Morphometric and Enzymatic Changes in Gills of Rainbow Trout After Exposure to Suboptimal Low Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish
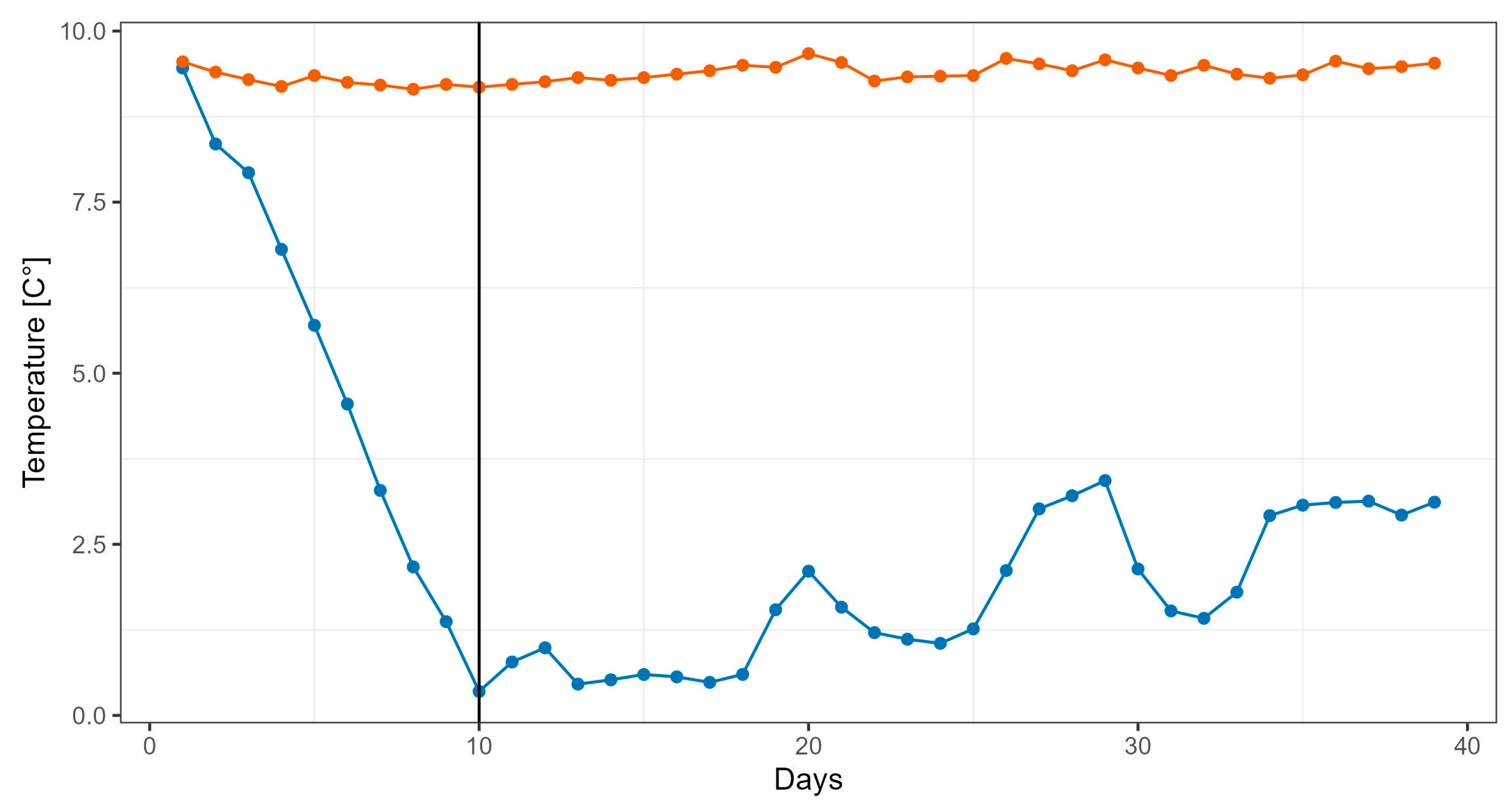
2.2. Temperature Exposure
2.3. Sampling
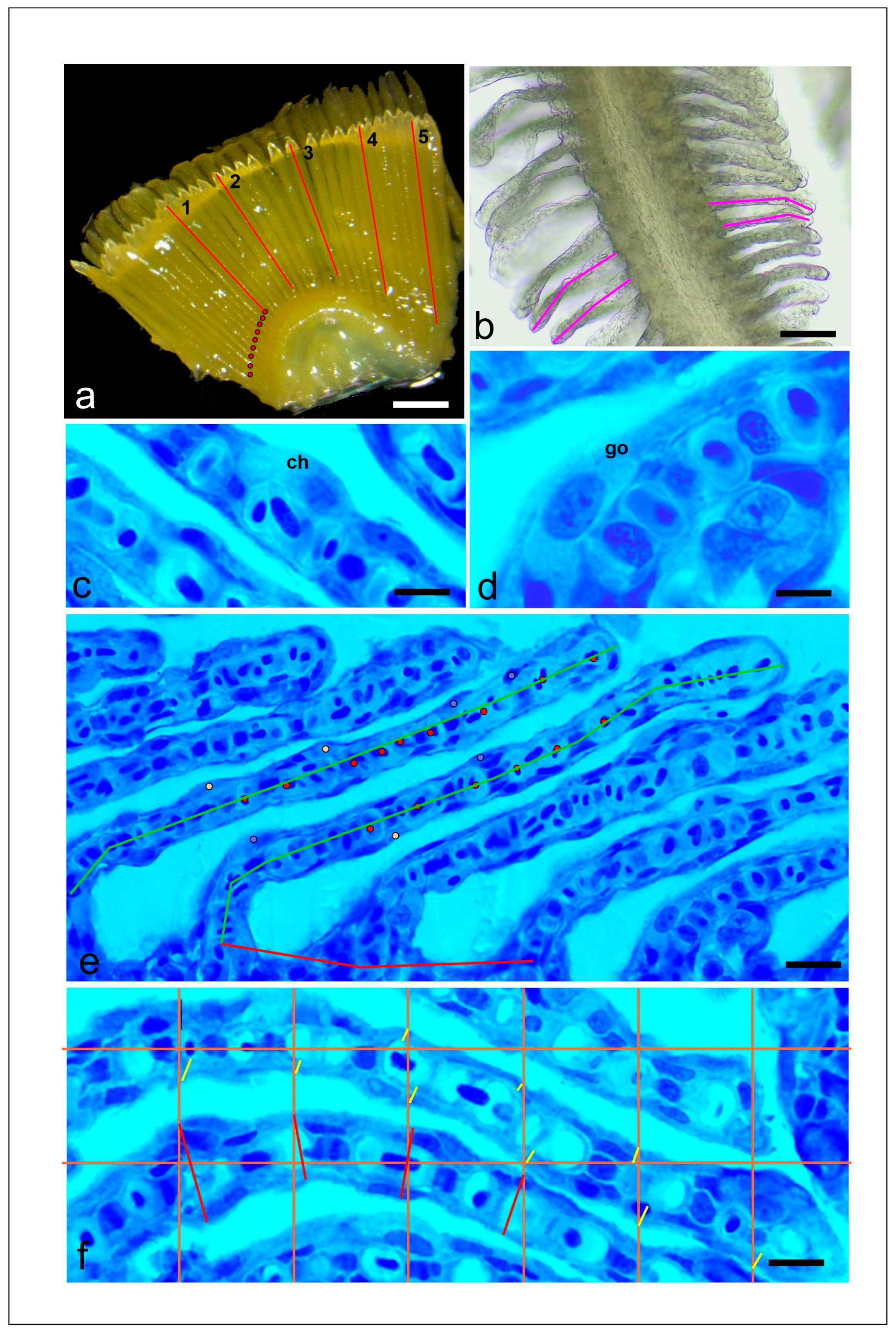
2.4. Gill Histology and Morphometry
2.5. Enzymatic Analyses
2.6. Gene Expression Analysis
2.7. Statistics
3. Results
3.1. Gill Morphometry
3.2. Enzymatic Activities and Gene Expressions
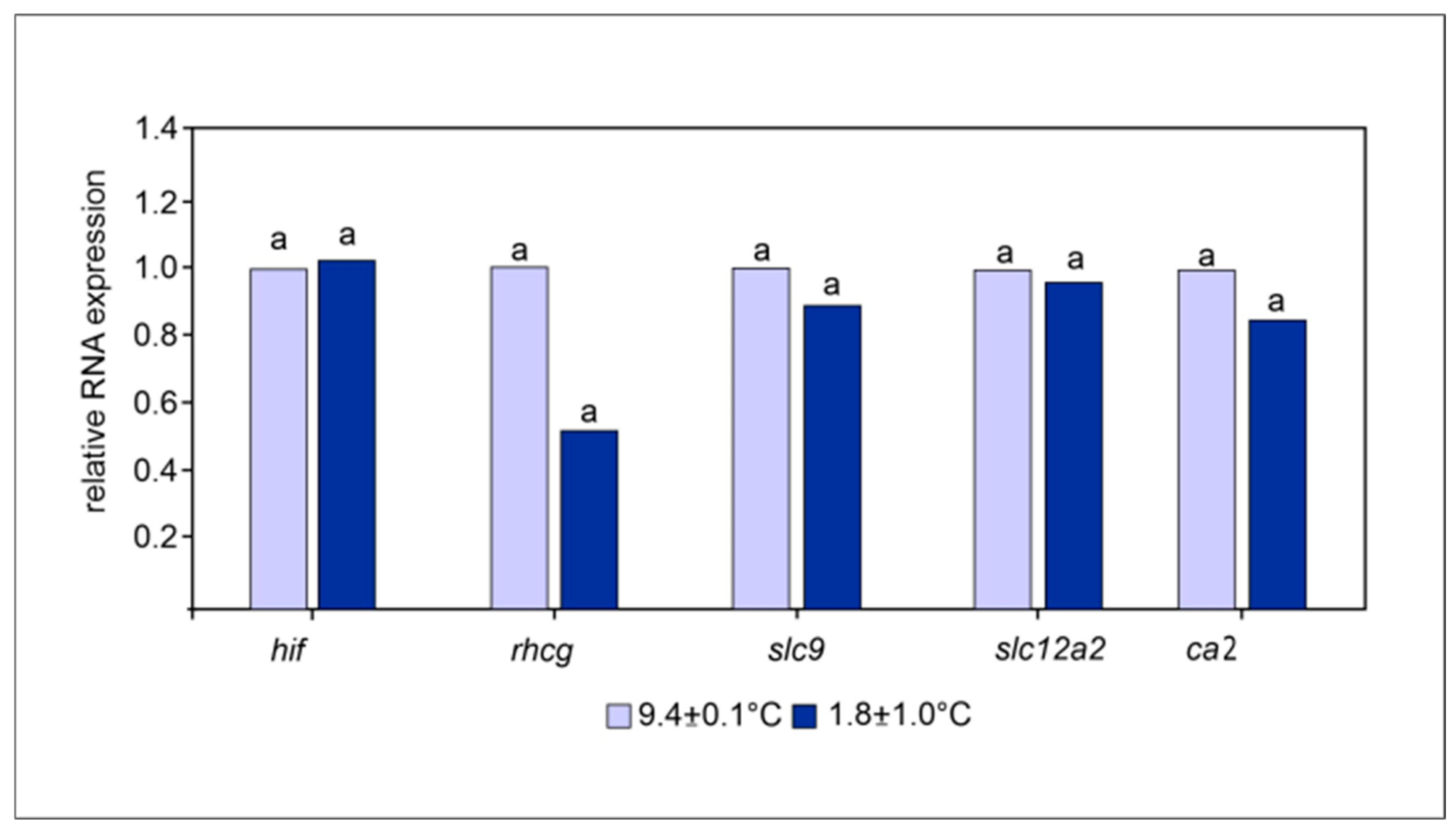
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MS222 | methanesulfonate or tricaine methanesulfonate |
| EDTA | ethylenediaminetetraacetic acid |
| KCL | potassium chloride |
| ATP | adenosine triphosphate |
| ADP | adenosine diphosphate |
| NCBI | National Center for Biotechnology Information |
| hif1a | hypoxia-inducible factor 1-α |
| ca2 | carbonic anhydrase II |
| rhCG | rhesus blood group C glycoprotein |
| slc9a1 | solute carrier family 9 |
| slc26a6 | solute carrier family 26 |
References
- Rodriguez-Dominguez, A.; Connell, S.D.; Leung, J.Y.S.; Nagelkerken, I. Adaptive responses of fishes to climate change: Feedback between physiology and behaviour. Sci. Total Environ. 2019, 692, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Agarwal, D.; Shanmugam, S.; Kathirvelpandian, A.; Eswaran, S.; Rather, A.; Rakkannan, G. Unraveling the Impact of Climate Change on Fish Physiology: A Focus on Temperature and Salinity Dynamics. J. Appl. Ichthyol. 2024, 2024, 5782274. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid–base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Foyle, K.L.; Hess, S.; Powell, M.D.; Herbert, N.A. What is gill health and what is its role in marine finfish aquaculture in the face of a changing climate? Front. Mar. Sci. 2020, 7, 400. [Google Scholar] [CrossRef]
- Tuurala, H.; Egginton, S.; Soivio, A. Cold exposure increases branchial water-blood barrier thickness in the eel. J. Fish Biol. 1998, 53, 451–455. [Google Scholar] [CrossRef]
- Sollid, J.; Weber, R.E.; Nilsson, G.E. Temperature alters the respiratory surface area of crucian carp Carassius carassius and goldfish Carassius auratus. J. Exp. Biol. 2005, 208, 1109–1116. [Google Scholar] [CrossRef]
- Mohamad, S.; Liew, H.J.; Zainuddin, R.A.; Rahmah, S.; Waiho, K.; Ghaffar, M.A.; Nhan, H.T.; Loh, J.-Y.; Lim, L.-S.; Chang, Y.; et al. High environmental temperature and low pH stress alter the gill phenotypic plasticity of Hoven’s carp Leptobarbus hoevenii. J. Fish Biol. 2021, 99, 206–218. [Google Scholar] [CrossRef]
- Das, S.K.; De, M.; Ghaffar, M.A.; Noor, N.M.; Mazumder, S.K.; Bakar, Y. Effects of temperature on the oxygen consumption rate and gill fine structure of hybrid grouper, Epinephelus fuscoguttatus ♀ × E. Lanceolatus ♂. J. King Saud Univ. Sci. 2021, 33, 101358. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Wei, S.; Li, W.; Li, W.; Wu, Z.; Jiang, S.; Lu, Y.; Xu, Q.; Chen, L. Reduced hypoxia tolerance and altered gill morphology at elevated temperatures may limit the survival of tilapia (GIFT, Oreochromis niloticus) under global warming. Fishes 2022, 7, 216. [Google Scholar] [CrossRef]
- Skeeles, M.R.; Clark, T.D. Fish gill surface area can keep pace with metabolic oxygen requirements across body mass and temperature. Funct. Ecol. 2024, 38, 755–764. [Google Scholar] [CrossRef]
- Lahnsteiner, F. Hematological adaptations in diploid and triploid Salvelinus fontinalis and diploid Oncorhynchus mykiss (Salmonidae, Teleostei) in response to long-term exposure to elevated temperature. J. Thermal. Biol. 2022, 106, 103256. [Google Scholar] [CrossRef]
- Farrell, A.P. Cardiovascular system in fish. In The Physiology of Fishes; Evans, D.H., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 119–152. [Google Scholar]
- Lahnsteiner, F.; Dünser, A. Hematological and physiological changes in rainbow trout, Oncorhynchus mykiss, exposed for 30 days to critical low temperature. Aquac. Res. 2024, 2024, 2925959. [Google Scholar] [CrossRef]
- Lahnsteiner, F. Morphometric and Enzymatic Changes in Gills of Rainbow Trout after Exposure to Elevated Temperature—Indications for Gill Remodeling. Animals 2024, 14, 919. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bergmeyer, H.U. Methods of Enzymatic Analysis; VCH Verlagsgesellschaft: Weinheim, Germany, 1985. [Google Scholar]
- Fowles, J.R.; Green, H.J.; Ouyang, J. Na+-K+-ATPase in rat skeletal muscle: Content, isoform, and activity characteristics. J. Appl. Physiol. 2004, 96, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Bystriansky, J.S.; Schulte, P.M. Changes in gill H+-ATPase and Na+/K+-ATPase expression and activity during freshwater acclimation of Atlantic salmon (Salmo salar). J. Exp. Biol. 2011, 214, 2435–2442. [Google Scholar] [CrossRef]
- Uda, N.R.; Seibert, V.; Stenner-Liewen, F.; Müller, P.; Herzig, P.; Gondi, G.; Zeidler, R.; van Dijk, M.; Zippelius, A.; Renner, C. Esterase activity of carbonic anhydrases serves as surrogate for selecting antibodies blocking hydratase activity. J. Enzym. Inhib. Med. Chem. 2015, 30, 955–960. [Google Scholar] [CrossRef]
- Talanian, R.V.; Quinlan, C.; Trautz, S.; Hackett, M.C.; Mankovich, J.A.; Banach, D.; Ghayur, T.; Brady, K.D.; Wong, W.W. Substrate specificities of caspase family proteases. J. Biol. Chem. 1997, 272, 9677–9682. [Google Scholar] [CrossRef]
- Gene [Internet]; National Library of Medicine (US), National Center for Biotechnology Information: Bethesda, MD, USA, 2004. Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 7 May 2025).
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Rombough, P. The functional ontogeny of the teleost gill: Which comes first, gas or ion exchange? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.E.; Dymowska, A.; Stecyk, J.A.W. New insights into the plasticity of gill structure. Respir. Physiol. Neurobiol. 2012, 184, 214–222. [Google Scholar] [CrossRef]
- Srivastava, N.; Kumari, U.; Rai, A.; Mittal, S.; Mittal, A. Alterations in the gill filaments and secondary lamellae of Cirrhinus mrigala exposed to Nuvan, an organophosphorus insecticide. J. Histol. 2014, 2014, 190139. [Google Scholar] [CrossRef]
- Hassaninezhad, L.; Safahieh, A.; Salamat, N.; Savari, A.; Majd, N.E. Assessment of gill pathological responses in the tropical fish yellowfin seabream of Persian Gulf under mercury exposure. Toxicol. Rep. 2014, 1, 621–628. [Google Scholar] [CrossRef]
- Strzyżewska, E.; Szarek, J.; Babinska, I. Morphologic evaluation of the gills as a tool in the diagnostics of pathological conditions in fish and pollution in the aquatic environment: A review. Vet. Med. 2016, 61, 123–132. [Google Scholar] [CrossRef]
- Fiedler, S.; Wünnemann, H.; Hofmann, I.; Theobalt, N.; Feuchtinger, A.; Walch, A.; Schwaiger, J.; Wanke, R.; Blutke, A. A practical guide to unbiased quantitative morphological analyses of the gills of rainbow trout (Oncorhynchus mykiss) in ecotoxicological studies. PLoS ONE 2020, 15, e0243462. [Google Scholar] [CrossRef]
- Xu, X.N.; Chen, S.L.; Jiang, Z.X.; Nissa, M.; Zou, S.M. Gill remodeling increases the respiratory surface area of grass carp (Ctenopharyngodon idella) under hypoxic stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2022, 272, 111278. [Google Scholar] [CrossRef] [PubMed]
- Brauner, C.J.; Matey, V.; Zhang, W.; Richards, J.G.; Dhillon, R.; Cao, Z.D.; Wang, Y.; Fu, S.J. Gill remodeling in crucian carp during sustained exercise and the effect on subsequent swimming performance. Physiol. Biochem. Zool. 2011, 84, 535–542. [Google Scholar] [CrossRef]
- Hwang, P.P.; Lee, T.H. New insights into fish ion regulation and mitochondrion-rich cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 479–497. [Google Scholar] [CrossRef]
- Kültz, D.; Podrabsky, J.E.; Stillman, J.H.; Tomanek, L. Physiological mechanisms used by fish to cope with salinity stress. J. Exp. Biol. 2015, 218, 1907–1914. [Google Scholar] [CrossRef]
- Persson, D.; Bjørgen, H.; Figenschou, A.; Hillestad, L.A.; Koppang, E.; Nødtvedt, A.; Stormoen, M. Variations in mucous cell numbers in gills of Atlantic salmon (Salmo salar) presmolt in commercial freshwater farms in Norway. J. Fish Dis. 2021, 44, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Olson, K. Vascular anatomy of the fish gill. J. Exp. Zool. 2002, 293, 214–231. [Google Scholar] [CrossRef]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, K.M. New insights into the many functions of carbonic anhydrase in fish gills. Respir. Physiol. Neurobiol. 2012, 184, 223–230. [Google Scholar] [CrossRef]
- Young, V.C.; Nakanishi, H.; Meyer, D.J.; Nishizawa, T.; Oshima, A.; Artigas, P.; Abe, K. Structure and function of H+/K+ pump mutants reveal Na+/K+ pump mechanisms. Nat. Commun. 2022, 13, 5270. [Google Scholar] [CrossRef]
- Xu, H.; Ghishan, F.K.; Kiela, P.R. SLC9 Gene Family: Function, Expression, and Regulation. Compr. Physiol. 2018, 26, 555–583. [Google Scholar]
- Alper, S.L.; Sharma, A.K. The SLC26 gene family of anion transporters and channels. Mol. Asp. Med. 2013, 34, 494–515. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.F.; Vulesevic, B.; Grosell, M.; Bayaa, M. Evidence that SLC26 anion transporters mediate branchial chloride uptake in adult zebrafish (Danio rerio). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, 988–997. [Google Scholar] [CrossRef]
- Finstad, B.; Staurnes, M.; Reite, O.B. Effect of low temperature on sea-water tolerance in rainbow trout, Salmo gairdneri. Aquaculture 1988, 72, 319–328. [Google Scholar] [CrossRef]
- Thomsen, D.S.; Koed, A.; Nielsen, C.; Madsen, S.S. Overwintering of sea trout (Salmo trutta) in freshwater: Escaping salt and low temperature or an alternate life strategy? Can. J. Fish. Aquat. Sci. 2007, 64, 793–802. [Google Scholar] [CrossRef]
- Handeland, S.; Imsland, A.K.; Nilsen, T.; Ebbesson, L.; Hosfeld, C.; Pedrosa, C.; Toften, H.; Stefansson, S. Osmoregulation in Atlantic salmon Salmo salar smolts transferred to seawater at different temperatures. J. Fish Biol. 2014, 85, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch, D.; Wilkie, M.P.; Walsh, P.J. Ammonia and urea transporters in gills of fish and aquatic crustaceans. J. Exp. Biol. 2009, 212, 1716–1730. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kurita, J.; Ozaki, A.; Sano, M.; Fukuda, H.; Ototake, M. Growth of cyprinid herpesvirus 2 (CyHV-2) in cell culture and experimental infection of goldfish Carassius auratus. Dis. Aquat. Org. 2013, 105, 193–202. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward and Reverse Primer (5′-3′) | Amplicon Length [bp] |
|---|---|---|
| ca2 (carbonic anhydrase II) | CAAGGAGTCAATCAGCGTCA TCCGTGTTTGGATCTCTTCC | 226 |
| hif1a (hypoxia-inducible factor 1-α) | CAGGTCCAGACTCCTTCAGC CAACATCTCCAGGTCCAGGT | 200 |
| ribS32 (28S ribosomal protein S32) | TCCTCGATGGTGGGGGCCTG AGACCCCCTGGCCAATCCGG | |
| rhCG (rhesus blood group C glycoprotein) | GAGCTTTTCCTGAGCATTGG GCCACAGAATGAGGATTGGT | 160 |
| slc26a6 (solute carrier family 26 member 6) | GTGACGTCATTTCGGGAGTT GCCATGCTCAGAACAACAGA | 233 |
| slc9a1 (solute carrier family 9 member A1) | TTCCTGGACCATCTCCTCAC AGCTCCATCTTGCGGTAGAA | 170 |
| Morphometric Parameters | 9.4 ± 0.1 °C | 1.8 ± 1.0 °C |
|---|---|---|
| Parameters with significant differnces | ||
| Width of secondary lamellae [µm] | 14.34 ± 2.15 a | 18.05 ± 2.04 b |
| Distance between secondary lamellae [µm] | 27.44 ± 2.46 a | 31.18 ± 2.79 b |
| Mean epithelial thickness [µm] | 2.99 ± 0.65 a | 3.49 ± 0.82 b |
| Parameters without differences | ||
| Length of primary lamellae [µm] | 5227.33 ± 505.50 a | 5269.67 ± 561.97 a |
| Distance between primary lamellae [µm] | 339.45 ± 25.73 a | 317.81 ± 28.43 a |
| Length of secondary lamellae [µm] | 270.45 ± 12.73 a | 282.44 ± 15.51 a |
| No. of mucus cells/mm | 8.97 ± 1.98 a | 9.67 ± 2.31 a |
| No. of chloride cells/mm | 10.50 ± 1.46 a | 12.12 ± 2.14 a |
| No. of capillaries/mm | 58.17 ± 3.13 a | 61.61 ± 4.00 a |
| No. of necrotic cells/mm | 4.32 ± 3.81 a | 5.11 ± 4.75 a |
| Enzyme Activities (µmol/min/g Protein) | 9.4 ± 0.1 °C | 1.8 ± 1.0 °C |
|---|---|---|
| Enzymes with significant differences (p ≤ 0.05) | ||
| Lactate dehydrogenase | 153.15 ± 42.09 a | 108.65 ± 40.70 b |
| Pyruvate kinase | 5.61 ± 2.39 a | 17.27 ± 3.69 b |
| Malate dehydrogenase | 7.13 ± 3.73 a | 15.15 ± 7.26 b |
| Enzymes without differences (p > 0.05) | ||
| Carbonic anhydrase | 4.75 ± 1.84 a | 4.19 ± 3.89 a |
| Caspase 3 | 0.95 ± 0.57 a | 1.11 ± 0.79 a |
| Na+/K+ ATPase | 1.47 ± 0.48 a | 4.20 ± 2.15 a |
| H+ ATPase | 1.05 ± 0.51 a | 1.11 ± 0.79 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahnsteiner, E.; Zamannejad, N.; Dünser, A.; Lahnsteiner, F. Morphometric and Enzymatic Changes in Gills of Rainbow Trout After Exposure to Suboptimal Low Temperature. Curr. Issues Mol. Biol. 2025, 47, 457. https://doi.org/10.3390/cimb47060457
Lahnsteiner E, Zamannejad N, Dünser A, Lahnsteiner F. Morphometric and Enzymatic Changes in Gills of Rainbow Trout After Exposure to Suboptimal Low Temperature. Current Issues in Molecular Biology. 2025; 47(6):457. https://doi.org/10.3390/cimb47060457
Chicago/Turabian StyleLahnsteiner, Elias, Nooshin Zamannejad, Anna Dünser, and Franz Lahnsteiner. 2025. "Morphometric and Enzymatic Changes in Gills of Rainbow Trout After Exposure to Suboptimal Low Temperature" Current Issues in Molecular Biology 47, no. 6: 457. https://doi.org/10.3390/cimb47060457
APA StyleLahnsteiner, E., Zamannejad, N., Dünser, A., & Lahnsteiner, F. (2025). Morphometric and Enzymatic Changes in Gills of Rainbow Trout After Exposure to Suboptimal Low Temperature. Current Issues in Molecular Biology, 47(6), 457. https://doi.org/10.3390/cimb47060457







