L-Ascorbic Acid (LAA) Supplementation as a Potential Treatment for Skin Aging: Regulation of Adipose Tissue Mesenchymal Stem Cells (AT-MSCs) Protein Secretion
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of AT-MSCs
2.2. Characterization of AT-MSCs
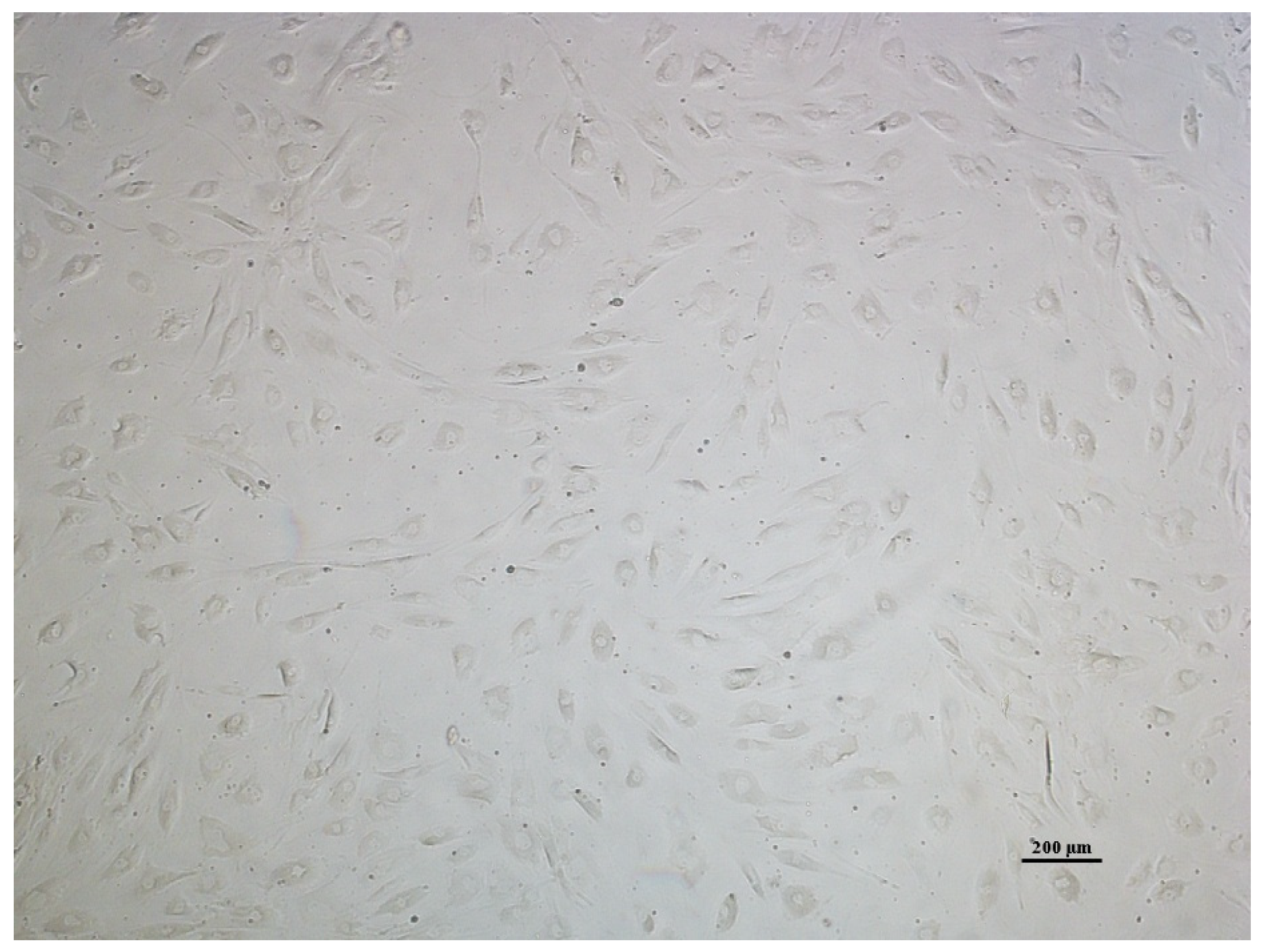
2.2.1. Cell Morphology Analysis of AT-MSCs
2.2.2. Immunophenotypic Analysis of AT-MSCs
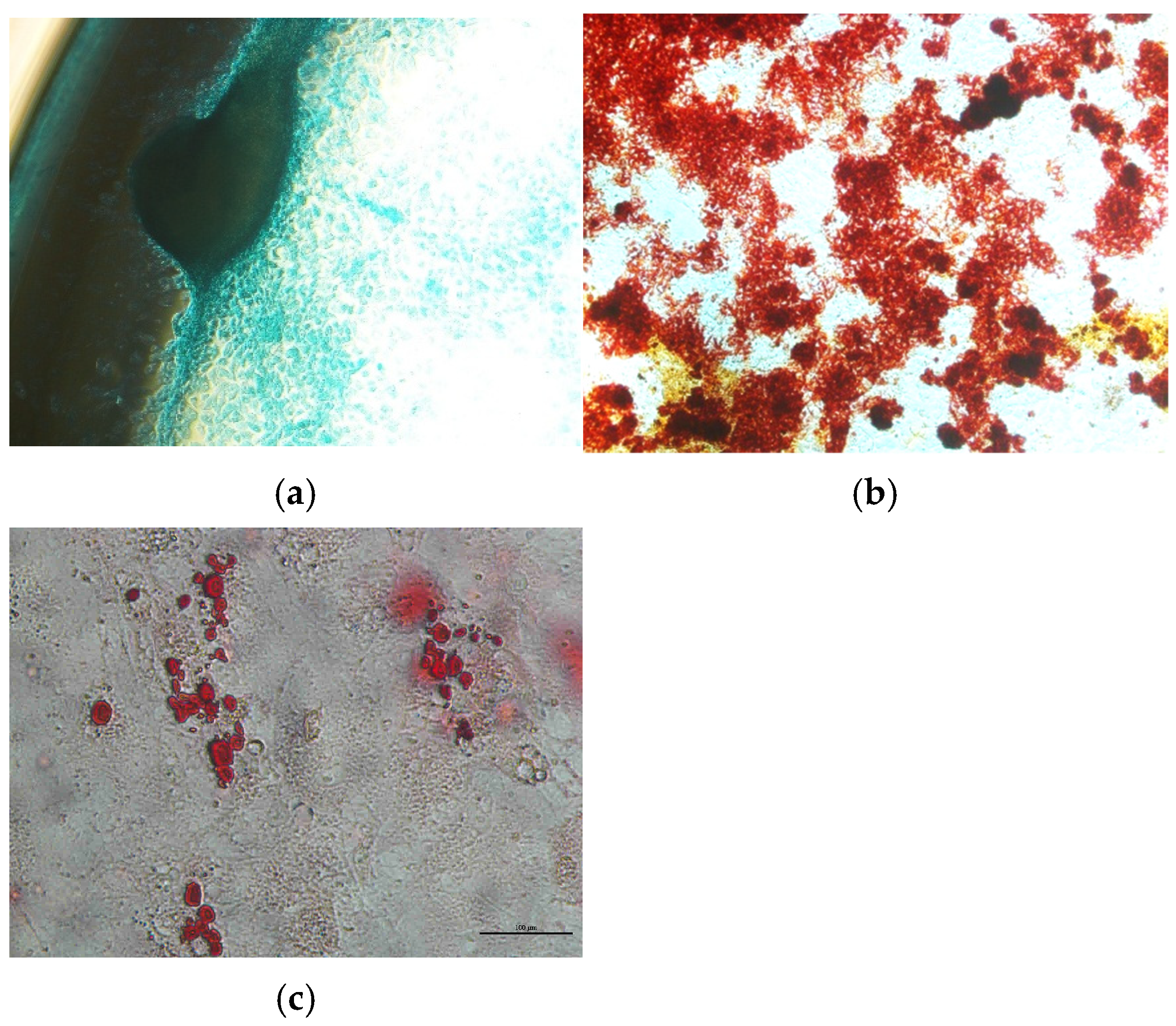
2.2.3. Differentiation of AT-MSCs
2.3. Preconditioning of AT-MSC Culture for Secretome Collection
2.4. Testing the Effects of L-Ascorbic Acid on AT-MSC Culture and Secretion
2.5. Statistical Analysis
3. Results
3.1. AT-MSC Characterization
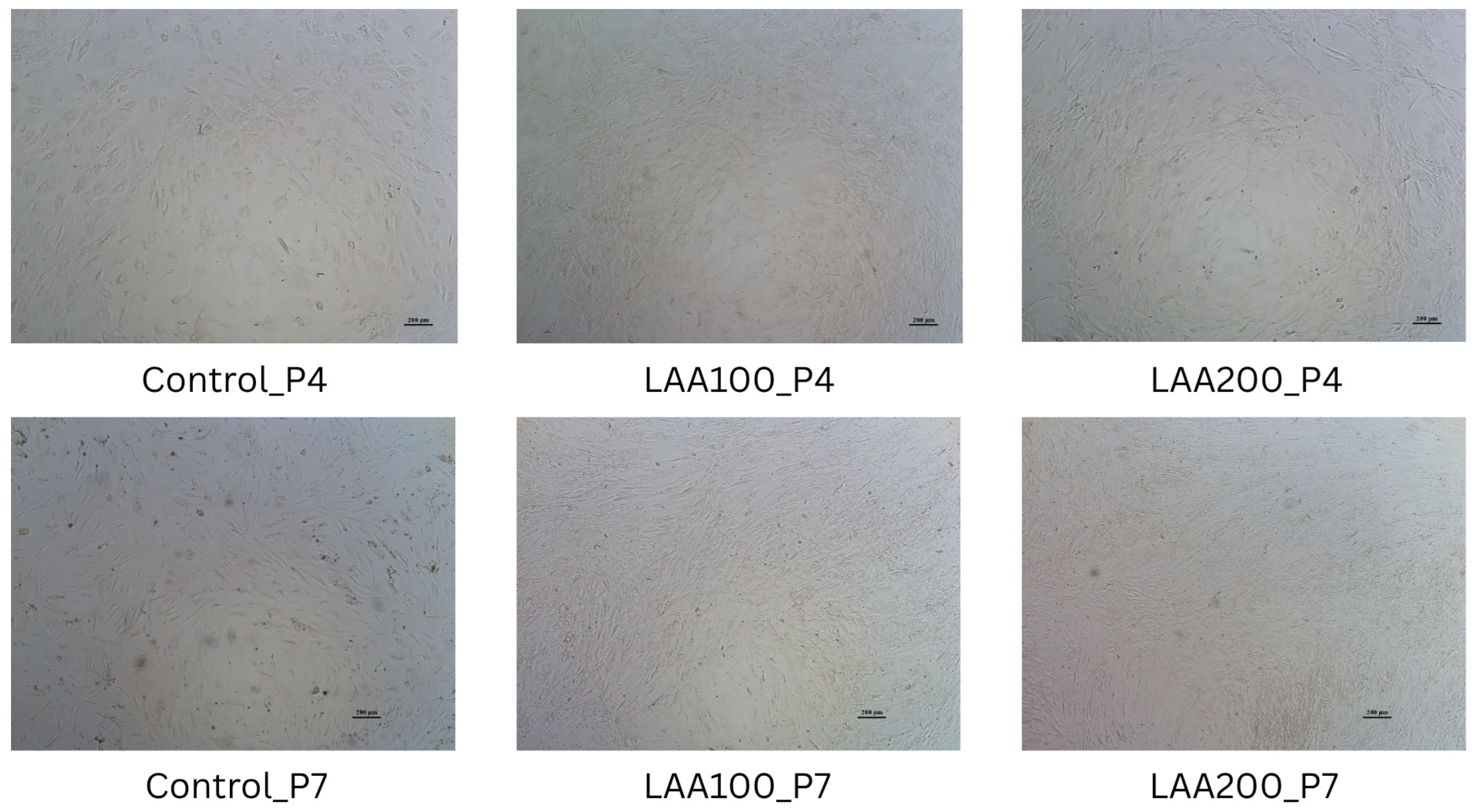
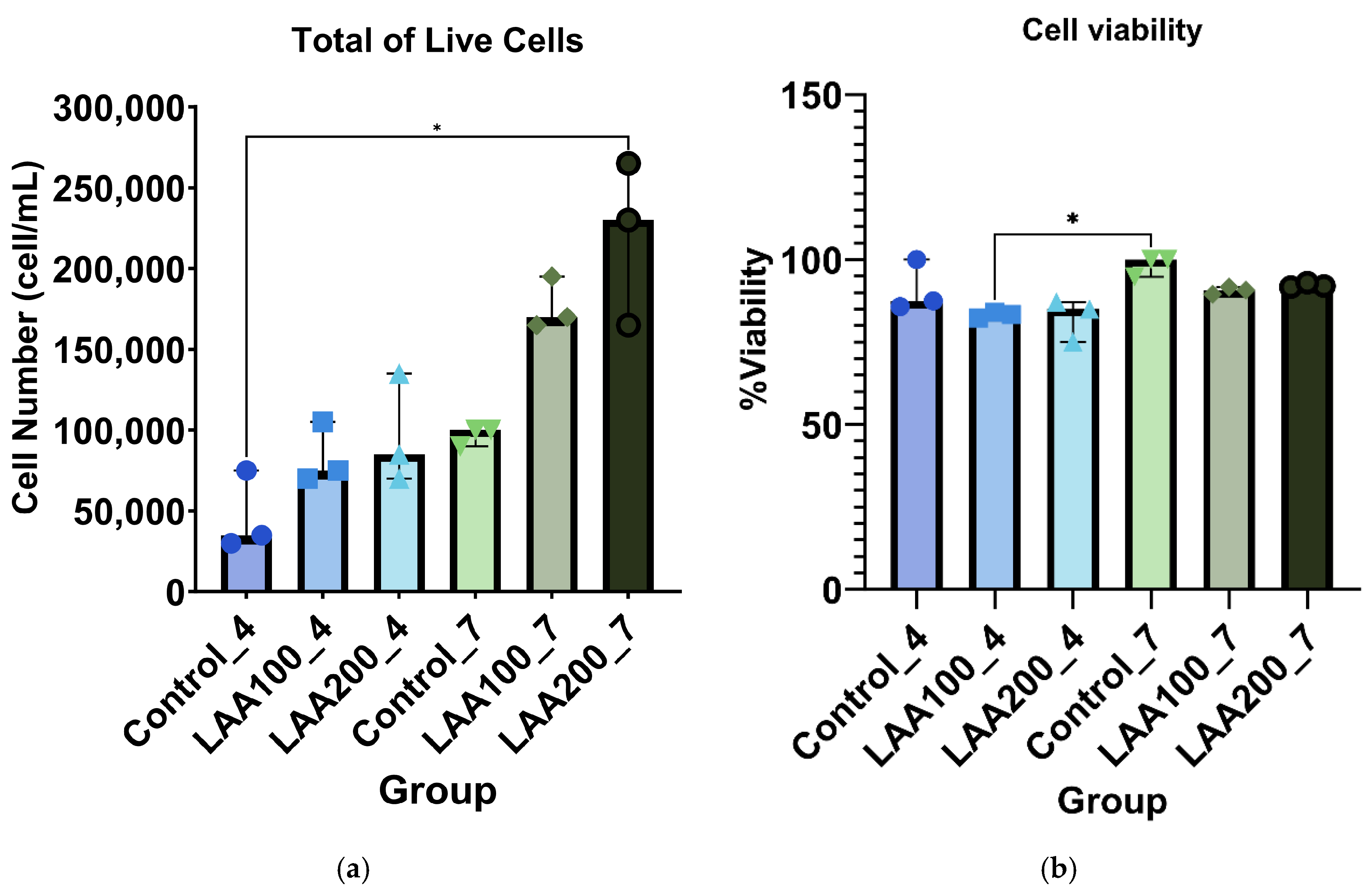
3.2. LAA Treatment in AT-MSCs: Improved Cell Morphology Without Affecting Cell Viability
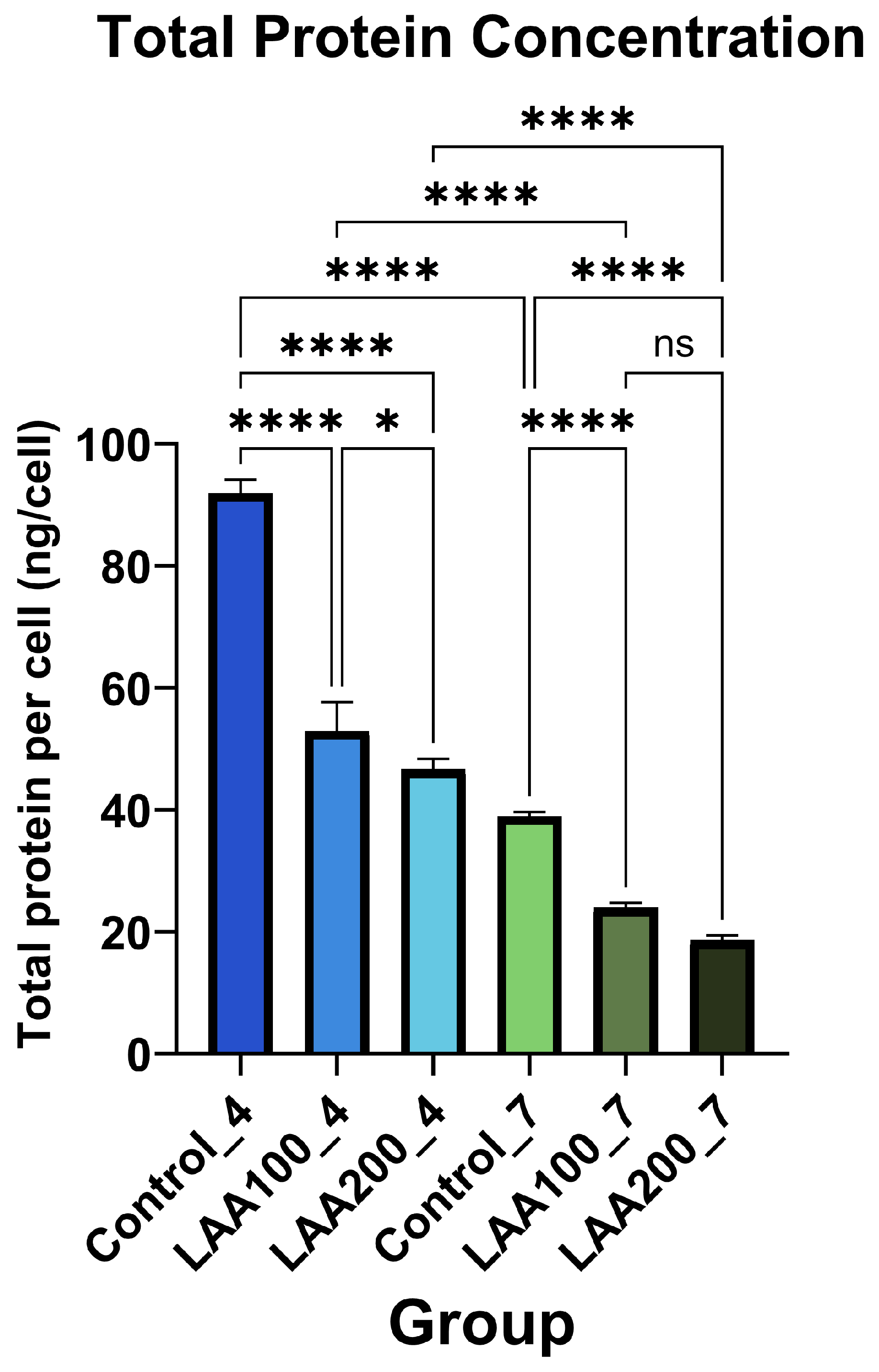
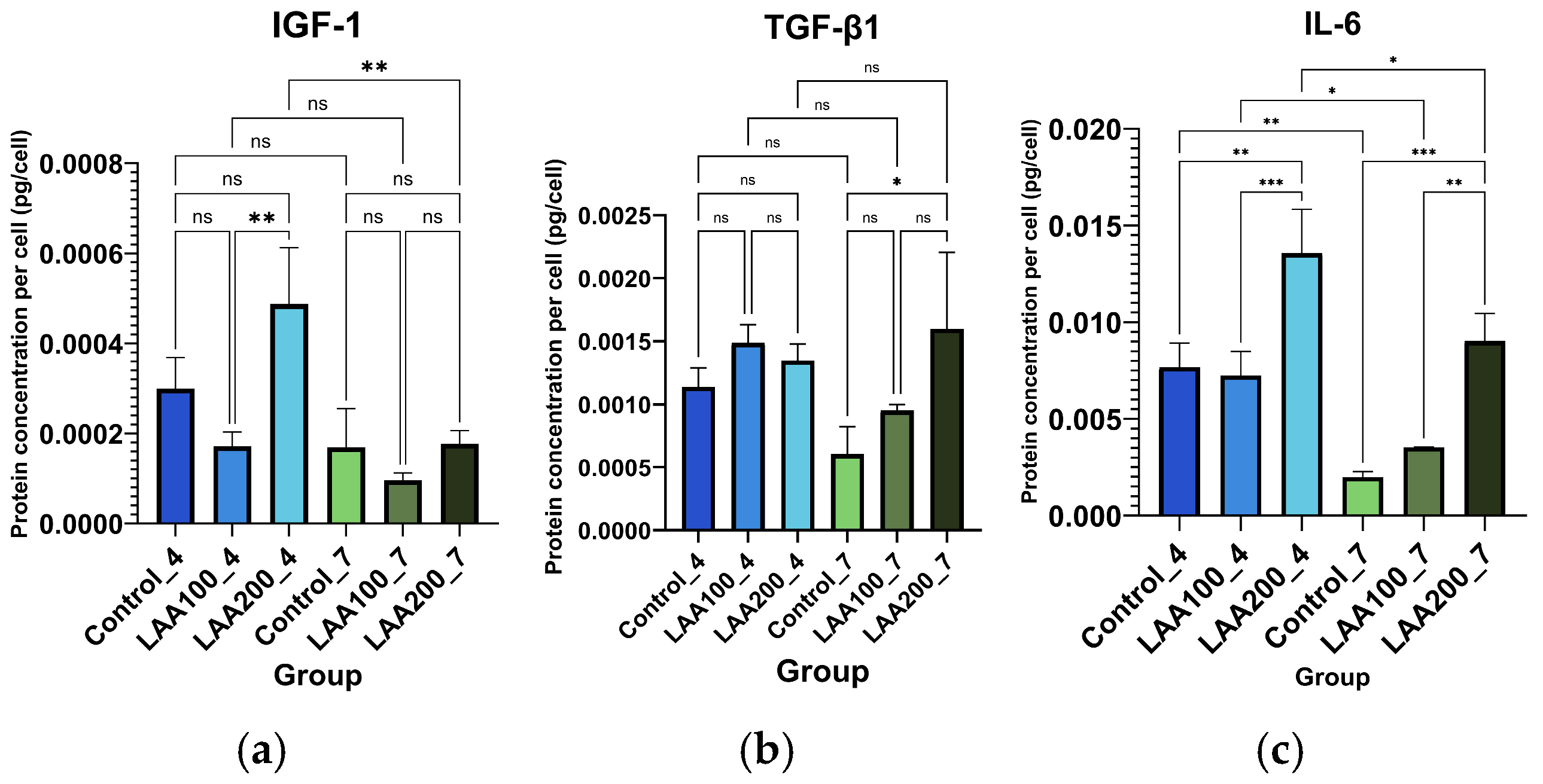
3.3. Effect of LAA Treatment on AT-MSC Protein Profile and Secretion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AT-MSCs | Adipose Tissue Mesenchymal Stem Cells |
| LAA | L-Ascorbic Acid |
| ROS | Reactive oxygen species |
| IGF-1 | Insulin-like growth factor 1 |
| TGF-β1 | Transforming growth factor beta 1 |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| TNF-α | Tumor Necrosis Factor alpha |
| VEGF | Vascular Endothelial Growth Factor |
| SOD | Superoxide dismutase |
| GSH-Px | Glutathione peroxidase |
| CAT | Catalase |
| miRNA | Micro–Ribonucleic Acid |
| DNA | Deoxyribonucleic Acid |
| HDF | Human dermal fibroblast |
| SVF | Stromal vascular fraction |
| SHEDs | Stem cells from human exfoliated deciduous teeth |
| UVB | Ultraviolet B |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| PBS | Phosphate-buffered saline |
| CO2 | Carbon dioxide |
| ECM | Extracellular Matrix |
| ISCT | International Society for Cell & Gene Therapy |
| CD | Cluster of differentiation |
| HLA-DR | Human Leukocyte Antigen DR |
| FITC | Fluorescein isothiocyanate |
| PE | R-phycoerythrin |
| APC | Allophycocyanin |
| PerCP-Cy5-5 | PerCP-Cyanine 5-5 |
| FACS | Fluorescence-Activated Cell Sorting |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ANOVA | Analysis of Variance |
| CDK | Cyclin-dependent kinases |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular Signal-Regulated Kinase |
| MEK | Mitogen-activated protein kinase kinase-MAPKK |
| STAT-JAK | Signal Transducer and Activator of Transcription—Janus Kinase |
| STAT3 | Signal transducer and activator of transcription 3 |
| Nrf2 | Nuclear factor-erythroid factor 2-related factor 2 |
References
- Shin, S.H.; Lee, Y.H.; Rho, N.K.; Park, K.Y. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Ali, S.; Tahir, H.M.; Kazmi, S.A.R.; Shakir, H.A.; Mughal, T.A.; Mumtaz, S.; Summer, M.; Farooq, M.A. Aging and its treatment with vitamin C: A comprehensive mechanistic review. Mol. Biol. Rep. 2021, 48, 8141–8153. [Google Scholar] [CrossRef]
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, S.; Fu, W.; Yang, Z.; Yao, H.; Zhang, Z. The Role and Prospects of Mesenchymal Stem Cells in Skin Repair and Regeneration. Biomedicines 2024, 12, 743. [Google Scholar] [CrossRef]
- Krawczenko, A.; Klimczak, A. Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Contribution to Angiogenic Processes in Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 2425. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Liu, X.; Dou, H.; Hou, Y. Characteristics and regulation of mesenchymal stem cell plasticity by the microenvironment —specific factors involved in the regulation of MSC plasticity. Genes. Dis. 2022, 9, 296–309. [Google Scholar] [CrossRef]
- Kemp, M.G.; Spandau, D.F.; Travers, J.B. Impact of age and insulin-like growth factor-1 on DNA damage responses in UV-irradiated human skin. Molecules 2017, 22, 356. [Google Scholar] [CrossRef] [PubMed]
- Surowiecka, A.; Strużyna, J. Adipose-Derived Stem Cells for Facial Rejuvenation. J. Pers. Med. 2022, 12, 117. [Google Scholar] [CrossRef]
- Pawitan, J.A.; Liem, I.K.; Suryani, D.; Bustamid, A.; Purwoko, R.Y. Simple lipoaspirate washing using a coffee filter. Asian Biomed. 2013, 7, 333–338. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krausem, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Prakoeswa, C.R.S.; Rindiastuti, Y.; Wirohadidjojo, Y.W.; Komaratih, E.; Nurwasis; Dinaryati, A.; Lestari, N.M.I.; Rantam, F.A. Resveratrol promotes secretion of wound healing related growth factors of mesenchymal stem cells originated from adult and fetal tissues. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- McCutchan, A.; Dobson, G.P.; Stewart, N.; Letson, H.L.; Grant, A.L.; Jovanovic, I.A.; Hazratwala, K.; Wilkinson, M.; McEwen, P.; Morris, J. Absence of cytotoxic and inflammatory effects following in vitro exposure of chondrogenically-differentiated human mesenchymal stem cells to adenosine, lidocaine and Mg2+ solution. J. Exp. Orthop. 2019, 6, 16. [Google Scholar] [CrossRef]
- KarbalaeiMahdi, A.; Moridi, K.; Ghollasi, M. Evaluation of osteogenic differentiation of human mesenchymal stem cells (hMSCs) on random and aligned polycaprolactone-polyaniline-gelatin scaffolds. BioImpacts 2022, 4, 149–166. [Google Scholar] [CrossRef]
- Fink, T.; Zachar, V. Adipogenic Differentiation of Human Mesenchymal Stem Cells. In Mesenchymal Stem Cell Assays and Applications; Vemuri, M., Chase, L., Rao, M., Eds.; Humana Press: Totowa, NJ, USA, 2011; Volume 698, pp. 243–251. Available online: https://link.springer.com/10.1007/978-1-60761-999-4_19 (accessed on 16 February 2025).
- Brito, K.d.N.L.d.; Trentin, A.G. Role of mesenchymal stromal cell secretome on recovery from cellular senescence: An overview. Cytotherapy 2024, 27, 422–437. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Teng, S.; Ma, C.; Yu, Y.; Wang, P.; Yi, C. Ascorbic acid inhibits senescence in mesenchymal stem cells through ROS and AKT/mTOR signaling. Cytotechnology 2018, 70, 1301–1313. [Google Scholar] [CrossRef]
- Bhandi, S.; Alkahtani, A.; Mashyakhy, M.; Abumelha, A.S.; Albar, N.H.M.; Renugalakshmi, A.; Alkahtany, M.F.; Robaian, A.; Almeslet, A.S.; Patil, V.R.; et al. Effect of ascorbic acid on differentiation, secretome and stemness of stem cells from human exfoliated deciduous tooth (Sheds). J. Pers. Med. 2021, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Hara, K.; Takami, T.; Okada, S.; Matsumoto, T.; Yamamoto, N.; Sakaida, I. Evaluation of the effects of ascorbic acid on metabolism of human mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Carrillo-Gálvez, A.B.; García-Pérez, A.; Cobo, M.; Martín, F. CD105 (Endoglin)-Negative Murine Mesenchymal Stromal Cells Define a New Multipotent Subpopulation with Distinct Differentiation and Immunomodulatory Capacities. PLoS ONE 2013, 8, e76979. [Google Scholar] [CrossRef]
- Cleary, M.A.; Narcisi, R.; Focke, K.; van der Linden, R.; Brama, P.A.J.; van Osch, G.J.V.M. Expression of CD105 on expanded mesenchymal stem cells does not predict their chondrogenic potential. Osteoarthr. Cartil. 2016, 24, 868–872. [Google Scholar] [CrossRef]
- Wahyuningsih, K.A.; Karina, K.; Rosadi, I.; Rosliana, I.; Subroto, W.R. Effect of ascorbic acid on morphology of post-thawed human adipose-derived stem cells. Stem Cell Investig. 2020, 7, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Segeritz, C.P.; Vallier, L. Cell Culture: Growing Cells as Model Systems In Vitro. In Basic Science Methods for Clinical Researchers; Jalali, M., Saldanha, F.Y.L., Jalali, M., Eds.; Elsevier Inc.: London, UK, 2017; pp. 151–172. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Qi, Y.; Zou, Y.; Liu, L.; Tang, X.; Duan, J.; Liu, H.; Zeng, G. Vitamin C promotes the proliferation of human adipose-derived stem cells via p53-p21 pathway. Organogenesis 2016, 12, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.K.; Tu, Y.K.; Yu, J.; Cheng, N.C. The Influence of Cell Culture Density on the Cytotoxicity of Adipose-Derived Stem Cells Induced by L-Ascorbic Acid-2-Phosphate. Sci. Rep. 2020, 10, 104. [Google Scholar] [CrossRef]
- Jobling, M.F.; Mott, J.D.; Finnegan, M.T.; Jurukovski, V.; Erickson, A.C.; Walian, P.J.; Taylor, S.E.; Ledbetter, S.; Lawrence, C.M.; Rifkin, D.B.; et al. Isoform-specific activation of latent transforming growth factor β (LTGF-β) by reactive oxygen species. Radiat. Res. 2006, 166, 839–848. [Google Scholar] [CrossRef]
- Kwack, M.H.; Shin, S.H.; Kim, S.R.; Im, S.U.; Han, I.S.; Kim, M.K.; Kim, J.; Sung, Y. l-Ascorbic acid 2-phosphate promotes elongation of hair shafts via the secretion of insulin-like growth factor-1 from dermal papilla cells through phosphatidylinositol 3-kinase. Br. J. Dermatol. 2009, 160, 1157–1162. [Google Scholar] [CrossRef]
- Piersma, B.; Wouters, O.Y.; de Rond, S.; Boersema, M.; Gjaltema, R.A.F.; Bank, R.A. Ascorbic acid promotes a TGFβ1-induced myofibroblast phenotype switch. Physiol. Rep. 2017, 5, e13324. [Google Scholar] [CrossRef] [PubMed]
- Chouw, A.; Sartika, C.R.; Milanda, T.; Faried, A. Interleukins Profiling in Umbilical Cord Mesenchymal Stem Cell-Derived Secretome. Stem Cells Cloning 2022, 15, 1–9. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- Dedier, M.; Magne, B.; Nivet, M.; Banzet, S.; Trouillas, M. Anti-inflammatory effect of interleukin-6 highly enriched in secretome of two clinically relevant sources of mesenchymal stromal cells. Front. Cell Dev. Biol. 2023, 11, 1244120. [Google Scholar] [CrossRef]
- Puschel, F.; Favaro, F.; Redondo-Pedraza, J.; Lucendo, E.; Iurlaro, R.; Marchetti, S.; Majem, B.; Eldering, E.; Nadal, E.; Ricci, J.-E.; et al. Starvation and antimetabolic therapy promote cytokine release and recruitment of immune cells. Proc. Natl. Acad. Sci. USA 2020, 117, 9932–9941. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Klemm, C.; Bruchhagen, C.; Van Krüchten, A.; Niemann, S.; Löffler, B.; Peters, G.; Ludwig, S.; Ehrhardt, C. Mitogen-activated protein kinases (MAPKs) regulate IL-6 over-production during concomitant influenza virus and Staphylococcus aureus infection. Sci. Rep. 2017, 7, srep42473. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef]
- Miyamae, Y.; Ohta, M.; Sato, K.; Ishisak, A.; Chosa, N. TGF-β1-induced IL-6 expression via MEK pathway in mesenchymal stem cells enhances NGF-dependent neurite extension in PC12 cells. J. Iwate Med. Univ. 2022, 47, 19–33. [Google Scholar]
- Luo, X.; Jiang, X.; Li, J.; Bai, Y.; Li, Z.; Wei, P.; Sun, S.; Liang, Y.; Han, S.; Li, X.; et al. Insulin-like growth factor-1 attenuates oxidative stress-induced hepatocyte premature senescence in liver fibrogenesis via regulating nuclear p53–progerin interaction. Cell Death Dis. 2019, 10, 451. [Google Scholar] [CrossRef]
- Herrera, M.L.; Champarini, L.G.; Oliveros, A.L.; Bellini, M.J.; Hereñú, C.B. Potentialities of IGF-1 for regulating oxidative stress in neuroinflammation and neurodegeneration: Theoretical review. Explor. Neuroprotect. Ther. 2024, 4, 442–458. [Google Scholar] [CrossRef]
- Jorgensen, P.; Rattan, S.I.S. Extracellular Matrix Modulates Morphology, Growth, Oxidative Stress Response and Functionality of Human Skin Fibroblasts during Aging In Vitro. J. Aging Sci. 2014, 2, 1–7. [Google Scholar] [CrossRef]
- Mirmira, R.G.; Linnemann, A.K.; Marasco, M.R.; Conteh, A.M.; Reissaus, C.A.; Cupit, J.E.; Appleman, E.M.; Mirmira, R.G.; Linnemann, A.K. Interleukin-6 reduces B-cell oxidative stress by linking autophagy with the antioxidant response. Diabetes 2018, 67, 1576–1588. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahyuningsih, K.A.; Wiratnaya, I.G.E.; Weta, I.W.; Widiana, I.G.R.; Pangkahila, W.I.; Wahyuniari, I.A.I.; Muliarta, I.M.; Sidharta, V.M.; Salwa, A. L-Ascorbic Acid (LAA) Supplementation as a Potential Treatment for Skin Aging: Regulation of Adipose Tissue Mesenchymal Stem Cells (AT-MSCs) Protein Secretion. Curr. Issues Mol. Biol. 2025, 47, 474. https://doi.org/10.3390/cimb47060474
Wahyuningsih KA, Wiratnaya IGE, Weta IW, Widiana IGR, Pangkahila WI, Wahyuniari IAI, Muliarta IM, Sidharta VM, Salwa A. L-Ascorbic Acid (LAA) Supplementation as a Potential Treatment for Skin Aging: Regulation of Adipose Tissue Mesenchymal Stem Cells (AT-MSCs) Protein Secretion. Current Issues in Molecular Biology. 2025; 47(6):474. https://doi.org/10.3390/cimb47060474
Chicago/Turabian StyleWahyuningsih, Komang Ardi, I. Gede Eka Wiratnaya, I. Wayan Weta, I. Gde Raka Widiana, Wimpie I. Pangkahila, Ida Ayu Ika Wahyuniari, I. Made Muliarta, Veronika Maria Sidharta, and Assyafiya Salwa. 2025. "L-Ascorbic Acid (LAA) Supplementation as a Potential Treatment for Skin Aging: Regulation of Adipose Tissue Mesenchymal Stem Cells (AT-MSCs) Protein Secretion" Current Issues in Molecular Biology 47, no. 6: 474. https://doi.org/10.3390/cimb47060474
APA StyleWahyuningsih, K. A., Wiratnaya, I. G. E., Weta, I. W., Widiana, I. G. R., Pangkahila, W. I., Wahyuniari, I. A. I., Muliarta, I. M., Sidharta, V. M., & Salwa, A. (2025). L-Ascorbic Acid (LAA) Supplementation as a Potential Treatment for Skin Aging: Regulation of Adipose Tissue Mesenchymal Stem Cells (AT-MSCs) Protein Secretion. Current Issues in Molecular Biology, 47(6), 474. https://doi.org/10.3390/cimb47060474





