Transgenerational Memory of Phenotypic Traits in Plants: Epigenetic Regulation of Growth, Hormonal Balance, and Stress Adaptation
Abstract
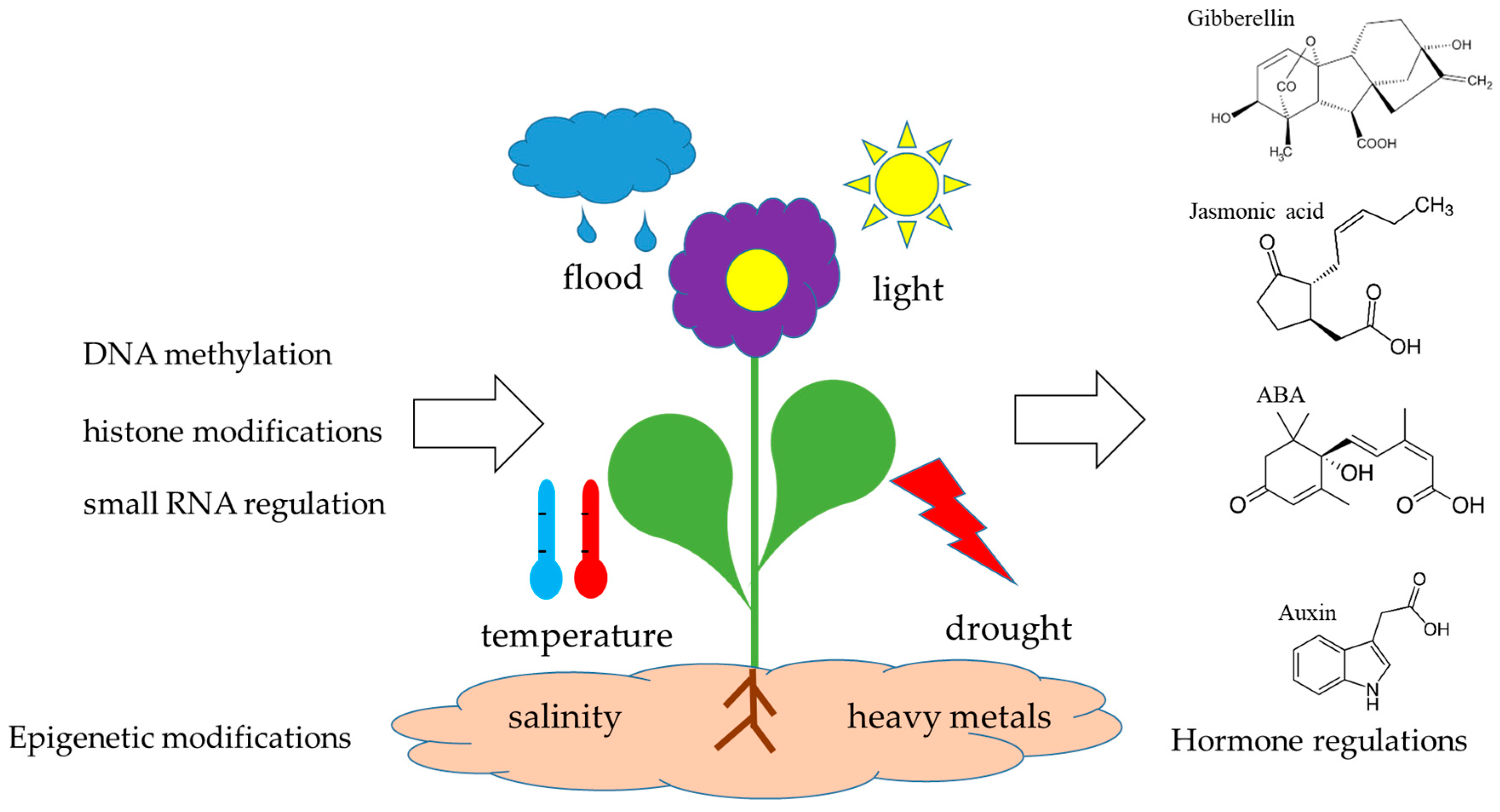
1. Introduction
2. Epigenetic Mechanisms Governing Hormonal Regulation
2.1. DNA Methylation Regulates Hormonal Synthesis and Signaling: A Crucial Epigenetic-Hormonal Interface in Plant Development and Stress Adaptation
- Auxin decreased DRM2 and DML3 expression in roots and shoots, while slightly upregulating CMT3.
- ABA upregulated VIM1 (a MET1 cofactor for CG methylation) in roots and shoots but suppressed DRM3 and DML3.
- GA treatment enhanced the expression of DML2 and DML3, suggesting active demethylation supports GA-mediated growth.
- SA broadly suppressed MET1, VIM1, DRM2, and demethylases like ROS1, reducing methylation turnover.
2.2. Histone Modifications in Hormonal Signaling: Epigenetic Regulation of Plant Development and Stress Responses
2.3. Small RNAs in Hormonal Pathways: Post-Transcriptional Regulation of Plant Development and Immunity
3. Hormonal Balance in Progeny: Epigenetic Effects on Fitness
3.1. Auxin and Root–Shoot Allocation: Epigenetic Regulation of Growth Plasticity
3.2. Abscisic Acid and Stress Tolerance: Epigenetic Memory of Drought Response
3.3. Gibberellins and Growth Plasticity: Epigenetic Regulation of Developmental Timing
3.4. Jasmonic Acid and Defense Responses: Epigenetic Regulation of Herbivore Resistance
4. Transgenerational Epigenetic Inheritance of Hormonal Balance: Implications for Crop Improvement
Potential for Crop Improvement Through Epigenetic Priming
5. Challenges and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| AM | Arbuscular mycorrhizal |
| ACS | 1-aminocyclopropane-1-carboxylate synthase |
| ACO | 1-aminocyclopropane-1-carboxylate oxidase |
| DME | DEMETER DNA glycosylase |
| DRM | Domains rearranged methyltransferase |
| GA | Gibberellin |
| GUS | β-glucuronidase |
| HAT | Histone acetyltransferase |
| HDAC | Histone deacetylase |
| HMT | Histone methyltransferase |
| JA | Jasmonic acid |
| LAFL | LEC1, ABI3, FUS3, and LEC2 gene network (seed maturation regulators) |
| miRNA | MicroRNA |
| PRC1/PRC2 | Polycomb repressive complex 1/2 |
| PTI | Pathogen-triggered immunity |
| RdDM | RNA-directed DNA methylation |
| RISC | RNA-induced silencing complex |
| RNA Pol II | RNA polymerase II |
| ROS | Reactive oxygen species |
| RSA | Root system architecture |
| SA | Salicylic acid |
| siRNA | Small interfering RNA |
| sRNA | Small RNA |
| TOR | Target of rapamycin |
References
- Boyko, A.; Kovalchuk, I. Transgenerational response to stress in Arabidopsis thaliana. Plant Signal. Behav. 2010, 5, 995–998. [Google Scholar] [CrossRef]
- Espinas, N.A.; Saze, H.; Saijo, Y. Epigenetic control of defense signaling and priming in plants. Front. Plant Sci. 2016, 7, 1201. [Google Scholar] [CrossRef]
- Kinoshita, T.; Seki, M. Epigenetic memory for stress response and adaptation in plants. Plant Cell Physiol. 2014, 55, 1859–1863. [Google Scholar] [CrossRef]
- Lämke, J.; Bäurle, I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Fromm, M.; Avramova, Z. Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nat. Commun. 2012, 3, 740. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Small RNAs and their targets are associated with the transgenerational effects of water-deficit stress in durum wheat. Sci. Rep. 2021, 11, 3613. [Google Scholar] [CrossRef] [PubMed]
- Gallusci, P.; Agius, D.R.; Moschou, P.N.; Dobránszki, J.; Kaiserli, E.; Martinelli, F. Deep inside the epigenetic memories of stressed plants. Trends Plant Sci. 2023, 28, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Ibragić, S.; Dahija, S.; Karalija, E. The Good, the Bad, and the Epigenetic: Stress-Induced Metabolite Regulation and Transgenerational Effects. Epigenomes 2025, 9, 10. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M. Foliar Fertilization: A Potential Strategy for Improving Plant Salt Tolerance. Crit. Rev. Plant Sci. 2024, 43, 94–115. [Google Scholar] [CrossRef]
- Coelho, F.S.; Miranda, S.S.; Moraes, J.L.; Hemerly, A.S.; Ballesteros, H.G.F.; Santa-Catarina, C.; dos Santos, R.C.; de Almeida, F.A.; Silveira, V.; Macedo, A.; et al. DNA methylation impacts soybean early development by modulating hormones and metabolic pathways. Physiol. Plant. 2024, 176, e14492. [Google Scholar] [CrossRef]
- Xiao, K.; Chen, J.; He, Q.; Wang, Y.; Shen, H.; Sun, L. DNA methylation is involved in the regulation of pepper fruit ripening and interacts with phytohormones. J. Exp. Bot. 2020, 71, 1928–1942. [Google Scholar] [CrossRef]
- Hemenway, C.A.; Gehring, M. Epigenetic regulation during plant development and the capacity for epigenetic memory. Annu. Rev. Plant Biol. 2023, 74, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Cleaves, K.; Hewezi, T. Expression patterns of DNA methylation and demethylation genes during plant development and in response to phytohormones. Int. J. Mol. Sci. 2021, 22, 9681. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Thakur, J.K.; Prasad, M. Histone acetylation dynamics regulating plant development and stress responses. Cell. Mol. Life Sci. 2021, 78, 4467–4486. [Google Scholar] [CrossRef]
- Sato, H.; Yamane, H. Histone modifications affecting plant dormancy and dormancy release: Common regulatory effects on hormone metabolism. J. Exp. Bot. 2024, 75, 6142–6158. [Google Scholar] [CrossRef]
- Yung, W.-S.; Li, M.-W.; Sze, C.-C.; Wang, Q.; Lam, H.-M. Histone modifications and chromatin remodeling in plants in response to salt stress. Physiol. Plant. 2021, 173, 1495–1513. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Fan, T.; Wu, J.; Zhu, Y.; Shen, W.-H. Histone modification and chromatin remodeling in plant response to pathogens. Front. Plant Sci. 2022, 13, 986940 . [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Peng, J.; Li, F.; Ali, F.; Wang, Z. Regulation of seed germination: ROS, epigenetic, and hormonal aspects. J. Adv. Res. 2024, 71, 107–125. [Google Scholar] [CrossRef]
- Smolikova, G.; Strygina, K.; Krylova, E.; Leonova, T.; Frolov, A.; Khlestkina, E.; Medvedev, S. Transition from seeds to seedlings: Hormonal and epigenetic aspects. Plants 2021, 10, 1884. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, M.; Wang, A. Recent advances in the regulation of climacteric fruit ripening: Hormone, transcription factor and epigenetic modifications. Front. Agric. Sci. Eng. 2021, 8, 314–334. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhang, Y.; Zhang, A.; You, C.-X. Regulation of fleshy fruit ripening: From transcription factors to epigenetic modifications. Hortic. Res. 2022, 9, uhac013. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, J.; Li, G. Dynamic epigenetic modifications in plant sugar signal transduction. Trends Plant Sci. 2022, 27, 379–391. [Google Scholar] [CrossRef]
- Dong, Q.; Hu, B.; Zhang, C. MicroRNAs and their roles in plant development. Front. Plant Sci. 2022, 13, 824240 . [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Ma, L.; Zhang, P.; Zhu, H. Small RNAs participate in plant–virus interaction and their application in plant viral defense. Int. J. Mol. Sci. 2022, 23, 696. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiong, X.; Wang, Q.; Zhu, L.; Wang, L.; He, Y.; Zeng, H. miR156, miR5488 and miR399 are involved in the regulation of male sterility in PTGMS rice. Int. J. Mol. Sci. 2021, 22, 2260. [Google Scholar] [CrossRef]
- Waheed, S.; Anwar, M.; Saleem, M.A.; Wu, J.; Tayyab, M.; Hu, Z. The critical role of small RNAs in regulating plant innate immunity. Biomolecules 2021, 11, 184. [Google Scholar] [CrossRef]
- Luján-Soto, E.; Dinkova, T.D. Time to wake up: Epigenetic and small-RNA-mediated regulation during seed germination. Plants 2021, 10, 236. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y. Current understanding of the interplays between host hormones and plant viral infections. PLoS Pathog. 2021, 17, e1009242. [Google Scholar] [CrossRef]
- Aerts, N.; Hickman, R.; Van Dijken, A.J.; Kaufmann, M.; Snoek, B.L.; Pieterse, C.M.; Van Wees, S.C. Architecture and dynamics of the abscisic acid gene regulatory network. Plant J. 2024, 119, 2538–2563. [Google Scholar] [CrossRef]
- Rudolf, J.; Tomovicova, L.; Panzarova, K.; Fajkus, J.; Hejatko, J.; Skalak, J. Epigenetics and plant hormone dynamics: A functional and methodological perspective. J. Exp. Bot. 2024, 75, 5267–5294. [Google Scholar] [CrossRef]
- Yun, P.; Kaya, C.; Shabala, S. Hormonal and epigenetic regulation of root responses to salinity stress. Crop J. 2024, 12, 1309–1320. [Google Scholar] [CrossRef]
- Rajpal, V.R.; Rathore, P.; Mehta, S.; Wadhwa, N.; Yadav, P.; Berry, E.; Raina, S.N. Epigenetic variation: A major player in facilitating plant fitness under changing environmental conditions. Front. Cell Dev. Biol. 2022, 10, 1020958. [Google Scholar] [CrossRef] [PubMed]
- Solanki, M.; Shukla, L.I. Recent advances in auxin biosynthesis and homeostasis. 3 Biotech 2023, 13, 290. [Google Scholar] [CrossRef]
- Tiwari, M.; Kumar, R.; Min, D.; Jagadish, S.K. Genetic and molecular mechanisms underlying root architecture and function under heat stress: A hidden story. Plant Cell Environ. 2022, 45, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Shelden, M.C.; Munns, R. Crop root system plasticity for improved yields in saline soils. Front. Plant Sci. 2023, 14, 1120583. [Google Scholar] [CrossRef]
- Pierik, R.; Sasidharan, R.; Voesenek, L.A. Growth control by ethylene: Adjusting phenotypes to the environment. J. Plant Growth Regul. 2007, 26, 188–200. [Google Scholar] [CrossRef]
- Bhoite, R.; Han, Y.; Chaitanya, A.K.; Varshney, R.K.; Sharma, D.L. Genomic approaches to enhance adaptive plasticity to cope with soil constraints amidst climate change in wheat. Plant Genome 2024, 17, e20358. [Google Scholar] [CrossRef]
- Aizaz, M.; Lubna; Jan, R.; Asaf, S.; Bilal, S.; Kim, K.M.; Al-Harrasi, A. Regulatory dynamics of plant hormones and transcription factors under salt stress. Biology 2024, 13, 673. [Google Scholar] [CrossRef]
- Ball, K.; Sadhukhan, S. Epigenetics—The molecular tool in understanding abiotic stress response in plants. In Biology and Biotechnology of Environment; Apple Academic Press: Palm Bay, FL, USA, 2023. [Google Scholar]
- Sousa, R.O.D.; Jesus, J.F.D.; da Silva, M.N.; Paula-Marinho, S.D.O.; Alcântara Neto, F.D.; Carvalho, H.H.D.; Miranda, R.D.S. Photosynthetic efficiency and water status as determinants for the performance of semiarid-adapted cotton cultivars under drought in greenhouse. Agronomy 2025, 15, 500. [Google Scholar] [CrossRef]
- Kambona, C.M. Memory of Past Drought Stress Exposure Effects Plant Responses in Subsequent Generations in Winter Wheat (Triticum aestivum L.). Ph.D. Thesis, Universität Bonn, Bonn, Germany, 2023. Available online: https://hdl.handle.net/20.500.11811/11150 (accessed on 16 March 2025).
- Zhang, H.; Mu, Y.; Zhang, H.; Yu, C. Maintenance of stem cell activity in plant development and stress responses. Front. Plant Sci. 2023, 14, 1302046. [Google Scholar] [CrossRef]
- Miryeganeh, M. Plants’ epigenetic mechanisms and abiotic stress. Genes 2021, 12, 1106. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Quan, W.; Bartels, D. Stress memory responses and seed priming correlate with drought tolerance in plants: An overview. Planta 2022, 255, 45. [Google Scholar] [CrossRef]
- Wang, G.L.; Que, F.; Xu, Z.S.; Wang, F.; Xiong, A.S. Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant Biol. 2015, 15, 290. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Waraich, E.A.; Skalicky, M.; Hussain, S.; Zulfiqar, U.; Anjum, M.Z.; El Sabagh, A. Adaptation strategies to improve the resistance of oilseed crops to heat stress under a changing climate: An overview. Front. Plant Sci. 2021, 12, 767150. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, Y.; Li, P.; Li, F.; Ali, S.; Hou, M. Silicon amendment is involved in the induction of plant defense responses to a phloem feeder. Sci. Rep. 2017, 7, 4232. [Google Scholar] [CrossRef]
- Zhao, J.H.; Guo, H.S. RNA silencing: From discovery and elucidation to application and perspectives. J. Integr. Plant Biol. 2022, 64, 476–498. [Google Scholar] [CrossRef]
- Ghosh, M. Climate-smart agriculture, productivity and food security in India. J. Dev. Policy Pract. 2019, 4, 166–187. [Google Scholar] [CrossRef]
- Puy, J.; Carmona, C.P.; Hiiesalu, I.; Öpik, M.; de Bello, F.; Moora, M. Mycorrhizal symbiosis alleviates plant water deficit within and across generations via phenotypic plasticity. J. Ecol. 2022, 110, 262–276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karalija, E.; Ibragić, S.; Dahija, S.; Šamec, D. Transgenerational Memory of Phenotypic Traits in Plants: Epigenetic Regulation of Growth, Hormonal Balance, and Stress Adaptation. Curr. Issues Mol. Biol. 2025, 47, 404. https://doi.org/10.3390/cimb47060404
Karalija E, Ibragić S, Dahija S, Šamec D. Transgenerational Memory of Phenotypic Traits in Plants: Epigenetic Regulation of Growth, Hormonal Balance, and Stress Adaptation. Current Issues in Molecular Biology. 2025; 47(6):404. https://doi.org/10.3390/cimb47060404
Chicago/Turabian StyleKaralija, Erna, Saida Ibragić, Sabina Dahija, and Dunja Šamec. 2025. "Transgenerational Memory of Phenotypic Traits in Plants: Epigenetic Regulation of Growth, Hormonal Balance, and Stress Adaptation" Current Issues in Molecular Biology 47, no. 6: 404. https://doi.org/10.3390/cimb47060404
APA StyleKaralija, E., Ibragić, S., Dahija, S., & Šamec, D. (2025). Transgenerational Memory of Phenotypic Traits in Plants: Epigenetic Regulation of Growth, Hormonal Balance, and Stress Adaptation. Current Issues in Molecular Biology, 47(6), 404. https://doi.org/10.3390/cimb47060404







