Molecular Regulation of Antioxidant Defense and Metabolic Reprogramming in Xiaozhan Rice Genotypes: Differential Roles of Salicylic Acid and Melatonin Under Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Materials
2.2. Experimental Design
2.3. Measurement of Rice Emergence Growth Parameters
2.4. Measurement of Rice Seedling Growth Parameters in Simulated Real Stresses
2.5. Measurement of Physiological and Biochemical Indicators
2.6. Statistical Analysis
3. Results
3.1. Analysis of Growth Differences and Mechanism in Warble Xiaozhan Rice Under Salt Stress
3.2. Effects of Different Exogenous Substances on Seed Germination Characteristics of Rice Under Salt Stress
3.3. Effects of Different Exogenous Substances on Seeding Growth of Rice Under Salt Stress
3.4. Effects of Different Exogenous Substances on Plant Morphogenesis of Rice Under Salt Stress
3.5. Regulatory Effects of Different Exogenous Substances on Physiological Metabolism in Salt-Stressed Rice Seedlings
3.5.1. Antioxidant Enzyme System Response
3.5.2. GSH and Soluble Sugar Content Dynamics
3.5.3. Lignin Biosynthesis and MDA Accumulation
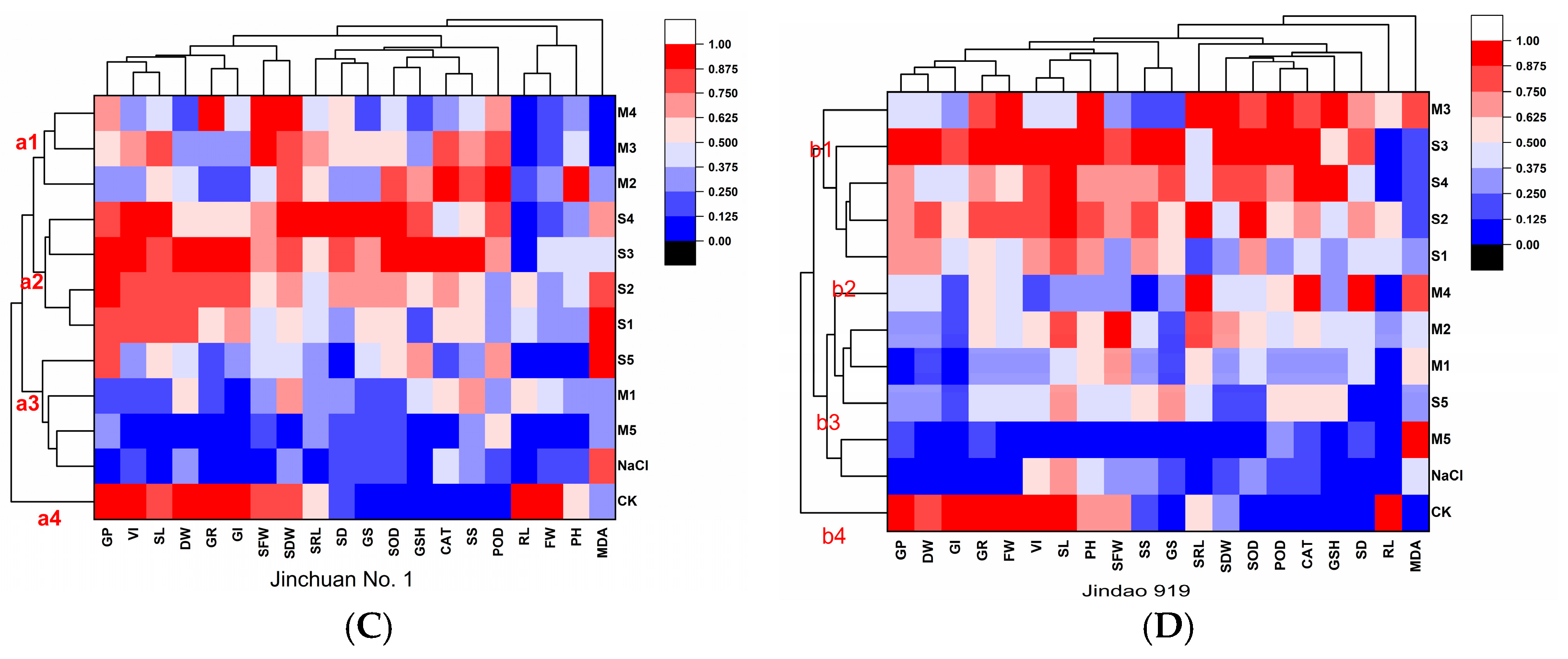
3.6. Correlation Data and Principal Component Analysis
3.7. Comprehensive Evaluation Methodology Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SA | Salicylic acid |
| MT | Melatonin |
| GP | Germination potential |
| GR | Germination rate |
| GI | Germination Index |
| VI | Vigour index |
| SRL | Seedling Root Length |
| SL | Seedling Length |
| SFW | Seedling Fresh Weight |
| SDW | Seedling Dry Weight |
| RL | Root length |
| PH | Plant height |
| FW | Fresh weight |
| DW | Dry weight |
| SD | Stem Diameter |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| GSH | Reduced glutathione |
| GS | Lignin |
| SS | Soluble sugar |
| MDA | Malondialdehyde |
| ROS | Reactive oxygen species |
| PCA | Principal Component Analysis |
| P5CS1 | Delta-1-pyrroline-5-carboxylate synthetase 1 |
| TGA1 | TGACG sequence-specific binding proteins 1 |
| ICS1 | ISOCHORISMATE SYNTHASE1 |
| HKT1 | High-affinity K transporter 1 |
References
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef]
- FAO. Global Status of Salt-Affected Soils—Main Report; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Shao, H.; Chu, L.; Lu, H.; Qi, W.; Chen, X.; Liu, J.; Kuang, S.; Tang, B.; Won, V. Towards sustainable agriculture for the salt-affected soil. Land Degrad. Dev. 2019, 30, 574–579. [Google Scholar] [CrossRef]
- Mohanavelu, A.; Naganna, S.R.; Al-Ansari, N. Irrigation Induced Salinity and Sodicity Hazards on Soil and Groundwater: An Overview of Its Causes, Impacts and Mitigation Strategies. Agriculture 2021, 11, 983. [Google Scholar] [CrossRef]
- Li, J.G.; Pu, L.J.; Han, M.F.; Zhu, M.; Zhang, R.S.; Xiang, Y.Z. Soil salinization research in China: Advances and prospects. J. Geogr. Sci. 2014, 24, 943–960. [Google Scholar] [CrossRef]
- Guo, Z.D.; Zhang, X.J.; Liu, Y.R. Researches on the coal fly ash applied to saline soil improvement in rural roads. In Proceedings of the 2nd International Conference on Civil, Architectural and Hydraulic Engineering (ICCAHE 2013), Zhuhai, China, 27–28 July 2013; p. 2616. [Google Scholar]
- Chen, S.; Guo, J.; Zhao, Y.; Li, X.; Liu, F.; Chen, Y. Evaluation and grading of climatic conditions on nutritional quality of rice: A case study of Xiaozhan rice in Tianjin. Meteorol. Appl. 2021, 28, e2021. [Google Scholar] [CrossRef]
- Zhao, H.X.; Gu, B.J.; Chen, D.C.; Tang, J.J.; Xu, X.L.; Qiao, Z.; Wang, J.Q. Physicochemical properties and salinization characteristics of soils in coastal land reclamation areas: A case study of China-Singapore Tianjin Eco-City. Heliyon 2022, 8, e12629. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Nasircilar, A.G.; Erkaymaz, T.; Ulukapi, K. Reflection of the synergistic/antagonistic effects of melatonin and salicylic acid on the biochemical profile of Allium cepa L. under drought stress. South Afr. J. Bot. 2024, 166, 1–13. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Moosa, A.; Ferrante, A.; Nafees, M.; Darras, A.; Nazir, M.M.; AlShaqhaa, M.A.; Elsaid, F.G. Melatonin and salicylic acid synergistically improve arsenic induced oxidative stress tolerance in ornamental sword lily. Sci. Hortic. 2023, 322, 112389. [Google Scholar] [CrossRef]
- Karimi, M.R.; Sabokdast, M.; Korang Beheshti, H.; Abbasi, A.R.; Bihamta, M.R. Seed priming with salicylic acid enhances salt stress tolerance by boosting antioxidant defense in Phaseolus vulgaris genotypes. BMC Plant Biol. 2025, 25, 489. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Banik, S.; Dutta, D. Membrane Proteins in Plant Salinity Stress Perception, Sensing, and Response. J. Membr. Biol. 2023, 256, 109–124. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, Y.; Wang, Y.; Zeng, Y.; Shang, X.; Meng, L.; Zhang, Y.; Fang, T.; Xiao, P.; Qu, J.; et al. The transcription factor TGA2 orchestrates salicylic acid signal to regulate cold-induced proline accumulation in Citrus. Plant Cell 2024, 37, koae290. [Google Scholar] [CrossRef]
- Andronachi, V.-C.; Simeanu, C.; Matei, M.; Radu-Rusu, R.-M.; Simeanu, D. Melatonin: An Overview on the Synthesis Processes and on Its Multiple Bioactive Roles Played in Animals and Humans. Agriculture 2025, 15, 273. [Google Scholar] [CrossRef]
- Spolaor, B.O.; Bertoli, S.C.; Sukert, D.S.; Sala, H.R.; Picoli de Oliveira, B.F.; de Freitas, I.R.; Lima-Moro, A. Exogenous melatonin induces tolerance to drought stress damage in seedlings and soybean plants. Chil. J. Agric. Res. 2022, 82, 515–526. [Google Scholar] [CrossRef]
- Ahmad, J.; Hayat, F.; Khan, U.; Ahmed, N.; Li, J.; Ercisli, S.; Iqbal, S.; Javed, H.U.; Alyas, T.; Tu, P.; et al. Melatonin: A promising approach to enhance abiotic stress tolerance in horticultural plants. South Afr. J. Bot. 2024, 164, 66–76. [Google Scholar] [CrossRef]
- Kumar, S.; Liu, Y.; Wang, M.; Khan, M.N.; Wang, S.; Li, Y.; Chen, Y.; Zhu, G. Alleviating sweetpotato salt tolerance through exogenous glutathione and melatonin: A profound mechanism for active oxygen detoxification and preservation of photosynthetic organs. Chemosphere 2024, 350, 141120. [Google Scholar] [CrossRef]
- Powers, J.; Zhang, X.; Reyes, A.; Zavaliev, R.; Ochakovski, R.; Xu, S.L.; Dong, X.N. Next-generation mapping of the salicylic acid signaling hub and transcriptional cascade. Mol. Plant 2024, 17, 1558–1572. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Zahan, I.; Hossain, M.S.; Imran, S.; Hasanuzzaman, M.; Dawood, M.F.A.; Dawood, A.F.A.; Asaduzzaman, M.; Rhaman, M.S.; Souri, Z.; et al. Melatonin-mediated ionic homeostasis in plants: Mitigating nutrient deficiency and salinity stress. Discov. Plants 2025, 2, 143. [Google Scholar] [CrossRef]
- Wang, J.; Yan, D.; Liu, R.; Wang, T.; Lian, Y.; Lu, Z.; Hong, Y.; Wang, Y.; Li, R. The Physiological and Molecular Mechanisms of Exogenous Melatonin Promote the Seed Germination of Maize (Zea mays L.) under Salt Stress. Plants 2024, 13, 2142. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A Review on Plant Responses to Salt Stress and Their Mechanisms of Salt Resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Zhao, X.; Lin, S.; Yu, S.; Zhang, Y.; Su, L.; Geng, L.; Cheng, C.; Jiang, X. Exogenous calcium enhances the physiological status and photosynthetic capacity of rose under drought stress. Hortic. Plant J. 2024, 10, 853–865. [Google Scholar] [CrossRef]
- GB/T 3543.4-1995; Rules for agricultural seed testing—Germination test. National Standard of the People’s Republic of China; S.A.O.: Beijing, China, 1995.
- Doerge, D.R.; Divi, R.L.; Churchwell, M.I. Identification of the colored guaiacol oxidation product produced by peroxidases. Anal. Biochem. 1997, 250, 10–17. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin. Chim. Acta 2000, 293, 157–166. [Google Scholar] [CrossRef]
- Johansson, L.H.; Borg, L.A. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Spitz, D.R.; Oberley, L.W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989, 179, 8–18. [Google Scholar] [CrossRef]
- Owens, C.W.; Belcher, R.V. A Colorimetric Micro-Method for the Determination of Glutathione. Biochem. J. 1965, 94, 705–711. [Google Scholar] [CrossRef]
- Bodelon, O.G.; Blanch, M.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. The effects of high CO2 levels on anthocyanin composition, antioxidant activity and soluble sugar content of strawberries stored at low non-freezing temperature. Food Chem. 2010, 122, 673–678. [Google Scholar] [CrossRef]
- Janshekar, H.; Brown, C.; Fiechter, A. Determination of biodegraded lignin by ultraviolet spectrophotometry. Anal. Chim. Acta 1981, 130, 81–91. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current Status and Future Perspectives in Plant Science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef]
- Shaki, F.; Maboud, H.E.; Niknam, V. Effects of salicylic acid on hormonal cross talk, fatty acids profile, and ions homeostasis from salt-stressed safflower. J. Plant Interact. 2019, 14, 340–346. [Google Scholar] [CrossRef]
- Imran, M.; Widemann, E.; Shafiq, S.; Bakhsh, A.; Chen, X.; Tang, X. Salicylic Acid and Melatonin Synergy Enhances Boron Toxicity Tolerance via AsA-GSH Cycle and Glyoxalase System Regulation in Fragrant Rice. Metabolites 2024, 14, 520. [Google Scholar] [CrossRef]
- Sidek, N.; Nulit, R.; Yap, C.K.; Yong, C.S.Y.; Sekeli, R. In vitro development of salt tolerant Malaysian indica rice ‘MARDI Siraj 297’ and enhancement of salinity tolerance using salicylic acid. Chil. J. Agric. Res. 2024, 84, 3–14. [Google Scholar] [CrossRef]
- Saharan, B.S.; Brar, B.; Duhan, J.S.; Kumar, R.; Marwaha, S.; Rajput, V.D.; Minkina, T. Molecular and Physiological Mechanisms to Mitigate Abiotic Stress Conditions in Plants. Life-Basel 2022, 12, 1634. [Google Scholar] [CrossRef]
- Swain, R.; Sahoo, S.; Behera, M.; Rout, G.R. Instigating prevalent abiotic stress resilience in crop by exogenous application of phytohormones and nutrient. Front. Plant Sci. 2023, 14, 1104874. [Google Scholar] [CrossRef]
- Vatanparast, M.; Merkel, L.; Amari, K. Exogenous Application of dsRNA in Plant Protection: Efficiency, Safety Concerns and Risk Assessment. Int. J. Mol. Sci. 2024, 25, 6530. [Google Scholar] [CrossRef]
- Saitanis, C.J.; Agathokleous, E. Exogenous application of chemicals for protecting plants against ambient ozone pollution: What should come next? Curr. Opin. Environ. Sci. Health 2021, 19, 100215. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Fu, L.; Wang, F.; Wu, D.; Shen, Q.; Zhang, G. GWAS and transcriptomic integrating analysis reveals key salt-responding genes controlling Na+ content in barley roots. Plant Physiol. Biochem. 2021, 167, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Ulhassan, Z.; Huang, Q.; Gill, R.A.; Ali, S.; Mwamba, T.M.; Ali, B.; Hina, F.; Zhou, W. Protective mechanisms of melatonin against selenium toxicity in Brassica napus: Insights into physiological traits, thiol biosynthesis and antioxidant machinery. BMC Plant Biol. 2019, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Colombage, R.; Singh, M.B.; Bhalla, P.L. Melatonin and Abiotic Stress Tolerance in Crop Plants. Int. J. Mol. Sci. 2023, 24, 7447. [Google Scholar] [CrossRef]
- Awan, S.A.; Khan, I.; Wang, Q.; Gao, J.; Tan, X.; Yang, F. Pre-treatment of melatonin enhances the seed germination responses and physiological mechanisms of soybean (Glycine max L.) under abiotic stresses. Front. Plant Sci. 2023, 14, 1149873. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, T.; Li, S.; Li, X.; Wang, W.; Fan, S. Transcriptomic analysis reveals that methyl jasmonate confers salt tolerance in alfalfa by regulating antioxidant activity and ion homeostasis. Front. Plant Sci. 2023, 14, 1258498. [Google Scholar] [CrossRef]
- Razmi, N.; Ebadi, A.; Daneshian, J.; Jahanbakhsh, S. Salicylic acid induced changes on antioxidant capacity, pigments and grain yield of soybean genotypes in water deficit condition. J. Plant Interact. 2017, 12, 457–464. [Google Scholar] [CrossRef]
- Wang, L.; Tanveer, M.; Wang, H.; Arnao, M.B. Melatonin as a key regulator in seed germination under abiotic stress. J. Pineal Res. 2024, 76, e12937. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Debnath, S.; Sikdar, P.; Bhattacharya, K.; Chanu, N.R. Melatonin in Plants: Biosynthesis, Occurrence and Role in plants. In Melatonin: Role in Plant Signaling, Growth and Stress Tolerance: Phytomelatonin in Normal and Challenging Environments; Mukherjee, S., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2023; Volume 4, pp. 29–44. [Google Scholar] [CrossRef]
- Faize, L.; Faize, M. Functional Analogues of Salicylic Acid and Their Use in Crop Protection. Agronomy 2018, 8, 5. [Google Scholar] [CrossRef]
- Zhao, D.; Luan, Y.; Xia, X.; Shi, W.; Tang, Y.; Tao, J. Lignin provides mechanical support to herbaceous peony (Paeonia lactiflora Pall.) stems. Hortic. Res. 2020, 7, 213. [Google Scholar] [CrossRef]
- Yang, M.; Lu, K.; Zhao, F.-J.; Xie, W.; Ramakrishna, P.; Wang, G.; Du, Q.; Liang, L.; Sun, C.; Zhao, H.; et al. Genome-Wide Association Studies Reveal the Genetic Basis of Ionomic Variation in Rice. Plant Cell 2018, 30, 2720–2740. [Google Scholar] [CrossRef] [PubMed]
- Sackey, O.K.; Feng, N.; Mohammed, Y.Z.; Dzou, C.F.; Zheng, D.; Zhao, L.; Shen, X. A comprehensive review on rice responses and tolerance to salt stress. Front. Plant Sci. 2025, 16, 1561280. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Synergistic Effects of Salicylic Acid and Melatonin on Modulating Ion Homeostasis in Salt-Stressed Wheat (Triticum aestivum L.) Plants by Enhancing Root H+-Pump Activity. Plants 2022, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzai, A.S.; Hu, C.; Zhang, C.; Li, Y. Mechanisms of anthocyanin-mediated salt stress alleviation and cellular homeostasis in plants. Plant Growth Regul. 2025, 105, 655–673. [Google Scholar] [CrossRef]
- Ali, M.; Malik, Z.; Abbasi, G.H.; Irfan, M.; Ahmad, S.; Ameen, M.; Ali, A.; Sohaib, M.; Rizwan, M.; Ali, S. Potential of melatonin in enhancing antioxidant defense system and yield of maize (Zea mays L.) hybrids under saline condition. Sci. Hortic. 2024, 325, 112665. [Google Scholar] [CrossRef]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New Insights on Plant Salt Tolerance Mechanisms and Their Potential Use for Breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef]
- Justamante, M.S.; Larriba, E.; Luque, A.; Nicolás-Albujer, M.; Pérez-Pérez, J.M. A systematic review to identify target genes that modulate root system architecture in response to abiotic stress. Sci. Rep. 2025, 15, 13219. [Google Scholar] [CrossRef] [PubMed]
- Rabeh, K.; Hnini, M.; Oubohssaine, M. A comprehensive review of transcription factor-mediated regulation of secondary metabolites in plants under environmental stress. Stress Biol. 2025, 5, 15. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Castro, C.B.; Whittock, L.D.; Whittock, S.P.; Leggett, G.; Koutoulis, A. DNA sequence and expression variation of hop (Humulus lupulus) valerophenone synthase (VPS), a key gene in bitter acid biosynthesis. Ann. Bot. 2008, 102, 265–273. [Google Scholar] [CrossRef]



| Code | Treatment | Code | Treatment |
|---|---|---|---|
| CK | 0 mM NaCl + 0 μM SA/MT | NaCl | 50 mM NaCl |
| S1 | 50 mM NaCl + 100 μM SA | M1 | 50 mM NaCl + 100 μM MT |
| S2 | 50 mM NaCl + 300 μM SA | M2 | 50 mM NaCl + 300 μM MT |
| S3 | 50 mM NaCl + 500 μM SA | M3 | 50 mM NaCl + 500 μM MT |
| S4 | 50 mM NaCl + 700 μM SA | M4 | 50 mM NaCl + 700 μM MT |
| S5 | 50 mM NaCl + 900 μM SA | M5 | 50 mM NaCl + 900 μM MT |
| Cultivar | Treatment | SOD Activity (U g−1 FW) | POD Activity (U g−1 FW) | CAT Activity (U g−1 min) | D Value | ΔD |
|---|---|---|---|---|---|---|
| Jinyuan U99 | CK | 214.125 ± 16.25 | 215.66 ± 41.90 | 776.49 ± 237.76 | 0.609 | 0.132 |
| NaCl | 272.275 ± 30.40 | 324.33 ± 47.92 | 978.725 ± 97.43 | 0.477 | ||
| Tianlongyou 619 | CK | 250.37 ± 29.66 | 272.00 ± 35.38 | 380.465 ± 120.81 | 0.656 | 0.216 |
| NaCl | 278.575 ± 39.23 | 386.33 ± 23.65 | 567.615 ± 174.79 | 0.440 | ||
| Jindao 919 | CK | 141.31 ± 21.68 | 319.33 ± 34.48 | 624.79 ± 310.25 | 0.462 | 0.122 |
| NaCl | 217.655 ± 59.46 | 383.66 ± 24.60 | 828.075 ± 180.73 | 0.340 | ||
| Jinchuan No.1 | CK | 157.50 ± 26.49 | 402.00 ± 35.38 | 442.18 ± 132.73 | 0.552 | 0.242 |
| NaCl | 186.41 ± 30.05 | 459.00 ± 14.28 | 927.53 ± 136.88 | 0.310 |
| Cultivar | Treatment | Germination Potential (GP) | Germination Rate (GR) | Germination Index (GI) | Vigour Index (VI) |
|---|---|---|---|---|---|
| Jinchuan No. 1 | CK | 95.33 ± 1.15 a | 98.66 ± 1.15 a | 14.88 ± 0.24 a | 140.49 ± 2.26 a |
| NaCl | 79.33 ± 3.05 b | 93.33 ± 1.15 b | 10.32 ± 0.31 d | 63.00 ± 0.88 e | |
| S1 | 90.00 ± 4.00 ab | 96.66 ± 1.15 ab | 13.39 ± 0.12 b | 121.08 ± 1.13 b | |
| S2 | 95.33 ± 1.15 a | 98.00 ± 2.00 a | 14.09 ± 0.69 ab | 128.34 ± 6.28 ab | |
| S3 | 96.66 ± 1.15 a | 99.33 ± 1.15 a | 15.02 ± 0.28 a | 143.16 ± 2.73 a | |
| S4 | 93.33 ± 3.05 a | 96.66 ± 1.15 ab | 12.96 ± 0.22 c | 139.85 ± 2.44 a | |
| S5 | 90.00 ± 4.00 a | 94.66 ± 1.15 b | 11.72 ± 0.27 cd | 82.56 ± 1.93 d | |
| M1 | 82.66 ± 3.05 bc | 94.66 ± 1.15 b | 10.62 ± 0.06 d | 68.29 ± 0.24 e | |
| M2 | 85.33 ± 5.03 bc | 94.66 ± 1.15 b | 10.94 ± 0.25 d | 82.47 ± 1.95 d | |
| M3 | 89.33 ± 3.05 ab | 95.33 ± 2.30 ab | 11.50 ± 0.22 cd | 109.25 ± 2.14 c | |
| M4 | 93.33 ± 1.15 a | 98.66 ± 1.15 a | 12.11 ± 0.31 c | 77.14 ± 1.98 de | |
| M5 | 86.00 ± 2.00 bc | 94.00 ± 0.00 b | 10.70 ± 0.31 d | 47.45 ± 1.07 f | |
| Jindao 919 | CK | 97.33 ± 1.15 a | 99.33 ± 1.15 a | 12.87 ± 0.28 a | 109.41 ± 2.43 a |
| NaCl | 72.00 ± 3.46 d | 90.66 ± 1.15 e | 9.58 ± 0.19 j | 73.94 ± 1.67 de | |
| S1 | 90.66 ± 3.05 ab | 96.00 ± 2.00 abcd | 11.08 ± 0.54 cd | 86.56 ± 4.29 c | |
| S2 | 91.33 ± 4.16 ab | 98.00 ± 2.00 ab | 11.45 ± 0.39 bc | 97.62 ± 3.40 b | |
| S3 | 98.00 ± 2.00 a | 99.33 ± 1.15 a | 12.08 ± 0.18 ab | 110.17 ± 1.73 s | |
| S4 | 90.00 ± 4.00 ab | 96.66 ± 1.15 abcd | 10.95 ± 0.28 cde | 95.26 ± 2.43 b | |
| S5 | 79.33 ± 4.16 cd | 94.00 ± 2.00 cd | 10.21 ± 0.20 gh | 71.01 ± 1.43 e | |
| M1 | 74.00 ± 2.00 d | 93.33 ± 1.15 d | 9.95 ± 0.06 hi | 55.75 ± 0.37 g | |
| M2 | 80.00 ± 2.00 cd | 96.00 ± 0.00 abcd | 10.27 ± 0.03 fgh | 79.12 ± 0.23 d | |
| M3 | 83.33 ± 3.05 bc | 97.33 ± 2.30 abc | 10.68 ± 0.25 def | 62.67 ± 1.46 f | |
| M4 | 84.66 ± 1.15 bc | 95.33 ± 1.15 bcd | 10.38 ± 0.23 efg | 51.25 ± 1.16 h | |
| M5 | 76.00 ± 2.00 d | 92.66 ± 3.05 d | 9.78 ± 0.19 i | 32.60 ± 0.66 i |
| Cultivar | Treatment | Seedling Root Length (SRL, cm) | Seedling Length (SL, cm) | Seedling Fresh Weight (SFW, mg) | Seedling Dry Weight (SDW, mg) |
|---|---|---|---|---|---|
| Jinchuan No. 1 | CK | 10.38 ± 1.53 bc | 8.94 ± 0.93 bc | 96.65 ± 8.70 ab | 19.63 ± 0.73 bc |
| NaCl | 4.70 ± 1.75 e | 2.76 ± 0.15 f | 46.00 ± 4.58 e | 17.00 ± 1.00 de | |
| S1 | 8.57 ± 1.15 cd | 9.04 ± 0.57 bc | 74.80 ± 6.62 cd | 18.43 ± 0.57 cd | |
| S2 | 8.93 ± 1.40 cd | 9.11 ± 0.54 bc | 79.21 ± 6.75 bcd | 19.06 ± 0.38 bcd | |
| S3 | 10.31 ± 1.36 bc | 9.53 ± 0.88 ab | 84.01 ± 8.51 abc | 19.56 ± 1.50 bc | |
| S4 | 14.24 ± 1.17 a | 10.79 ± 0.73 a | 87.53 ± 11.76 ab | 21.02 ± 0.18 a | |
| S5 | 7.38 ± 0.80 d | 7.04 ± 0.91 de | 74.68 ± 10.22 cd | 17.46 ± 0.87 de | |
| M1 | 8.03 ± 1.61 cd | 4.13 ± 0.96 ef | 66.66 ± 13.79 de | 19.00 ± 2.64 bcd | |
| M2 | 10.13 ± 1.72 bc | 7.53 ± 1.84 cde | 69.00 ± 7.21 de | 19.66 ± 2.08 abc | |
| M3 | 11.76 ± 1.78 ab | 9.50 ± 0.75 ab | 104.33 ± 12.50 a | 20.00 ± 1.00 ab | |
| M4 | 9.13 ± 2.28 cd | 6.36 ± 0.32 de | 98.33 ± 15.04 ab | 20.66 ± 1.52 a | |
| M5 | 7.70 ± 2.10 d | 3.43 ± 0.51 ef | 55.66 ± 11.93 e | 15.00 ± 4.00 e | |
| Jindao 919 | CK | 8.82 ± 1.19 bcd | 8.50 ± 0.92 abc | 101.08 ± 8.53 abc | 19.03 ± 0.29 bcd |
| NaCl | 6.27 ± 1.37 e | 7.11 ± 0.56 de | 86.71 ± 3.78 cd | 18.28 ± 0.15 de | |
| S1 | 7.28 ± 1.11 de | 7.81 ± 0.45 bcd | 87.05 ± 5.26 cd | 18.60 ± 0.28 cde | |
| S2 | 10.47 ± 1.85 ab | 8.52 ± 0.83 abc | 100.71 ± 10.84 abc | 19.31 ± 1.06 abcd | |
| S3 | 8.44 ± 1.51 cd | 9.12 ± 0.67 a | 105.05 ± 8.21 ab | 21.32 ± 0.46 a | |
| S4 | 8.30 ± 0.77 cd | 8.70 ± 0.67 ab | 101.58 ± 13.34 abc | 20.44 ± 0.28 ab | |
| S5 | 8.03 ± 0.60 d | 6.95 ± 0.64 def | 90.88 ± 7.6 b bc | 18.26 ± 1.41 de | |
| M1 | 8.90 ± 0.60 bcd | 5.60 ± 0.45 fg | 102.66 ± 11.50 abc | 18.66 ± 2.08 cde | |
| M2 | 10.20 ± 0.20 abc | 7.70 ± 0.78 cd | 115.66 ± 11.59 a | 20.00 ± 3.60 abc | |
| M3 | 10.93 ± 0.80 a | 5.86 ± 0.77 ef | 88.33 ± 8.38 cd | 21.33 ± 3.05 a | |
| M4 | 10.43 ± 0.66 ab | 4.93 ± 0.41 g | 84.00 ± 8.18 d | 19.33 ± 5.85 abcd | |
| M5 | 6.23 ± 1.23 e | 3.33 ± 0.92 h | 73.00 ± 13.22 e | 17.66 ± 2.51 e |
| Cultivar | Treatment | Root Length (RL, cm) | Plant Height (PH, cm) | Fresh Weight (FW, mg) | Dry Weight (DW, mg) | Stem Diameter (SD, mm) |
|---|---|---|---|---|---|---|
| Jinchuan No. 1 | CK | 14.89 ± 1.37 a | 17.78 ± 0.38 b | 218.66 ± 4.61 a | 22.00 ± 0.00 a | 1.72 ± 0.14 cd |
| NaCl | 4.52 ± 0.84 cd | 13.10 ± 1.76 efg | 110.25 ± 37.62 ef | 14.66 ± 0.57 e | 1.66 ± 0.13 d | |
| S1 | 8.36 ± 0.70 b | 15.44 ± 1.07 cde | 128.00 ± 5.56 cd | 19.66 ± 0.57 b | 1.92 ± 0.12 bcd | |
| S2 | 10.63 ± 0.46 ab | 15.69 ± 1.09 cde | 135.66 ± 1.52 c | 20.33 ± 0.57 ab | 2.60 ± 0.20 a | |
| S3 | 5.36 ± 0.28 cd | 16.97 ± 0.13 bc | 141.33 ± 3.21 bc | 22.33 ± 0.57 a | 2.89 ± 0.18 a | |
| S4 | 4.79 ± 0.89 cd | 14.61 ± 0.82 def | 113.33 ± 4.16 ef | 18.00 ± 1.00 bc | 3.21 ± 0.34 a | |
| S5 | 4.05 ± 0.27 d | 12.12 ± 0.65 fg | 92.33 ± 24.58 fg | 16.00 ± 1.00 cd | 1.36 ± 0.13 e | |
| M1 | 9.67 ± 1.88 ab | 14.23 ± 1.98 def | 154.00 ± 19.16 b | 17.00 ± 1.00 cd | 1.97 ± 0.09 bc | |
| M2 | 5.82 ± 0.61 cd | 23.10 ± 2.15 a | 129.25 ± 26.66 cd | 16.66 ± 0.57 cd | 2.05 ± 0.21 ab | |
| M3 | 5.23 ± 0.97 cd | 16.00 ± 1.74 bcd | 119.00 ± 9.30 de | 15.33 ± 0.57 de | 2.51 ± 0.24 a | |
| M4 | 4.81 ± 0.66 cd | 14.55 ± 2.27 def | 111.75 ± 18.57 ef | 13.00 ± 1.00 ef | 2.48 ± 0.13 a | |
| M5 | 4.25 ± 1.02 d | 10.86 ± 2.71 g | 90.25 ± 20.12 g | 11.33 ± 1.15 f | 1.78 ± 0.14 cd | |
| Jindao 919 | CK | 10.29 ± 0.56 a | 13.82 ± 0.42 cd | 149.66 ± 17.03 a | 21.33 ± 2.51 ab | 1.85 ± 0.09 f |
| NaCl | 4.66 ± 0.14 d | 11.87 ± 1.20 ef | 99.00 ± 16.69 e | 14.66 ± 1.52 f | 1.85 ± 0.08 f | |
| S1 | 7.14 ± 0.46 b | 13.70 ± 0.46 cd | 122.66 ± 8.14 cd | 20.00 ± 1.00 bc | 2.54 ± 0.18 de | |
| S2 | 7.49 ± 0.54 b | 15.13 ± 0.36 b | 138.33 ± 5.13 ab | 21.00 ± 1.00 ab | 3.05 ± 0.22 bc | |
| S3 | 4.97 ± 0.70 d | 16.92 ± 0.60 a | 147.00 ± 7.54 a | 23.00 ± 1.00 a | 3.44 ± 0.21 ab | |
| S4 | 4.73 ± 0.26 d | 14.48 ± 0.60 bc | 130.00 ± 9.53 bc | 18.13 ± 0.80 cd | 2.72 ± 0.21 cd | |
| S5 | 4.42 ± 0.21 d | 11.04 ± 0.64 fg | 122.66 ± 4.72 cd | 17.66 ± 2.08 de | 1.84 ± 0.17 f | |
| M1 | 4.83 ± 1.02 d | 12.49 ± 1.63 de | 113.00 ± 7.48 d | 16.33 ± 1.52 e | 2.64 ± 0.38 cd | |
| M2 | 6.47 ± 0.43 c | 12.60 ± 1.59 de | 121.00 ± 13.24 cd | 17.33 ± 0.57 de | 2.69 ± 0.30 cd | |
| M3 | 7.37 ± 1.15 b | 16.63 ± 0.47 a | 147.00 ± 16.79 a | 18.00 ± 1.00 cd | 3.14 ± 0.82 abc | |
| M4 | 4.86 ± 0.37 d | 10.48 ± 0.64 g | 119.75 ± 15.08 cd | 18.66 ± 1.15 cd | 3.70 ± 0.21 a | |
| M5 | 4.58 ± 0.04 d | 7.36 ± 2.26 h | 105.25 ± 35.96 de | 14.66 ± 0.57 f | 2.24 ± 0.11 e |
| Cultivar | Treatment | Membership Function | D Value | Rank | |||
|---|---|---|---|---|---|---|---|
| Jinchuan No. 1 | CK | 0.636 | 0.000 | 0.877 | 0.366 | 0.452 | 7 |
| NaCl | 0.157 | 0.552 | 0.013 | 0.623 | 0.240 | 11 | |
| S1 | 0.565 | 0.511 | 0.196 | 0.131 | 0.432 | 8 | |
| S2 | 0.785 | 0.620 | 0.414 | 0.405 | 0.611 | 3 | |
| S3 | 1.000 | 0.770 | 0.102 | 0.130 | 0.701 | 2 | |
| S4 | 0.733 | 0.804 | 0.043 | 0.521 | 0.588 | 4 | |
| S5 | 0.405 | 0.661 | 0.000 | 0.397 | 0.376 | 10 | |
| M1 | 0.294 | 0.704 | 0.422 | 0.763 | 0.400 | 9 | |
| M2 | 0.535 | 0.691 | 0.489 | 1.000 | 0.543 | 5 | |
| M3 | 0.656 | 1.000 | 1.000 | 0.595 | 0.708 | 1 | |
| M4 | 0.411 | 0.958 | 0.781 | 0.068 | 0.515 | 6 | |
| M5 | 0.000 | 0.725 | 0.505 | 0.000 | 0.217 | 12 | |
| Jindao 919 | CK | 0.620 | 1.000 | 0.230 | 0.406 | 0.571 | 3 |
| NaCl | 0.003 | 0.251 | 0.796 | 0.651 | 0.215 | 11 | |
| S1 | 0.583 | 0.423 | 0.965 | 0.500 | 0.518 | 4 | |
| S2 | 0.758 | 0.396 | 0.908 | 0.507 | 0.584 | 2 | |
| S3 | 1.000 | 0.173 | 0.808 | 0.618 | 0.640 | 1 | |
| S4 | 0.912 | 0.076 | 0.662 | 0.022 | 0.504 | 5 | |
| S5 | 0.340 | 0.185 | 1.000 | 0.201 | 0.330 | 8 | |
| M1 | 0.292 | 0.325 | 0.329 | 0.761 | 0.317 | 9 | |
| M2 | 0.584 | 0.000 | 0.105 | 1.000 | 0.371 | 6 | |
| M3 | 0.662 | 0.102 | 0.010 | 0.299 | 0.349 | 7 | |
| M4 | 0.500 | 0.249 | 0.000 | 0.000 | 0.280 | 10 | |
| M5 | 0.000 | 0.196 | 0.541 | 0.100 | 0.117 | 12 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Duan, Y.; Luo, X.; Li, M.; Gao, H.; Zhu, W.; Zhao, F.; Liu, J.; Zhang, W. Molecular Regulation of Antioxidant Defense and Metabolic Reprogramming in Xiaozhan Rice Genotypes: Differential Roles of Salicylic Acid and Melatonin Under Salt Stress. Curr. Issues Mol. Biol. 2025, 47, 432. https://doi.org/10.3390/cimb47060432
Wu Y, Duan Y, Luo X, Li M, Gao H, Zhu W, Zhao F, Liu J, Zhang W. Molecular Regulation of Antioxidant Defense and Metabolic Reprogramming in Xiaozhan Rice Genotypes: Differential Roles of Salicylic Acid and Melatonin Under Salt Stress. Current Issues in Molecular Biology. 2025; 47(6):432. https://doi.org/10.3390/cimb47060432
Chicago/Turabian StyleWu, Yang, Yongbo Duan, Xifan Luo, Mingjun Li, Hengjie Gao, Wei Zhu, Fei Zhao, Jian Liu, and Wenzhong Zhang. 2025. "Molecular Regulation of Antioxidant Defense and Metabolic Reprogramming in Xiaozhan Rice Genotypes: Differential Roles of Salicylic Acid and Melatonin Under Salt Stress" Current Issues in Molecular Biology 47, no. 6: 432. https://doi.org/10.3390/cimb47060432
APA StyleWu, Y., Duan, Y., Luo, X., Li, M., Gao, H., Zhu, W., Zhao, F., Liu, J., & Zhang, W. (2025). Molecular Regulation of Antioxidant Defense and Metabolic Reprogramming in Xiaozhan Rice Genotypes: Differential Roles of Salicylic Acid and Melatonin Under Salt Stress. Current Issues in Molecular Biology, 47(6), 432. https://doi.org/10.3390/cimb47060432





