Miyako Bidens pilosa Extract Ameliorates Allodynia and Suppresses Spinal Microglial Activation in Mice with Partial Sciatic Nerve Ligation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
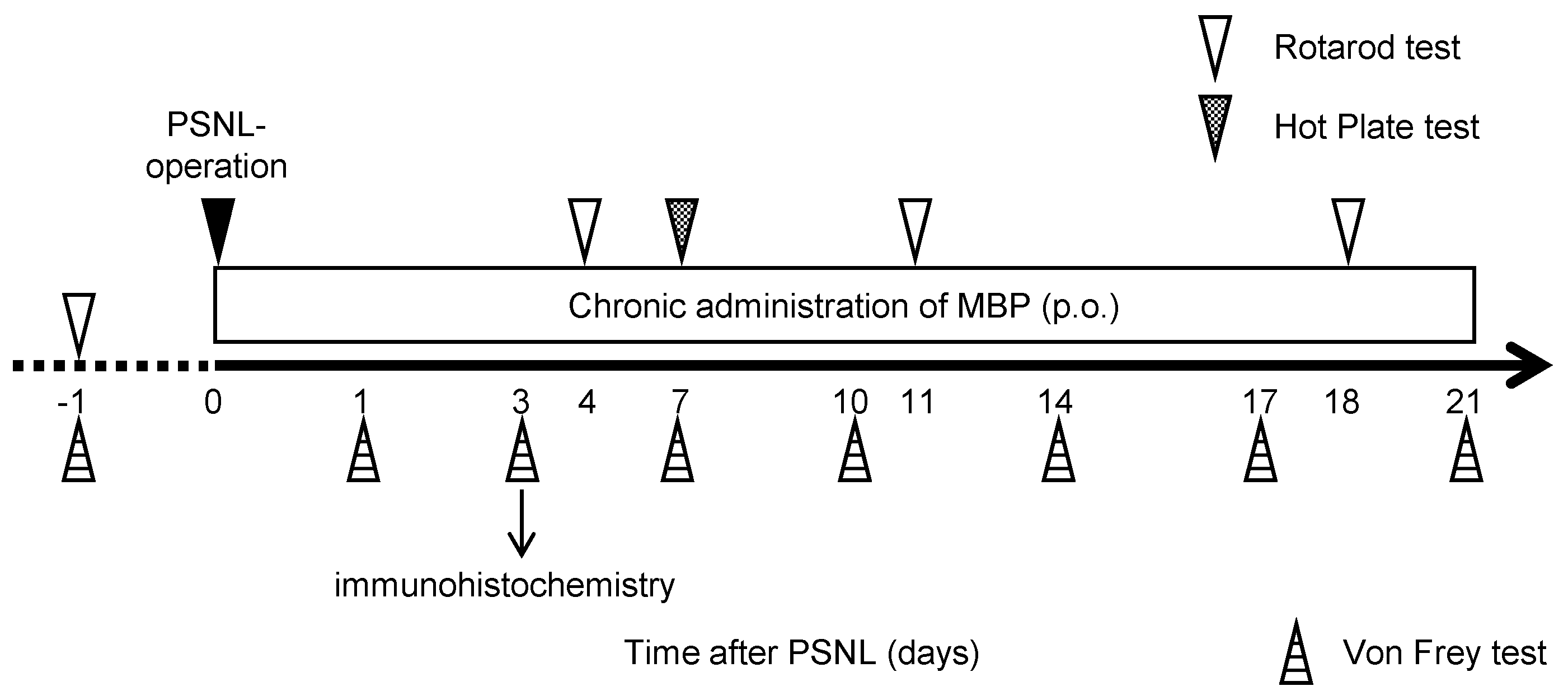
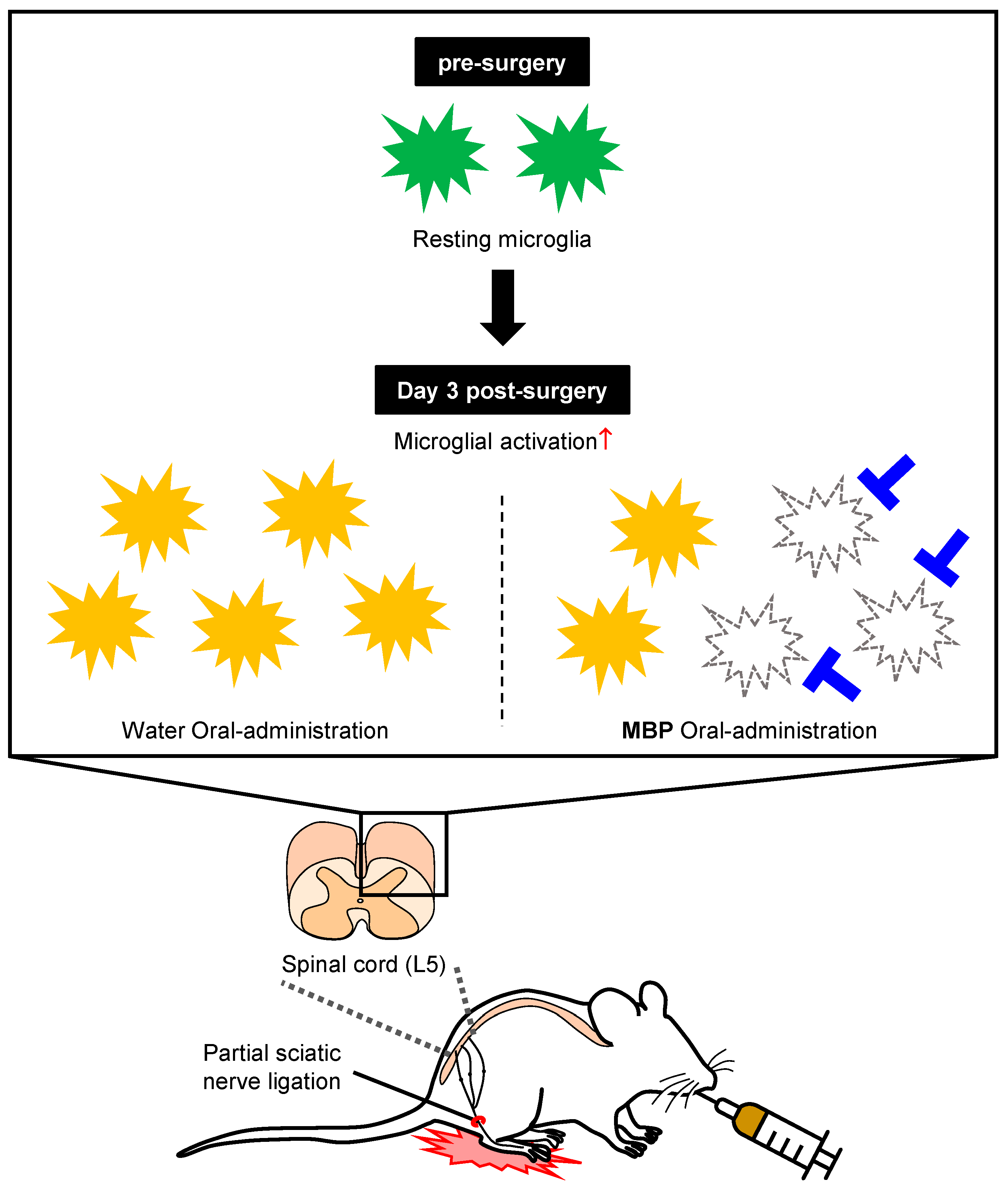
2.2. Neuropathic Pain Model and MBP Treatment
2.3. Mouse Motor Performance
2.4. Assessment of Mechanical Withdrawal Thresholds
2.5. Assessment of Thermal Hyperalgesia
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
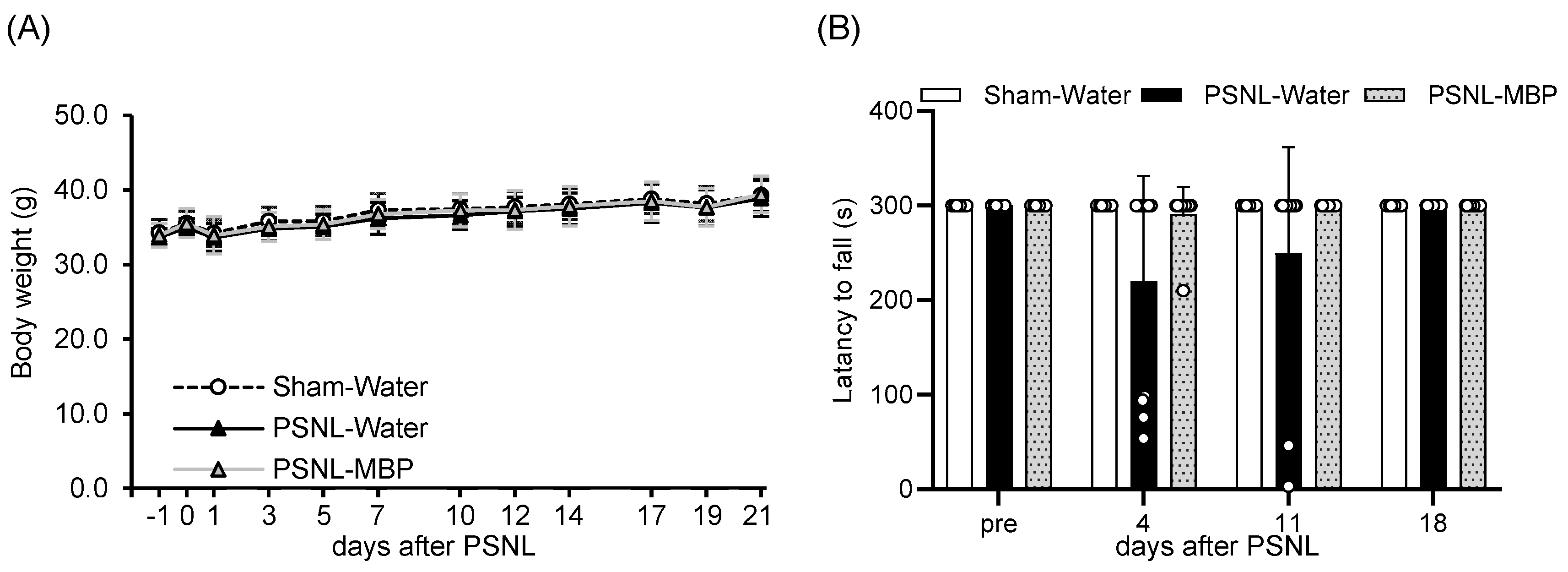
3.1. MBP Had No Effect on Body Weight Gain and Motor Performance in PSNL Mice
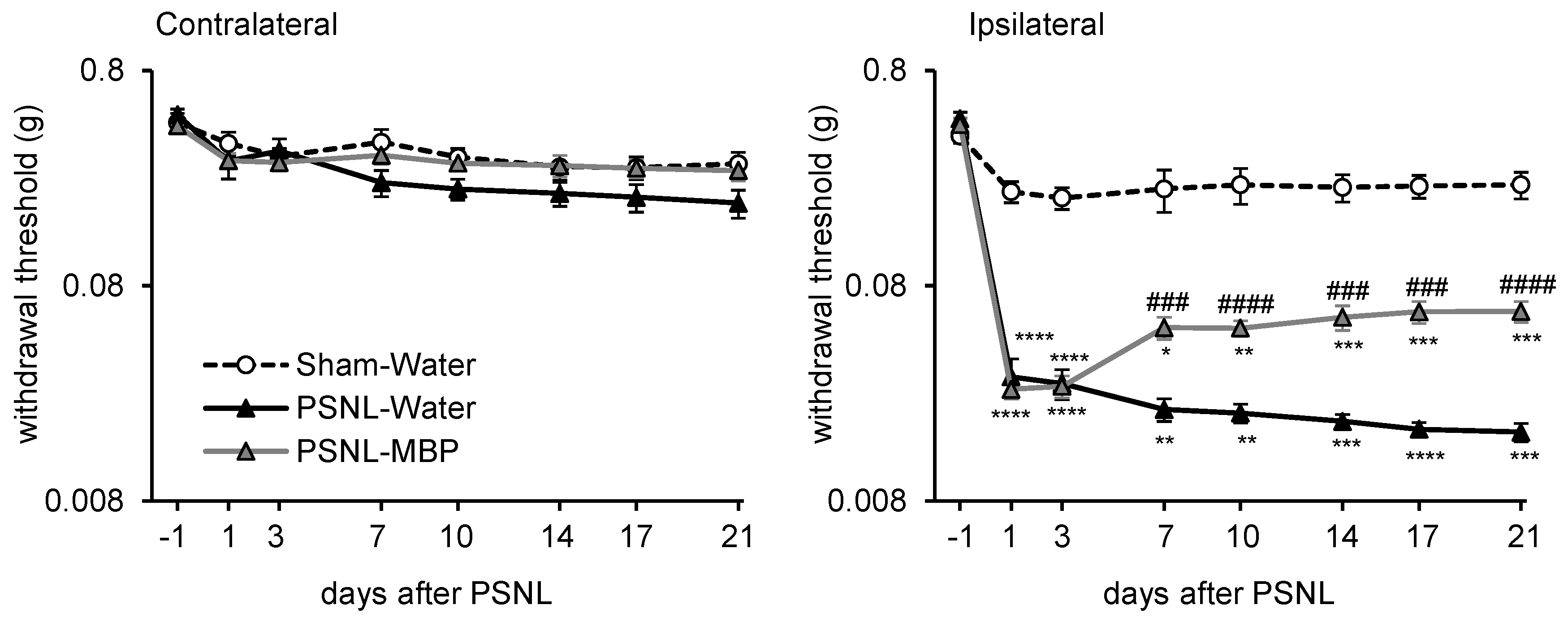
3.2. MBP Ameliorated the PSNL-Induced Mechanical Allodynia in Mice
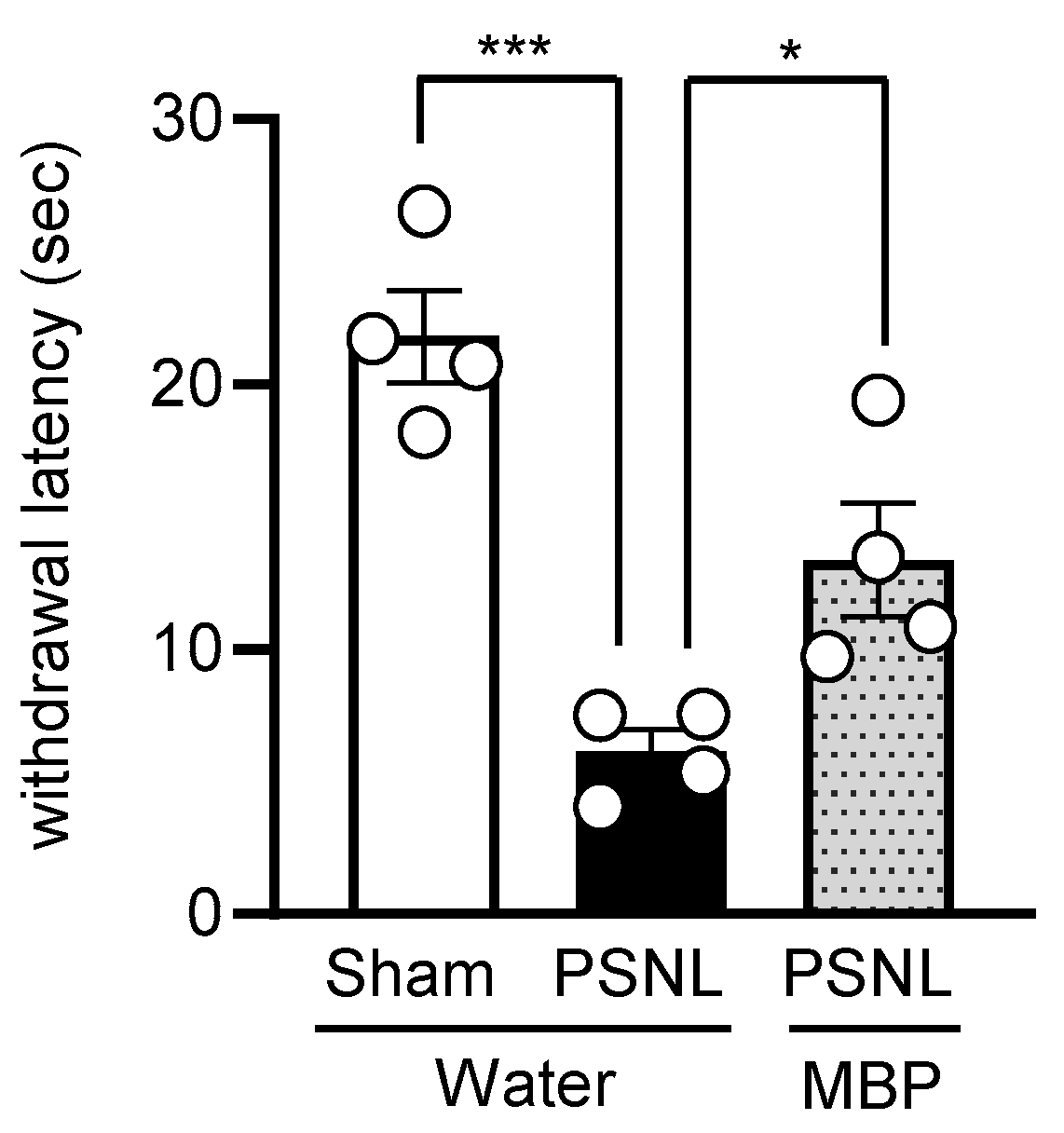
3.3. MBP Ameliorated the PSNL-Induced Thermal Hyperalgesia in Mice
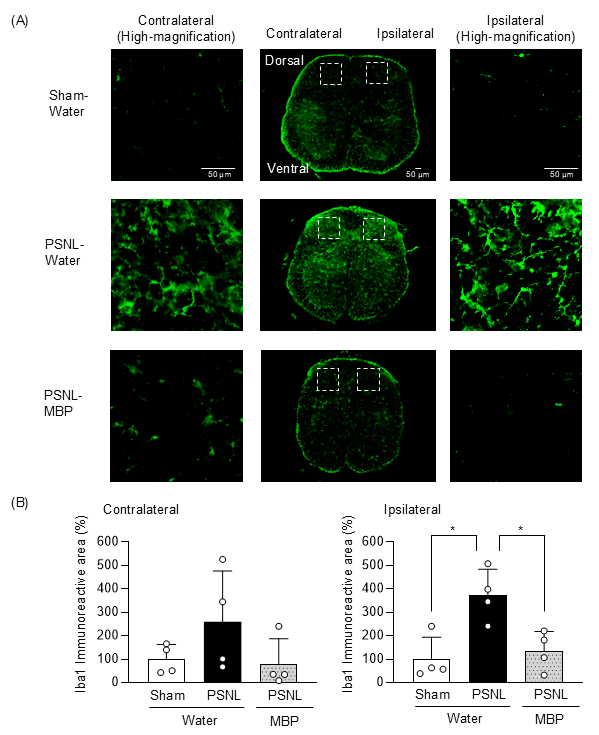
3.4. MBP Reduced Microglia Expression in the Dorsal Horn of the Spinal Cord at the Ipsilateral Site of Nerve Injury in PSNL Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic Pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Finnerup, N.B. Allodynia and Hyperalgesia in Neuropathic Pain: Clinical Manifestations and Mechanisms. Lancet Neurol. 2014, 13, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Moehring, F.; Halder, P.; Seal, R.P.; Stucky, C.L. Uncovering the Cells and Circuits of Touch in Normal and Pathological Settings. Neuron 2018, 100, 349–360. [Google Scholar] [CrossRef]
- Liu, Y.; Latremoliere, A.; Li, X.; Zhang, Z.; Chen, M.; Wang, X.; Fang, C.; Zhu, J.; Alexandre, C.; Gao, Z.; et al. Touch and Tactile Neuropathic Pain Sensitivity Are Set by Corticospinal Projections. Nature 2018, 561, 547–550. [Google Scholar] [CrossRef]
- Shanti, B.F.; Shanti, I.F.; Al-Abbadi, K.; Al Rjoub, A.; Otom, A. The Ambivalent Role of Glial Cells in Neuroinflammation and Neuropathic Pain. Acta Sci. Neurol. 2020, 3, 51–58. [Google Scholar] [CrossRef]
- Raghavendra, V.; Tanga, F.; Deleo, J.A. Inhibition of Microglial Activation Attenuates the Development but Not Existing Hypersensitivity in a Rat Model of Neuropathy. J. Pharmacol. Exp. Ther. 2003, 306, 624–630. [Google Scholar] [CrossRef]
- Yang, L.; Gomm, A.; Bai, P.; Ding, W.; Tanzi, R.E.; Wang, C.; Shen, S.; Zhang, C. The Effect of Pexidartinib on Neuropathic Pain via Influences on Microglia and Neuroinflammation in Mice. Anesth. Analg. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Lee, W.C.; Peng, C.C.; Chang, C.H.; Huang, S.H.; Chyau, C.C. Extraction of Antioxidant Components from Bidens pilosa Flowers and Their Uptake by Human Intestinal Caco-2 Cells. Molecules 2013, 18, 1582–1601. [Google Scholar] [CrossRef]
- Horiuchi, M.; Seyama, Y. Antiinflammatory and Antiallergic Activity of Bidens pilosa L. Var. Radiata SCHERFF. J. Health Sci. 2006, 52, 711–717. [Google Scholar] [CrossRef][Green Version]
- Horiuchi, M.; Wachi, H.; Seyama, Y. Effects of Bidens pilosa L. Var. Radiata Scherff on Experimental Gastric Lesion. J. Nat. Med. 2010, 64, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Nakama, S.; Ishikawa, C.; Nakachi, S.; Mori, N. Anti-Adult T-Cell Leukemia Effects of Bidens pilosa. Int. J. Oncol. 2011, 38, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Nakama, S.; Tamaki, K.; Ishikawa, C.; Tadano, M.; Mori, N. Efficacy of Bidens pilosa Extract against Herpes Simplex Virus Infection In Vitro and In Vivo. Evid.-Based Complement. Altern. Med. 2012, 2012, 413453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kosuge, Y.; Kaneko, E.; Nango, H.; Miyagishi, H.; Ishige, K.; Ito, Y. Bidens pilosa Extract Administered after Symptom Onset Attenuates Glial Activation, Improves Motor Performance, and Prolongs Survival in a Mouse Model of Amyotrophic Lateral Sclerosis. Oxidative Med. Cell. Longev. 2020, 2020, 1020673. [Google Scholar] [CrossRef]
- Tsuruta, K.; Shidara, T.; Miyagishi, H.; Nango, H.; Nakatani, Y.; Suzuki, N.; Amano, T.; Suzuki, T.; Kosuge, Y. Anti-Inflammatory Effects of Miyako Bidens pilosa in a Mouse Model of Amyotrophic Lateral Sclerosis and Lipopolysaccharide-Stimulated BV-2 Microglia. Int. J. Mol. Sci. 2023, 24, 13698. [Google Scholar] [CrossRef]
- Nango, H.; Takahashi, A.; Suzuki, N.; Kurano, T.; Sakamoto, S.; Nagatomo, T.; Suzuki, T.; Kanazawa, T.; Kosuge, Y.; Miyagishi, H. Therapeutic Efficacy of Intranasal N-Acetyl-L-Cysteine with Cell-Penetrating Peptide-Modified Polymer Micelles on Neuropathic Pain in Partial Sciatic Nerve Ligation Mice. Pharmaceutics 2025, 17, 44. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Nakai, K.; Ozaki, M.; Hayashi, M.; Tanaka, H.; Yahagi, T.; Kosuge, Y.; Kawato, T. Inhibitory Effect of Bidens pilosa Extract on RANKL-Induced Osteoclast Differentiation of RAW264.7 Cells. J. Hard Tissue Biol. 2024, 33, 147–154. [Google Scholar] [CrossRef]
- Narita, M.; Yoshida, T.; Nakajima, M.; Narita, M.; Miyatake, M.; Takagi, T.; Yajima, Y.; Suzuki, T. Direct Evidence for Spinal Cord Microglia in the Development of a Neuropathic Pain-like State in Mice. J. Neurochem. 2006, 97, 1337–1348. [Google Scholar] [CrossRef]
- De La Puente, B.; Zamanillo, D.; Romero, L.; Carceller, A.; Vela, J.M.; Merlos, M.; Portillo-Salido, E. Comprehensive Preclinical Assessment of Sensory, Functional, Motivational-Affective, and Neurochemical Outcomes in Neuropathic Pain: The Case of the Sigma-1 Receptor. ACS Pharmacol. Transl. Sci. 2022, 5, 240–254. [Google Scholar] [CrossRef]
- Lai, B.Y.; Chen, T.Y.; Huang, S.H.; Kuo, T.F.; Chang, T.H.; Chiang, C.K.; Yang, M.T.; Chang, C.L.T. Bidens pilosa Formulation Improves Blood Homeostasis and β-Cell Function in Men: A Pilot Study. Evid.-Based Complement. Altern. Med. 2015, 2015, 832314. [Google Scholar] [CrossRef]
- Frida, L.; Rakotonirina, S.; Rakotonirina, A.; Savineau, J.-P. In Vivo and In Vitro Effects of Bidens pilosa L. (Asteraceae) Leaf Aqueous and Ethanol Extracts on Primed-Oestrogenized Rat Uterine Muscle. Afr. J. Tradit. Complement. Altern. Med. 2007, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Ezeonwumelu, J.O.C.; Julius, A.K.; Muhoho, C.N.; Ajayi, A.M.; Oyewale, A.A.; Tanayen, J.K.; Balogun, S.O.; Ibrahim, A.; Adzu, B.; Adiukwu, C.P.; et al. Biochemical and Histological Studies of Aqueous Extract of Bidens pilosa Leaves from Ugandan Rift Valley in Rats. Br. J. Pharmacol. Toxicol. 2011, 2, 302–309. [Google Scholar]
- Liang, Y.C.; Yang, M.T.; Lin, C.J.; Chang, C.L.T.; Yang, W.C. Bidens pilosa and Its Active Compound Inhibit Adipogenesis and Lipid Accumulation via Down-Modulation of the C/EBP and PPARγ Pathways. Sci. Rep. 2016, 6, 24285. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Lin, C.J.; Yang, C.Y.; Chen, Y.H.; Yang, M.T.; Chou, F.S.; Yang, W.C.; Chang, C.L.T. Toxicity Study of Bidens pilosa in Animals. J. Tradit. Complement. Med. 2019, 10, 150. [Google Scholar] [CrossRef]
- Shan, L.; Xu, K.; Ji, L.; Zeng, Q.; Liu, Y.; Wu, Y.; Chen, Y.; Li, Y.; Hu, Q.; Wu, J.; et al. Injured Sensory Neurons-Derived Galectin-3 Contributes to Neuropathic Pain via Programming Microglia in the Spinal Dorsal Horn. Brain Behav. Immun. 2024, 117, 80–99. [Google Scholar] [CrossRef]
- Chen, Y.N.; Liu, H.F.; Sun, H.; Xiao, C.J.; Jiang, B.; Shen, L. Antinociceptive Effect of Ethanolic Extract of Bauhinia brachycarpa Benth on Neuropathic Pain Model Induced by Partial Sciatic Nerve Ligation. J. Ethnopharmacol. 2022, 295, 115412. [Google Scholar] [CrossRef]
- Lee, S.H.; Shi, X.Q.; Fan, A.; West, B.; Zhang, J. Targeting Macrophage and Microglia Activation with Colony Stimulating Factor 1 Receptor Inhibitor Is an Effective Strategy to Treat Injury-Triggered Neuropathic Pain. Mol. Pain 2018, 14, 1744806918764979. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kiguchi, N.; Maeda, T.; Ozaki, M.; Kishioka, S. The Critical Role of Spinal Ceramide in the Development of Partial Sciatic Nerve Ligation-Induced Neuropathic Pain in Mice. Biochem. Biophys. Res. Commun. 2012, 421, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Tansley, S.; Gu, N.; Guzmán, A.U.; Cai, W.; Wong, C.; Lister, K.C.; Muñoz-Pino, E.; Yousefpour, N.; Roome, R.B.; Heal, J.; et al. Microglia-Mediated Degradation of Perineuronal Nets Promotes Pain. Science 2022, 377, 80–86. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia States and Nomenclature: A Field at Its Crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef]
- Kusano, A.; Seyama, Y.; Usami, E.; Katayose, T.; Shibano, M.; Tsukamoto, D.; Kusano, G. Studies on the Antioxidant Actiye Constituents of the Dried Powder from Bidens pilosa L. var. radiata SCH. Nat. Med. 2003, 57, 100–104. [Google Scholar]
- Matsumoto, T.; Horiuchi, M.; Kamata, K.; Seyama, Y. Effects of Bidens pilosa L. Var. Radiata SCHERFF Treated with Enzyme on Histamine-Induced Contraction of Guinea Pig Ileum and on Histamine Release from Mast Cells. J. Smooth Muscle Res. 2009, 45, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Cinkilic, N.; Ozboluk, H.Y.; Ozyigit, M.O.; Gurun, M.S. Antihyperalgesic Activity of Chlorogenic Acid in Experimental Neuropathic Pain. J. Nat. Med. 2013, 67, 698–704. [Google Scholar] [CrossRef]
- El Gabbas, Z.; Bezza, K.; Laadraoui, J.; Ait Laaradia, M.; Kebbou, A.; Oufquir, S.; Boukhira, A.; Aboufatima, R.; Chait, A. Salvia Officinalis, Rosmarinic and Caffeic Acids Attenuate Neuropathic Pain and Improve Function Recovery after Sciatic Nerve Chronic Constriction in Mice. Evid.-Based Complement. Altern. Med. 2019, 2019, 1702378. [Google Scholar] [CrossRef]
- Shen, W.; Qi, R.; Zhang, J.; Wang, Z.; Wang, H.; Hu, C.; Zhao, Y.; Bie, M.; Wang, Y.; Fu, Y.; et al. Chlorogenic Acid Inhibits LPS-Induced Microglial Activation and Improves Survival of Dopaminergic Neurons. Brain Res. Bull. 2012, 88, 487–494. [Google Scholar] [CrossRef]
- Bettoni, I.; Comelli, F.; Rossini, C.; Granucci, F.; Giagnoni, G.; Peri, F.; Costa, B. Glial TLR4 Receptor as New Target to Treat Neuropathic Pain: Efficacy of a New Receptor Antagonist in a Model of Peripheral Nerve Injury in Mice. Glia 2008, 56, 1312–1319. [Google Scholar] [CrossRef]
- Kuang, X.; Huang, Y.; Gu, H.F.; Zu, X.Y.; Zou, W.Y.; Song, Z.B.; Guo, Q.L. Effects of Intrathecal Epigallocatechin Gallate, an Inhibitor of Toll-like Receptor 4, on Chronic Neuropathic Pain in Rats. Eur. J. Pharmacol. 2012, 676, 51–56. [Google Scholar] [CrossRef]
- Lewis, S.S.; Loram, L.C.; Hutchinson, M.R.; Li, C.M.; Zhang, Y.; Maier, S.F.; Huang, Y.; Rice, K.C.; Watkins, L.R. (+)-Naloxone, an Opioid-Inactive Toll-like Receptor 4 Signaling Inhibitor, Reverses Multiple Models of Chronic Neuropathic Pain in Rats. J. Pain 2012, 13, 498–506. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, A.; Miyagishi, H.; Tsuruta, K.; Nango, H.; Hirose, D.; Aono, Y.; Tanigawa, M.; Nishimura, K.; Saito, M.; Kawato, T.; et al. Miyako Bidens pilosa Extract Ameliorates Allodynia and Suppresses Spinal Microglial Activation in Mice with Partial Sciatic Nerve Ligation. Curr. Issues Mol. Biol. 2025, 47, 453. https://doi.org/10.3390/cimb47060453
Takahashi A, Miyagishi H, Tsuruta K, Nango H, Hirose D, Aono Y, Tanigawa M, Nishimura K, Saito M, Kawato T, et al. Miyako Bidens pilosa Extract Ameliorates Allodynia and Suppresses Spinal Microglial Activation in Mice with Partial Sciatic Nerve Ligation. Current Issues in Molecular Biology. 2025; 47(6):453. https://doi.org/10.3390/cimb47060453
Chicago/Turabian StyleTakahashi, Ai, Hiroko Miyagishi, Komugi Tsuruta, Hiroshi Nango, Dai Hirose, Yuri Aono, Minoru Tanigawa, Katsushi Nishimura, Minoru Saito, Takayuki Kawato, and et al. 2025. "Miyako Bidens pilosa Extract Ameliorates Allodynia and Suppresses Spinal Microglial Activation in Mice with Partial Sciatic Nerve Ligation" Current Issues in Molecular Biology 47, no. 6: 453. https://doi.org/10.3390/cimb47060453
APA StyleTakahashi, A., Miyagishi, H., Tsuruta, K., Nango, H., Hirose, D., Aono, Y., Tanigawa, M., Nishimura, K., Saito, M., Kawato, T., Saigusa, T., & Kosuge, Y. (2025). Miyako Bidens pilosa Extract Ameliorates Allodynia and Suppresses Spinal Microglial Activation in Mice with Partial Sciatic Nerve Ligation. Current Issues in Molecular Biology, 47(6), 453. https://doi.org/10.3390/cimb47060453





