Mitochondrial Genomes of Four Millipedes (Diplopoda: Spirostreptida and Spirobolida) Unveil Phylogenetic Novelty and Gene Rearrangement Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
2.2. Next-Generation Sequencing and Assembling
2.3. Annotation and Sequence Analysis
2.4. Phylogenetic Analysis
3. Results and Discussion
3.1. Mitochondrial Genome Organization
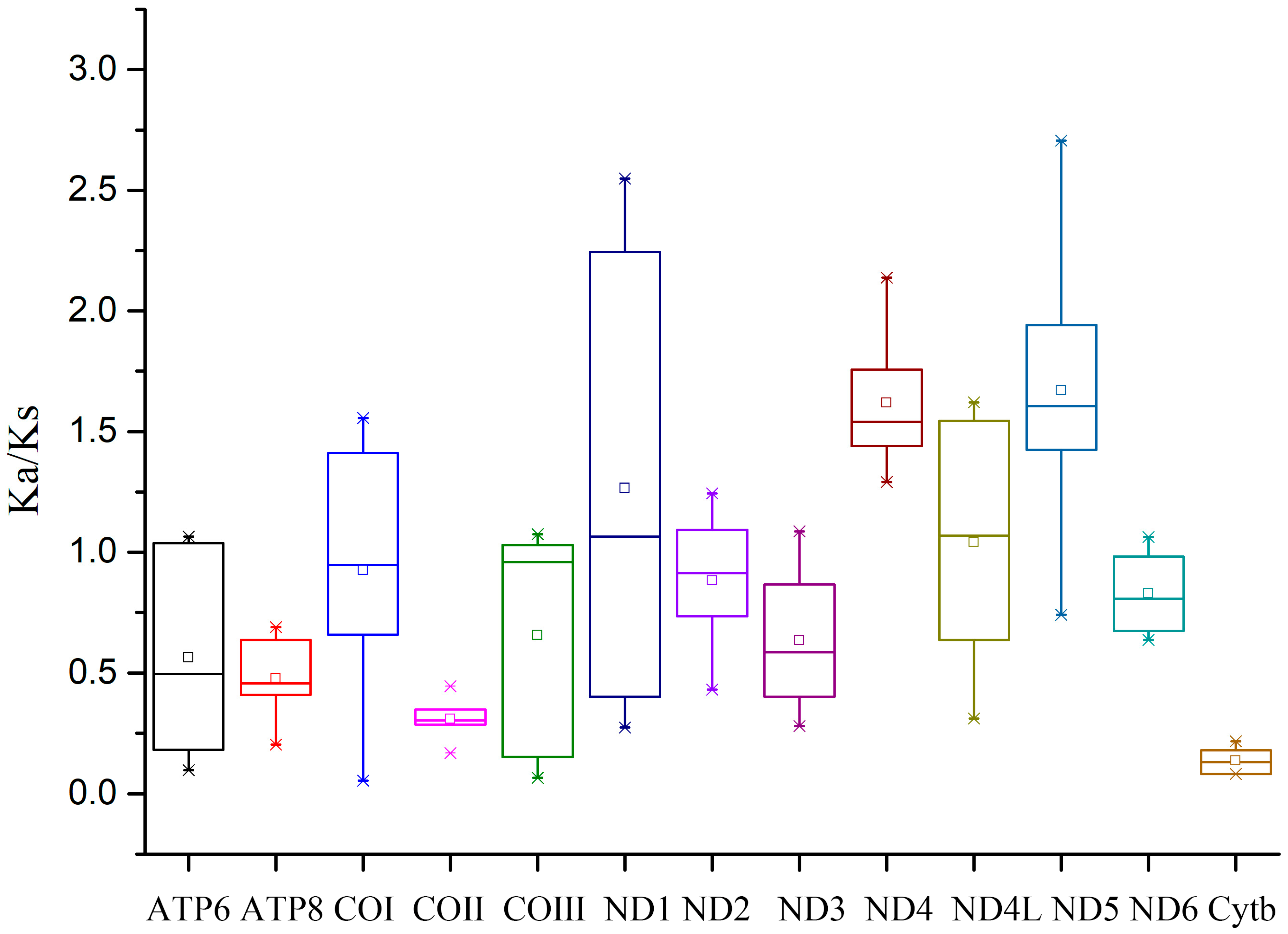
3.2. Protein-Coding Genes and Codon Usage
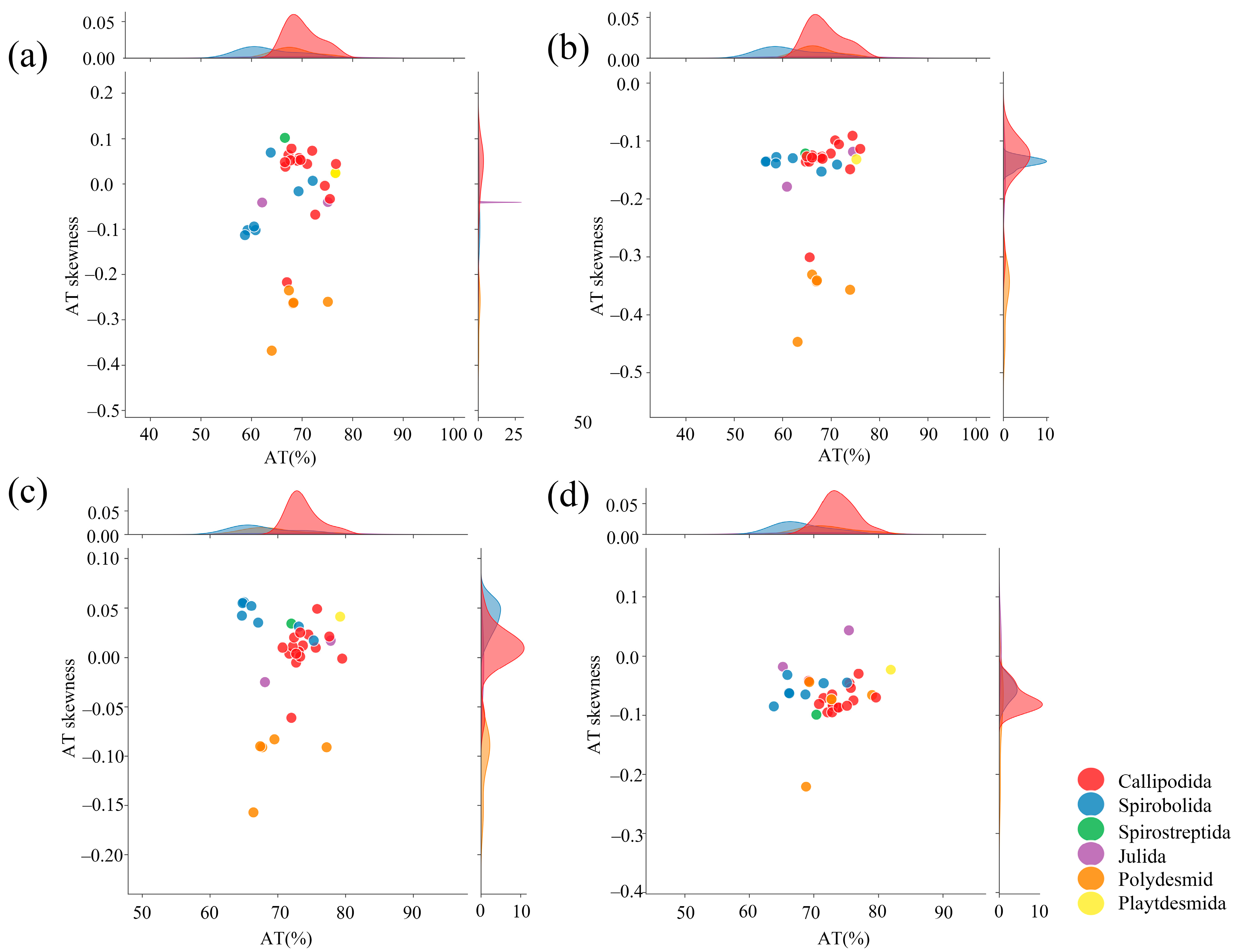
3.3. tRNAs, rRNAs, and D-Loops
3.4. Phylogenetic Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinwandter, M.; Seeber, J. The buffet is open: Alpine soil macro-decomposers feed on a wide range of litter types in a microcosm cafeteria experiment. Soil Biol. Biochem. 2020, 144, 107786. [Google Scholar] [CrossRef]
- Culliney, T.W. Role of arthropods in maintaining soil fertility. Agriculture 2013, 3, 629–659. [Google Scholar] [CrossRef]
- Coleman, D.C.; Wall, D.H. Soil fauna: Occurrence, biodiversity, and roles in ecosystem function. In Soil Microbiology, Ecology and Biochemistry; Paul, E., Ed.; Academic Press: London, UK, 2015; pp. 111–150. [Google Scholar]
- Bray, N.; Kao-Kniffin, J.; Frey, S.D.; Fahey, T.; Wickings, K. Soil macroinvertebrate presence alters microbial community composition and activity in the Rhizosphere. Front. Microbiol. 2019, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, O.Y.; Pokhylenko, A.; Maltseva, I.R.; Kulbachko, Y.L. Participation of Rossiulus kessleri (Diplopoda, Julida) in the Formation of Algae Assemblages of Urbanized Territories. Diversity 2022, 14, 508. [Google Scholar] [CrossRef]
- Shear, W. Class Diplopoda de Blainville in Gervais, 1844. In: Zhang, Z.-Q. (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 2011, 3148, 159–164. [Google Scholar] [CrossRef]
- Sierwald, P.; Bond, J.E. Current status of the Myriapod class diplopoda (millipedes): Taxonomic diversity and phylogeny. Annu. Rev. Entomol. 2007, 52, 401–420. [Google Scholar] [CrossRef]
- Liu, W.; Golovatch, S.; Wesener, T.; Tian, M. Convergent evolution of unique morphological adaptations to a subterranean environment in cave millipedes (Diplopoda). PLoS ONE 2017, 12, e0170717. [Google Scholar] [CrossRef]
- Wesener, T.; Enghoff, H.; Wägele, J.-W. Pachybolini-A tribe of giant Afrotropical millipedes: Arguments for monophyly and the description of a new genus from Madagascar (Diplopoda: Spirobolida: Pachybolidae). Invertebr. Syst. 2008, 22, 37–53. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef]
- Prada, C.F.; Hazzi, N.A.; Hormiga, G.; Cabarcas, F.; Franco, L.M. Complete mitochondrial genome of Phoneutria depilata (Araneae, Ctenidae): New insights into the phylogeny and evolution of spiders. Gene 2023, 850, 146925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, L.; Ding, Y.; Zhang, F.; Zhang, J. Comparative mitogenomics of jumping spiders with first complete mitochondrial genomes of Euophryini (Araneae:Salticidae). Insects 2023, 14, 517. [Google Scholar] [CrossRef]
- Zhang, W.; Gan, T.; Xu, T.; Wang, P.; Tai, J.; Ma, F. Characterization of the complete mitochondrial genome of Spirobolus grahami (Diplopoda: Spirobolidae) with phylogenetic analysis. Sci. Rep. 2024, 14, 7541. [Google Scholar] [CrossRef]
- Pimvichai, P.; Enghoff, H.; Panha, S.; Backeljau, T. Integrative taxonomy of the new millipede genus Coxobolellus, gen. nov.(Diplopoda: Spirobolida: Pseudospirobolellidae), with descriptions of ten new species. Invertebr. Syst. 2020, 34, 591–617. [Google Scholar] [CrossRef]
- VandenSpiegel, D.; Henrard, A.; Mathys, A. Sechelleptus arborivagus sp. nov., a new arboreal spirostreptid millipede (Diplopoda, Spirostreptidae) endemic to Mayotte Island (Comoros Archipelago), Indian Ocean. Eur. J. Taxon. 2021, 755, 1–21. [Google Scholar] [CrossRef]
- Pitz, K.M.; Sierwald, P. Phylogeny of the millipede order Spirobolida (Arthropoda: Diplopoda: Helminthomorpha). Cladistics 2010, 26, 497–525. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Zhang, N.G. An interesting new millipede, Agaricogonopus acrotrifoliolatus gen. et sp. nov., from the tropical rain-forest region of Xishuangbanna, Yunnan (Diplopoda: Spirostreptida: Harpagophoridae). Acta Zootaxonomica Sin. 1997, 22, 349–352. [Google Scholar]
- Liu, W.X.; Wynne, J.J. Cave millipede diversity with the description of six new species from Guangxi, China. Subterr. Biol. 2019, 30, 57–94. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Yamahama, Y. An anatomical and ultrastructural study of the eye of the luminescent millipede Paraspirobolus lucifugus (Gervais 1836) (Diplopoda, Spirobolida, Spiroboleliidae). Arthropod Struct. Dev. 2022, 69, 101171. [Google Scholar] [CrossRef]
- Shelley, R.M.; Lehtinen, P.T. Diagnoses, synonymies and occurrences of the pantropical millipeds, Leptogoniulus sorornus (Butler) and Trigoniulus corallinus (Gervais) (Spirobolida: Pachybolidae: Trigoniulinae). J. Nat. Hist. 1999, 33, 1379–1401. [Google Scholar] [CrossRef]
- Meng, G.; Li, Y.; Yang, C.; Liu, S. MitoZ: A toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 2019, 47, e63. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-Q.; Sun, Y.-B. AutoSeqMan: Batch assembly of contigs for Sanger sequences. Zool. Res. 2018, 39, 123–126. [Google Scholar] [PubMed]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq-versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rozewicki, J.; Li, S.; Amada, K.M.; Standley, D.M.; Katoh, K. MAFFT-DASH: Integrated protein sequence and structural alignment. Nucleic Acids Res. 2019, 47, W5–W10. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Liddle, A.R. Information criteria for astrophysical model selection. Mon. Not. R. Astron. Soc. Lett. 2007, 377, L74–L78. [Google Scholar] [CrossRef]
- Xiang, C.Y.; Gao, F.; Jakovlić, I.; Lei, H.; Hu, Y.; Zhang, H.; Zhang, D. Using PhyloSuite for molecular phylogeny and tree-based analyses. IMeta 2023, 2, e87. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.J.; Lee, Y.S.; Park, S.J.; Lim, J.T.; Jang, K.H.; Choi, E.H.; Choi, Y.G.; Hwang, U.W. Complete mitochondrial genome of a troglobite millipede Antrokoreana gracilipes (Diplopoda, Juliformia, Julida), and juliformian phylogeny. Mol. Cells 2007, 23, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Fang, Y.; Cao, G.; Shen, C.; Liu, H.; Ruan, H. The Complete Mitochondrial Genome of Spirobolus bungii (Diplopoda, Spirobolidae): The First Sequence for the Genus Spirobolus. Genes 2022, 13, 1587. [Google Scholar] [CrossRef]
- Yuan, M.L.; Zhang, Q.L. The complete mitochondrial genome of Gynaephora menyuanensis (Lepidoptera: Lymantriidae) from the Qinghai-Tibetan Plateau. Mitochondrial DNA 2013, 24, 328–330. [Google Scholar] [CrossRef]
- Zhu, K.; Lü, Z.; Liu, B.; Gong, L.; Jiang, L.; Liu, L. The complete mitochondrial genome of Hyporhamphus quoyi (Beloniformes; Hemiramphidae). Mitochondrial DNA Part B 2018, 3, 1235–1236. [Google Scholar] [CrossRef]
- Ojala, D.; Montoya, J.; Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef]
- Hassanin, A.; Léger, N.; Deutsch, J. Evidence for Multiple Reversals of Asymmetric Mutational Constraints during the Evolution of the Mitochondrial Genome of Metazoa, and Consequences for Phylogenetic Inferences. Syst. Biol. 2005, 54, 277–298. [Google Scholar] [CrossRef]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486–487. [Google Scholar] [CrossRef]
- Cook, C.E. The complete mitochondrial genome of the stomatopod crustacean Squilla mantis. BMC Genom. 2005, 6, 105. [Google Scholar] [CrossRef]
- Stoccoro, A.; Coppedè, F. Mitochondrial DNA methylation and human diseases. Int. J. Mol. Sci. 2021, 22, 4594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.X.; Yu, G.; Li, T.J.; He, Q.Y.; Zhou, Y.; Si, F.L.; Ren, S.; Chen, B. The complete mitochondrial genome of Delia antiqua and its implications in dipteran phylogenetics. PLoS ONE 2015, 10, e0139736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Chen, H.; Huang, J. Comparative mitochondrial genomes between the genera Amiota and Phortica (Diptera: Drosophilidae) with evolutionary insights into D-Loop sequence variability. Genes 2023, 14, 1240. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.L.; Zhang, Q.L.; Guo, Z.L.; Wang, J.; Shen, Y.Y. The complete mitochondrial genome of Corizus tetraspilus (Hemiptera: Rhopalidae) and phylogenetic analysis of pentatomomorpha. PLoS ONE 2015, 10, e0129003. [Google Scholar] [CrossRef]
- Hua, J.; Li, M.; Dong, P.; Cui, Y.; Xie, Q.; Bu, W. Comparative and phylogenomic studies on the mitochondrial genomes of Pentatomomorpha (Insecta: Hemiptera: Heteroptera). BMC Genom. 2008, 9, 610. [Google Scholar] [CrossRef]
- Zuo, Q.; Zhang, Z.; Shen, Y. Novel mitochondrial gene rearrangements pattern in the millipede Polydesmus sp. GZCS-2019 and phylogenetic analysis of the Myriapoda. Ecol. Evol. 2022, 12, e8764. [Google Scholar] [CrossRef]
- Enghoff, H.; Dohle, W.; Blower, J.G. Anamorphosis in millipedes (Diplopoda)-The present state of knowledge with some developmental and phylogenetic considerations. Zool. J. Linn. Soc. 1993, 109, 103–234. [Google Scholar] [CrossRef]
- Sierwald, P.; Shear, W.A.; Shelley, R.M.; Bond, J.E. Millipede phylogeny revisited in the light of the enigmatic order Siphoniulida. J. Zool. Syst. Evol. Res. 2003, 41, 87–99. [Google Scholar] [CrossRef]
- Jiang, X.K.; Shear, W.A.; Ye, L.P.; Chen, H.M.; Xie, Z.C. Recovery of the family status of Pericambalidae silvestri, 1909, stat. nov. (Diplopoda: Spirostreptida: Cambalidea), with a revision of the genera and species from China. Invertebr. Syst. 2023, 37, 78–100. [Google Scholar] [CrossRef]
- Zhang, G.; Gao, M.; Chen, Y.; Wang, Y.; Gan, T.; Zhu, F.; Liu, H. The first complete mitochondrial genome of the genus Litostrophus: Insights into the rearrangement and evolution of mitochondrial genomes in Diplopoda. Genes 2024, 15, 254. [Google Scholar] [CrossRef]
- Funk, D.J.; Omland, K.E. Species-level paraphyly and polyphyly: Frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 397–423. [Google Scholar] [CrossRef]
- Zhang, D.; Zou, H.; Hua, C.J.; Li, W.X.; Mahboob, S.; Al-Ghanim, K.A.; Wang, G.T. Mitochondrial architecture rearrangements produce asymmetrical nonadaptive mutational pressures that subvert the phylogenetic reconstruction in Isopoda. Genome Biol. Evol. 2019, 11, 1797–1812. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xu, W.; Zhang, G.; Liu, Z.; Liu, H. Characterization of the complete mitochondrial genomes of four tarantulas (Arachnida: Theraphosidae) with phylogenetic analysis. Gene 2025, 933, 148954. [Google Scholar] [CrossRef] [PubMed]
- Wesener, T.; Rühr, P.T. Internal gonopod reconstruction in an amber-preserved millipede from the Cretaceous: Laeviglyphiulus patrickmuelleri n. gen., n. sp.(Diplopoda, Spirostreptida, Cambalopsidae). Swiss J. Palaeontol. 2025, 144, 16. [Google Scholar] [CrossRef]



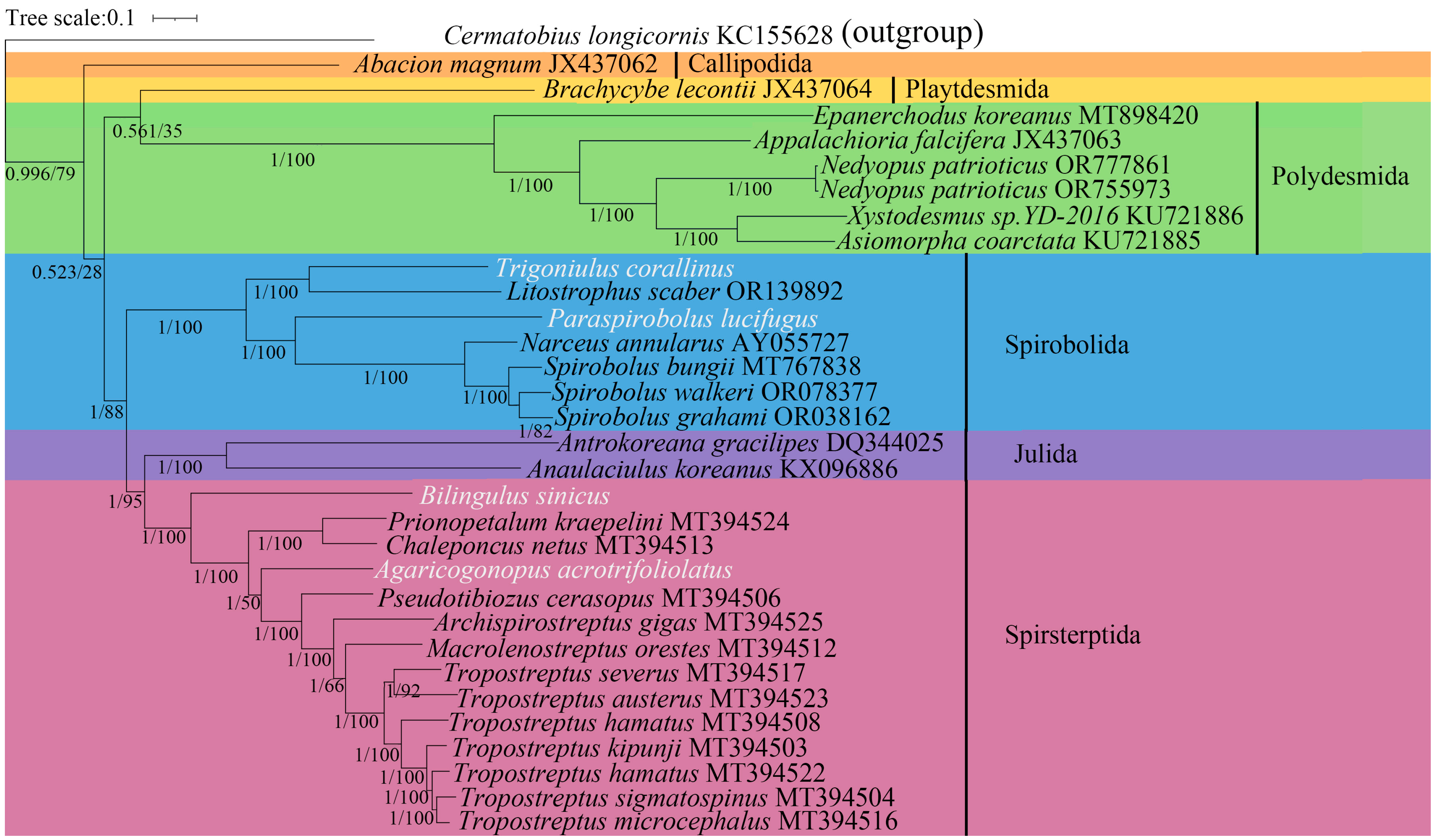
| Class | Order | Family | Species | Length(bp) | Accession |
|---|---|---|---|---|---|
| Diplopoda | Callipodida | Callipodidae | Abacion magnum Loomis, 1943 | 15,160 | JX437062.1 |
| Playtdesmida | Andrognathidae | Brachycybe lecontii Wood, 1864 | 15,115 | JX437064.1 | |
| polydesmida | Xystodesmidae | Appalachioria falcifera Keeton, 1959 | 15,282 | JX437063.1 | |
| Xystodesmus sp. YD-2016 Cook, 1895 | 15,791 | KU721886.1 | |||
| Paradoxosomatidae | Asiomorpha coarctata DeSaussure, 1860 | 15,644 | KU721885.1 | ||
| Nedyopu patrioticus Attems, 1898 | 15,814 | OR755973.1 | |||
| Polydesmidae | Epanerchodus koreanus Verhoeff, 1937 | 15,581 | MT898420.1 | ||
| Nedyopus patrioticus unicolor Carl, 1902 | 15,798 | OR777861.1 | |||
| Spirobolida | Trigoniulidae | Trigoniulus corallinus Gervais, 1847 | 14,906 | PQ459337 | |
| Pachybolidae | Litostrophus scaber Verhoeff, 1938 | 15,081 | OR139892.1 | ||
| Spirobolellidae | Paraspirobolus lucifugus Gervais, 1836 | 14,929 | PQ625794 | ||
| Spirobolidae | Narceus annularus Rafinesque, 1820 | 14,868 | AY055727.1 | ||
| Spirobolus bungii Keeton, 1960 | 14,879 | MT767838.1 | |||
| Spirobolus grahami Keeton, 1960 | 14,875 | OR038162.1 | |||
| Spirobolus walkeri Pocock, 1895 | 14,879 | OR078377.1 | |||
| Julida | Nemasomatidae | Antrokoreana gracilipes Verhoeff, 1938 | 14,747 | DQ344025.1 | |
| Julidae | Anaulaciulus koreanus Verhoeff, 1937 | 14,916 | KX096886.1 | ||
| Spirostreptida | Cambalopsidae | Bilingulus sinicus Zhang & Li, 1981 | 15,879 | PQ568882 | |
| Odontopygidae | Prionopetalum kraepelini Attems, 1896 | 15,114 | MT394524.1 | ||
| Chaleponcus netus Enghoff, 2014 | 15,093 | MT394513.1 | |||
| Harpagophoridae | Agaricogonopus acrotrifoliolatus Zhang, 1997 | 15,016 | PQ602473 | ||
| Spirostreptidae | Pseudotibiozus cerasopus Attems, 1914 | 15,121 | MT394506.1 | ||
| Archispirostreptus gigas Peters, 1985 | 15,177 | MT394525.1 | |||
| Macrolenostreptus oreste Hoffman & Howell, 1996 | 15,367 | MT394512.1 | |||
| Tropostreptus severus Enghoff, 2017 | 15,209 | MT394517.1 | |||
| Tropostreptus austerus Attems, 1950 | 15,261 | MT394523.1 | |||
| Tropostreptus hamatus Demange, 1977 | 15,156 | MT394508.1 | |||
| Tropostreptus kipunji Enghoff, 2017 | 15,170 | MT394503.1 | |||
| Tropostreptus droides Enghoff, 2017 | 15,172 | MT394522.1 | |||
| Tropostreptus sigmatospinus Enghoff, 2017 | 15,176 | MT394504.1 | |||
| Tropostreptus microcephalus Enghoff, 2017 | 15,169 | MT394516.1 | |||
| Chilooda | Lithobiomorpha | Henicopidae | Cermatobius longicornis Takakuwa, 1939 | 16,833 | KC155628.1 |
| Methods | Subset Partitions | Best Model |
|---|---|---|
| Cytb+ATP6+COII+COIII | TIM2+F+I+G4 | |
| ML | ATP8 | GTR+F+I+G4 |
| COI | ||
| ND2+ND3+ND6 | ||
| 12S rRNA+16S rRNA | ||
| ND1+ND4L+ND4+ND5 | GTR+F+I+I+R5 | |
| BI | ATP8 | GTR+F+I+G4 |
| COI | ||
| Cytb+ATP6+CO+COII | ||
| ND1+ND4L+ND4+ND5 | ||
| ND2+ND3+ND6 | ||
| Cytb+ATP6+COII+COIII | ||
| 12S rRNA+16S rRNA |
| Gene | Location | Intergenic Nucleotides | Size (bp) | |
|---|---|---|---|---|
| From | To | |||
| 12S rRNA(+) | 1/1/1/1 | 768/758/784/764 | 0/0/0/0 | 768/758/784/764 |
| tRNA-Val(+) | 769/760/789/766 | 829/821/849/820 | 0/1/4/1 | 61/62/61/55 |
| 16S rRNA(+) | 826/810/850/816 | 2081/2045/2138/2080 | −4/−12/0/−5 | 1256/1236/1289/1265 |
| tRNA-Leu1(+) | 2059/2058/2139/2107 | 2120/2118/2202/2170 | −23/12/0/26 | 62/61/64/64 |
| tRNA-Leu2(+) | 2122/2120/2204/2171 | 2183/2184/2269/2234 | 1/1/1/0 | 62/65/66/64 |
| ND1(+) | 2184/2185/2270/2235 | 3102/3109/3193/3150 | 0/0/0/0 | 919/925/924/916 |
| tRNA-Pro(+) | 3103/3110/3198/3151 | 3163/3171/3264/3213 | 0/0/4/0 | 61/62/67/63 |
| ND4L(+) | 3169/3173/3256/3223 | 3441/3445/3546/3495 | 5/1/0/9 | 273/273/282/273 |
| ND4(+) | 3447/3469/3540/3489 | 4764/4788/4839/4820 | 5/23/−7/−7 | 1318/1320/1300/1332 |
| tRNA-His(+) | 4765/4848/4844/4820 | 4829/4909/4909/4884 | 0/59/4/−1 | 65/62/66/65 |
| ND5(+) | 4830/4910/4910/4885 | 6534/6629/6608/6588 | 0/0/0/0 | 1705/1720/1699/1704 |
| tRNA-Phe(+) | 6535/6630/6609/6589 | 6592/6687/6671/6649 | 0/0/0/0 | 58/58/63/61 |
| tRNA-Tyr(+) | 6539/6687/6670/6646 | 6657/6749/6735/6709 | 0/−1/−2/−4 | 65/63/66/64 |
| tRNA-Gln(+) | 6658/7972/6736/6711 | 6724/8027/6802/6775 | 0/0/0/1 | 67/56/67/65 |
| tRNA-Thr(−) | 7206/8028/6853/6825 | 7268/8090/6921/6887 | 0/0/50/49 | 63/63/69/63 |
| tRNA-Ser1(−) | 7269/8091/6928/6905 | 7334/8157/6996/6973 | 0/0/6/17 | 66/67/69/69 |
| Cytb(−) | 7335/8156/6995/6972 | 8453/9268/8113/8090 | 0/−2/−2/−2 | 1119/1113/1119/1119 |
| ND6(−) | 8454/9268/8114/8044 | 8910/9734/8564/8544 | 0/−1/0/−47 | 457/467/451/501 |
| tRNA-Glu(−) | 8914/9741/8565/8542 | 8977/9806/8629/8603 | 3/6/0/−3 | 64/66/65/62 |
| tRNA-Ser2(−) | 8978/9807/8630/8603 | 9037/9867/8687/8663 | 0/0/0/−1 | 60/61/58/61 |
| tRNA-Asn(−) | 9038/9867/8688/8664 | 9105/9929/8754/8728 | 0/−1/0/0 | 68/63/67/65 |
| tRNA-Arg(−) | 9106/9928/8754/8728 | 9172/9988/8815/8790 | 0/−2/−1/−1 | 67/61/62/63 |
| tRNA-Ala(−) | 9173/9987/8816/8794 | 9236/10,050/8883/8858 | 0/−2/0/3 | 64/64/68/65 |
| ND3(−) | 9235/10,050/8882/8857 | 9579/10,393/9232/9210 | −2/−1/−2/−2 | 345/344/351/354 |
| tRNA-Gly(−) | 9580/10,394/9233/9205 | 9646/10,457/9295/9270 | 0/0/0/−6 | 67/64/63/66 |
| COIII(−) | 9646/10,456/9299/9271 | 10,431/11,241/10,076/10,048 | −1/−2/3/0 | 786/786/778/778 |
| ATP6(−) | 10,432/11,228/10,076/10,049 | 11,107/11,911/10,752/10,724 | 0/−14/−1/0 | 676/684/677/676 |
| ATP8(−) | 11,101/11,905/10,746/10,718 | 11,256/12,060/10,901/10,873 | −7/−7/−7/−7 | 156/156/156/156 |
| tRNA-Asp(−) | 11,257/12,061/10,902/10,874 | 11,319/12,126/10,965/10,937 | 0/0/0/0 | 63/66/64/64 |
| tRNA-Lys(−) | 11,320/12,126/10,966/10,937 | 11,387/12,190/11,031/11,003 | 0/−1/0/−1 | 68/65/66/67 |
| COII(−) | 11,388/12,192/11,032/11,004 | 12,069/12,872/11,707/11,682 | 0/1/0/0 | 682/681/676/679 |
| COI(−) | 12,073/12,873/11,711/11,683 | 13,605/14,411/13,243/13,213 | 3/0/3/0 | 1533/1539/1533/1531 |
| tRNA-Cys(+) | 13,616/14,412/13,248/13,219 | 13,680/14,473/13,310/13,281 | 10/0/4/5 | 65/62/63/63 |
| tRNA-Trp(−) | 13,617/14,474/13,303/13,274 | 13,681/14,536/13,364/13,336 | −64/0/−8/−8 | 65/63/62/63 |
| ND2(−) | 13,680/14,535/13,365/13,335 | 14,687/15,536/14,361/14,327 | −2/−2/0/−2 | 1008/1002/997/993 |
| tRNA-Met(−) | 14,688/15,537/14,362/14,328 | 14,755/15,601/14,425/14,393 | 0/0/0/0 | 68/65/64/66 |
| tRNA-Ile(−) | 14,756/15,601/14,425/14,393 | 14,821/15,666/14,491/14,457 | 0/−1/−1/−1 | 66/66/67/65 |
| D-loop | 6725/6750/14,426/14,458 | 7205/7971/14,929/14,906 | 0/0/0/0 | 481/1222/504/449 |
| Species | Region | Size (bp) | A+T % | AT-Skew | GC-Skew |
|---|---|---|---|---|---|
| Agaricogonopus acrotrifoliolatus | Whole genome | 15,016 | 72.57 | −0.068 | 0.342 |
| PCGs | 10,971 | 71.58 | −0.106 | −0.066 | |
| tRNAs | 1408 | 75.81 | 0.049 | 0.079 | |
| rRNAs | 2024 | 75.10 | −0.084 | 0.385 | |
| D-loop | 481 | 67.57 | 0.003 | 0.295 | |
| Bilingulus sinicus | Whole genome | 15,879 | 75.43 | 0.033 | 0.323 |
| PCGs | 11,022 | 74.31 | 0.091 | 0.025 | |
| tRNAs | 1385 | 77.24 | 0.021 | 0.129 | |
| rRNAs | 1994 | 75.68 | −0.054 | 0.381 | |
| D-loop | 1222 | 78.47 | 0.051 | 0.353 | |
| Paraspirobolus lucifugus | Whole genome | 15,016 | 60.50 | −0.068 | 0.342 |
| PCGs | 10,971 | 58.54 | −0.106 | −0.066 | |
| tRNAs | 1415 | 64.68 | 0.049 | 0.07 | |
| rRNAs | 2024 | 65.90 | −0.084 | 0.385 | |
| D-loop | 504 | 68.65 | 0.069 | 0.265 | |
| Trigoniulus corallinus | Whole genome | 14,906 | 72.05 | 0.007 | 0.317 |
| PCGs | 11,007 | 71.21 | −0.141 | 0.013 | |
| tRNAs | 1402 | 75.32 | 0.017 | 0.092 | |
| rRNAs | 2029 | 75.11 | 0.045 | 0.386 | |
| D-loop | 449 | 71.04 | 0.065 | 0.246 |
| Gene | Codon | |
|---|---|---|
| Start | Stop | |
| ND1 | ATG/ATT/ATT/ATA | TAA/T/TAA/T |
| ND4L | ATG/ATG/GTG/ATG | TAA/TAA/TAG/TAA |
| ND4 | ATA/ATG/ATG/ATG | T/T/T/TAA |
| ND5 | TTA/GTG/GTG/ATT | T/T/T/TAG |
| Cytb | ATG/ATG/ATG/ATG | TAA/TAG/TAA/TAA |
| ND6 | ATA/ATT/ATC/ATA | T/TA/T/TAA |
| ND3 | ATA/ATT/ATA/ATA | TAG/TA/TAG/TAG |
| COIII | ATG/ATG/ATG/ATG | TAG/TAA/T/T |
| ATP6 | ATG/ATG/ATG/ATG | T/TAA/TA/T |
| ATP8 | ATT/ATT/ATA/ATT | TAA/TAA/TAA/TAA |
| COII | ATG/ATG/ATG/ATG | T/TAG/T/T |
| COI | ACG/ATT/ACG/CGA | TAA/TAA/TAA/TTT |
| ND2 | ATT/ATT/ATC/ATT | TAA/TAA/TAA/TAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, G.; Xu, W.; Xu, T.; Li, L.; Gao, M.; Wang, J.; Liu, H. Mitochondrial Genomes of Four Millipedes (Diplopoda: Spirostreptida and Spirobolida) Unveil Phylogenetic Novelty and Gene Rearrangement Patterns. Curr. Issues Mol. Biol. 2025, 47, 476. https://doi.org/10.3390/cimb47060476
Li Y, Zhang G, Xu W, Xu T, Li L, Gao M, Wang J, Liu H. Mitochondrial Genomes of Four Millipedes (Diplopoda: Spirostreptida and Spirobolida) Unveil Phylogenetic Novelty and Gene Rearrangement Patterns. Current Issues in Molecular Biology. 2025; 47(6):476. https://doi.org/10.3390/cimb47060476
Chicago/Turabian StyleLi, Yingzhu, Gaoji Zhang, Wei Xu, Tangjun Xu, Lingna Li, Ming Gao, Jiachen Wang, and Hongyi Liu. 2025. "Mitochondrial Genomes of Four Millipedes (Diplopoda: Spirostreptida and Spirobolida) Unveil Phylogenetic Novelty and Gene Rearrangement Patterns" Current Issues in Molecular Biology 47, no. 6: 476. https://doi.org/10.3390/cimb47060476
APA StyleLi, Y., Zhang, G., Xu, W., Xu, T., Li, L., Gao, M., Wang, J., & Liu, H. (2025). Mitochondrial Genomes of Four Millipedes (Diplopoda: Spirostreptida and Spirobolida) Unveil Phylogenetic Novelty and Gene Rearrangement Patterns. Current Issues in Molecular Biology, 47(6), 476. https://doi.org/10.3390/cimb47060476




