Molecular Effects of Cornelian Cherry Fruit (Cornus mas L.) Extract on Sleep Deprivation-Induced Oxidative Stress, Cytokine Dysregulation, and Behavioural Changes in Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Cornelian Cherry Fruit Extract: Preparation, Characterisation, and In Vitro Antioxidant Activity
2.1.1. Fruit Extract Preparation
2.1.2. Total Polyphenol Content Evaluation
2.1.3. Liquid Chromatography Tandem Mass Spectrometry Analysis (LC-MS/MS)
2.1.4. In Vitro Antioxidant Activity—DPPH Radical Scavenging Activity
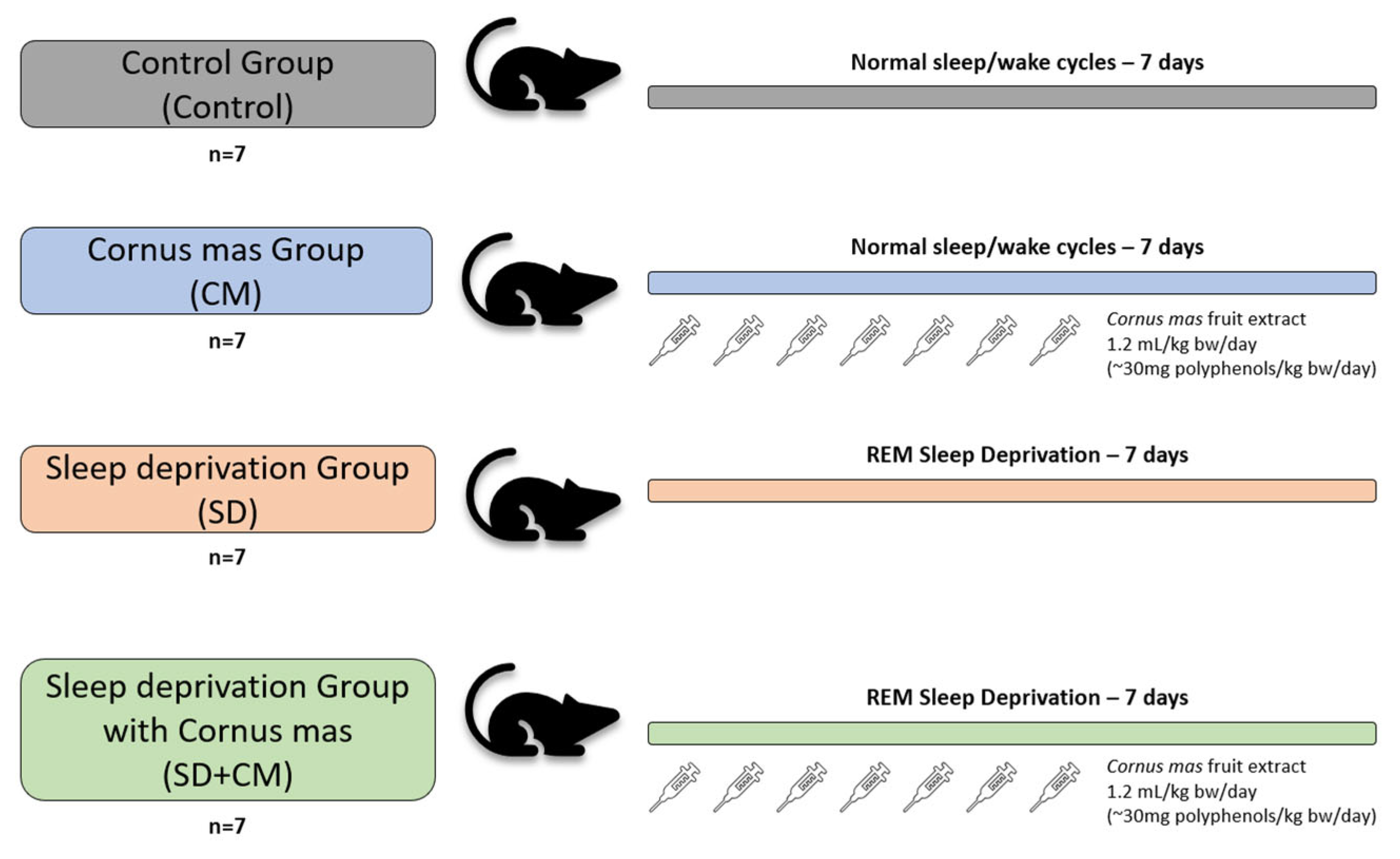
2.2. Experimental Animals
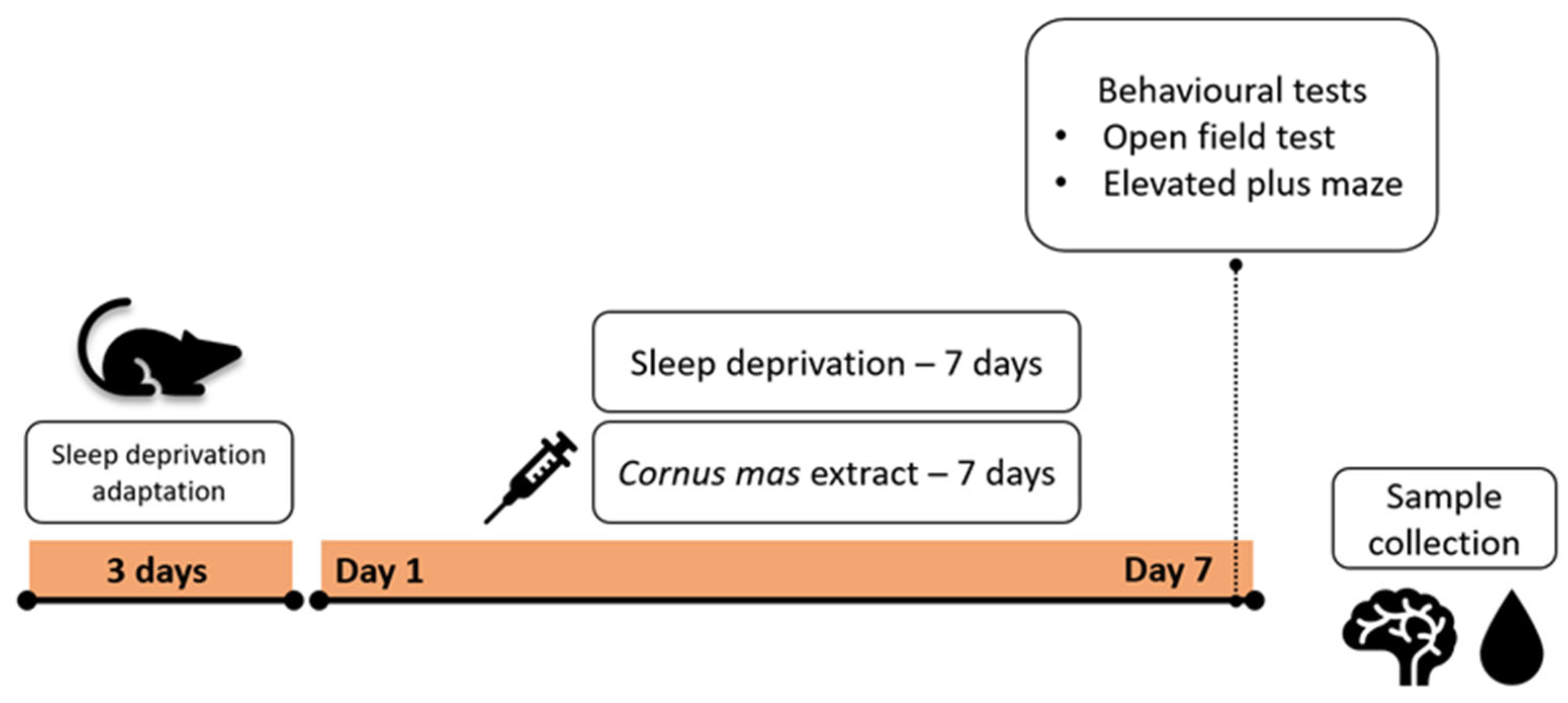
2.3. Experimental Design
2.4. Sleep Deprivation Induction
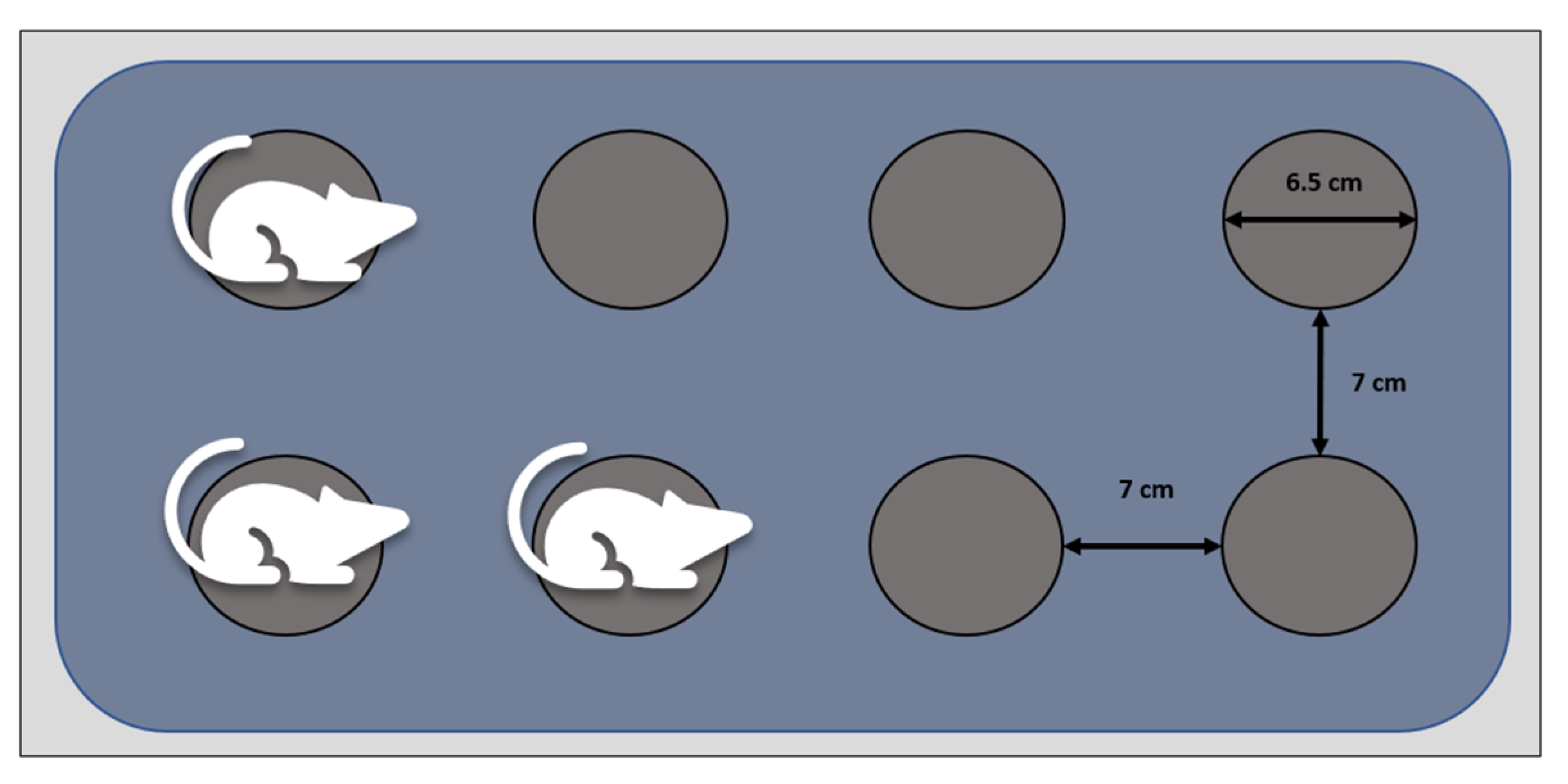
2.5. Behavioural Tests—Locomotor and Emotionality-like Behaviour
2.6. Sample Collection
2.7. Histopathological Examination
2.8. Transmission Electron Microscopy (TEM)
2.9. Oxidative Stress Parameters, Inflammatory Cytokines and Corticosterone, BDNF, GABA
2.10. Statistical Analysis
3. Results
3.1. Cornelian Cherry Fruit Extract Characterisation
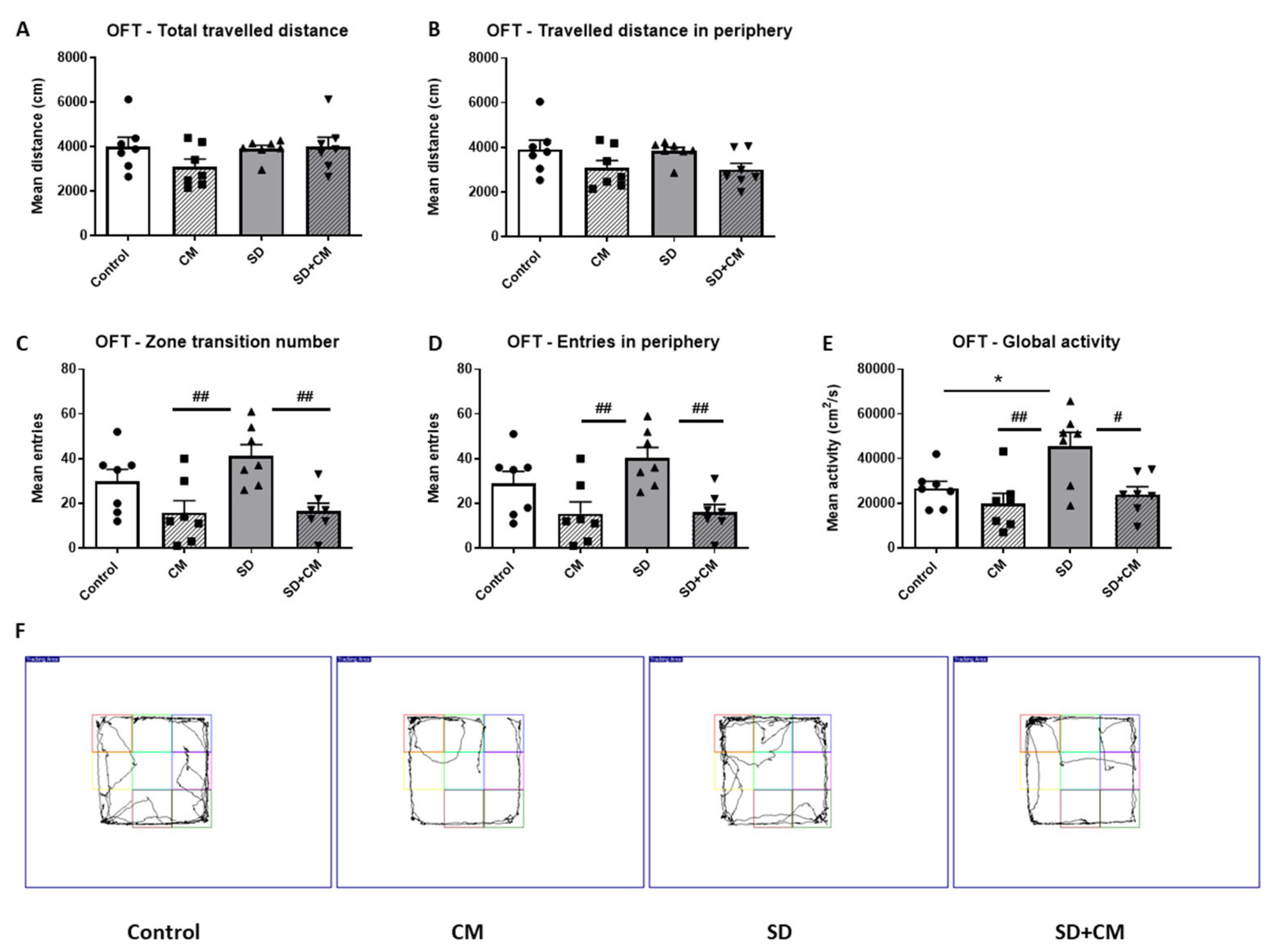
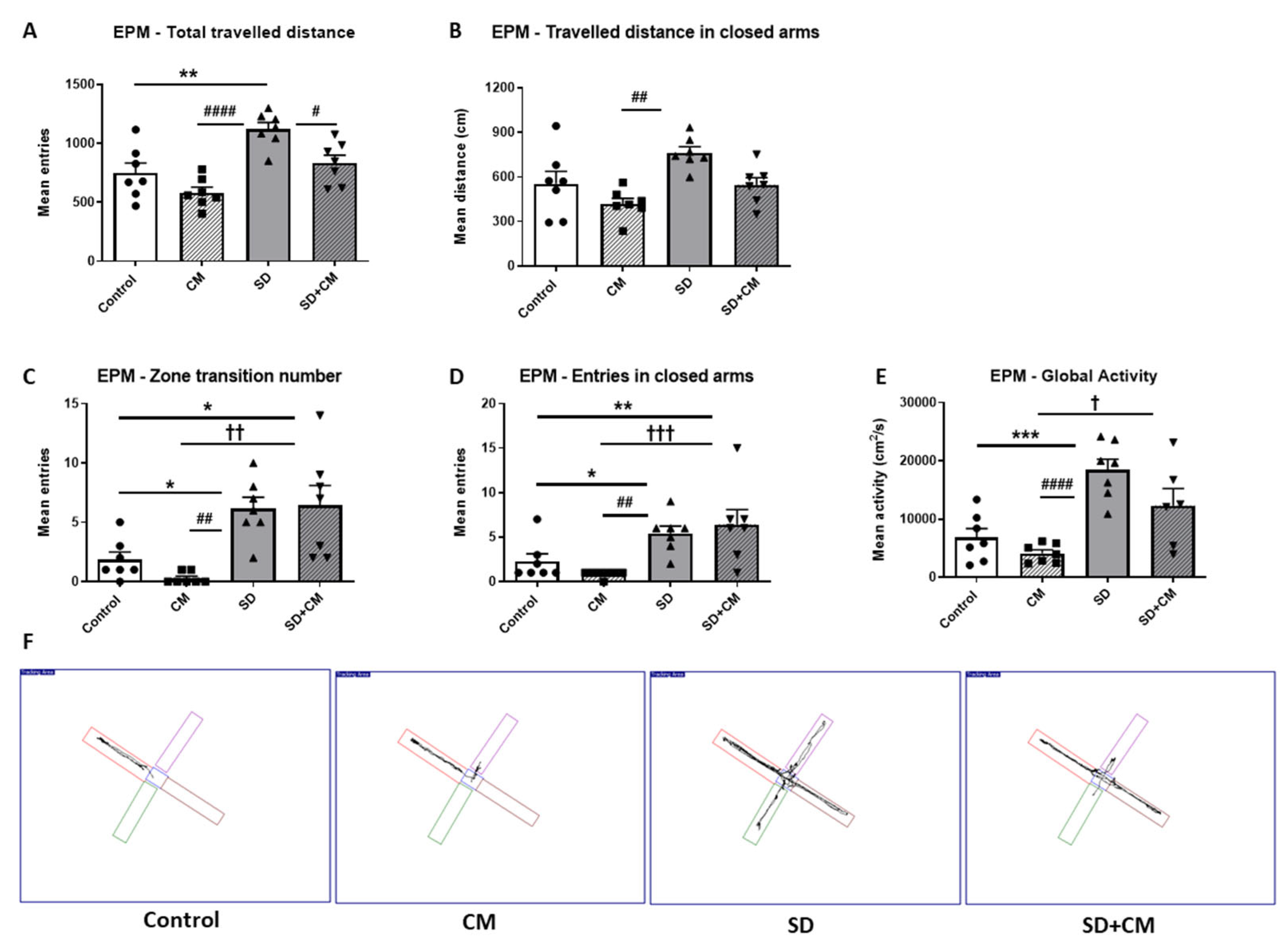
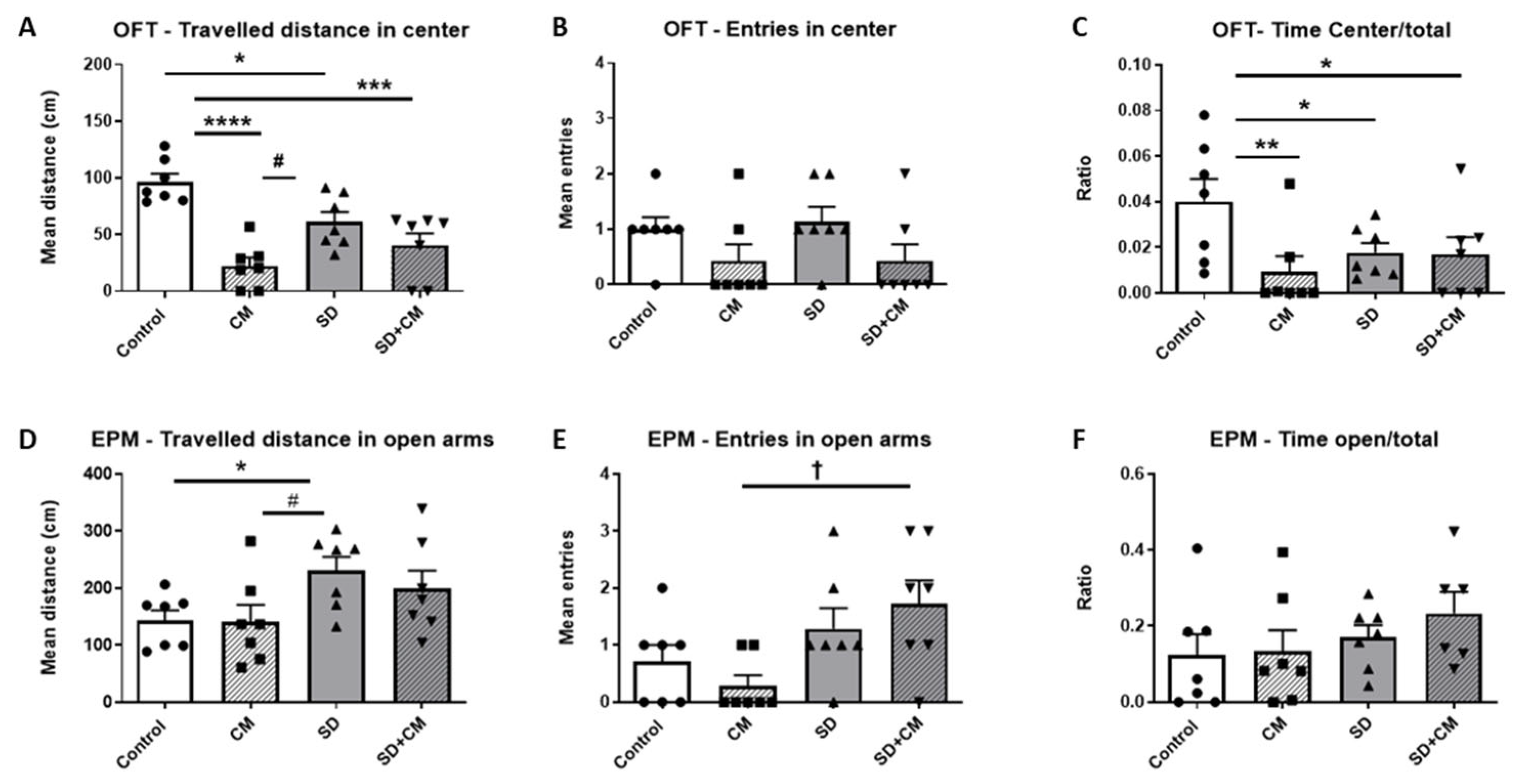
3.2. Behavioural Tests
3.2.1. Locomotor Activity
3.2.2. Emotionality-like Behaviour
3.3. Brain Histopathology
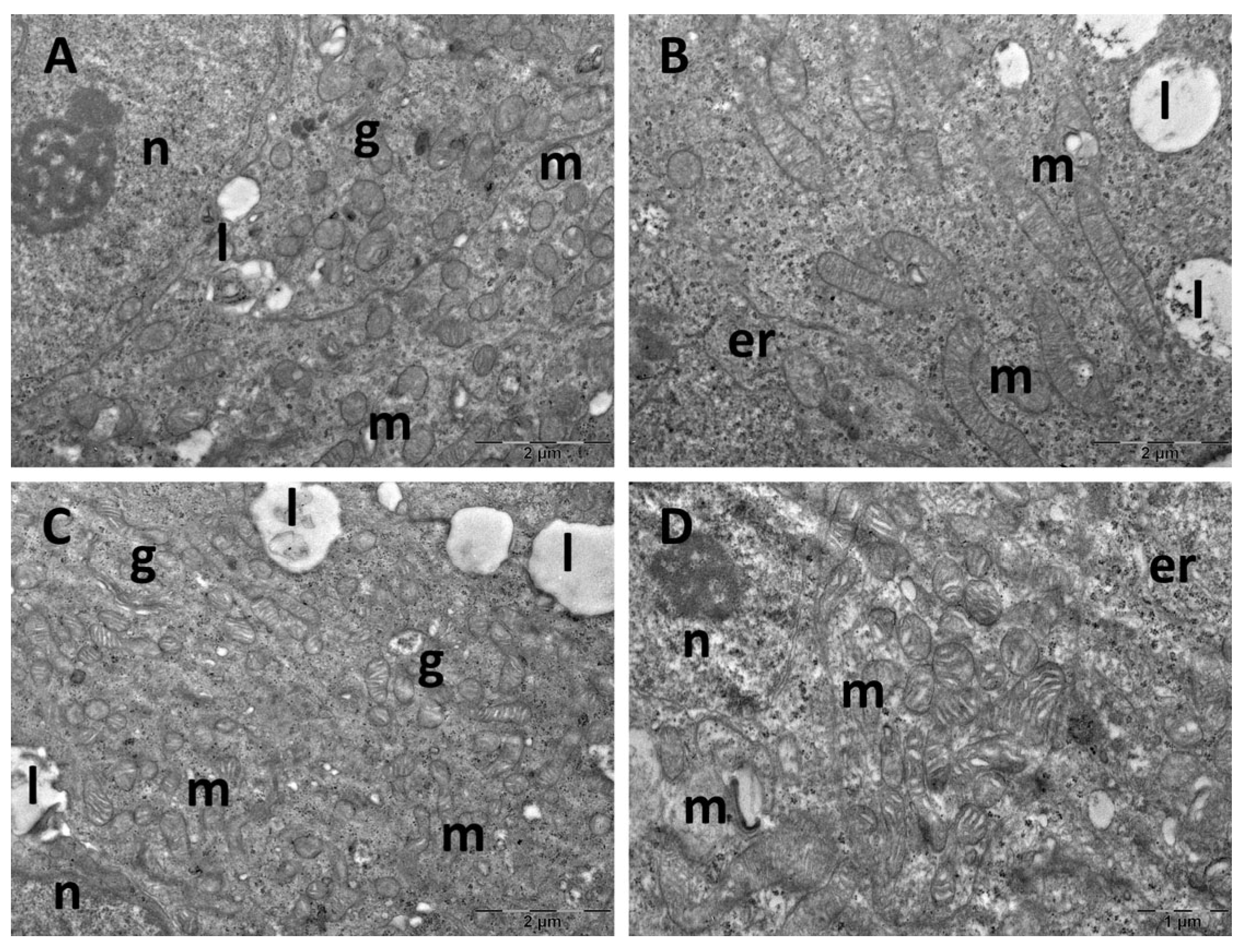
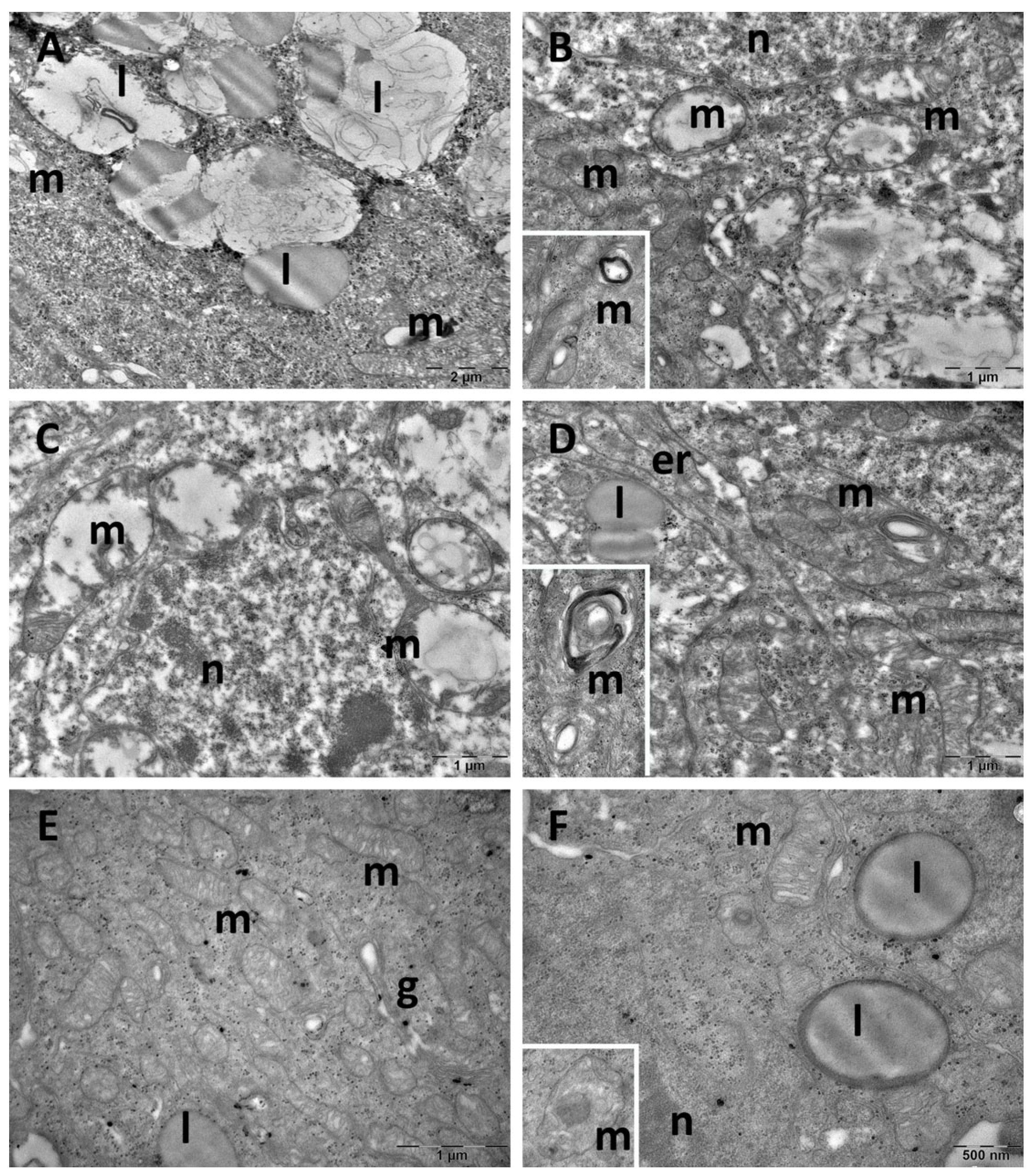
3.4. Brain Transmission Electron Microscopy (TEM) Ultrastructure Changes
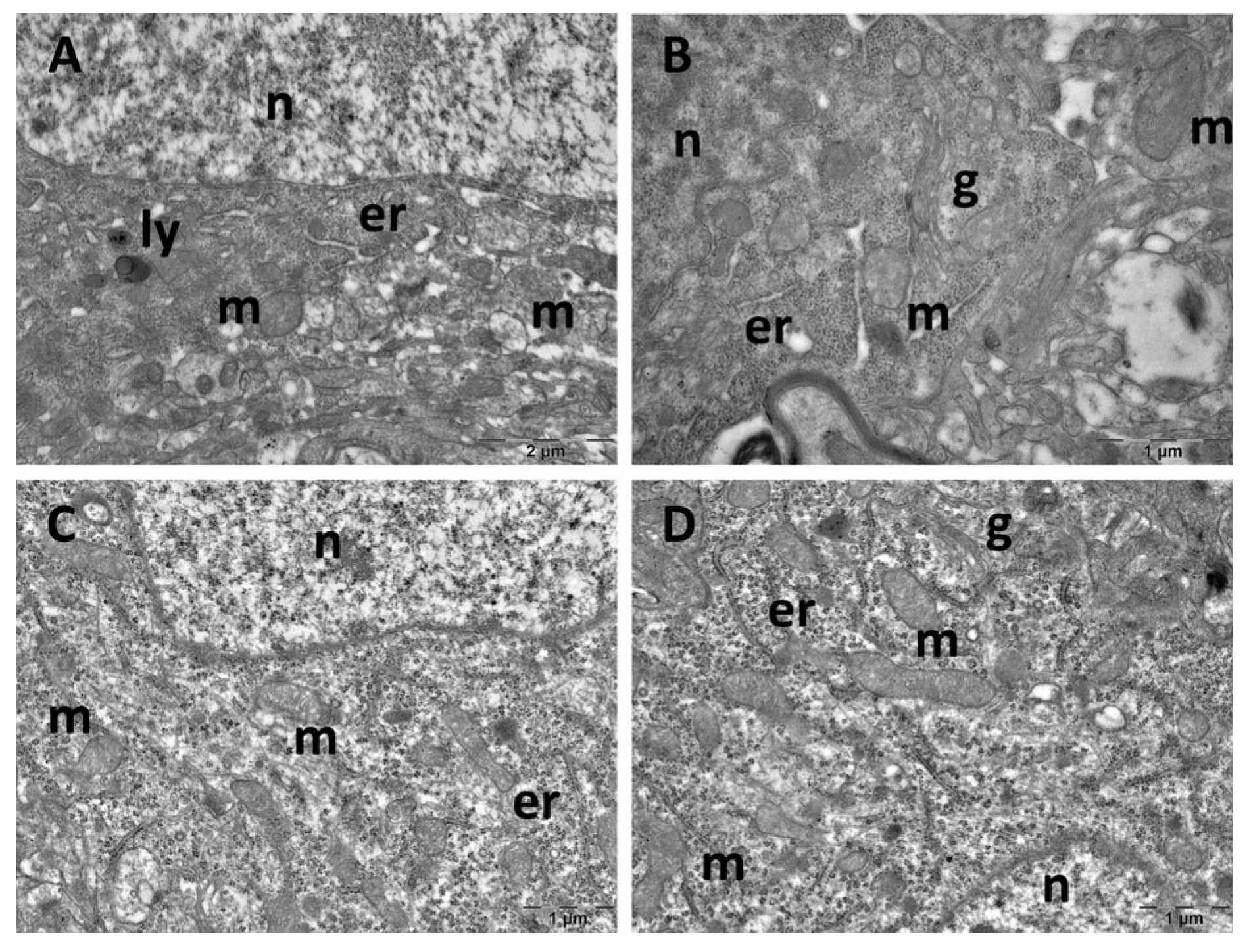
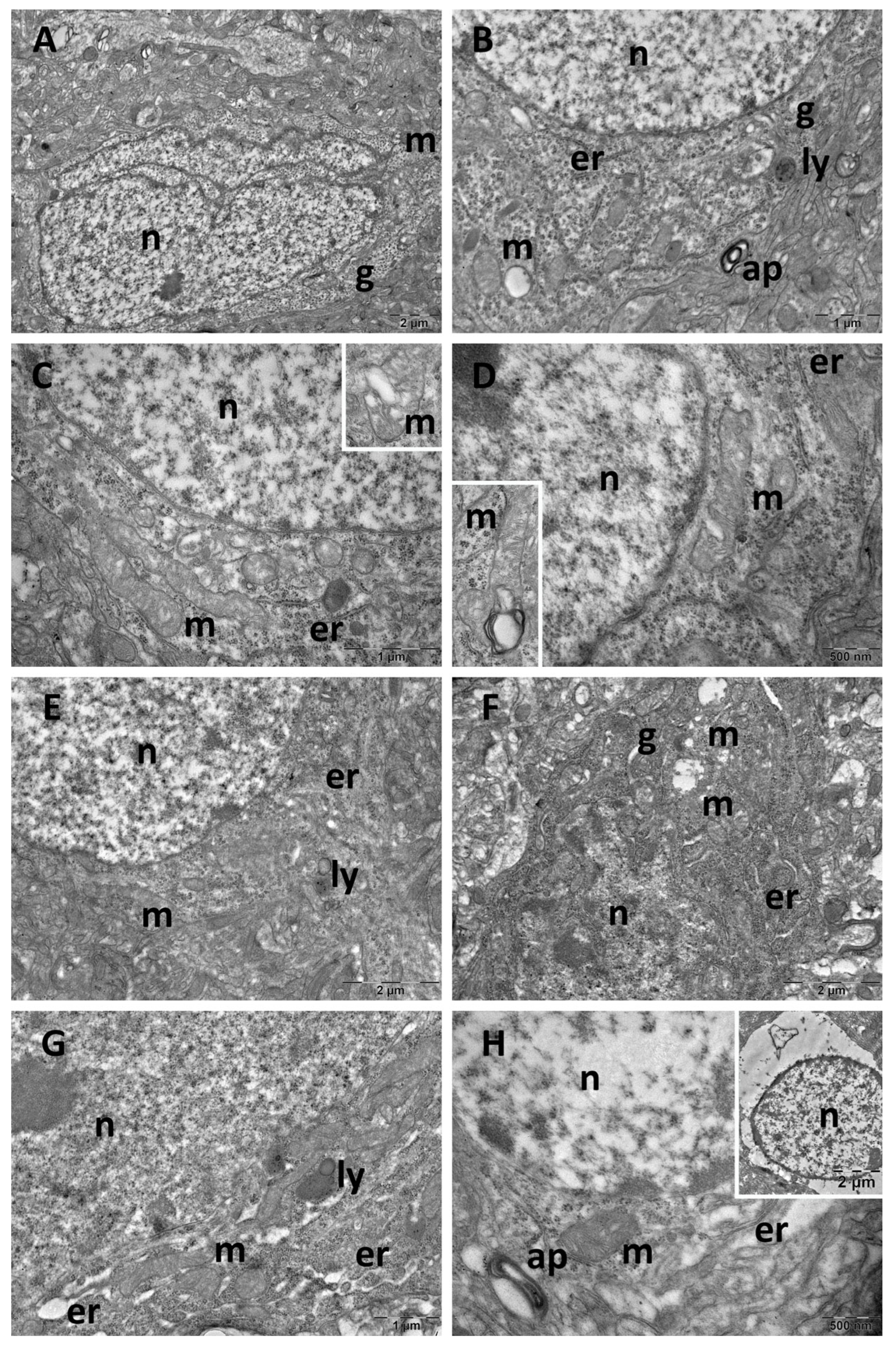
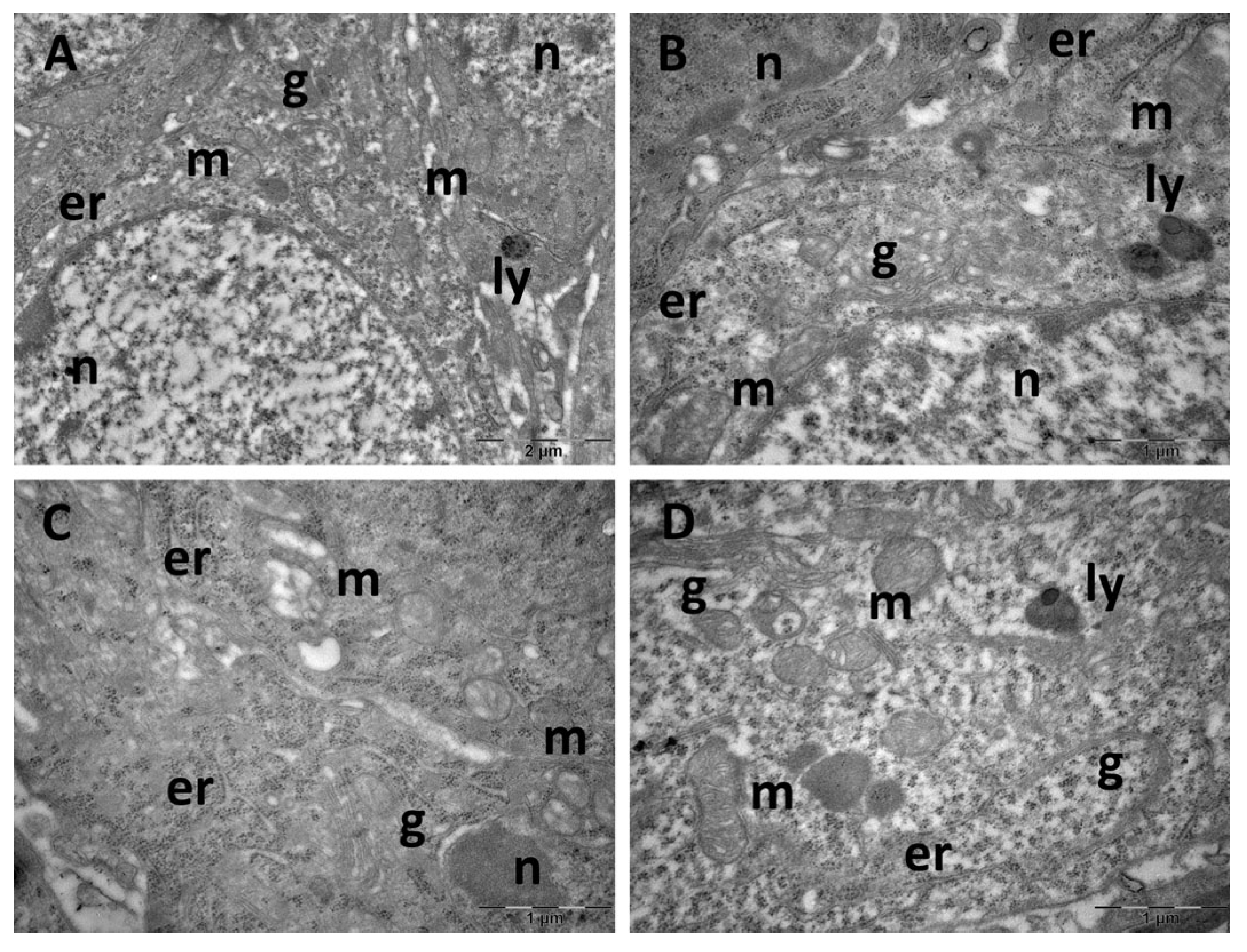
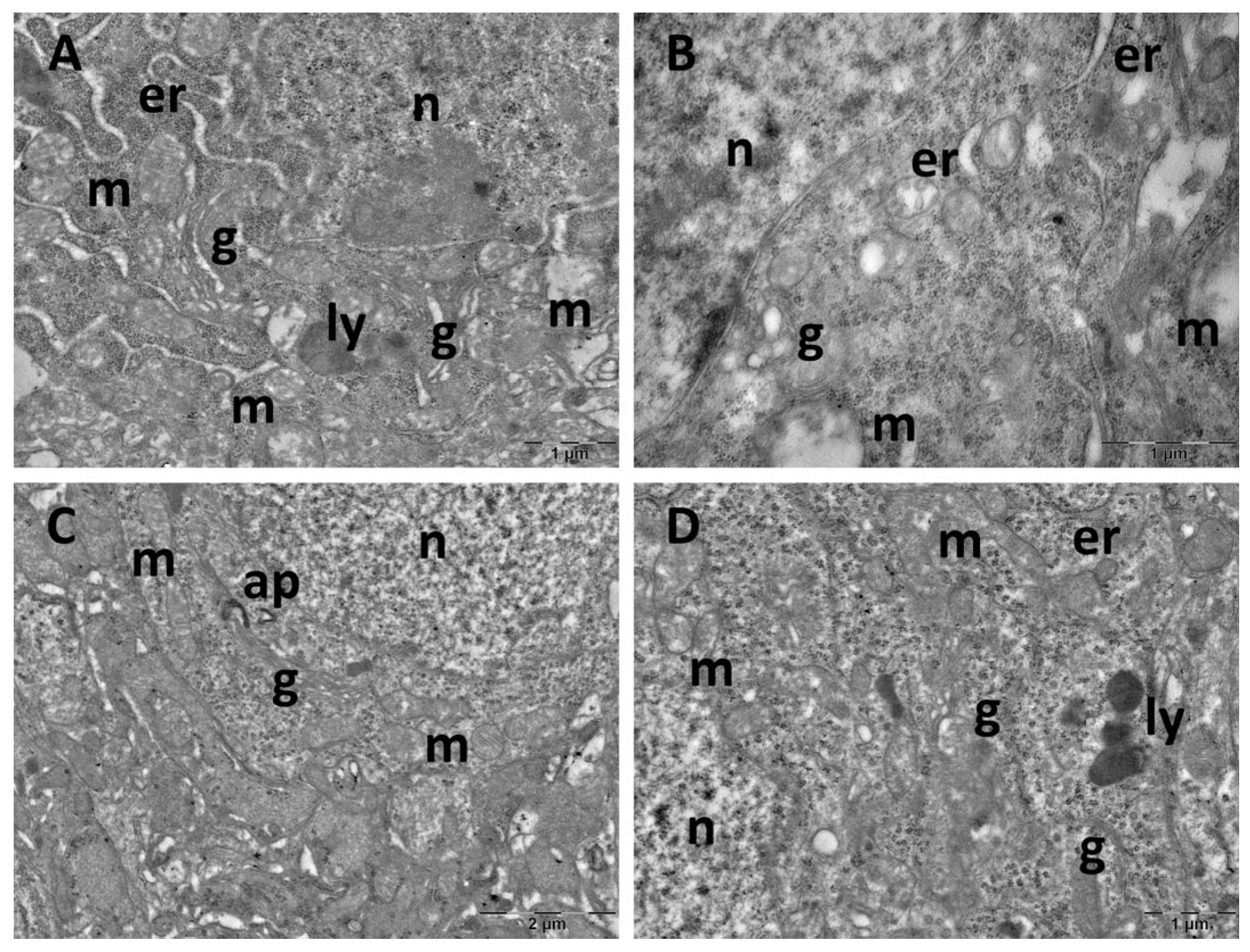
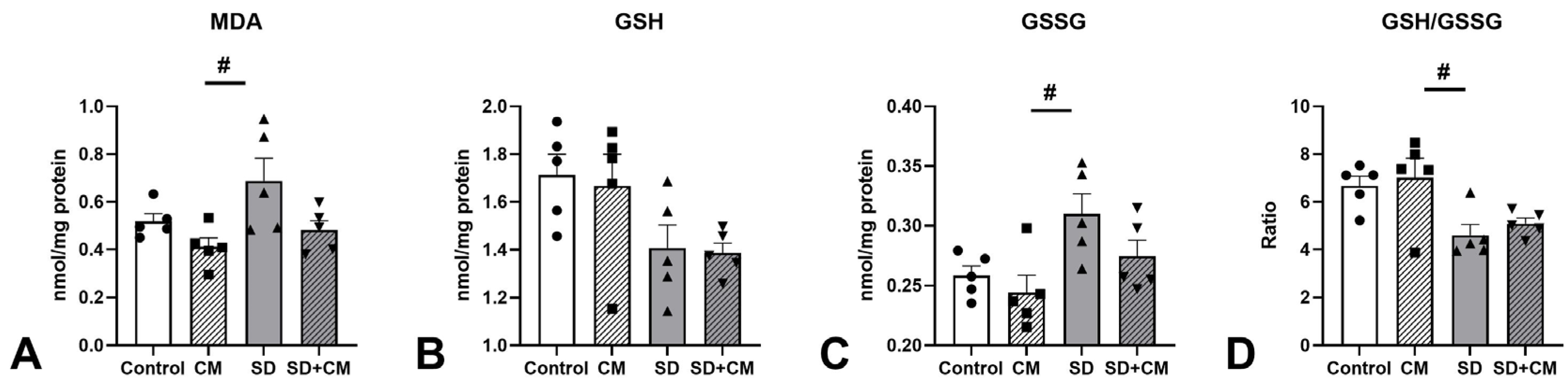
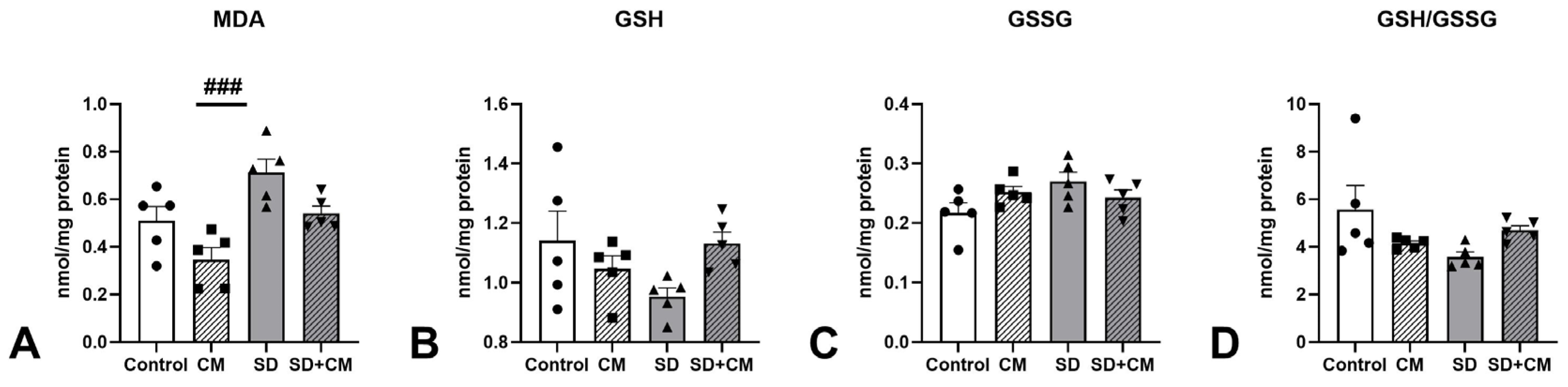
3.5. Oxidative Stress and Inflammatory Cytokine Changes


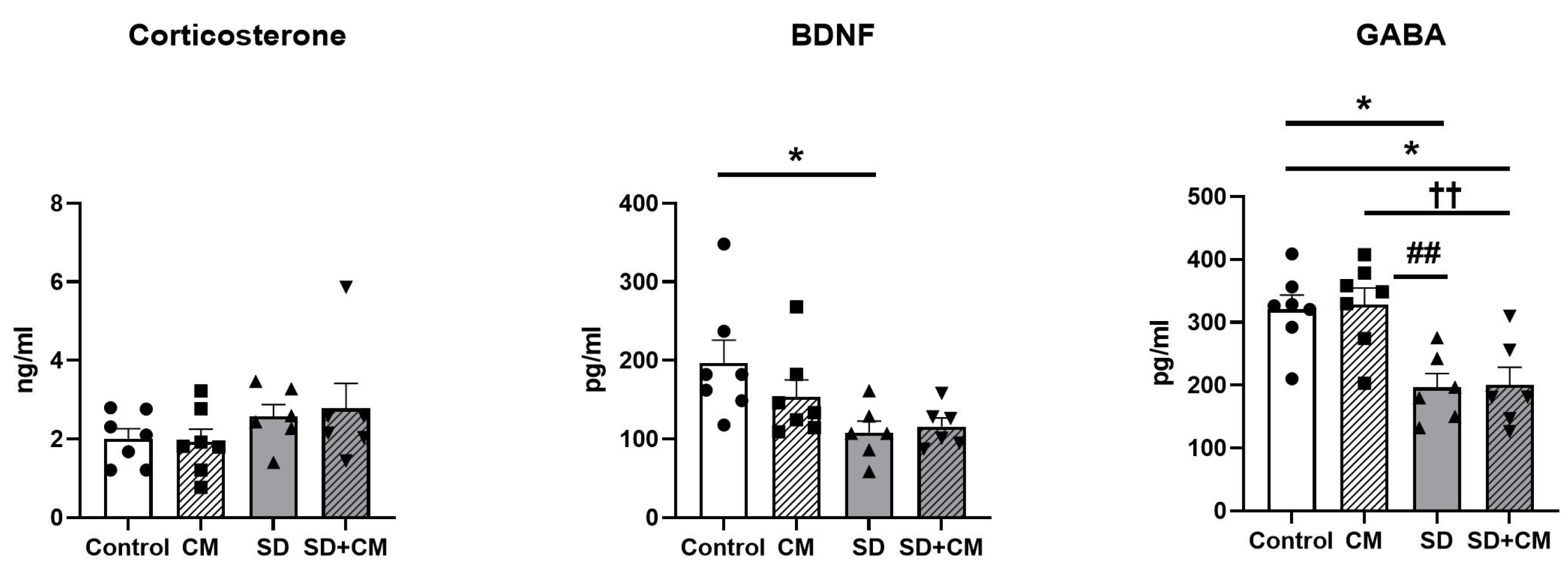
3.6. Serum BDNF, GABA and Corticosterone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neculicioiu, V.S.; Colosi, I.A.; Costache, C.; Sevastre-Berghian, A.; Clichici, S. Time to Sleep?—A Review of the Impact of the COVID-19 Pandemic on Sleep and Mental Health. Int. J. Environ. Res. Public Health 2022, 19, 3497. [Google Scholar] [CrossRef] [PubMed]
- Pankowska, M.M.; Lu, H.; Wheaton, A.G.; Liu, Y.; Lee, B.; Greenlund, K.J.; Carlson, S.A. Prevalence and Geographic Patterns of Self-Reported Short Sleep Duration Among US Adults, 2020. Prev. Chronic Dis. 2023, 20, E53. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, S.A.; Wheaton, A.G.; Li, J.; Michael, S.L. Sleep Duration, Mental Health, and Increased Difficulty Doing Schoolwork Among High School Students During the COVID-19 Pandemic. Prev. Chronic Dis. 2023, 20, E14. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. High School Youth Risk Behavior Survey Data. Available online: https://nccd.cdc.gov/Youthonline (accessed on 11 November 2024).
- Basner, M.; Rao, H.; Goel, N.; Dinges, D.F. Sleep Deprivation and Neurobehavioral Dynamics. Curr. Opin. Neurobiol. 2013, 23, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short Sleep Duration and Health Outcomes: A Systematic Review, Meta-Analysis, and Meta-Regression. Sleep Med. 2017, 32, 246–256. [Google Scholar] [CrossRef]
- Holmer, B.J.; Lapierre, S.S.; Jake-Schoffman, D.E.; Christou, D.D. Effects of Sleep Deprivation on Endothelial Function in Adult Humans: A Systematic Review. Geroscience 2021, 43, 137–158. [Google Scholar] [CrossRef]
- Antza, C.; Kostopoulos, G.; Mostafa, S.; Nirantharakumar, K.; Tahrani, A. The Links between Sleep Duration, Obesity and Type 2 Diabetes Mellitus. J. Endocrinol. 2021, 252, 125–141. [Google Scholar] [CrossRef]
- Zamore, Z.; Veasey, S.C. Neural Consequences of Chronic Sleep Disruption. Trends Neurosci. 2022, 45, 678–691. [Google Scholar] [CrossRef]
- Hill, V.M.; O’Connor, R.M.; Sissoko, G.B.; Irobunda, I.S.; Leong, S.; Canman, J.C.; Stavropoulos, N.; Shirasu-Hiza, M. A Bidirectional Relationship between Sleep and Oxidative Stress in Drosophila. PLoS Biol. 2018, 16, e2005206. [Google Scholar] [CrossRef]
- Neculicioiu, V.S.; Colosi, I.A.; Costache, C.; Toc, D.A.; Sevastre-Berghian, A.; Colosi, H.A.; Clichici, S. Sleep Deprivation-Induced Oxidative Stress in Rat Models: A Scoping Systematic Review. Antioxidants 2023, 12, 1600. [Google Scholar] [CrossRef]
- Villafuerte, G.; Miguel-Puga, A.; Rodríguez, E.M.; Machado, S.; Manjarrez, E.; Arias-Carrión, O. Sleep Deprivation and Oxidative Stress in Animal Models: A Systematic Review. Oxid. Med. Cell. Longev. 2015, 2015, 234952. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Medoro, A.; Savino, R.; Scapagnini, G. Sleep and Oxidative Stress: Current Perspectives on the Role of NRF2. Cell. Mol. Neurobiol. 2024, 44, 52. [Google Scholar] [CrossRef] [PubMed]
- Krsnik, S.; Erjavec, K. Factors Influencing Use of Medicinal Herbs. J. Patient Exp. 2024, 11, 23743735241241180. [Google Scholar] [CrossRef]
- Imtiaz, I.; Schloss, J.; Bugarcic, A. Traditional and Contemporary Herbal Medicines in Management of Cancer: A Scoping Review. J. Ayurveda Integr. Med. 2024, 15, 100904. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Kramarova, D.; Jurikova, T. Selected Cultivars of Cornelian Cherry (Cornus mas L.) as a New Food Source for Human Nutrition. Afr. J. Biotechnol. 2010, 9, 1205–1210. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Cuyamendous, C.; Bonesi, M.; Sicari, V.; Trabalzini, L.; Mitaine-Offer, A.-C.; Xiao, J.; Tundis, R. An Overview of Traditional Uses, Phytochemical Compositions and Biological Activities of Edible Fruits of European and Asian Cornus Species. Foods 2022, 11, 1240. [Google Scholar] [CrossRef]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (Cornelian Cherry), an Important European and Asian Traditional Food and Medicine: Ethnomedicine, Phytochemistry and Pharmacology for Its Commercial Utilization in Drug Industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef]
- Bayram, H.M.; Iliaz, R.; Gunes, F.E. Effects of Cornus mas L. on Anthropometric and Biochemical Parameters among Metabolic Associated Fatty Liver Disease Patients: Randomized Clinical Trial. J. Ethnopharmacol. 2024, 318, 117068. [Google Scholar] [CrossRef]
- Yarhosseini, F.; Sangouni, A.A.; Sangsefidi, Z.S.; Hosseinzadeh, M.; Akhondi-Meybodi, M.; Ranjbar, A.; Fallahzadeh, H.; Mozaffari-Khosravi, H. Effect of Cornus mas L. Fruit Extract on Blood Pressure, Anthropometric and Body Composition Indices in Patients with Non-Alcoholic Fatty Liver Disease: A Double-Blind Randomized Controlled Trial. Clin. Nutr. ESPEN 2023, 56, 18–24. [Google Scholar] [CrossRef]
- Aryaeian, N.; Amiri, F.; Rahideh, S.T.; Abolghasemi, J.; Jazayeri, S.; Gholamrezayi, A.; Motevalian, M.; Solaymani-Dodaran, M.; Taghizadeh, M.; Heshmati, E.; et al. The Effect of Cornus mas Extract Consumption on Bone Biomarkers and Inflammation in Postmenopausal Women: A Randomized Clinical Trial. Phytother. Res. 2021, 35, 4425–4432. [Google Scholar] [CrossRef]
- Asgary, S.; Kelishadi, R.; Rafieian-Kopaei, M.; Najafi, S.; Najafi, M.; Sahebkar, A. Investigation of the Lipid-Modifying and Antiinflammatory Effects of Cornus mas L. Supplementation on Dyslipidemic Children and Adolescents. Pediatr. Cardiol. 2013, 34, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M. Synergy and Other Interactions in Phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole Plant Extracts versus Single Compounds for the Treatment of Malaria: Synergy and Positive Interactions. Malar. J. 2011, 10 (Suppl. S1), S4. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, Food and Pharma. Current Knowledge and Directions for Future Research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef]
- Tubaş, F.; Per, S.; Taşdemir, A.; Bayram, A.K.; Yıldırım, M.; Uzun, A.; Saraymen, R.; Gümüş, H.; Elmalı, F.; Per, H. Effects of Cornus mas L. and Morus rubra L. Extracts on Penicillin-Induced Epileptiform Activity: An Electrophysiological and Biochemical Study. Acta Neurobiol. Exp. 2017, 77, 45–56. [Google Scholar] [CrossRef]
- Moldovan, B.; Filip, A.; Clichici, S.; Suharoschi, R.; Bolfa, P.; David, L. Antioxidant Activity of Cornelian Cherry (Cornus mas L.) Fruits Extract and the in Vivo Evaluation of Its Anti-Inflammatory Effects. J. Funct. Foods 2016, 26, 77–87. [Google Scholar] [CrossRef]
- Filip, G.A.; Moldovan, B.; Baldea, I.; Olteanu, D.; Suharoschi, R.; Decea, N.; Cismaru, C.M.; Gal, E.; Cenariu, M.; Clichici, S.; et al. UV-Light Mediated Green Synthesis of Silver and Gold Nanoparticles Using Cornelian Cherry Fruit Extract and Their Comparative Effects in Experimental Inflammation. J. Photochem. Photobiol. B Biol. 2019, 191, 26–37. [Google Scholar] [CrossRef]
- Saei, H.; Hatami, H.; Azarmi, M.; Dehghan, G. Hepatoprotective Effect of Cornus mas Fruits Extract on Serum Biomarkers in Methotrexate-Induced Liver Injury in Male Rats. Pharmacologyonline 2016, 1, 91–98. [Google Scholar]
- Francik, R.; Kryczyk, J.; Krośniak, M.; Berköz, M.; Sanocka, I.; Francik, S. The Neuroprotective Effect of Cornus mas on Brain Tissue of Wistar Rats. Sci. World J. 2014, 2014, 847368. [Google Scholar] [CrossRef]
- Darbandi, N.; Hashemi, A.; Noori, M.; Momeni, H.R. Effect of Cornus mas Fruit Flavonoids on Memory Retention, Level of Plasma Glucose and Lipids in an Intracerebroventricular Streptozotocin-Induced Experimental Alzheimer’s Disease Model in Wistar Rats. Environ. Exp. Biol. 2016, 14, 113–120. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Packer, L., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Moldovan, B.; David, L. Influence of Different Sweeteners on the Stability of Anthocyanins from Cornelian Cherry Juice. Foods 2020, 9, 1266. [Google Scholar] [CrossRef] [PubMed]
- Vlase, A.-M.; Toiu, A.; Tomuță, I.; Vlase, L.; Muntean, D.; Casian, T.; Fizeșan, I.; Nadăș, G.C.; Novac, C.Ș.; Tămaș, M.; et al. Epilobium Species: From Optimization of the Extraction Process to Evaluation of Biological Properties. Antioxidants 2022, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.-M.; Nadăș, G.C.; Filip, G.A.; Vlase, L.; Crișan, G. Influences of Different Extraction Techniques and Their Respective Parameters on the Phytochemical Profile and Biological Activities of Xanthium spinosum L. Extracts. Plants 2022, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Adehuwa-Olabode, A.A.; Sautreau, A.; Vlase, L.; Vlase, A.-M.; Muntean, D. The Phytochemical Analysis and Antioxidant Capacity Determination of Five Hypericum Species. Stud. Univ. Babes-Bolyai Chem. 2022, 67, 17–35. [Google Scholar] [CrossRef]
- Parvu, M.; Vlase, L.; Parvu, A.E.; Rosca-Casian, O.; Gheldiu, A.-M.; Parvu, O. Phenolic Compounds and Antifungal Activity of Hedera helix L. (Ivy) Flowers and Fruits. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 53–58. [Google Scholar] [CrossRef]
- Solcan, M.-B.; Fizeșan, I.; Vlase, L.; Vlase, A.-M.; Rusu, M.E.; Mateș, L.; Petru, A.-E.; Creștin, I.-V.; Tomuțǎ, I.; Popa, D.-S. Phytochemical Profile and Biological Activities of Extracts Obtained from Young Shoots of Blackcurrant (Ribes nigrum L.), European Blueberry (Vaccinium myrtillus L.), and Mountain Cranberry (Vaccinium vitis-idaea L.). Horticulturae 2023, 9, 1163. [Google Scholar] [CrossRef]
- Pașca, D.; Frangiamone, M.; Mangiapelo, L.; Vila-Donat, P.; Mîrza, O.; Vlase, A.-M.; Miere, D.; Filip, L.; Mañes, J.; Loghin, F.; et al. Phytochemical Characterization of Bilberries and Their Potential as a Functional Ingredient to Mitigate Ochratoxin A Toxicity in Cereal-Based Products. Nutrients 2024, 16, 3137. [Google Scholar] [CrossRef]
- Toiu, A.; Vlase, L.; Gheldiu, A.-M.; Vodnar, D.; Oniga, I. Evaluation of the Antioxidant and Antibacterial Potential of Bioactive Compounds from Ajuga reptans Extracts. Farmacia 2017, 65, 351–355. [Google Scholar]
- Hanganu, D.; Benedec, D.; Vlase, L.; Popica, I.; Bele, C.; Raita, O.; Gheldiu, A.-M.; Mihali, C.V.; Țărmure, V. Polyphenolic Content and Antioxidant Activity of Chrysanthemum parthenium Extract. Farmacia 2016, 64, 498–501. [Google Scholar]
- Machado, R.B.; Hipólide, D.C.; Benedito-Silva, A.A.; Tufik, S. Sleep Deprivation Induced by the Modified Multiple Platform Technique: Quantification of Sleep Loss and Recovery. Brain Res. 2004, 1004, 45–51. [Google Scholar] [CrossRef]
- Yujra, V.Q.; Moreira Antunes, H.K.; Mônico-Neto, M.; Quintana, H.T.; de Oliveira, F.; Galvani, M.G.; Lee, K.S.; Oshima, C.T.F.; Ribeiro, D.A. Paradoxical Sleep Deprivation Induces Differential Biological Response in Rat Masticatory Muscles: Inflammation, Autophagy and Myogenesis. J. Oral Rehabil. 2020, 47, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Song, J.; Wang, H.; Shi, F.; Zhou, N.; Jiang, J.; Xu, Y.; Zhang, L.; Yang, L.; Zhou, M. Chronic Paradoxical Sleep Deprivation-Induced Depression-like Behavior, Energy Metabolism and Microbial Changes in Rats. Life Sci. 2019, 225, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, M.; Lee, S.J.; Lee, E.; Lee, S.A.; Lee, J.D.; Choi, J.H.; Kim, B.G. Effect of Sleep Deprivation on Hearing Levels in Rats. Int. J. Pediatr. Otorhinolaryngol. 2018, 112, 169–175. [Google Scholar] [CrossRef]
- Gamberini, M.T.; Rodrigues, D.S.; Rodrigues, D.; Pontes, V.B. Effects of the Aqueous Extract of Pimpinella anisum L. Seeds on Exploratory Activity and Emotional Behavior in Rats Using the Open Field and Elevated plus Maze Tests. J. Ethnopharmacol. 2015, 168, 45–49. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The Use of the Elevated Plus Maze as an Assay of Anxiety-Related Behavior in Rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Conti, M.; Morand, P.C.; Levillain, P.; Lemonnier, A. Improved Fluorometric Determination of Malonaldehyde. Clin. Chem. 1991, 37, 1273–1275. [Google Scholar] [CrossRef]
- Vats, P.; Singh, V.K.; Singh, S.N.; Singh, S.B. Glutathione Metabolism under High-Altitude Stress and Effect of Antioxidant Supplementation. Aviat. Space Environ. Med. 2008, 79, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.Z.; Szumny, A.; Sokół-Łętowska, A.; Piórecki, N.; Klymenko, S.V. Iridoids and Anthocyanins in Cornelian Cherry (Cornus mas L.) Cultivars. J. Food Compos. Anal. 2015, 40, 95–102. [Google Scholar] [CrossRef]
- Lidiková, J.; Čeryová, N.; Grygorieva, O.; Bobková, A.; Bobko, M.; Árvay, J.; Šnirc, M.; Brindza, J.; Ňorbová, M.; Harangozo, Ľ.; et al. Cornelian Cherry (Cornus mas L.) as a Promising Source of Antioxidant Phenolic Substances and Minerals. Eur. Food Res. Technol. 2024, 250, 1745–1754. [Google Scholar] [CrossRef]
- Andrabi, M.; Andrabi, M.M.; Kunjunni, R.; Sriwastva, M.K.; Bose, S.; Sagar, R.; Srivastava, A.K.; Mathur, R.; Jain, S.; Subbiah, V. Lithium Acts to Modulate Abnormalities at Behavioral, Cellular, and Molecular Levels in Sleep Deprivation-Induced Mania-like Behavior. Bipolar Disord. 2020, 22, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Mirabzadeh, Y.; Khesali, G.; Ebrahimkhani, Z.; Karimi, H.; Vaseghi, S. Chronic REM Sleep Deprivation Leads to Manic- and OCD-Related Behaviors, and Decreases Hippocampal BDNF Expression in Female Rats. Psychopharmacology 2024, 241, 1345–1363. [Google Scholar] [CrossRef] [PubMed]
- Abrial, E.; Bétourné, A.; Etiévant, A.; Lucas, G.; Scarna, H.; Lambás-Señas, L.; Haddjeri, N. Protein Kinase C Inhibition Rescues Manic-like Behaviors and Hippocampal Cell Proliferation Deficits in the Sleep Deprivation Model of Mania. Int. J. Neuropsychopharmacol. 2014, 18, pyu031. [Google Scholar] [CrossRef]
- Benedetti, F.; Fresi, F.; Maccioni, P.; Smeraldi, E. Behavioural Sensitization to Repeated Sleep Deprivation in a Mice Model of Mania. Behav. Brain Res. 2008, 187, 221–227. [Google Scholar] [CrossRef]
- Valvassori, S.S.; Resende, W.R.; Dal-Pont, G.; Sangaletti-Pereira, H.; Gava, F.F.; Peterle, B.R.; Carvalho, A.F.; Varela, R.B.; Dal-Pizzol, F.; Quevedo, J. Lithium Ameliorates Sleep Deprivation-Induced Mania-like Behavior, Hypothalamic-Pituitary-Adrenal (HPA) Axis Alterations, Oxidative Stress and Elevations of Cytokine Concentrations in the Brain and Serum of Mice. Bipolar Disord. 2017, 19, 246–258. [Google Scholar] [CrossRef]
- Pires, G.N.; Bezerra, A.G.; Tufik, S.; Andersen, M.L. Effects of Experimental Sleep Deprivation on Anxiety-like Behavior in Animal Research: Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2016, 68, 575–589. [Google Scholar] [CrossRef]
- Pires, G.N.; Bezerra, A.G.; Tufik, S.; Andersen, M.L. Effects of Acute Sleep Deprivation on State Anxiety Levels: A Systematic Review and Meta-Analysis. Sleep Med. 2016, 24, 109–118. [Google Scholar] [CrossRef]
- Jung, T.; Noh, J. Alteration of Fear Behaviors in Sleep-Deprived Adolescent Rats: Increased Fear Expression and Delayed Fear Extinction. Animal Cells Syst. 2021, 25, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, F.; Liu, Y.; Ma, J.; Yu, X.; Wu, X.; Zhang, W.; Li, D.; Chen, D.; Dai, N.; et al. Modified Si-Ni-San Decoction Ameliorates Central Fatigue by Improving Mitochondrial Biogenesis in the Rat Hippocampus. Evidence-Based Complement. Altern. Med. 2018, 2018, 9452127. [Google Scholar] [CrossRef]
- Dai, W.; Xiao, Y.; Tu, Y.; Xiao, F.; Lu, Y.; Qin, Y.; Xie, Y. Propofol Protects Hippocampal Neurons in Sleep-Deprived Rats by Inhibiting Mitophagy and Autophagy. Ann. Transl. Med. 2021, 9, 1427. [Google Scholar] [CrossRef]
- Lu, Y.; Xiao, Y.; Tu, Y.; Dai, W.; Xie, Y. Propofol-Induced Sleep Ameliorates Cognition Impairment in Sleep-Deprived Rats. Sleep Breath. 2023, 27, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-H.; Yan, Y.-N.; Williams, J.P.; Guo, J.; Ma, B.-F.; An, J.-X. Dexmedetomidine Prevents Spatial Learning and Memory Impairment Induced by Chronic REM Sleep Deprivation in Rats. Sleep Biol. Rhythms 2023, 21, 347–357. [Google Scholar] [CrossRef]
- Wang, L.; Gu, Y.; Zhang, J.; Gong, L. Effects of Sleep Deprivation (SD) on Rats via ERK1/2 Signaling Pathway. Med. Sci. Monit. 2019, 25, 2886–2895. [Google Scholar] [CrossRef] [PubMed]
- Aboufares El Alaoui, A.; Buhl, E.; Galizia, S.; Hodge, J.J.L.; de Vivo, L.; Bellesi, M. Increased Interaction between Endoplasmic Reticulum and Mitochondria Following Sleep Deprivation. BMC Biol. 2023, 21, 1. [Google Scholar] [CrossRef]
- De Vivo, L.; Nelson, A.B.; Bellesi, M.; Noguti, J.; Tononi, G.; Cirelli, C. Loss of Sleep Affects the Ultrastructure of Pyramidal Neurons in the Adolescent Mouse Frontal Cortex. Sleep 2016, 39, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Abushov, B.M. Morphofunctional Analysis of the Effects of Total Sleep Deprivation on the CNS in Rats. Neurosci. Behav. Physiol. 2010, 40, 403–409. [Google Scholar] [CrossRef]
- Bellesi, M.; Haswell, J.D.; de Vivo, L.; Marshall, W.; Roseboom, P.H.; Tononi, G.; Cirelli, C. Myelin Modifications after Chronic Sleep Loss in Adolescent Mice. Sleep 2018, 41, zsy034. [Google Scholar] [CrossRef]
- Elvsåshagen, T.; Norbom, L.B.; Pedersen, P.Ø.; Quraishi, S.H.; Bjørnerud, A.; Malt, U.F.; Groote, I.R.; Westlye, L.T. Widespread Changes in White Matter Microstructure after a Day of Waking and Sleep Deprivation. PLoS ONE 2015, 10, e0127351. [Google Scholar] [CrossRef]
- Simayi, R.; Ficiarà, E.; Faniyan, O.; Cerdán Cerdá, A.; Aboufares El Alaoui, A.; Fiorini, R.; Cutignano, A.; Piscitelli, F.; Andújar, P.; Santos, A.; et al. Sleep Loss Impairs Myelin Function by Altering Cholesterol Metabolism in Oligodendroglia. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lan, C.T.; Hsu, J.C.; Ling, E.A. Influence of Sleep Deprivation Coupled with Administration of Melatonin on the Ultrastructure of Rat Pineal Gland. Brain Res. 2001, 910, 1–11. [Google Scholar] [CrossRef]
- Kus, I.; Sarsilmaz, M.; Ozen, O.A.; Turkoglu, A.O.; Pekmez, H.; Songur, A.; Kelestimur, H. Light and Electron Microscopic Examination of Pineal Gland in Rats Exposed to Constant Light and Constant Darkness. Neuro Endocrinol. Lett. 2004, 25, 102–108. [Google Scholar] [PubMed]
- Chen, L.-Y.; Tiong, C.; Tsai, C.-H.; Liao, W.-C.; Yang, S.-F.; Youn, S.-C.; Mai, F.-D.; Chang, H.-M. Early-Life Sleep Deprivation Persistently Depresses Melatonin Production and Bio-Energetics of the Pineal Gland: Potential Implications for the Development of Metabolic Deficiency. Brain Struct. Funct. 2015, 220, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-M.; Lin, H.-C.; Cheng, H.-L.; Liao, C.-K.; Tseng, T.-J.; Renn, T.-Y.; Lan, C.-T.; Chen, L.-Y. Melatonin Successfully Rescues the Hippocampal Molecular Machinery and Enhances Anti-Oxidative Activity Following Early-Life Sleep Deprivation Injury. Antioxidants 2021, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- Reimund, E. The Free Radical Flux Theory of Sleep. Med. Hypotheses 1994, 43, 231–233. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The Importance of Glutathione in Human Disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Everson, C.A.; Henchen, C.J.; Szabo, A.; Hogg, N. Cell Injury and Repair Resulting from Sleep Loss and Sleep Recovery in Laboratory Rats. Sleep 2014, 37, 1929–1940. [Google Scholar] [CrossRef]
- Lingappan, K. NF-ΚB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence Between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Xu, Y.-P.; Tao, Y.-N.; Wu, Y.-P.; Zhang, J.; Jiao, W.; Wang, Y.-H.; Chen, T. Sleep Deprivation Aggravates Brain Injury after Experimental Subarachnoid Hemorrhage via TLR4-MyD88 Pathway. Aging 2021, 13, 3101–3111. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Zhang, M.; Cheng, L.; Ding, L.; Lv, Q.; Huang, Z.; Zhou, J.; Chen, J.; Wang, P.; Zhang, S.; et al. Melatonin Modulates TLR4/MyD88/NF-ΚB Signaling Pathway to Ameliorate Cognitive Impairment in Sleep-Deprived Rats. Front. Pharmacol. 2024, 15, 1430599. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, B.; Wei, M.; Li, Y.; Liu, J.; Ma, L.; Shang, S.; Huo, K.; Wang, J.; Li, R.; et al. Activation of Inflammation Is Associated with Amyloid-β Accumulation Induced by Chronic Sleep Restriction in Rats. J. Alzheimer’s Dis. 2020, 74, 759–773. [Google Scholar] [CrossRef]
- Irwin, M.R. Sleep Disruption Induces Activation of Inflammation and Heightens Risk for Infectious Disease: Role of Impairments in Thermoregulation and Elevated Ambient Temperature. Temperature 2023, 10, 198–234. [Google Scholar] [CrossRef]
- Uğur, Y. Extraction and Quantification of Melatonin in Cornelian Cherry (Cornus mas L.) by Ultra-Fast Liquid Chromatography Coupled to Fluorescence Detector (UFLC-FD). Acta Chromatogr. 2023, 35, 219–226. [Google Scholar] [CrossRef]
- Garrido, M.; Paredes, S.D.; Cubero, J.; Lozano, M.; Toribio-Delgado, A.F.; Muñoz, J.L.; Reiter, R.J.; Barriga, C.; Rodríguez, A.B. Jerte Valley Cherry-Enriched Diets Improve Nocturnal Rest and Increase 6-Sulfatoxymelatonin and Total Antioxidant Capacity in the Urine of Middle-Aged and Elderly Humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 909–914. [Google Scholar] [CrossRef]
- Garrido, M.; Espino, J.; González-Gómez, D.; Lozano, M.; Barriga, C.; Paredes, S.D.; Rodríguez, A.B. The Consumption of a Jerte Valley Cherry Product in Humans Enhances Mood, and Increases 5-Hydroxyindoleacetic Acid but Reduces Cortisol Levels in Urine. Exp. Gerontol. 2012, 47, 573–580. [Google Scholar] [CrossRef]
- Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of Tart Cherry Juice (Prunus cerasus) on Melatonin Levels and Enhanced Sleep Quality. Eur. J. Nutr. 2012, 51, 909–916. [Google Scholar] [CrossRef]
- Chuang, J.I.; Lin, M.T. Pharmacological Effects of Melatonin Treatment on Both Locomotor Activity and Brain Serotonin Release in Rats. J. Pineal Res. 1994, 17, 11–16. [Google Scholar] [CrossRef]
- Shanmugasundaram, J.; Subramanian, V.; Nadipelly, J.S.; Kathirvelu, P.; Sayeli, V.; Cheriyan, B.V. Sedative-Hypnotic like Effect of 5-Methoxyflavone in Mice and Investigation on Possible Mechanisms by In Vivo and In Silico Methods. Biomed. Pharmacother. 2018, 108, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Al Hasan, M.S.; Bhuia, M.S.; Chowdhury, R.; Husain, Z.; Saifuzzaman, M.; Mia, E.; Akbor, M.S.; Yana, N.T.; Islam, M.A.; Ansari, S.A.; et al. Tangeretin Enhances Sedative Activity of Diazepam in Swiss Mice through GABAA Receptor Interaction: In Vivo and in Silico Approaches. Neuroscience 2025, 572, 1–10. [Google Scholar] [CrossRef]
- Nogueira Neto, J.D.; de Almeida, A.A.C.; da Silva Oliveira, J.; Dos Santos, P.S.; de Sousa, D.P.; de Freitas, R.M. Antioxidant Effects of Nerolidol in Mice Hippocampus after Open Field Test. Neurochem. Res. 2013, 38, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. Medicinal Plants Possess Sedative and Anxiolytic Effect with Emphasis on Their Mechanisms of Action. GSC Biol. Pharm. Sci. 2021, 17, 61–77. [Google Scholar] [CrossRef]
- Zamyad, M.; Abasnejad, M.; Esmaeili-Mahani, S.; Mostafavi, A. Alpha-Pinene as the Main Component of Ducrosia anethifolia (Boiss) Essential Oil Is Responsible for Its Effect on Locomotor Activity in Rats. Avicenna J. Neuro Psycho Physiol. 2016, 3, 29–34. [Google Scholar] [CrossRef]
- Fernández, S.P.; Wasowski, C.; Loscalzo, L.M.; Granger, R.E.; Johnston, G.A.R.; Paladini, A.C.; Marder, M. Central Nervous System Depressant Action of Flavonoid Glycosides. Eur. J. Pharmacol. 2006, 539, 168–176. [Google Scholar] [CrossRef]
- Czerwińska, M.E.; Melzig, M.F. Cornus mas and Cornus officinalis—Analogies and Differences of Two Medicinal Plants Traditionally Used. Front. Pharmacol. 2018, 9, 894. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qiao, H.; Han, Y.; Shi, R.; He, J.; Zhang, W. Loganin, a Major Iridoid Glycoside Obtained from Corni fructus, Exhibits Sleep-Enhancing Effects in Rodents. In Proceedings of the Annual Meeting of the Japanese Pharmacological Society, WCP2018_PO2-12-15, Kyoto, Japan, 1–6 July 2018. [Google Scholar] [CrossRef]
- Shi, R.; Han, Y.; Yan, Y.; Qiao, H.-Y.; He, J.; Lian, W.-W.; Xia, C.-Y.; Li, T.-L.; Zhang, W.-K.; Xu, J.-K. Loganin Exerts Sedative and Hypnotic Effects via Modulation of the Serotonergic System and GABAergic Neurons. Front. Pharmacol. 2019, 10, 409. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; ALSalamat, H.A.; Bashatwah, R.M. Reactive Oxygen Species: The Dual Role in Physiological and Pathological Conditions of the Human Body. Eurasian J. Med. 2018, 50, 193–201. [Google Scholar] [CrossRef]
- Vicente-Gutierrez, C.; Bonora, N.; Bobo-Jimenez, V.; Jimenez-Blasco, D.; Lopez-Fabuel, I.; Fernandez, E.; Josephine, C.; Bonvento, G.; Enriquez, J.A.; Almeida, A.; et al. Astrocytic Mitochondrial ROS Modulate Brain Metabolism and Mouse Behaviour. Nat. Metab. 2019, 1, 201–211. [Google Scholar] [CrossRef]
- Chen, P.; Wu, H.; Yao, H.; Zhang, J.; Fan, W.; Chen, Z.; Su, W.; Wang, Y.; Li, P. Multi-Omics Analysis Reveals the Systematic Relationship Between Oral Homeostasis and Chronic Sleep Deprivation in Rats. Front. Immunol. 2022, 13, 847132. [Google Scholar] [CrossRef] [PubMed]
- Torabi-Nami, M.; Nasehi, M.; Razavi, S.; Zarrindast, M.R. Aversive Memory, Anxiety-Related Behaviors, and Serum Neurochemical Levels in a Rat Model of Labored Sleep Loss. Shiraz E-Med. J. 2014, 15, e22358. [Google Scholar] [CrossRef]
- Schmitt, K.; Holsboer-Trachsler, E.; Eckert, A. BDNF in Sleep, Insomnia, and Sleep Deprivation. Ann. Med. 2016, 48, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.S.; Molendijk, M.L.; Köhler, C.A.; Soares, J.C.; Leite, C.M.G.S.; Machado-Vieira, R.; Ribeiro, T.L.; Silva, J.C.; Sales, P.M.G.; Quevedo, J.; et al. Peripheral Brain-Derived Neurotrophic Factor (BDNF) as a Biomarker in Bipolar Disorder: A Meta-Analysis of 52 Studies. BMC Med. 2015, 13, 289. [Google Scholar] [CrossRef]
- Ballesio, A.; Zagaria, A.; Curti, D.G.; Moran, R.; Goadsby, P.J.; Rosenzweig, I.; Lombardo, C. Peripheral Brain-Derived Neurotrophic Factor (BDNF) in Insomnia: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2023, 67, 101738. [Google Scholar] [CrossRef]
- Romeo, B.; Choucha, W.; Fossati, P.; Rotge, J.-Y. Meta-Analysis of Central and Peripheral γ-Aminobutyric Acid Levels in Patients with Unipolar and Bipolar Depression. J. Psychiatry Neurosci. 2018, 43, 58–66. [Google Scholar] [CrossRef]
- Nollet, M.; Wisden, W.; Franks, N.P. Sleep Deprivation and Stress: A Reciprocal Relationship. Interface Focus 2020, 10, 20190092. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Hu, M. Commentary: Bioavailability of Flavonoids and Polyphenols: Call to Arms. Mol. Pharm. 2007, 4, 803–806. [Google Scholar] [CrossRef]
- Wang, P.; Zou, L.; Han, H.; Zhang, T. A Simple and Sensitive LC–MS/MS Method for the Determination of Loganic Acid in Rat Plasma and Its Pharmacokinetic Analysis. Biomed. Chromatogr. 2024, 38, e5951. [Google Scholar] [CrossRef]
- Isabel, U.-V.; de la Riera, M.B.A.; Dolores, R.S.; Elena, G.-B. A New Frontier in Neuropharmacology: Recent Progress in Natural Products Research for Blood–Brain Barrier Crossing. Curr. Res. Biotechnol. 2024, 8, 100235. [Google Scholar] [CrossRef]


| Total Polyphenol Content (g GAE/1000 mL) | Antioxidant Activity—DPPH (mg TE/mL) | |
|---|---|---|
| C. mas fruit extract | 25.5 ± 0.6639 | 4.8497 ± 0.0094 |
| Chemical Class | Individual Compounds | Concentration (µg/mL) | |
|---|---|---|---|
| Phenolic acids | Hydroxycinnamic acids | Caftaric acid | 35.992 ± 0.71984 |
| Gentisic acid | 2.922 ± 0.35064 | ||
| Caffeic acid | 70.998 ± 7.80978 | ||
| Chlorogenic acid | 70.647 ± 9.18411 | ||
| Hydroxybenzoic acids | Gallic acid | 49.49 ± 6.4337 | |
| Protocatechuic acid | 13.97 ± 1.5367 | ||
| Flavonoids | Flavonols | Quercetin | <LOQ * |
| Kaempferol | <LOQ * | ||
| Flavonol glycosides | Hyperoside | 3.6 ± 0.324 | |
| Isoquercitrin | 37.476 ± 1.12428 | ||
| Rutoside | 4.74 ± 0.0948 | ||
| Quercitrin | 2.315 ± 0.20835 | ||
| Flavan-3-ols (catechins) | Epicatechin | 0.62 ± 0.0372 | |
| Catechin | 0.05 ± 0.003 | ||
| Epigallocatechin | 0.58 ± 0.0696 | ||
| Epigallocatechin gallate | 1.08 ± 0.0648 | ||
| Anthocyanins | Cyanidins | Cyanidin 3-glucoside | 1162.241 ± 34.867 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neculicioiu, V.S.; Colosi, I.; Sevastre-Berghian, A.; Toc, D.A.; Colosi, H.A.; David, L.; Muntean, M.; Moldovan, R.; Vlase, A.-M.; Toma, V.A.; et al. Molecular Effects of Cornelian Cherry Fruit (Cornus mas L.) Extract on Sleep Deprivation-Induced Oxidative Stress, Cytokine Dysregulation, and Behavioural Changes in Wistar Rats. Curr. Issues Mol. Biol. 2025, 47, 399. https://doi.org/10.3390/cimb47060399
Neculicioiu VS, Colosi I, Sevastre-Berghian A, Toc DA, Colosi HA, David L, Muntean M, Moldovan R, Vlase A-M, Toma VA, et al. Molecular Effects of Cornelian Cherry Fruit (Cornus mas L.) Extract on Sleep Deprivation-Induced Oxidative Stress, Cytokine Dysregulation, and Behavioural Changes in Wistar Rats. Current Issues in Molecular Biology. 2025; 47(6):399. https://doi.org/10.3390/cimb47060399
Chicago/Turabian StyleNeculicioiu, Vlad Sever, Ioana Colosi, Alexandra Sevastre-Berghian, Dan Alexandru Toc, Horațiu Alexandru Colosi, Luminita David, Mara Muntean, Remus Moldovan, Ana-Maria Vlase, Vlad Alexandru Toma, and et al. 2025. "Molecular Effects of Cornelian Cherry Fruit (Cornus mas L.) Extract on Sleep Deprivation-Induced Oxidative Stress, Cytokine Dysregulation, and Behavioural Changes in Wistar Rats" Current Issues in Molecular Biology 47, no. 6: 399. https://doi.org/10.3390/cimb47060399
APA StyleNeculicioiu, V. S., Colosi, I., Sevastre-Berghian, A., Toc, D. A., Colosi, H. A., David, L., Muntean, M., Moldovan, R., Vlase, A.-M., Toma, V. A., Costache, C., Suciu, Ş. M., & Clichici, S. (2025). Molecular Effects of Cornelian Cherry Fruit (Cornus mas L.) Extract on Sleep Deprivation-Induced Oxidative Stress, Cytokine Dysregulation, and Behavioural Changes in Wistar Rats. Current Issues in Molecular Biology, 47(6), 399. https://doi.org/10.3390/cimb47060399











