsen, G.H.; Owen, L.; Sakai, O.; Sneider, J.T.; Nyer, M.B.; Silveri, M.M. Thalamic Gamma Aminobutyric Acid Level Changes in Major Depressive Disorder After a 12-Week Iyengar Yoga and Coherent Breathing Intervention. J. Altern. Complement. Med. 2020, 26, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Özkan, S.A.; Kücükkelepce, D.S.; Korkmaz, B.; Yılmaz, G.; Bozkurt, M.A. The effectiveness of an exercise intervention in reducing the severity of postpartum depression: A randomized controlled trial. Perspect. Psychiatr. Care 2020, 56, 844–850. [Google Scholar] [CrossRef]
- Drust, B.; Waterhouse, J. Exercise at altitude. Scott. Med. J. 2010, 55, 31–34. [Google Scholar] [CrossRef]
- Chen, B.X.; Wu, Z.S.; Huang, X.; Li, Z.C.; Wu, Q.J.; Chen, Z.C. Effect of altitude training on the aerobic capacity of athletes: A systematic review and meta-analysis. Heliyon 2023, 9, e20188. [Google Scholar] [CrossRef]
| Years | Subject Population | Microbiota | Intervention Duration | Effect |
|---|---|---|---|---|
| 2002 [163] | Adults suffering from stress or exhaustion (n = 34) | Lactobacillus acidophilus, Bifidobacterium bifidum and Bifidobacterium longum | 6 months | The subjects’ stress improved by 40.7% overall |
| 2004 [164] | College students under exam pressure (n = 136) | Lactobacillus casei, Lactobacillus delbrueckii subspecies bulgaricus and Streptococcus salivarius subspecies thermophiles | 6 weeks | Increased the number of lymphocytes and CD56 cells in subjects |
| 2009 [148] | Chronic Fatigue Syndrome (CFS) patients (n = 35) | Lactobacillus casei strain Shirota | 8 weeks | The anxiety scores of CFS patients were significantly reduced |
| 2010 [165] | Irritable Bowel Syndrome (IBS) patients (n = 74) | Lactobacillus paracasei, ssp. paracasei F19, Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 | 8 weeks | Reduce Anxiety and Depression (HAD) scale scores in IBS subjects |
| 2011 [166] | Subjects with urine free cortisol (UFC) levels below 50 ng/mL (n = 25) | Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 | 30 days | Significantly reduced HAD scores in UFS subjects |
| 2013 [167] | College students who exercise vigorously every day (n = 44) | Lactobacillus gasseri OLL2809 | 4 weeks | The effects of increasing the activity of natural killer cells reduced by intense exercise in subjects and improving mood in depressed states may help athletes maintain physical and mental health |
| 2015 [168] | Adults with IBS and diarrhea or mixed stool patterns (based on the Rome III criteria) and mild to moderate anxiety and/or depression (based on the Hospital Anxiety and Depression Scale) (n = 44) | Bifidobacterium longum NCC3001 | 6 weeks | HAD scores were significantly reduced, and negative emotional stimulus responses in the amygdala and frontal limbic regions were reduced. |
| 2016 [145] | Laryngeal Cancer (LC) patients (n = 20) | Clostridium butyricum | 2 weeks | After taking Cb, the anxiety level of LC patients was relieved, and the serum adrenocorticotropin-releasing factor was inhibited |
| 2016 [146] | MDD patients (n = 40) | Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum | 8 weeks | The total score of the Baker Depression Scale (BDI) was significantly reduced |
| 2018 [169] | Patients with moderate depression (n = 40) | Bifidobacterium breve, Bifidobacterium longum, Lactobacillus acidofilus, Lactobacillus bulgarigus, Lactobacillus casaei, Lactobacillus rhamnosus and Streptococus thermophilus | 10 weeks | Patients with MD had significantly lower Hamilton Depression Scale (HAM-D) scores |
| 2019 [170] | Patients with clinically diagnosed depression (n = 110) | Lactobacillus helveticus and Bifidobacterium longum | 8 weeks | Patients with depression had significantly lower total BDI scores and significantly lower serum kynurenine/tryptophan ratios |
| 2021 [171] | MDD patients (n = 10) | Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 | 6 weeks | Overall early depressive symptoms improved significantly in MDD patients as measured by MADRS and QIDS-SR16, as well as anhedonia-like symptoms as measured by SHAPS. |
| 2022 [172] | Patients with mild to moderate depression (n = 47) | Streptococcus thermophilus NCIMB 30438, Bifidobacterium breve NCIMB 30441, Bifidobacterium longum NCIMB 30435, Bifidobacterium infantis NCIMB 30436, Lactobacillus acidophilus NCIMB 30442, Lactobacillus plantarum NCIMB 30437, Lactobacillus paracasei NCIMB 30439, Lactobacillus delbrueckii subsp and Bulgaricus NCIMB 30440. | 8 weeks | Patients with depression had significantly lower HAM-D scores and significantly lower activation of the left and right putamen. |
| 2023 [173] | Adults with MDD aged 18 to 55 years taking antidepressants with an incomplete response (n = 49) | Bacillus subtilis, Bifidobacterium bifidum, Bifidobacterium breve, Bifidobacterium infantis, Bifidobacterium longum, Lactobacillus acidophilus, Lactobacillus delbrueckii subsp bulgaricus, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus helveticus, Lactobacillus salivarius, Lactococcus lactis, and Streptococcus thermophilus | 8 weeks | Probiotics can assist selective serotonin reuptake inhibitors to improve clinical outcomes in patients with depression. |
| 2025 [147] | Male volunteers with a body mass index (BMI) greater than 25 and aged over 65 years (n = 67) | Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 | 8 weeks | Probiotics were able to significantly reduce geriatric depression Scale 15 scores in obese older adults and significantly improve total antioxidant capacity. |
| Population | Key Microbiota Changes | Metabolic/Clinical Correlates |
|---|---|---|
| Acute exposure | - Increased aerobic bacteria (Escherichia, Enterococcus) - Reduced anaerobes (Blautia, Bifidobacterium) [18] | Gut barrier dysfunction, acute mountain sickness (AMS) [170] |
| Chronic adaptation | - Elevated Firmicutes, Actinomyces, Clostridium- Higher SCFA production (Lachnospiraceae, Clostridiaceae) [147,174] | Improved hypoxia tolerance, reduced inflammation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Cheng, R.; Li, X.; Zheng, H.; Guo, J.; Wei, L.; Gao, T.; Bi, H. Plateau Environment, Gut Microbiota, and Depression: A Possible Concealed Connection? Curr. Issues Mol. Biol. 2025, 47, 487. https://doi.org/10.3390/cimb47070487
Qiao Y, Cheng R, Li X, Zheng H, Guo J, Wei L, Gao T, Bi H. Plateau Environment, Gut Microbiota, and Depression: A Possible Concealed Connection? Current Issues in Molecular Biology. 2025; 47(7):487. https://doi.org/10.3390/cimb47070487
Chicago/Turabian StyleQiao, Yajun, Ruiying Cheng, Xiaohui Li, Huimin Zheng, Juan Guo, Lixin Wei, Tingting Gao, and Hongtao Bi. 2025. "Plateau Environment, Gut Microbiota, and Depression: A Possible Concealed Connection?" Current Issues in Molecular Biology 47, no. 7: 487. https://doi.org/10.3390/cimb47070487
APA StyleQiao, Y., Cheng, R., Li, X., Zheng, H., Guo, J., Wei, L., Gao, T., & Bi, H. (2025). Plateau Environment, Gut Microbiota, and Depression: A Possible Concealed Connection? Current Issues in Molecular Biology, 47(7), 487. https://doi.org/10.3390/cimb47070487


_Kim.png)



