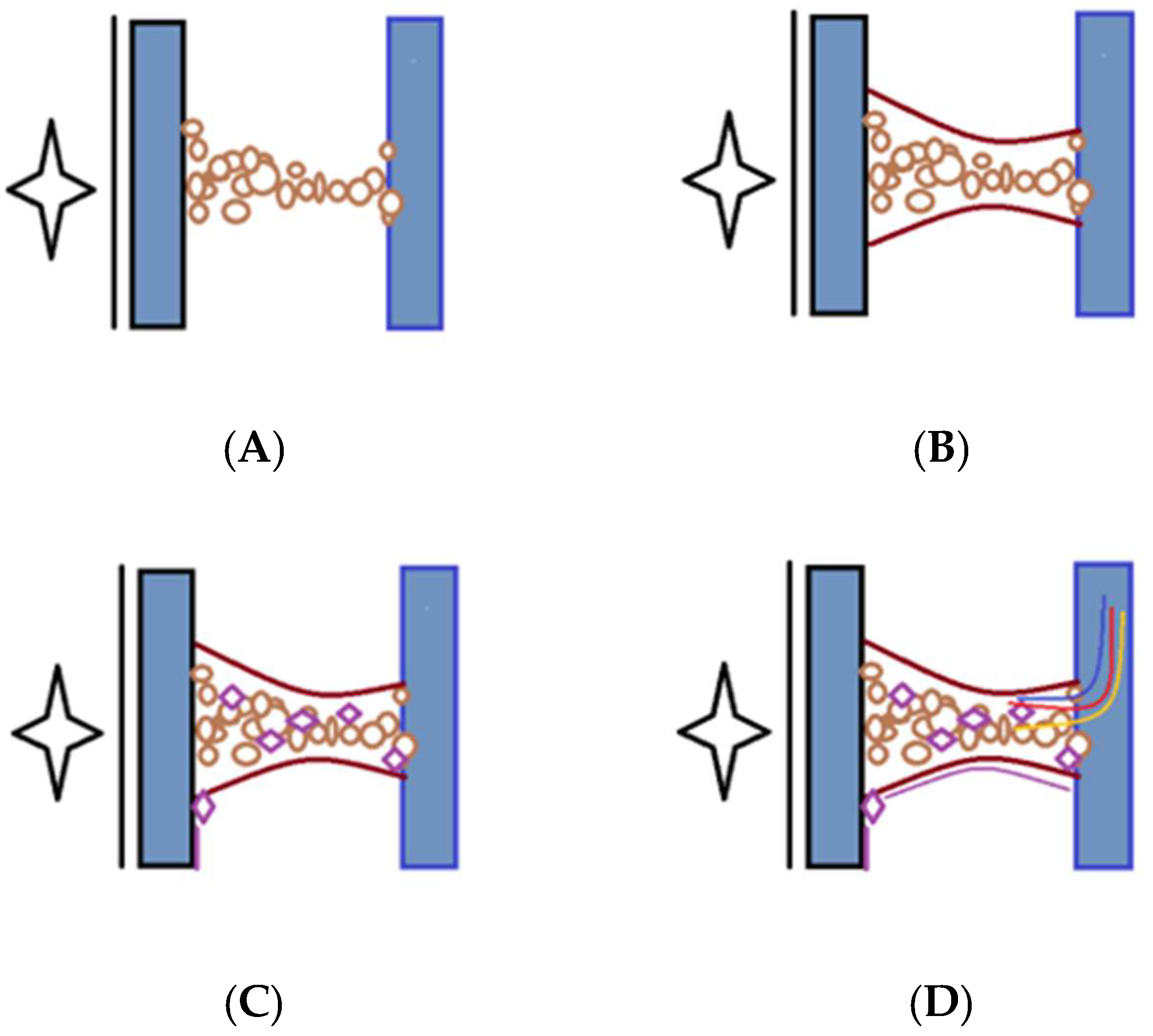
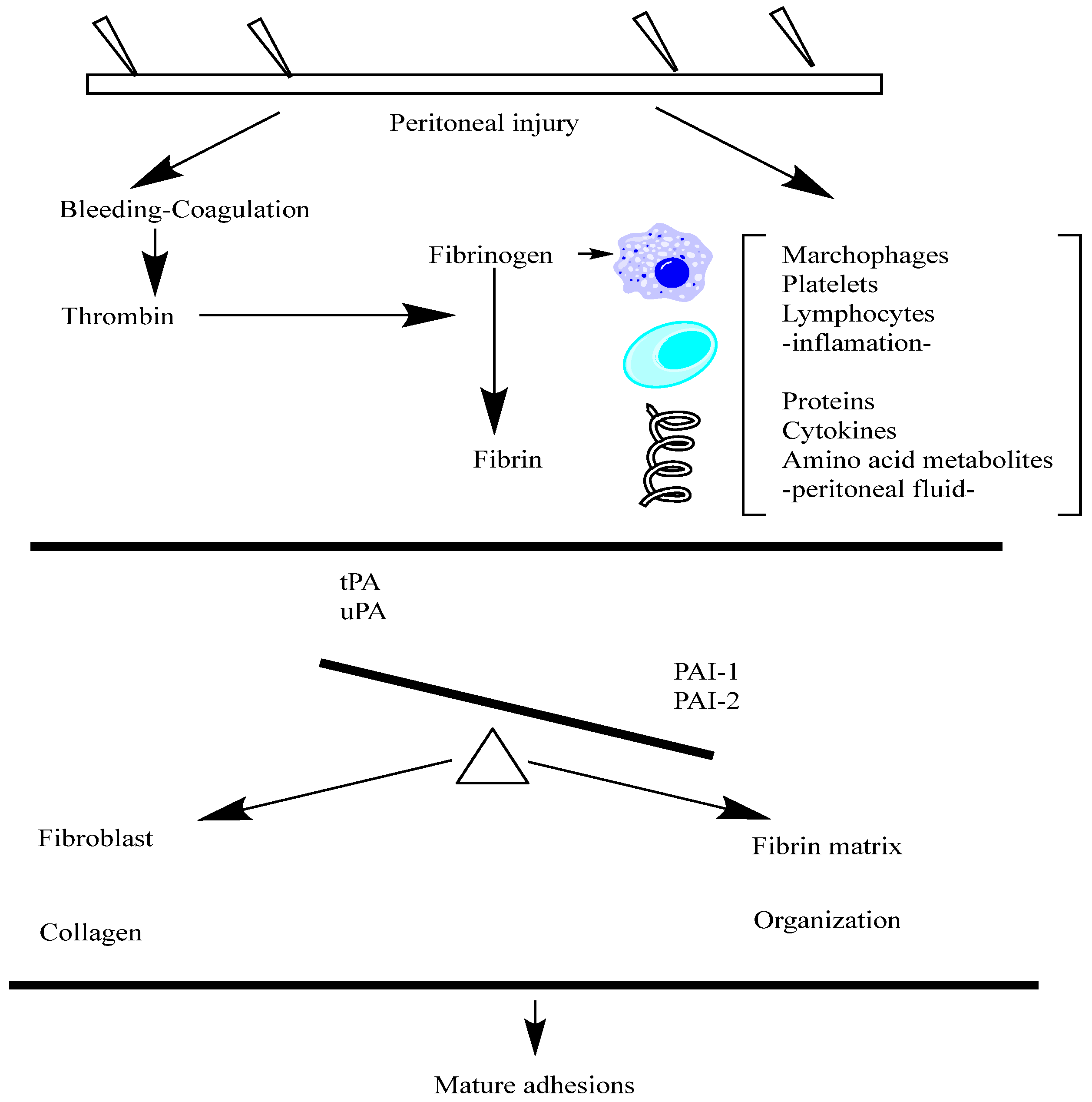
Peritoneal adhesion formation is a complex biological process involving multiple molecular mechanisms, prominently featuring inflammation, coagulation, fibrinolysis, angiogenesis, and extracellular matrix (ECM) remodeling. At the molecular level, several critical pathways, including transforming growth factor-beta (TGF-β), cyclooxygenase-2 (COX-2), tissue plasminogen activator (tPA)/plasminogen activator inhibitor-1 (PAI-1), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), matrix metalloproteinases (MMPs), and tissue inhibitors of metalloproteinases (TIMPs), orchestrate this intricate process. Understanding the dynamics and interactions within these molecular pathways is essential for identifying potential therapeutic targets and developing effective strategies to prevent or mitigate peritoneal adhesion formation following surgical intervention or injury.
Each protein is then briefly explained in the paragraph below.
1.2.3. Comparative Analysis Across Studies
Critical comparative analyses of multiple transcriptomic and proteomic studies reveal common and unique molecular signatures across different models and clinical scenarios. For instance, the increased expression of TGF-β signaling components (TGFBR1, SMAD3), inflammatory markers (IL-1β, TNF-α), and hypoxia-related genes (HIF1α) have consistently emerged across several independent studies, reinforcing their fundamental role in adhesion biology [
43].
The recent utilization of bioinformatics resources, including GEO for accessing transcriptomic data, STRING for understanding protein interactions, and KEGG for pathway analysis, has facilitated an integrative understanding of adhesion mechanisms. Leveraging these tools can identify novel biomarkers, therapeutic targets, and mechanistic insights into peritoneal adhesion formation [
44]. The summary of genomic and molecular insights are listed in
Table 2.
In contrast, inside-out signaling involves the regulation of integrin affinity and clustering by intracellular signaling molecules. Various mechanisms, including changes in ligand binding affinity, clustering, and intracellular signaling, tightly regulate integrin activity. E-cadherin is essential for maintaining epithelial tissue integrity and barrier function. The loss or dysfunction of E-cadherin can lead to epithelial-to-mesenchymal transition (EMT), a process characterized by the loss of cell–cell adhesion and the acquisition of migratory and invasive properties, which is associated with tumor progression and metastasis. E-cadherin expression in peritoneal mesothelial cells is critical in regulating peritoneal adhesion formation. Decreased E-cadherin expression or altered localization has been observed in response to peritoneal E-cadherin expression, and various signaling pathways and transcription factors regulate function. For example, the Wnt/β-catenin signaling pathway can modulate E-cadherin expression and cell adhesion by regulating β-catenin localization and transcriptional activity. Injury may contribute to disrupting mesothelial cell junctions and adhesion formation. Targeting E-cadherin and its associated signaling pathways may represent a potential therapeutic strategy for preventing or reducing peritoneal adhesion formation. Approaches to restoring E-cadherin expression or function could help preserve mesothelial barrier integrity and mitigate adhesion formation following abdominal surgery or injury. Overall, E-cadherin is a crucial regulator of cell–cell adhesion and tissue integrity in epithelial tissues, including the peritoneum, and its dysregulation has implications for peritoneal adhesion formation and related pathologies [
51,
52].
They mediate the initial interactions between circulating leukocytes and endothelial cells during inflammation and immune responses. P-selectin is stored in secretory granules (Weibel–Palade bodies in endothelial cells and α-granules in platelets) and rapidly translocated to the cell surface upon activation. E-selectin is induced on endothelial cells by inflammatory cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α). Low affinity and rapid association/dissociation kinetics characterize this interaction, allowing leukocyte rolling adhesion on the endothelial surface under fluid shear stress conditions. Selectins are vital regulators of leukocyte–endothelial interactions during inflammation and immune responses, with implications for peritoneal adhesion formation and other inflammatory conditions [
53,
54].
Immunoglobulin superfamily molecules, including Intercellular Adhesion Molecule-1 (ICAM-1) and Vascular Cell Adhesion Molecule-1 (VCAM-1), are cell adhesion molecules expressed on the surface of endothelial cells and immune cells. ICAM-1 and VCAM-1 are transmembrane glycoproteins belonging to the immunoglobulin superfamily. They consist of extracellular immunoglobulin-like domains, a transmembrane domain, and a cytoplasmic tail. The extracellular domains of ICAM-1 and VCAM-1 contain binding sites for their respective ligands on leukocytes. ICAM-1 is constitutively expressed at low levels on the surface of endothelial cells. However, its expression can be upregulated by inflammatory cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α). VCAM-1 expression is induced on endothelial cells in response to inflammatory stimuli, particularly cytokines such as TNF-α and interleukin-4 (IL-4). These interactions are essential for the adhesion and transendothelial migration of leukocytes during inflammation. ICAM-1 and VCAM-1 play crucial roles in the recruitment of leukocytes to sites of inflammation. They facilitate the adhesion of circulating leukocytes to endothelial cells, leading to their extravasation from the bloodstream into inflamed tissues [
55]. This process is essential for initiating and propagating immune responses and inflammatory reactions. ICAM-1 and VCAM-1 expression on peritoneal mesothelial and endothelial cells may contribute to peritoneal adhesion formation by promoting leukocyte adhesion and infiltration into the peritoneal cavity. Targeting ICAM-1 and VCAM-1 interaction could represent a potential therapeutic approach for preventing or reducing peritoneal adhesion formation following abdominal surgery or injury. Lastly, ICAM-1 and VCAM-1 are essential mediators of leukocyte adhesion and recruitment during inflammation, which have implications for various inflammatory conditions, including peritoneal adhesion formation [
56,
57,
58,
59].
Hyaluronic acid (HA), a non-sulfated glycosaminoglycan and significant component of the extracellular matrix, plays a vital role in maintaining peritoneal homeostasis and modulating postsurgical healing responses. Due to its high molecular weight and viscoelastic properties, HA contributes to mesothelial cell lubrication, hydration, and barrier function, thereby reducing friction and mechanical trauma between peritoneal surfaces during surgery. More importantly, HA possesses anti-inflammatory, antifibrotic, and immunomodulatory properties that make it particularly effective in mitigating adhesion formation. Mechanistically, HA inhibits leukocyte and fibroblast adhesion to the mesothelial surface by masking cell adhesion molecules and reducing the exposure of fibrinous substrates. It also downregulates pro-inflammatory cytokines, such as IL-1β and TNF-α, and can reduce the expression of fibrogenic mediators, like TGF-β1. Additionally, HA modulates mesothelial-to-mesenchymal transition (MMT), a process implicated in fibrosis and adhesion development, by maintaining mesothelial phenotype integrity [
60].
Clinically, HA-based biomaterials—including HA-carboxymethylcellulose membranes (e.g., Seprafilm
®) and cross-linked HA hydrogels—have been successfully used as physical barriers to prevent tissue apposition in the critical postoperative window. These formulations degrade gradually, maintaining peritoneal separation during the peak of fibrin deposition and early fibroblast infiltration, thus allowing natural reperitonealization without fibrotic bridging. Several randomized clinical trials have shown that HA-based agents significantly reduce both the incidence and severity of adhesions in abdominal and pelvic surgeries. Overall, HA serves not only as a mechanical separator but also as a bioactive modulator of the healing response, making it one of the most promising adjuncts in adhesion prevention strategies [
61].
It is a central extracellular matrix (ECM) component and is widely distributed throughout connective tissues, including the peritoneum. MMPs are structurally characterized by a conserved catalytic domain containing a zinc ion essential for enzymatic activity. They also typically possess additional domains, such as a propeptide domain that regulates enzyme activation, a catalytic domain responsible for substrate cleavage, and hemopexin-like domains involved in substrate recognition and binding. MMPs are classified based on their substrate specificity and domain structure into several subfamilies, including collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs (MT-MMPs), and others. Each MMP subtype exhibits distinct substrate preferences and tissue localization. MMPs are synthesized as inactive zymogens (pro-MMPs) that require the proteolytic cleavage of the propeptide domain for activation. Various mechanisms can regulate this activation, including other MMPs, serine proteases, and tissue inhibitors of metalloproteinases (TIMPs). By cleaving these substrates, MMPs facilitate tissue remodeling, cell migration, and the release of bioactive ECM fragments that modulate cell behavior and signaling pathways. MMPs are implicated in peritoneal adhesion formation by mediating ECM degradation and remodeling processes. Dysregulated MMP activity, characterized by excessive MMP expression or insufficient inhibition by TIMPs, may disrupt the balance of ECM turnover and contribute to fibrosis, adhesion formation, and tissue dysfunction in the peritoneum. Modulating MMP activity represents a potential therapeutic strategy for preventing or reducing peritoneal adhesion formation. Approaches targeting MMP activation, expression, or enzymatic activity could help restore ECM homeostasis and mitigate adhesion formation following abdominal surgery or injury. MMPs are critical regulators of ECM turnover and tissue remodeling processes with essential implications for peritoneal adhesion formation and related pathologies [
62,
63,
64,
65,
66].
Fibrinogen is a soluble plasma glycoprotein that plays a central role in the blood clotting cascade. The liver synthesizes and circulates it in the blood at relatively high concentrations. Fibrinogen is cleaved by thrombin during blood clotting to form insoluble fibrin, which polymerizes into a meshwork that stabilizes blood clots. Fibrinogen is a large, multi-subunit protein composed of six polypeptide chains—two sets of three different chains, named Aα, Bβ, and γ. These chains are linked by disulfide bonds, forming a symmetrical dimeric structure with a central region called the E domain. Fibrinogen also contains N-terminal fibrinopeptides cleaved by thrombin to initiate fibrin polymerization. Fibrinogen is a critical component of the blood clotting cascade, which is converted into fibrin by the proteolytic action of thrombin. Fibrin monomers then polymerize to form insoluble fibrin strands, which aggregate to create a stable blood clot.
Fibrinogen also interacts with various proteins and cell surface receptors, playing roles in platelet aggregation, wound healing, inflammation, and angiogenesis. Fibrinogen is involved in the initial stages of peritoneal adhesion formation following surgery or injury. The exudation of fibrinogen-rich fluid into the peritoneal cavity leads to the deposition of fibrin matrices on injured peritoneal surfaces [
67]. These fibrin matrices serve as scaffolds for the recruitment and adhesion of inflammatory cells and fibroblasts, ultimately contributing to the formation of fibrous peritoneal adhesions. Targeting fibrinogen and its interactions in the peritoneal cavity may represent a potential therapeutic strategy for preventing or reducing peritoneal adhesion formation. Approaches could include using fibrinolytic agents to promote fibrin degradation, anticoagulants to inhibit fibrin formation, or agents that interfere with fibrinogen binding to cell surface receptors involved in adhesion and inflammation. Fibrinogen is a critical mediator of blood clotting and wound healing processes, with implications for peritoneal adhesion formation and a potential drug [
68,
69,
70,
71].
In the context of peritoneal adhesion formation, the roles of Tissue Plasminogen Activator (tPA) and Plasminogen Activator Inhibitor-1 (PAI-1) are relevant, albeit less studied compared to their roles in other physiological and pathological processes. tPA is involved in the dissolution of fibrin, a key component of blood clots. In the peritoneal cavity, fibrin deposition occurs as part of the wound-healing response following surgery or injury. Increased tPA activity may facilitate the breakdown of fibrin and prevent the accumulation of fibrin-rich adhesions between peritoneal surfaces. Enhancing tPA activity or expression in the peritoneal cavity could represent a therapeutic approach for preventing or reducing peritoneal adhesion formation. Strategies to promote fibrinolysis, such as local delivery of recombinant tPA or tPA-stimulating agents, may help mitigate adhesion formation following abdominal surgery. Inhibiting tPA and urokinase-type plasminogen activator (uPA) effectively, PAI-1 suppresses fibrinolysis and enhances clot stability. Increased fibrinolysis and the survival of fibrin-rich adhesions in the peritoneal cavity may be caused by elevated PAI-1 levels. Targeting PAI-1 activity or expression could be a therapeutic strategy for promoting fibrinolysis and reducing peritoneal adhesion formation. Inhibiting PAI-1 function, either locally or systemically, may enhance fibrinolytic activity and facilitate the resolution of peritoneal adhesions [
72,
73,
74]. Overall, the balance between tPA-mediated fibrinolysis and the PAI-1-mediated inhibition of fibrinolysis will likely influence peritoneal adhesion formation. Modulating these factors could hold promise for developing novel therapeutic interventions aimed at preventing or treating peritoneal adhesions. However, further research is needed to fully elucidate the roles of tPA and PAI-1 in this context and explore their potential as targets for drug design [
75,
76].
The multifunctional cytokine known as transforming growth factor-beta, or TGF-β, is essential for numerous physiological and pathological processes, including as immune control, tissue repair, apoptosis, cell proliferation, differentiation, and migration. TGF-β belongs to a superfamily of cytokines that have structural similarities. Three isoforms of TGF-β (TGF-β, TGF-β2, and TGF-β3) exist in mammals, and a different gene expresses each. Initially produced as precursor proteins, TGF-β isoforms are cleaved by proteases to produce active TGF-β dimers. Numerous cell types, including fibroblasts, tumor cells, endothelial cells, epithelial cells, and immunological cells (such as T cells and macrophages), produce TGF-β. In order to have biological effects, it must be activated after being secreted as a latent complex [
77].
TGF-β exerts its effects by binding to specific cell surface receptors (TGF-β receptors) and activating intracellular signaling pathways, such as the Smad signaling pathway. TGF-β signaling regulates diverse cellular processes, including cell proliferation, differentiation, migration, extracellular matrix production, and immune responses. TGF-β has been implicated in peritoneal adhesion formation following abdominal surgery or injury. It promotes the activation of fibroblasts and myofibroblasts, producing collagen and other extracellular matrix proteins that contribute to the formation of fibrous adhesions between peritoneal surfaces. TGF-β can modulate inflammatory responses and immune cell functions, which may influence the development and resolution of peritoneal adhesions. Targeting TGF-β signaling pathways represents a potential therapeutic strategy for preventing or reducing peritoneal adhesion formation. Approaches aimed at inhibiting TGF-β activity or downstream signaling pathways could help mitigate fibrosis and promote tissue repair in the peritoneal cavity. TGF-β is a crucial mediator of tissue repair, fibrosis, and inflammation with essential implications for peritoneal adhesion formation and potential therapy [
78,
79,
80,
81].
An angiogenic signaling protein forms new blood vessels from pre-existing vasculature. VEGF is a glycoprotein belonging to the PDGF/VEGF family of growth factors. Multiple isoforms of VEGF exist, resulting from alternative splicing of the VEGF gene. The most common isoforms include VEGF-A, VEGF-B, VEGF-C, and VEGF-D. VEGF-A is the predominant isoform and is often referred to in various cell types, including endothelial cells, macrophages, fibroblasts, and tumor cells that produce VEGF. Multiple factors regulate its expression, including hypoxia, growth factors, cytokines, and oncogenes. VEGF exerts its effects primarily by binding to VEGF receptors (VEGFRs) on endothelial cells, leading to endothelial cell proliferation, migration, and survival. VEGF also promotes the vascular permeability, vasodilation, and recruitment of endothelial progenitor cells. These actions are crucial for angiogenesis during embryonic development, wound healing, and pathological conditions, such as cancer and ischemic diseases. VEGF has been implicated in peritoneal adhesion formation following surgery or injury. It promotes angiogenesis and neovascularization in the peritoneal tissues, which may contribute to the development and persistence of peritoneal adhesions [
82,
83].
Additionally, VEGF-mediated vascular permeability and endothelial cell activation may facilitate the recruitment of inflammatory cells and fibroblasts to the injury site, further promoting adhesion formation. Targeting VEGF signaling pathways represents a potential therapeutic strategy for preventing or reducing peritoneal adhesion formation. Approaches aimed at inhibiting VEGF activity or blocking its receptors could help mitigate angiogenesis and neovascularization in the peritoneal cavity, thereby reducing the formation of fibrous adhesions between peritoneal surfaces [
84,
85,
86].
Various stimuli regulate its expression, including growth factors, cytokines, and mechanical stress. PDGF has been implicated in peritoneal adhesion formation following surgery or injury. It stimulates the proliferation and migration of fibroblasts and myofibroblasts, leading to the deposition of extracellular matrix proteins and the formation of fibrous adhesions between peritoneal surfaces [
87].
Additionally, PDGF stimulates angiogenesis, which may contribute to the development and persistence of peritoneal adhesions. Targeting PDGF signaling pathways represents a potential therapeutic strategy for preventing or reducing peritoneal adhesion formation. Approaches aimed at inhibiting PDGF activity or blocking its receptors could help mitigate fibrosis, angiogenesis, and tissue remodeling in the peritoneal cavity, thereby reducing the formation of adhesions between peritoneal surfaces [
88,
89,
90].
Interleukins (ILs) are a group of cytokines that regulate immune responses, inflammation, hematopoiesis, and various physiological processes. Here are some details about interleukins. Interleukins are a diverse group of proteins, ranging from small secreted molecules to larger glycoproteins. They are typically produced by immune cells, such as leukocytes, macrophages, and lymphocytes, as well as by other cell types, including endothelial cells and fibroblasts. Interleukins exert their effects by binding to specific cell surface receptors expressed on target cells. Interleukins are numbered sequentially based on their discovery, such as IL-1, IL-2, IL-6, IL-10, etc. [
91].
However, the classification system has expanded as more interleukins have been discovered, leading to subgroups and families of interleukins with similar functions or structural features. Interleukins mediate communication between immune cells and regulate immune responses in various ways. They can stimulate or suppress immune cell proliferation, differentiation, and activity, including T cells, B cells, natural killer cells, macrophages, and dendritic cells. Interleukins modulate inflammatory responses, tissue repair, hematopoiesis, and other physiological processes. Several interleukins have been implicated in peritoneal adhesion formation following surgery or injury. Interleukins, such as IL-1, IL-6, and IL-8, initiate and amplify inflammatory responses, which contribute to the recruitment of immune cells, fibroblasts, and other cell types to the injury site [
92,
93].
Additionally, interleukins may influence extracellular matrix remodeling, angiogenesis, and tissue repair processes, contributing to adhesion formation. Targeting interleukin signaling pathways represents a potential therapeutic strategy for preventing or reducing peritoneal adhesion formation. Approaches aimed at inhibiting specific interleukins or blocking their receptors could help modulate inflammatory responses, immune cell activation, and tissue remodeling processes involved in adhesion formation [
94,
95].
The family of growth factors known as fibroblast growth factors (FGFs) is essential to many biological processes, such as angiogenesis, migration, differentiation, and cell proliferation. The family of structurally related proteins known as FGFs is distinguished by a high degree of sequence homology and conserved amino acid sequences. There are now 22 FGF family members known to exist in mammals. Usually, these tiny proteins produced in the body, FGFs, have either an autocrine or paracrine effect locally. Numerous cell types, such as fibroblasts, endothelial cells, epithelial cells, and immunological cells, produce FGFs. They are released into the extracellular matrix, where they bind with particular target cell surface receptors to start signaling cascades. Tyrosine kinase receptors called FGF receptors (FGFRs), which are expressed on the surface of target cells, are bound to FGFs and then activated. Upon ligand binding, FGFRs undergo dimerization and autophosphorylation, activating downstream signaling pathways, such as the Ras-MAPK and PI3K-Akt pathways. These pathways regulate cellular processes, including cell proliferation, survival, differentiation, and migration. FGFs have been implicated in peritoneal adhesion formation following surgery or injury. They promote the proliferation and migration of fibroblasts, endothelial cells, and other cell types involved in tissue repair and remodeling [
96,
97,
98,
99].
Additionally, FGFs stimulate angiogenesis, the formation of new blood vessels, which may contribute to the development and persistence of peritoneal adhesions. Targeting FGF signaling pathways represents a potential therapeutic strategy for preventing or reducing peritoneal adhesion formation. Approaches aimed at inhibiting FGF activity or blocking its receptors could help mitigate fibrosis, angiogenesis, and tissue remodeling in the peritoneal cavity, thereby reducing the formation of adhesions between peritoneal surfaces [
100].
The innate immune system relies heavily on Toll-like receptors (TLRs), a family of pattern recognition receptors (PRRs), to identify conserved molecular patterns linked to infections, or pathogen-associated molecular patterns (PAMPs). Type I transmembrane proteins known as Toll-like receptors are defined by the following three domains: an intracellular Toll/interleukin-1 receptor (TIR) domain that initiates downstream signaling pathways, a transmembrane domain that recognizes ligands, and an extracellular domain that contains leucine-rich repeat (LRR) motifs. Numerous cell types, including immune cells like neutrophils, dendritic cells, and macrophages as well as non-immune cells like fibroblasts and epithelial cells, express Toll-like receptors. PAMPs originating from bacteria, viruses, fungi, and other microbes are recognized differently by different TLRs. Numerous PAMPs are recognized by Toll-like receptors, including as lipoproteins, flagellin, viral nucleic acids (dsRNA, ssRNA), bacterial DNA with unmethylated CpG patterns, and components of fungal cell walls (β-glucans, for example) [
101]. Upon ligand binding, initiating downstream signaling cascades, Toll-like receptors dimerize and enlist adaptor proteins with TIR domains, such as MyD88 (myeloid differentiation primary response 88) or TRIF (TIR-domain-containing adapter-inducing interferon-β). Pro-inflammatory cytokines, chemokines, and type I interferons are produced as a result of these pathways’ activation of transcription factors, which include NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and IRF (interferon regulatory factor). The inflammatory response that follows surgery or damage and results in the production of peritoneal adhesions has been linked to Toll-like receptors [
66]. The activation of TLR signaling pathways by endogenous ligands released from damaged tissues or by the microbial contamination of the peritoneal cavity can lead to the production of pro-inflammatory cytokines and chemokines, recruitment of immune cells, and activation of fibroblasts, contributing to tissue remodeling and adhesion formation. Targeting Toll-like receptor signaling pathways represents a potential therapeutic strategy for modulating inflammation and tissue repair processes associated with peritoneal adhesion formation. Approaches aimed at inhibiting TLR activation or downstream signaling cascades could help mitigate adhesion formation and promote tissue healing [
102,
103,
104].
Tenascin-C is an extracellular matrix glycoprotein that plays diverse roles in tissue development, wound healing, inflammation, and remodeling. Various cell types, including fibroblasts, endothelial cells, immune cells, and cancer cells, express tenascin-C during embryonic development and tissue repair processes. Its expression is highly regulated and is induced in response to tissue injury, inflammation, and mechanical stress.
Tenascin-C is involved in peritoneal adhesion formation following surgery or injury. It is upregulated in response to tissue damage and inflammation in the peritoneal cavity, contributing to fibrous adhesions between peritoneal surfaces. Tenascin-C promotes the migration and activation of fibroblasts and myofibroblasts, stimulates extracellular matrix deposition, and modulates immune cell functions, thereby promoting adhesion formation and tissue remodeling. Targeting tenascin-C signaling pathways represents a potential therapeutic strategy for preventing or reducing peritoneal adhesion formation. Approaches aimed at inhibiting tenascin-C expression or blocking its interactions with cell surface receptors could help mitigate fibrosis, inflammation, and tissue remodeling in the peritoneal cavity, thereby reducing the formation of adhesions between peritoneal surfaces [
93,
105,
106,
107,
108].
Multiple stimuli regulate its expression, including growth factors, cytokines, and mechanical stress. PDGF has been implicated in peritoneal adhesion formation following surgery or injury. It stimulates the proliferation and migration of fibroblasts and myofibroblasts, leading to the deposition of extracellular matrix proteins and the formation of fibrous adhesions between peritoneal surfaces [
109,
110].
Additionally, PDGF stimulates angiogenesis, which may contribute to the development and persistence of peritoneal adhesions. Targeting PDGF signaling pathways represents a potential therapeutic strategy for preventing or reducing peritoneal adhesion formation. Approaches aimed at inhibiting PDGF activity or blocking its receptors could help mitigate fibrosis, angiogenesis, and tissue remodeling in the peritoneal cavity, thereby reducing the formation of adhesions between peritoneal surfaces [
111].
VEGF is a homodimeric glycoprotein consisting of multiple isoforms, including VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF). Each isoform is generated through the alternative splicing of the VEGF gene and exhibits distinct biological activities. Various cell types produce VEGF, including endothelial cells, macrophages, fibroblasts, smooth muscle cells, and tumor cells. Multiple stimuli induce its expression, including hypoxia, growth factors, cytokines, and mechanical stress. VEGF exerts its effects primarily by binding to and activating VEGF receptors (VEGFRs) expressed on the surface of endothelial cells. VEGFR activation triggers intracellular signaling pathways involved in endothelial cell proliferation, migration, survival, and vascular permeability. These processes are essential for angiogenesis, vasculogenesis, and vascular remodeling during development, wound healing, and pathological conditions, such as cancer and ischemic diseases. VEGF has been implicated in peritoneal adhesion formation following surgery or injury. It promotes angiogenesis within the peritoneal cavity, forming new blood vessels that supply nutrients and oxygen to the developing adhesions [
112,
113,
114].
Additionally, VEGF may enhance vascular permeability and inflammatory responses, contributing to tissue edema and fibrosis associated with adhesion formation. Targeting VEGF signaling pathways represents a potential therapeutic strategy for preventing or reducing peritoneal adhesion formation. Approaches aimed at inhibiting VEGF expression or blocking its receptors could help mitigate angiogenesis, inflammation, and tissue remodeling in the peritoneal cavity, thereby reducing the formation and severity of adhesions between peritoneal surfaces [
115,
116].
TIMPs are small proteins typically composed of around 200 amino acids. They contain a conserved N-terminal domain responsible for binding to the active site of MMPs, inhibiting their proteolytic activity. TIMPs also possess a C-terminal domain that mediates interactions with other proteins and ECM components. Various cell types, including fibroblasts, endothelial cells, smooth muscle cells, and immune cells, produce TIMPs. Multiple stimuli, including growth factors, cytokines, and mechanical stress, regulate their expression. TIMPs regulate ECM homeostasis by inhibiting the activity of MMPs, which are responsible for degrading ECM components, such as collagen, elastin, and proteoglycans. By inhibiting MMPs, TIMPs help maintain the structural integrity of tissues and prevent excessive ECM degradation [
47,
117,
118].
Additionally, TIMPs have been shown to modulate cell proliferation, migration, and survival through MMP-independent mechanisms. TIMPs have been implicated in peritoneal adhesion formation following surgery or injury. The dysregulation of TIMP expression and MMP/TIMP imbalance can disrupt ECM remodeling processes, leading to aberrant tissue repair and fibrosis. Both inadequate and excessive TIMP activity can contribute to pathological conditions associated with peritoneal adhesions, highlighting the importance of maintaining proper MMP/TIMP balance. Modulating TIMP expression or activity represents a potential therapeutic strategy for preventing or reducing peritoneal adhesion formation. Approaches to restoring MMP/TIMP balance could help mitigate excessive ECM remodeling, fibrosis, and tissue adhesion in the peritoneal cavity, thereby improving surgical outcomes and patient recovery [
119,
120].
Fibroblast Growth Factor 2 (FGF2), or essential fibroblast growth factor (bFGF), is a member of the fibroblast growth factor family. It plays diverse roles in various biological processes, including cell proliferation, differentiation, migration, and angiogenesis. GF2 is a small, secreted protein consisting of around 155 amino acids. It contains a conserved core region responsible for binding to fibroblast growth factor receptors (FGFRs) and heparan sulfate proteoglycans (HSPGs) on the cell surface. FGF2 exists in several isoforms generated by alternative splicing, with the most common forms being low-molecular-weight (LMW) and high-molecular-weight (HMW) variants. Various cell types, including fibroblasts, endothelial cells, smooth muscle cells, and tumor cells, produce FGF2. Multiple stimuli regulate its expression, including growth factors, cytokines, and mechanical stress.
FGF2 exerts its effects by binding to and activating FGFRs, receptor tyrosine kinases expressed on the surface of target cells. Upon ligand binding, FGFRs undergo dimerization and autophosphorylation, activating downstream signaling pathways, such as the Ras-MAPK and PI3K-Akt pathways. These pathways regulate cellular processes, including cell proliferation, survival, differentiation, and migration. FGF2 also stimulates angiogenesis, forming new blood vessels by promoting endothelial cell proliferation and migration. FGF2 has been implicated in peritoneal adhesion formation following surgery or injury. It promotes the proliferation and migration of fibroblasts, endothelial cells, and other cell types involved in tissue repair and remodeling [
121,
122,
123].
Additionally, FGF2 stimulates angiogenesis, which may contribute to the development and persistence of peritoneal adhesions. Targeting FGF2 signaling pathways represents a potential therapeutic strategy for preventing or reducing peritoneal adhesion formation. Approaches aimed at inhibiting FGF2 activity or blocking its receptors could help mitigate fibrosis, angiogenesis, and tissue remodeling in the peritoneal cavity, thereby reducing the formation of adhesions between peritoneal surfaces. In summary, exploring strategies to modulate FGF2 signaling pathways may hold promise for managing peritoneal adhesions and improving patient outcomes [
124,
125].
In conclusion, peritoneal adhesion formation is a multifaceted process involving diverse molecular pathways and regulatory mechanisms, including integrin-mediated signaling, the regulation of E-cadherin expression and function, selectin-dependent leukocyte-endothelial interactions, and immunoglobulin superfamily molecules, such as ICAM-1 and VCAM-1, which facilitate leukocyte recruitment during inflammatory responses. Hyaluronan (HA), a critical extracellular matrix component, modulates inflammatory, fibrotic, and immune responses, providing a protective barrier that can mitigate adhesion formation. Matrix metalloproteinases (MMPs), regulated by tissue inhibitors of metalloproteinases (TIMPs), play a crucial role in extracellular matrix remodeling and turnover, influencing fibrosis and adhesion severity. The coagulation cascade, especially fibrinogen and its conversion to fibrin, along with the delicate fibrinolytic balance between tissue plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1), further governs the extent and persistence of adhesions. Key growth factors, such as transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF), along with interleukins and fibroblast growth factors (FGFs), actively participate in promoting angiogenesis, inflammation, and fibroblast proliferation, significantly influencing adhesion development and severity. Furthermore, Toll-like receptor (TLR)-mediated innate immune activation and tenascin-C expression critically shape the inflammatory environment, perpetuating fibrotic processes. Given the complexity and interconnectivity of these molecular mechanisms, therapeutic strategies that simultaneously or selectively target these pathways hold promise for effectively reducing or preventing postoperative adhesions. A deeper understanding of these interactions, combined with targeted molecular therapies, could significantly enhance clinical outcomes, ultimately reducing adhesion-related complications and improving patient quality of life following abdominal surgery or peritoneal injuries.