Mitigation of 3.5 GHz Electromagnetic Field-Induced BV2 Microglial Cytotoxicity by Polydeoxyribonucleotide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
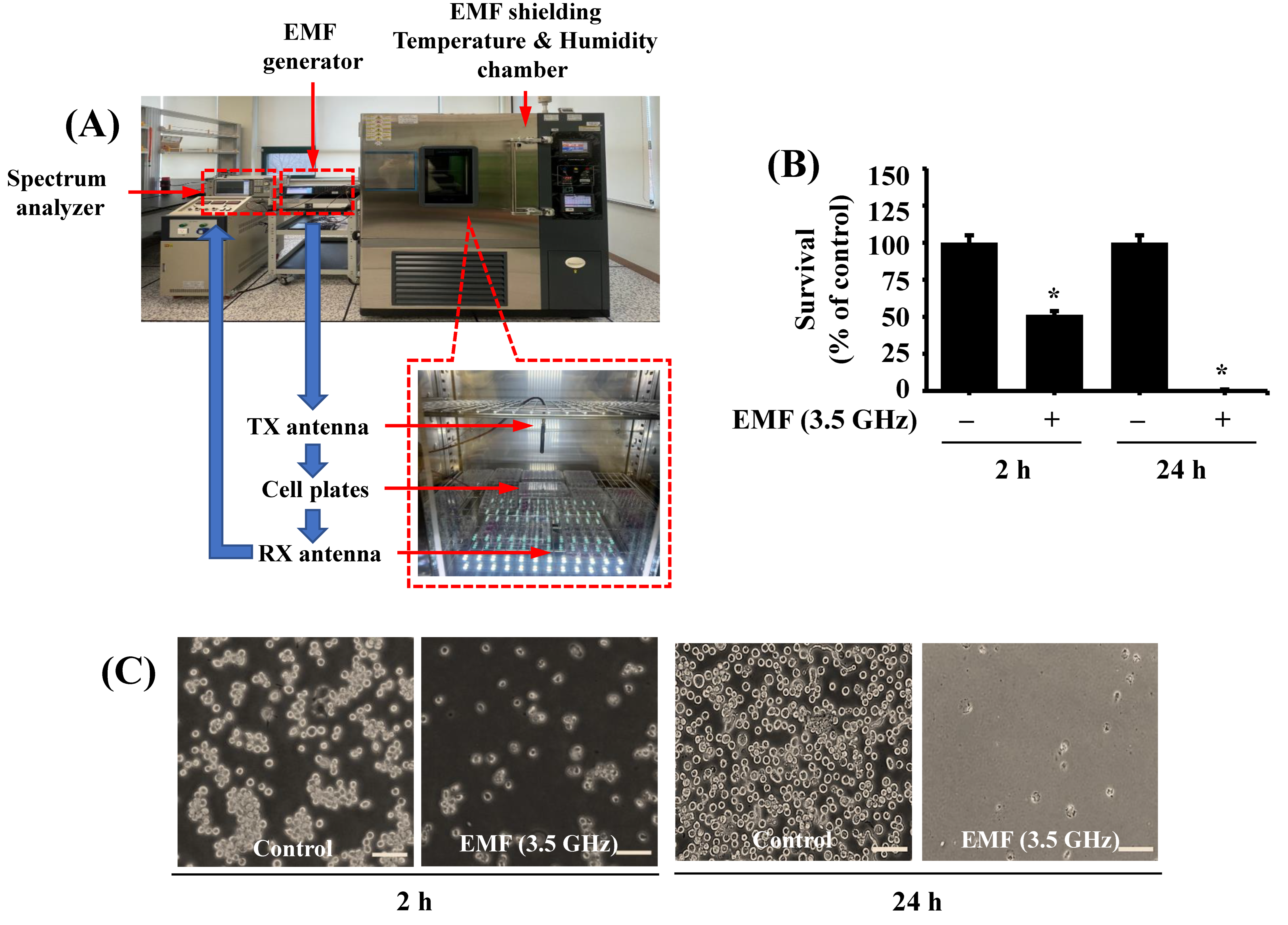
2.3. EMF Exposure Setup
2.4. 3.5 GHz EMF Exposure to BV2 Cells
2.5. Cell Count Analysis
2.6. Measurement of DNA Fragmentation
2.7. Preparation of Whole-Cell Lysates
2.8. Western Blot Analysis
2.9. A2A Receptor Inhibition Using ZM241385
2.10. Measurement of Cellular ROS Levels
2.11. Statistical Analysis
3. Results
3.1. Exposure to 3.5 GHz EMFs for Either 2 or 24 h Significantly Inhibits the Growth of BV2 Microglial Cells
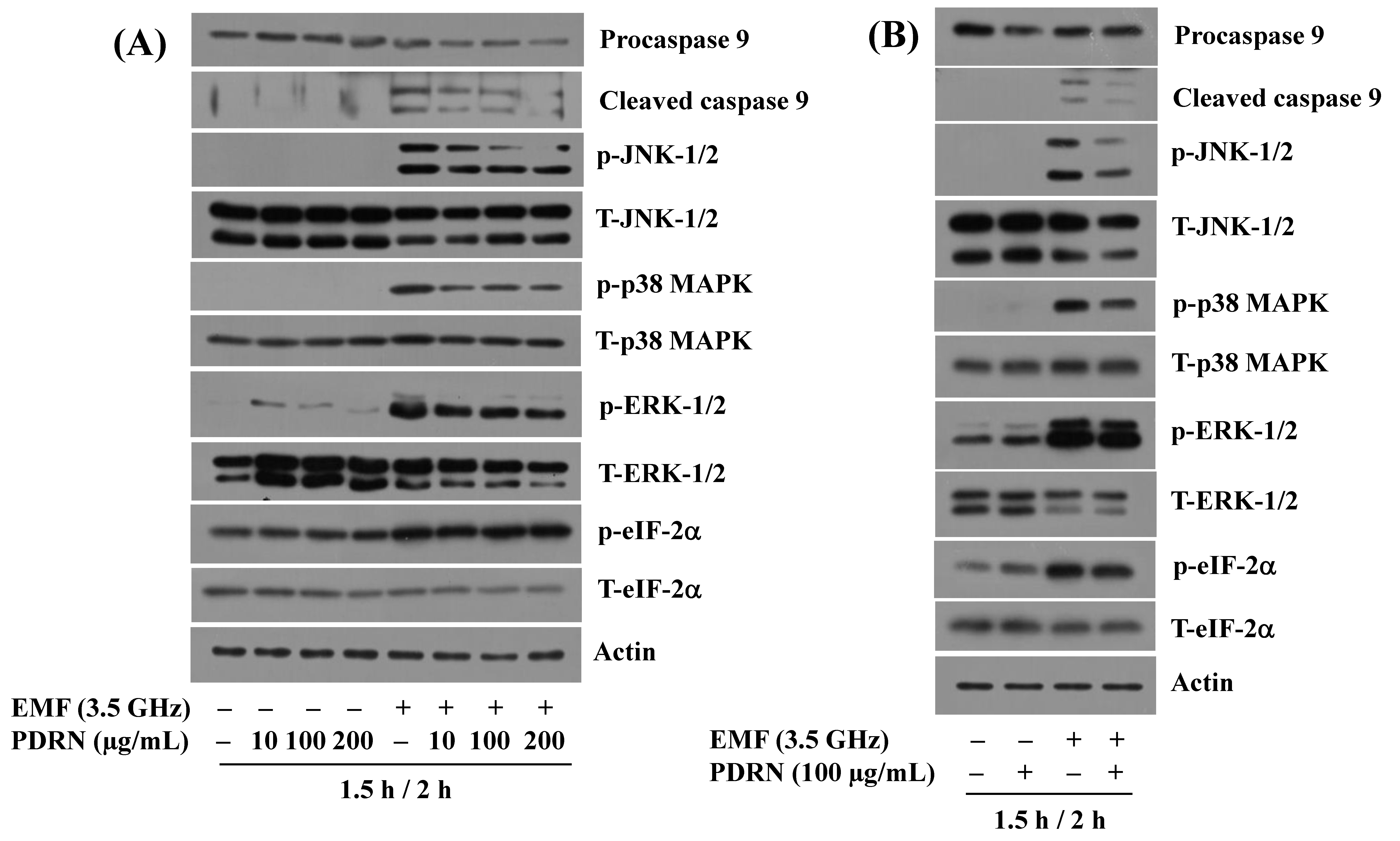
3.2. Exposure to 3.5 GHz EMFs Leads to the Altered Phosphorylation and Expression Levels of JNK-1/2, ERK-1/2, p38 MAPK, eIF-2α, and Procaspase-9 in BV2 Murine Microglial Cells
3.3. PDRN Blocks 3.5 GHz EMF-Induced Growth Inhibition and Apoptosis in BV2 Murine Microglial Cells
3.4. PDRN Inhibits 3.5 GHz EMF-Induced Generation of Cleaved Caspase-9, Phosphorylation of JNK-1/2, p38 MAPK, and ERK-1/2 in BV2 Murine Microglial Cells
3.5. Activation of JNK-1/2 and p38 MAPK Is Crucial for 3.5 GHz EMF-Induced Growth Inhibition of BV2 Murine Microglial Cells
3.6. PDRN’s Blockage Effect on 3.5 GHz EMF-Induced Growth Inhibition of BV2 Murine Microglial Cells Is Dependent on the A2AR Pathway
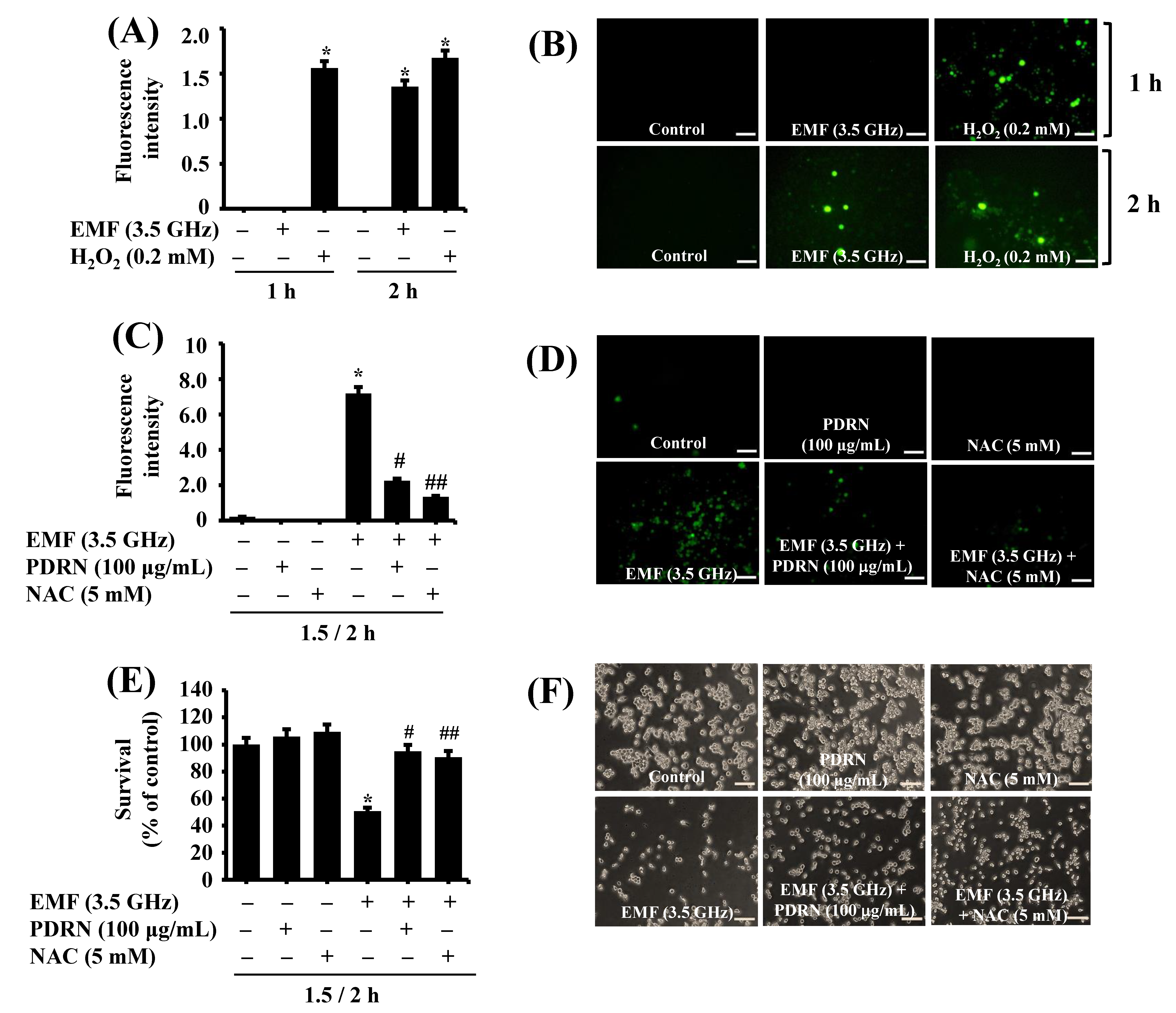
3.7. PDRN Significantly Inhibits 3.5 GHz EMF-Induced ROS Production and Growth Suppression of BV2 Murine Microglial Cells
4. Discussion
Study Limitations and Future Directions
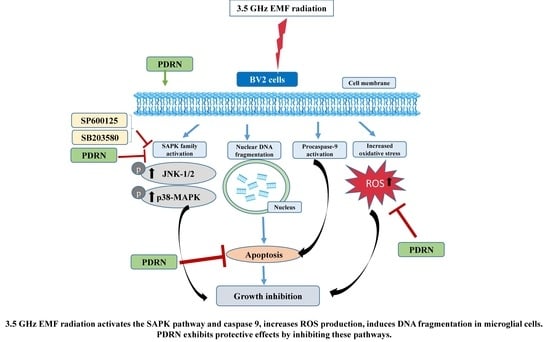
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Kapoor, N. Health implications of electromagnetic fields, mechanisms of action, and research needs. Adv. Biol. 2014, 2014, 198609. [Google Scholar] [CrossRef]
- Kıvrak, E.G.; Yurt, K.K.; Kaplan, A.A.; Alkan, I.; Altun, G. Effects of electromagnetic fields exposure on the antioxidant defense system. J. Microsc. Ultrastruct. 2017, 5, 167–176. [Google Scholar] [CrossRef]
- Terzi, M.; Ozberk, B.; Deniz, O.G.; Kaplan, S. The role of electromagnetic fields in neurological disorders. J. Chem. Neuroanat. 2016, 75, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Ge, H.; Zhao, H.; Zou, Y.; Chen, Y.; Feng, H. Electromagnetic fields for the regulation of neural stem cells. Stem Cells Int. 2017, 2017, 9898439. [Google Scholar] [CrossRef]
- van der Meer, J.N.; Eisma, Y.B.; Meester, R.; Jacobs, M.; Nederveen, A.J. Effects of mobile phone electromagnetic fields on brain waves in healthy volunteers. Sci. Rep. 2023, 13, 21758. [Google Scholar] [CrossRef] [PubMed]
- Maskey, D.; Kim, H.G.; Suh, M.-W.; Roh, G.S.; Kim, M.J. Alteration of glycine receptor immunoreactivity in the auditory brainstem of mice following three months of exposure to radiofrequency radiation at SAR 4.0 W/kg. Int. J. Mol. Med. 2014, 34, 409–419. [Google Scholar] [CrossRef]
- Hardell, L.; Sage, C. Biological effects from electromagnetic field exposure and public exposure standards. Biomed. Pharmacother. 2008, 62, 104–109. [Google Scholar] [CrossRef]
- Su, L.; Yimaer, A.; Xu, Z.; Chen, G. Effects of 1800 MHz RF-EMF exposure on DNA damage and cellular functions in primary cultured neurogenic cells. Int. J. Radiat. Biol. 2018, 94, 295–305. [Google Scholar] [CrossRef]
- He, G.-L.; Luo, Z.; Shen, T.-T.; Li, P.; Yang, J.; Luo, X.; Chen, C.-H.; Gao, P.; Yang, X.-S. Inhibition of STAT3-and MAPK-dependent PGE 2 synthesis ameliorates phagocytosis of fibrillar β-amyloid peptide (1-42) via EP2 receptor in EMF-stimulated N9 microglial cells. J. Neuroinflammation 2016, 13, 296. [Google Scholar] [CrossRef]
- Zuo, H.; Lin, T.; Wang, D.; Peng, R.; Wang, S.; Gao, Y.; Xu, X.; Li, Y.; Wang, S.; Zhao, L. Neural cell apoptosis induced by microwave exposure through mitochondria-dependent caspase-3 pathway. Int. J. Med. Sci. 2014, 11, 426. [Google Scholar] [CrossRef]
- Budziosz, J.; Stanek, A.; Sieroń, A.; Witkoś, J.; Cholewka, A.; Sieroń, K. Effects of Low-Frequency Electromagnetic Field on Oxidative Stress in Selected Structures of the Central Nervous System. Oxidative Med. Cell. Longev. 2018, 2018, 1427412. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological activity and clinical use of PDRN. Front. Pharmacol. 2017, 8, 224. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, M.-J.; Kweon, D.-K.; Lim, S.-T.; Lee, S.-J. Polydeoxyribonucleotide activates mitochondrial biogenesis but reduces MMP-1 activity and melanin biosynthesis in cultured skin cells. Appl. Biochem. Biotechnol. 2020, 191, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, M.T.; Galli, C.; Guizzardi, S. Polydeoxyribonucleotide regulation of inflammation. Adv. Wound Care 2020, 9, 576–589. [Google Scholar] [CrossRef]
- Bitto, A.; Oteri, G.; Pisano, M.; Polito, F.; Irrera, N.; Minutoli, L.; Squadrito, F.; Altavilla, D. Adenosine receptor stimulation by polynucleotides (PDRN) reduces inflammation in experimental periodontitis. J. Clin. Periodontol. 2013, 40, 26–32. [Google Scholar] [CrossRef]
- Irrera, N.; Arcoraci, V.; Mannino, F.; Vermiglio, G.; Pallio, G.; Minutoli, L.; Bagnato, G.; Anastasi, G.P.; Mazzon, E.; Bramanti, P.; et al. Activation of A2A receptor by PDRN reduces neuronal damage and stimulates WNT/β-CATENIN driven neurogenesis in spinal cord injury. Front. Pharmacol. 2018, 9, 506. [Google Scholar] [CrossRef]
- Ko, I.-G.; Jin, J.-J.; Hwang, L.; Kim, S.-H.; Kim, C.-J.; Jeon, J.W.; Chung, J.-Y.; Han, J.H. Adenosine A2A receptor agonist polydeoxyribonucleotide ameliorates short-term memory impairment by suppressing cerebral ischemia-induced inflammation via MAPK pathway. PLoS ONE 2021, 16, e0248689. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jeon, S.B.; Pack, J.-K.; Kim, N.; Choi, H.-D. An in vitro experimental system for 5G 3.5 GHz exposures. IEEE Access 2022, 10, 94832–94840. [Google Scholar] [CrossRef]
- Jia, H.L.; Chao, W.; Yue, L.; Yan, L.; Wang, P.P.; Pan, W.D.; Tao, S. Combined effects of 50 Hz magnetic field and magnetic nanoparticles on the proliferation and apoptosis of PC12 cells. Biomed. Environ. Sci. 2014, 27, 97–105. [Google Scholar]
- Cuenda, A.; Rouse, J.; Doza, Y.N.; Meier, R.; Cohen, P.; Gallagher, T.F.; Young, P.R.; Lee, J.C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995, 364, 229–233. [Google Scholar]
- Katsoulidis, E.; Li, Y.; Mears, H.; Platanias, L.C. The p38 mitogen-activated protein kinase pathway in interferon signal transduction. J. Interferon Cytokine Res. 2005, 25, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H. Exogenous H2O2 induces growth inhibition and cell death of human pulmonary artery smooth muscle cells via glutathione depletion. Mol. Med. Rep. 2016, 14, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Halasi, M.; Wang, M.; Chavan, T.S.; Gaponenko, V.; Hay, N.; Gartel, A.L. ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochem. J. 2013, 454, 201–208. [Google Scholar] [CrossRef]
- Nittby, H.; Grafström, G.; Eberhardt, J.L.; Malmgren, L.; Brun, A.; Persson, B.R.; Salford, L.G. Radiofrequency and extremely low-frequency electromagnetic field effects on the blood-brain barrier. Electromagn. Biol. Med. 2008, 27, 103–126. [Google Scholar] [CrossRef]
- Jamal, L. Effects of the 5th Generation Radio Frequencies of 3.5 GHz on the Electrical Activity of the Brain and on the Autonomic Nervous System in Healthy Volunteers. Ph.D. thesis, Université de Picardie Jules Verne, Amiens, France, 2023. [Google Scholar]
- Varghese, R.; Majumdar, A.; Kumar, G.; Shukla, A. Rats exposed to 2.45 GHz of non-ionizing radiation exhibit behavioral changes with increased brain expression of apoptotic caspase 3. Pathophysiology 2018, 25, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Consales, C.; Merla, C.; Marino, C.; Benassi, B. Electromagnetic fields, oxidative stress, and neurodegeneration. Int. J. Cell Biol. 2012, 2012, 683897. [Google Scholar] [CrossRef]
- Joushomme, A.; Orlacchio, R.; Patrignoni, L.; Canovi, A.; Chappe, Y.L.; Poulletier De Gannes, F.; Hurtier, A.; Garenne, A.; Lagroye, I.; Moisan, F. Effects of 5G-modulated 3.5 GHz radiofrequency field exposures on HSF1, RAS, ERK, and PML activation in live fibroblasts and keratinocytes cells. Sci. Rep. 2023, 13, 8305. [Google Scholar] [CrossRef]
- Keshet, Y.; Seger, R. The MAP kinase signaling cascades: A system of hundreds of components regulates a diverse array of physiological functions. MAP Kinase Signal. Protoc. Second Ed. 2010, 661, 3–38. [Google Scholar]
- Tsoy, A.; Saliev, T.; Abzhanova, E.; Turgambayeva, A.; Kaiyrlykyzy, A.; Akishev, M.; Saparbayev, S.; Umbayev, B.; Askarova, S. The effects of mobile phone radiofrequency electromagnetic fields on β-amyloid-induced oxidative stress in human and rat primary astrocytes. Neuroscience 2019, 408, 46–57. [Google Scholar] [CrossRef]
- Guo, Y.-J.; Pan, W.-W.; Liu, S.-B.; Shen, Z.-F.; Xu, Y.; Hu, L.-L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.L.; Sonenberg, N.; Parker, R. Neuronal regulation of eIF2α function in health and neurological disorders. Trends Mol. Med. 2018, 24, 575–589. [Google Scholar] [CrossRef]
- McAllister, C.S.; Taghavi, N.; Samuel, C.E. Protein kinase PKR amplification of interferon β induction occurs through initiation factor eIF-2α-mediated translational control. J. Biol. Chem. 2012, 287, 36384–36392. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Cuziol, C.; Philpott, D.J.; Arnoult, D. The eIF2α kinase HRI in innate immunity, proteostasis, and mitochondrial stress. FEBS J. 2021, 288, 3094–3107. [Google Scholar] [CrossRef]
- Baird, T.D.; Wek, R.C. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Adv. Nutr. 2012, 3, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef]
- Troy, C.M.; Akpan, N.; Jean, Y.Y. Regulation of caspases in the nervous system: Implications for functions in health and disease. Prog. Mol. Biol. Transl. Sci. 2011, 99, 265–305. [Google Scholar]
- Kaszuba-Zwoinska, J.; Wojcik, K.; Bereta, M.; Ziomber, A.; Pierzchalski, P.; Rokita, E.; Marcinkiewicz, J.; Zaraska, W.; Thor, P. Pulsating electromagnetic field stimulation prevents cell death of puromycin treated U937 cell line. J. Physiol. Pharmacol. 2010, 61, 201. [Google Scholar]
- Benassi, B.; Filomeni, G.; Montagna, C.; Merla, C.; Lopresto, V.; Pinto, R.; Marino, C.; Consales, C. Extremely low frequency magnetic field (ELF-MF) exposure sensitizes SH-SY5Y cells to the pro-Parkinson’s disease toxin MPP+. Mol. Neurobiol. 2016, 53, 4247–4260. [Google Scholar] [CrossRef]
- Rohn, T.T.; Rissman, R.A.; Davis, M.C.; Kim, Y.E.; Cotman, C.W.; Head, E. Caspase-9 activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol. Dis. 2002, 11, 341–354. [Google Scholar] [CrossRef]
- Gao, Z. Adenosine A2A receptor and glia. Int. Rev. Neurobiol. 2023, 170, 29–48. [Google Scholar] [PubMed]
- Cheng, Y.; Cao, P.; Geng, C.; Chu, X.; Li, Y.; Cui, J. The adenosine A (2A) receptor antagonist SCH58261 protects photoreceptors by inhibiting microglial activation and the inflammatory response. Int. Immunopharmacol. 2022, 112, 109245. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Park, T.J.; Ali, T.; Kim, M.O. Antioxidant and neuroprotective effects of caffeine against Alzheimer’s and Parkinson’s disease: Insight into the role of Nrf-2 and A2AR signaling. Antioxidants 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Pall, M.L. Microwave frequency electromagnetic fields (EMFs) produce widespread neuropsychiatric effects including depression. J. Chem. Neuroanat. 2016, 75, 43–51. [Google Scholar] [CrossRef]
- Kesari, K.K.; Siddiqui, M.; Meena, R.; Verma, H.; Kumar, S. Cell phone radiation exposure on brain and associated biological systems. Indian J. Exp. Biol. 2013, 51, 187–200. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pachhapure, S.; Mufida, A.; Wei, Q.; Choi, J.-S.; Jang, B.-C. Mitigation of 3.5 GHz Electromagnetic Field-Induced BV2 Microglial Cytotoxicity by Polydeoxyribonucleotide. Curr. Issues Mol. Biol. 2025, 47, 386. https://doi.org/10.3390/cimb47060386
Pachhapure S, Mufida A, Wei Q, Choi J-S, Jang B-C. Mitigation of 3.5 GHz Electromagnetic Field-Induced BV2 Microglial Cytotoxicity by Polydeoxyribonucleotide. Current Issues in Molecular Biology. 2025; 47(6):386. https://doi.org/10.3390/cimb47060386
Chicago/Turabian StylePachhapure, Shailashree, Amila Mufida, Qun Wei, Jong-Soon Choi, and Byeong-Churl Jang. 2025. "Mitigation of 3.5 GHz Electromagnetic Field-Induced BV2 Microglial Cytotoxicity by Polydeoxyribonucleotide" Current Issues in Molecular Biology 47, no. 6: 386. https://doi.org/10.3390/cimb47060386
APA StylePachhapure, S., Mufida, A., Wei, Q., Choi, J.-S., & Jang, B.-C. (2025). Mitigation of 3.5 GHz Electromagnetic Field-Induced BV2 Microglial Cytotoxicity by Polydeoxyribonucleotide. Current Issues in Molecular Biology, 47(6), 386. https://doi.org/10.3390/cimb47060386