Neurotransmission Sex Dichotomy in the Rat Hypothalamic Paraventricular Nucleus in Healthy and Infantile Spasm Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Treatments, and Tissue Collection
2.2. Microarray and Data Processing
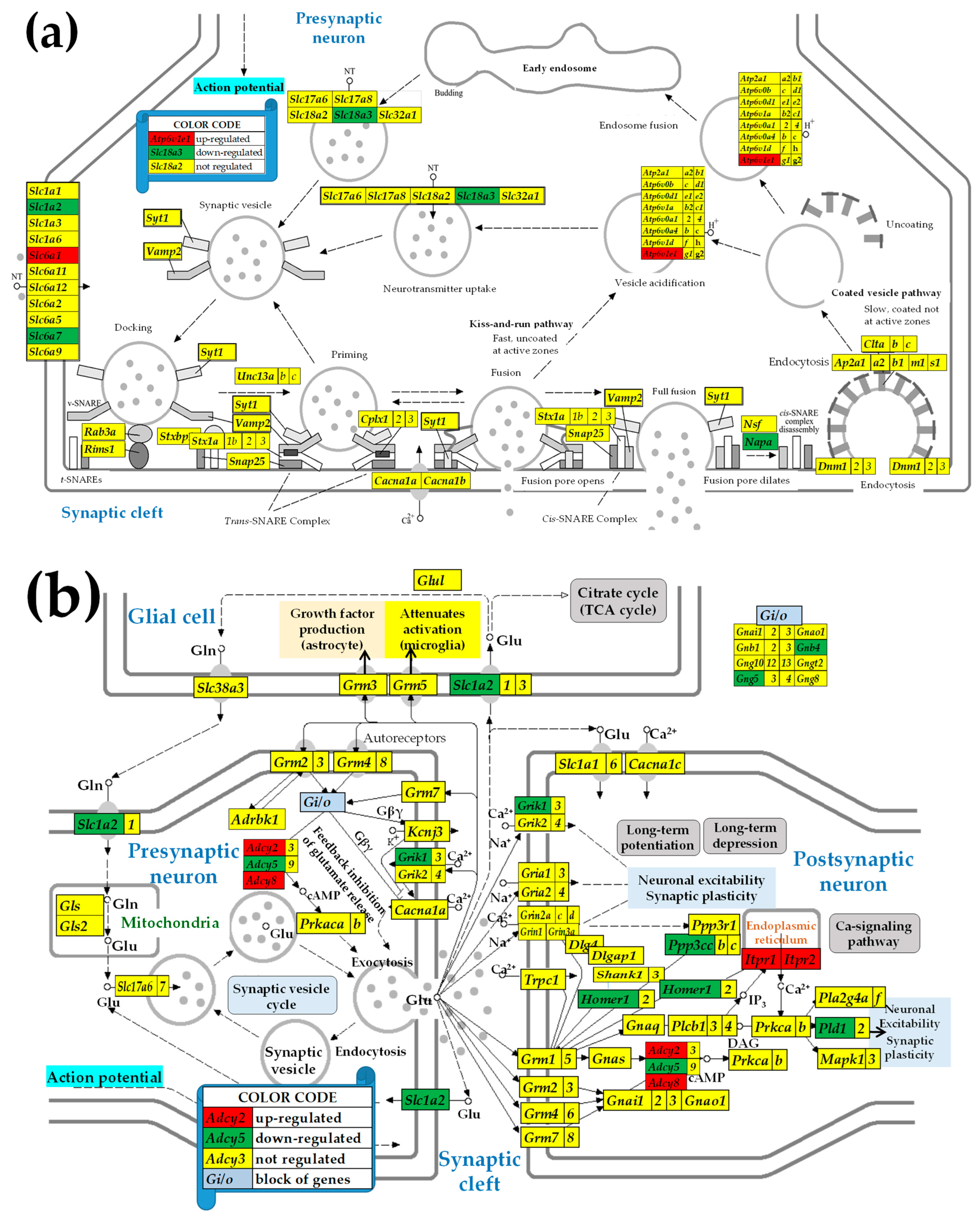
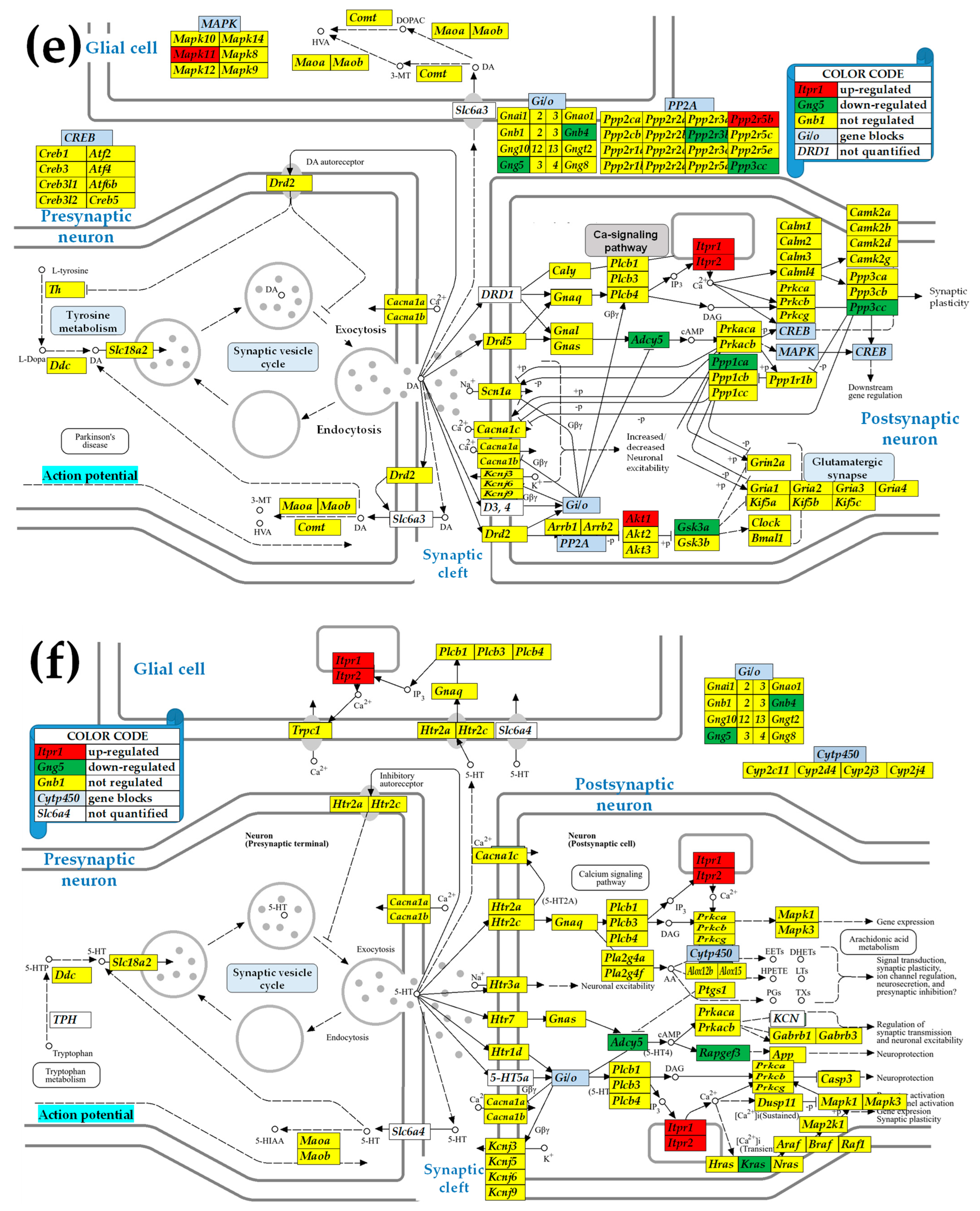
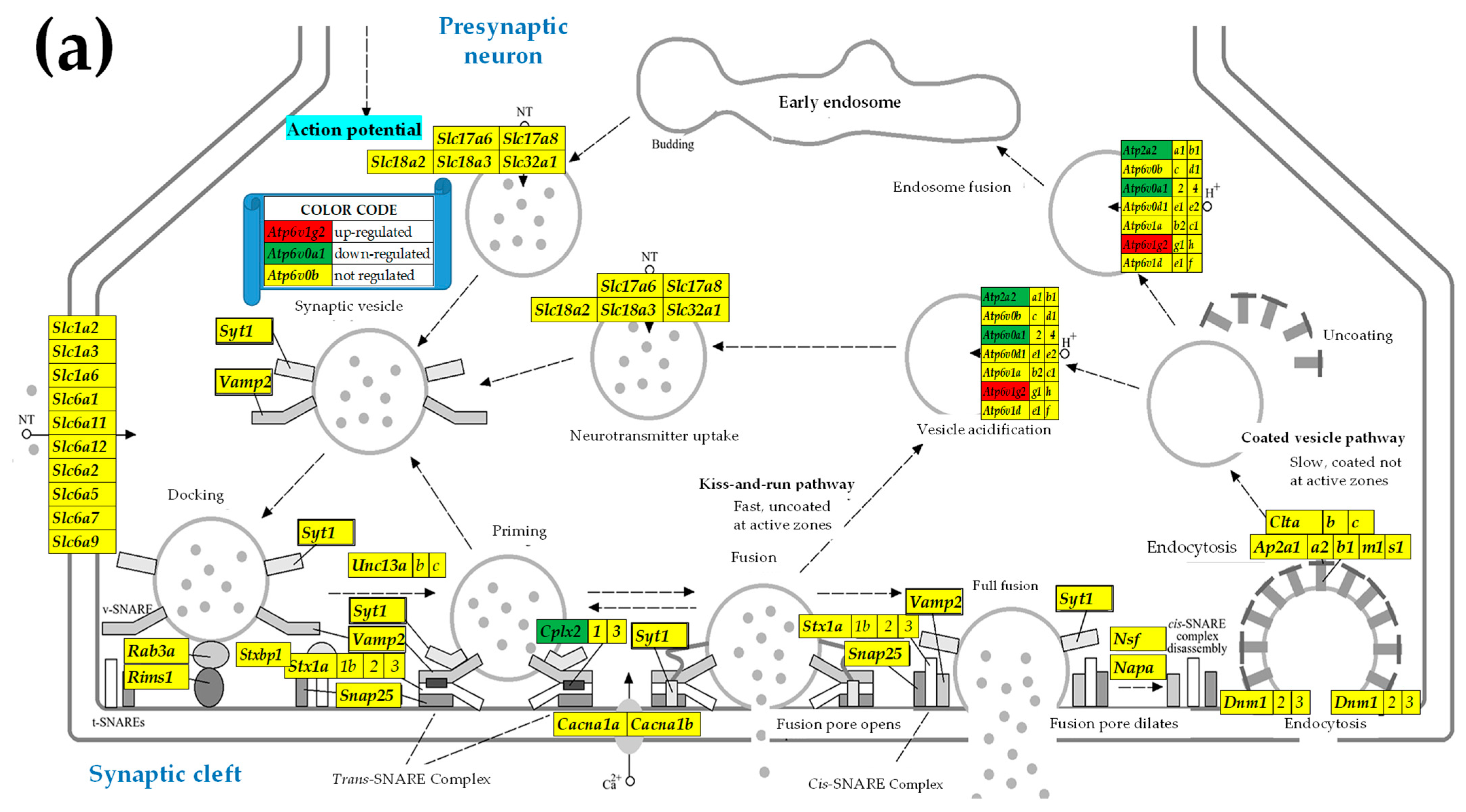
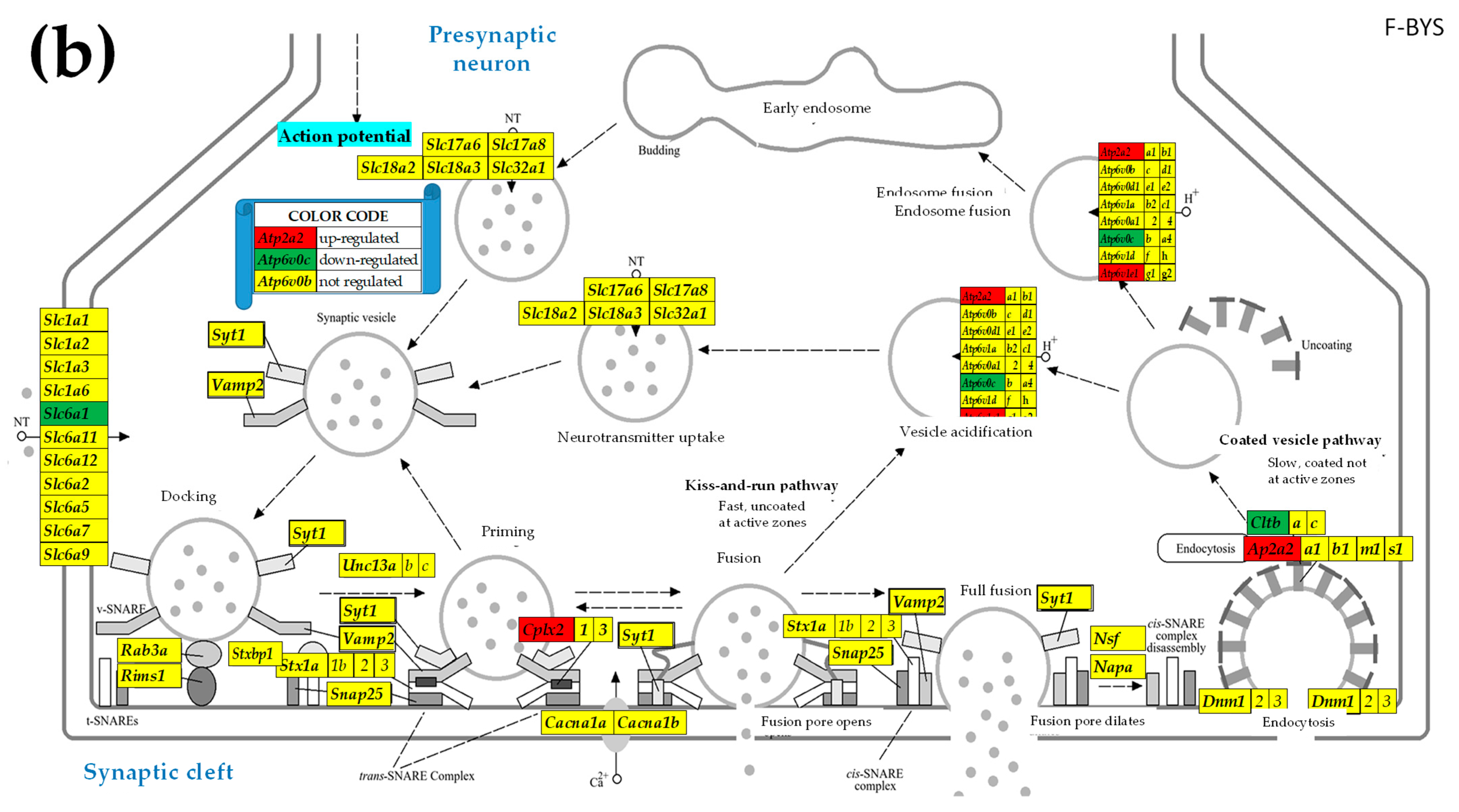
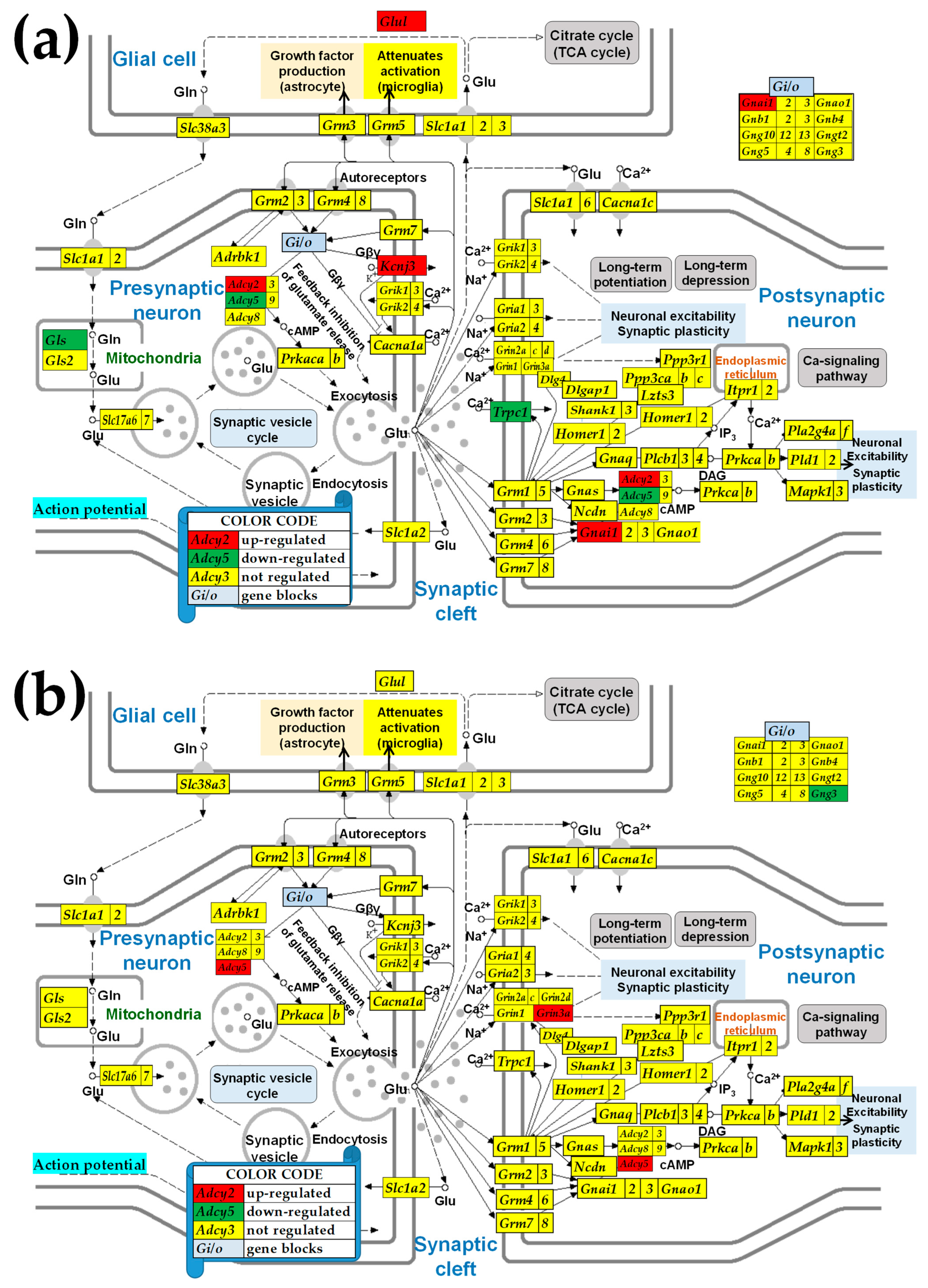
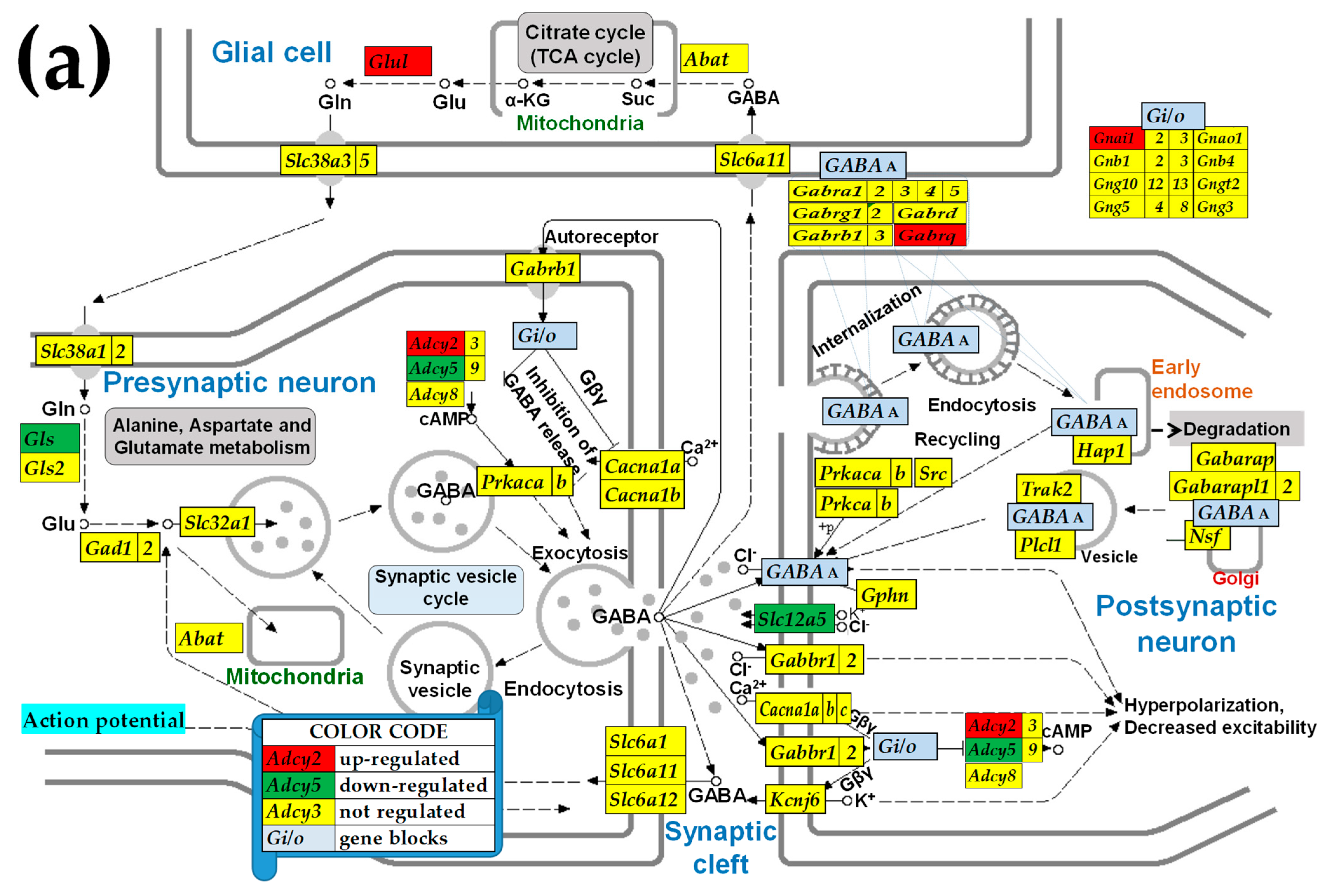
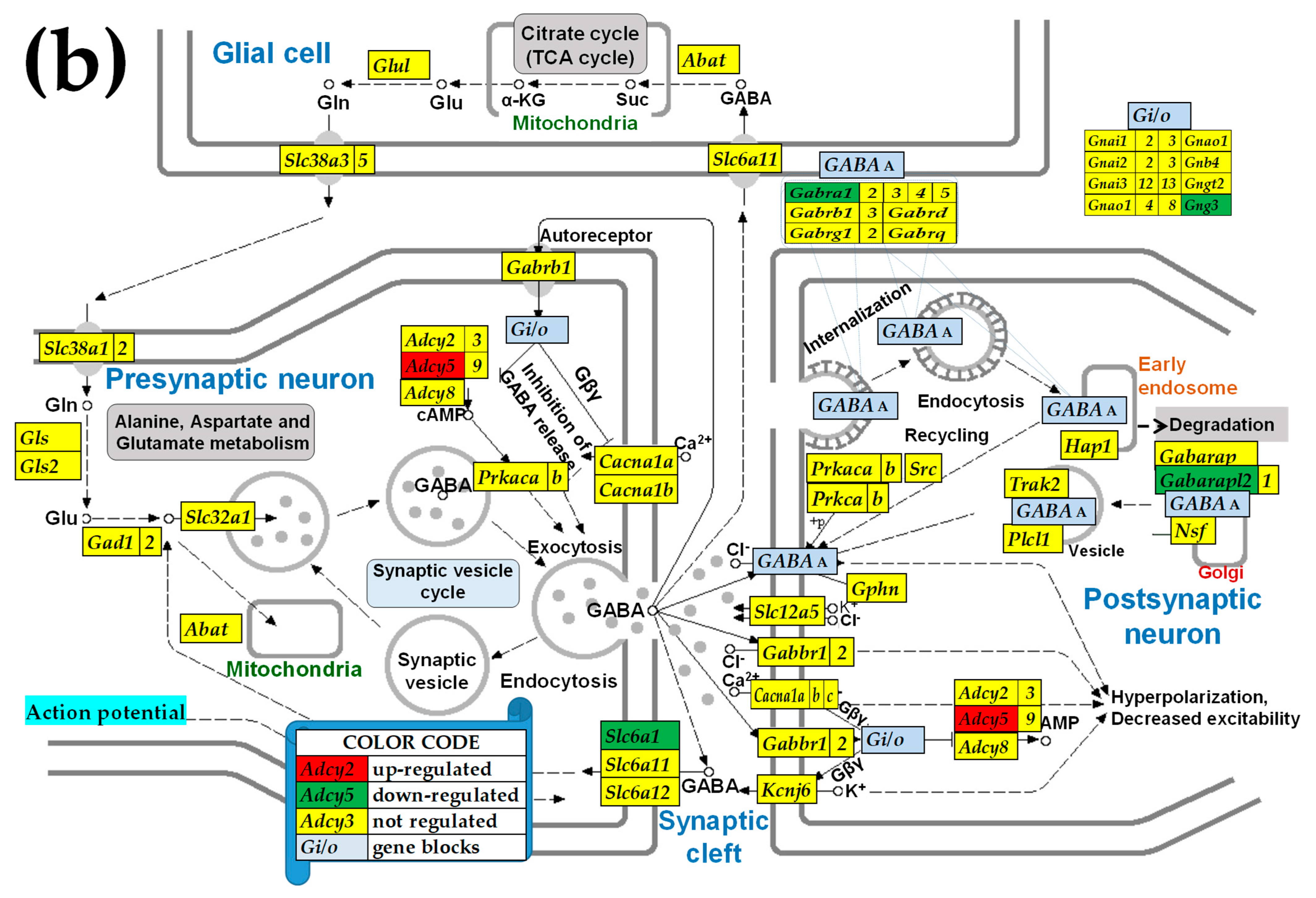
2.3. KEGG-Constructed Functional Neurotransmission Pathways
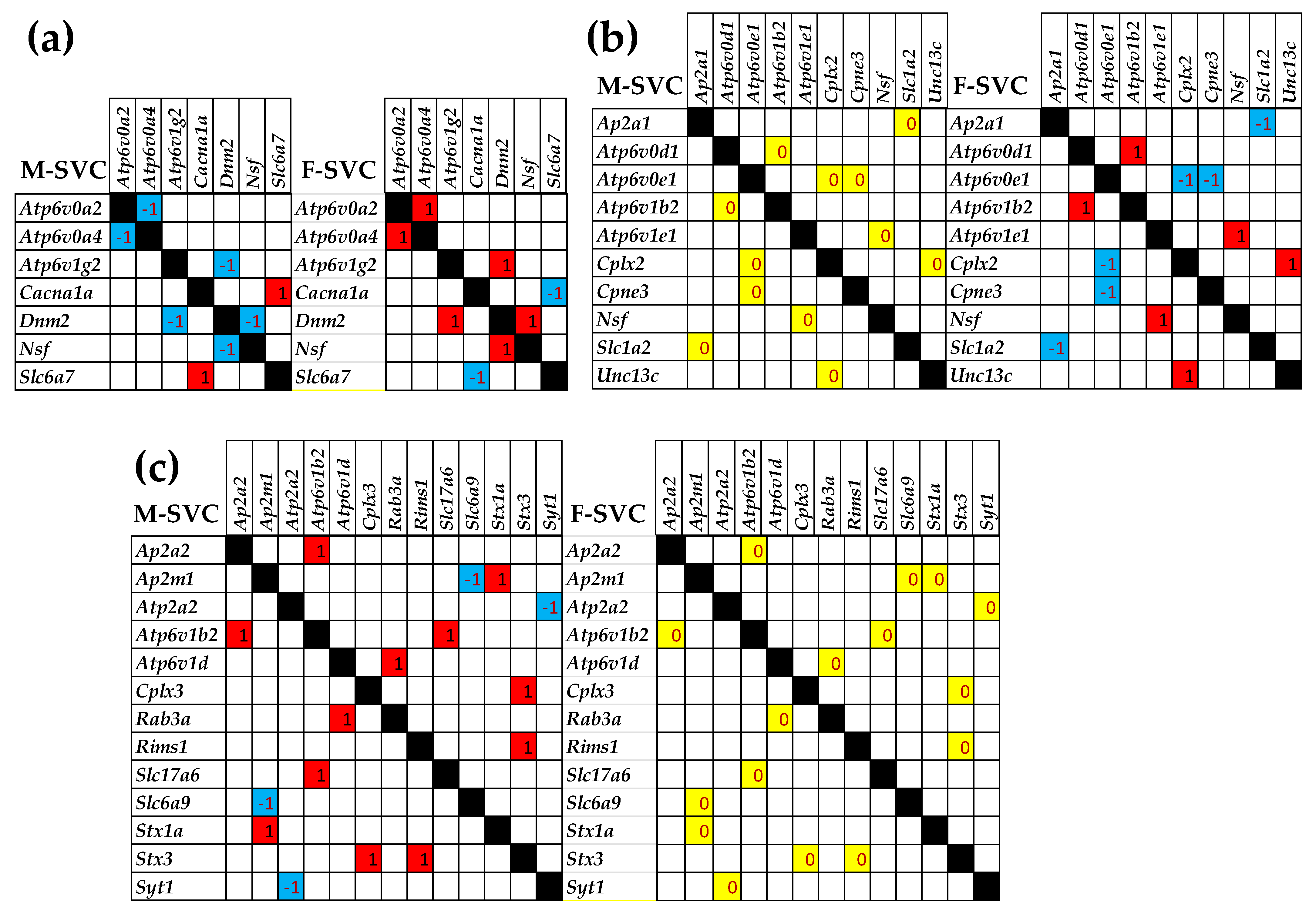
3. Results
3.1. There Is Little Sex Dichotomy of the Most Highly Expressed Neurotransmission Genes
3.2. Sex Dichotomy in the Expression Control and Alteration by IESS
3.3. Sex Differences in the Unaltered State of the Six Neurotransmission Pathways
3.4. Sex Differences Between the Significantly Regulated SVC Genes in the PVNs by the Induction of Spasms in the Betamethasone-Primed Rats
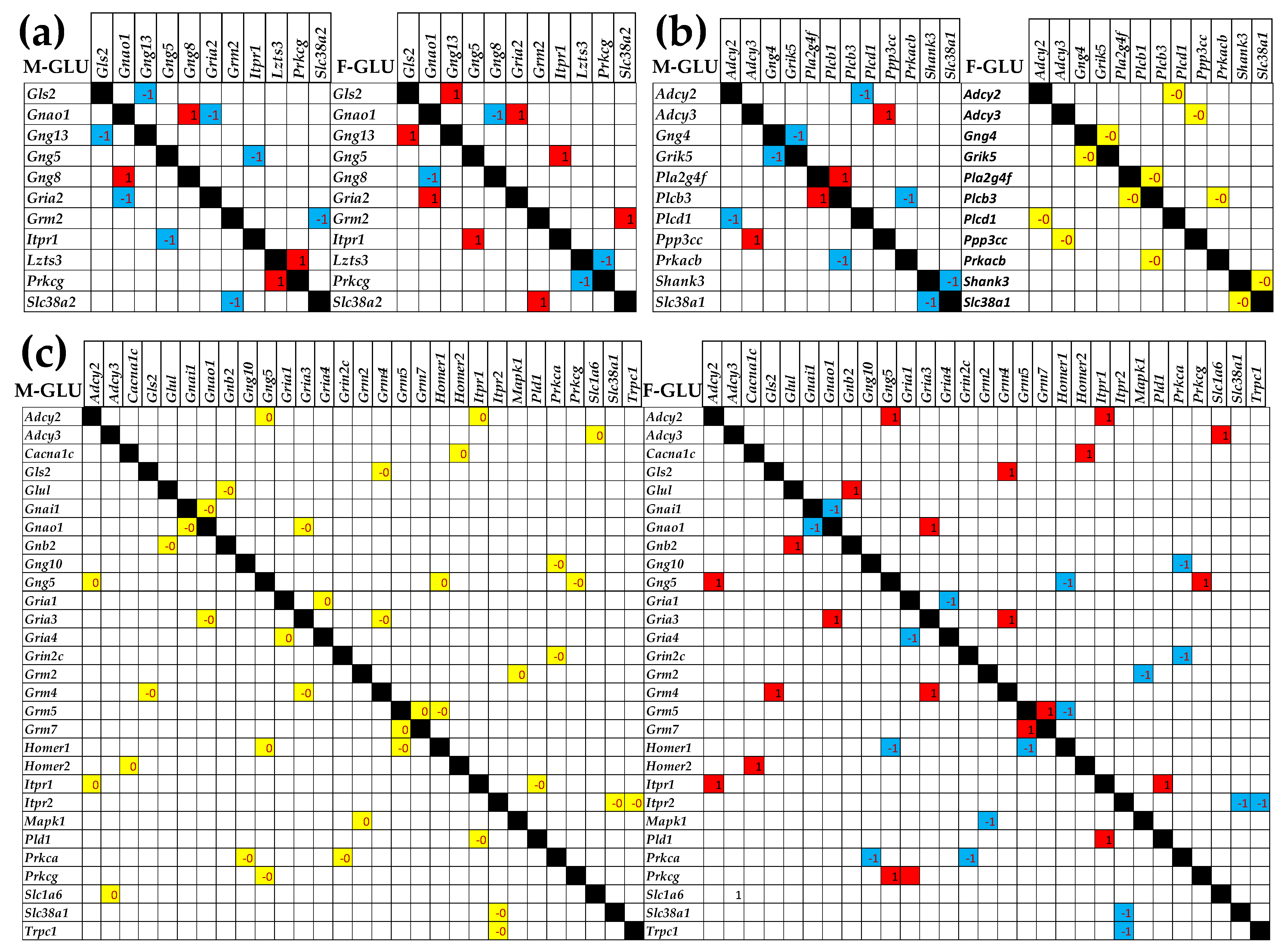
3.5. Sex Differences Between the Significantly Regulated GLU Genes in the PVN by the Induction of Infantile Spasms in the Betamethasone-Primed Rats
3.6. Sex Differences Between the Significantly Regulated GABA Genes in the PVN by the Induction of Infantile Spasms in the Betamethasone-Primed Rats
3.7. Sex Dichotomy of the Genes’ Transcriptomic Networks
3.8. Sex Dichotomy of the Genes’ Hierarchy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Synaptic Vesicle Cycle. Available online: https://www.genome.jp/kegg-bin/show_pathway?rno04721 (accessed on 12 December 2024).
- Glutamatergic Synapse. Available online: https://www.genome.jp/pathway/rno04724 (accessed on 12 December 2024).
- GABAergic Synapse. Available online: https://www.genome.jp/kegg-bin/show_pathway?rno04727 (accessed on 12 December 2024).
- Cholinergic Synapse. Available online: https://www.genome.jp/kegg-bin/show_pathway?rno04725 (accessed on 12 December 2024).
- Dopaminergic Synapse. Available online: https://www.genome.jp/kegg-bin/show_pathway?rno04728 (accessed on 12 December 2024).
- Serotonergic Synapse. Available online: https://www.genome.jp/kegg-bin/show_pathway?rno04726 (accessed on 12 December 2024).
- Velíšek, L.; Jehle, K.; Asche, S.; Velíšková, J. Model of infantile spasms induced by N-methyl-D-aspartic acid in prenatally impaired brain. Ann. Neurol. 2007, 61, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Iacobas, D.A.; Iacobas, S.; Chachua, T.; Goletiani, C.; Sidyelyeva, G.; Veliskova, J.; Velisek, L. Prenatal corticosteroids modify glutamatergic and GABAergic synapse genomic fabric: Insights from a novel animal model of infantile spasms. J. Neuroendocrinol. 2013, 25, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Iacobas, D.A.; Chachua, T.; Iacobas, S.; Benson, M.J.; Borges, K.; Veliskova, J.; Velisek, L. ACTH and PMX53 recover synaptic transcriptome alterations in a rat model of infantile spasms. Sci. Rep. 2018, 8, 5722. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Velisek, L. Regeneration of neurotransmission transcriptome in a model of epileptic encephalopathy after antiinflammatory treatment. Neural Regen. Res. 2018, 13, 1715–1718. [Google Scholar] [CrossRef]
- Hrachovy, R.A. West’s syndrome (infantile spasms). Clinical description and diagnosis. Adv. Exp. Med. Biol. 2002, 497, 33–50. [Google Scholar]
- Dulac, O.; Soufflet, C.; Chiron, C.; Kaminska, A. What is West syndrome? Int. Rev. Neurobiol. 2002, 49, 1–22. [Google Scholar]
- Hrachovy, R.A.; Frost, J.D., Jr. Infantile spasms. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 111, pp. 611–618. [Google Scholar] [CrossRef]
- Pavone, P.; Striano, P.; Falsaperla, R.; Pavone, L.; Ruggieri, M. Infantile spasms syndrome, West syndrome and related phenotypes: What we know in 2013. Brain Dev. 2014, 36, 739–751. [Google Scholar] [CrossRef]
- Mackay, M.T.; Weiss, S.K.; Adams-Webber, T.; Ashwal, S.; Stephens, D.; Ballaban-Gill, K.; Baram, T.Z.; Duchowny, M.; Hirtz, D.; Pellock, J.M.; et al. Practice parameter: Medical treatment of infantile spasms: Report of the American Academy of Neurology and the Child Neurology Society. Neurology 2004, 62, 1668–1681. [Google Scholar] [CrossRef]
- Go, C.Y.; Mackay, M.T.; Weiss, S.K.; Stephens, D.; Adams-Webber, T.; Ashwal, S.; Snead, O.C., 3rd; Child Neurology Society; American Academy of Neurology. Evidence-based guideline update: Medical treatment of infantile spasms: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2012, 78, 1974–1980. [Google Scholar] [CrossRef]
- Riikonen, R. The latest on infantile spasms. Curr. Opin. Neurol. 2005, 18, 91–95. [Google Scholar] [CrossRef]
- Lux, A.L.; Edwards, S.W.; Hancock, E.; Johnson, A.L.; Kennedy, C.R.; Newton, R.W.; O’Callaghan, F.J.; Verity, C.M.; Osborne, J.P. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: A multicentre randomised trial. Lancet Neurol. 2005, 4, 712–717. [Google Scholar] [CrossRef]
- Riikonen, R. Long-term outcome of patients with West syndrome. Brain Dev. 2001, 23, 683–687. [Google Scholar] [CrossRef]
- Luthvigsson, P.; Olafsson, E.; Sigurthardottir, S.; Hauser, W.A. Epidemiologic features of infantile spasms in Iceland. Epilepsia 1994, 35, 802–805. [Google Scholar] [CrossRef]
- Harini, C.; Nagarajan, E.; Bergin, A.M.; Pearl, P.; Loddenkemper, T.; Takeoka, M.; Morrison, P.F.; Coulter, D.; Harappanahally, G.; Marti, C.; et al. Mortality in infantile spasms: A hospital-based study. Epilepsia 2020, 61, 702–713. [Google Scholar] [CrossRef]
- Hancock, E.C.; Osborne, J.P.; Edwards, S.W. Treatment of infantile spasms. Cochrane Database Syst. Rev. 2013, CD001770. [Google Scholar] [CrossRef]
- Chachua, T.; Yum, M.-S.; Velíšková, J.; Velíšek, L. Validation of the rat model of cryptogenic infantile spasms. Epilepsia 2011, 52, 1666–1677. [Google Scholar] [CrossRef]
- Mareš, P.; Velíšek, L. N-methyl-D-aspartate (NMDA)-induced seizures in developing rats. Dev. Brain Res. 1992, 65, 185–189. [Google Scholar] [CrossRef]
- Yum, M.S.; Chachua, T.; Velíšková, J.; Velíšek, L. Prenatal stress promotes development of spasms in infant rats. Epilepsia 2012, 53, e46–e49. [Google Scholar] [CrossRef]
- Tsuji, M.; Takahashi, Y.; Watabe, A.M.; Kato, F. Enhanced long-term potentiation in mature rats in a model of epileptic spasms with betamethasone-priming and postnatal N-methyl-d-aspartate administration. Epilepsia 2016, 57, 495–505. [Google Scholar] [CrossRef]
- Baek, H.; Yi, M.H.; Pandit, S.; Park, J.B.; Kwon, H.H.; Zhang, E.; Kim, S.; Shin, N.; Kim, E.; Lee, Y.H.; et al. Altered expression of KCC2 in GABAergic interneuron contributes prenatal stress-induced epileptic spasms in infant rat. Neurochem. Int. 2016, 97, 57–64. [Google Scholar] [CrossRef]
- Janicot, R.; Shao, L.R.; Stafstrom, C.E. 2-deoxyglucose and beta-hydroxybutyrate fail to attenuate seizures in the betamethasone-NMDA model of infantile spasms. Epilepsia Open 2022, 7, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Savic, B.; Murphy, D.; Japundzic-Zigon, N. The Paraventricular Nucleus of the Hypothalamus in Control of Blood Pressure and Blood Pressure Variability. Front. Physiol. 2022, 13, 858941. [Google Scholar] [CrossRef]
- Velíšková, J. Sex matters in epilepsy. In Developmental Epilepsy: From Clinical Medicine to Neurobiological Mechanisms; Stafstrom, C.E., Velíšek, L., Eds.; World Scientific Publishing: Singapore, 2019; pp. 387–405. [Google Scholar]
- Koh, P.O.; Bergson, C.; Undie, A.S.; Goldman-Rakic, P.S.; Lidow, M.S. Up-regulation of the D1 dopamine receptor-interacting protein, calcyon, in patients with schizophrenia. Arch. Gen. Psychiatry 2003, 60, 311–319. [Google Scholar] [CrossRef]
- Ha, C.M.; Park, D.; Han, J.K.; Jang, J.I.; Park, J.Y.; Hwang, E.M.; Seok, H.; Chang, S. Calcyon forms a novel ternary complex with dopamine D1 receptor through PSD-95 protein and plays a role in dopamine receptor internalization. J. Biol. Chem. 2012, 287, 31813–31822. [Google Scholar] [CrossRef]
- Tian, Y.; Huang, W.; Yang, J.; Wen, Z.; Geng, Y.; Zhao, C.; Zhang, H.; Wang, Y. Systematic identification of hepatitis E virus ORF2 interactome reveals that TMEM134 engages in ORF2-mediated NF-kappaB pathway. Virus Res. 2017, 228, 102–108. [Google Scholar] [CrossRef]
- Gold, M.S.; Blum, K.; Oscar-Berman, M.; Braverman, E.R. Low dopamine function in attention deficit/hyperactivity disorder: Should genotyping signify early diagnosis in children? Postgrad. Med. 2014, 126, 153–177. [Google Scholar] [CrossRef]
- Puig, M.V.; Gulledge, A.T. Serotonin and prefrontal cortex function: Neurons, networks, and circuits. Mol. Neurobiol. 2011, 44, 449–464. [Google Scholar] [CrossRef]
- Lei, S. Serotonergic modulation of Neural activities in the entorhinal cortex. Int. J. Physiol. Pathophysiol. Pharmacol. 2012, 4, 201–210. [Google Scholar]
- Seyedabadi, M.; Fakhfouri, G.; Ramezani, V.; Mehr, S.E.; Rahimian, R. The role of serotonin in memory: Interactions with neurotransmitters and downstream signaling. Exp. Brain Res. 2014, 232, 723–738. [Google Scholar] [CrossRef]
- Bakker, J. The role of steroid hormones in the sexual differentiation of the human brain. J. Neuroendocrinol. 2022, 34, e13050. [Google Scholar] [CrossRef]
- Cutia, C.A.; Christian-Hinman, C.A. Mechanisms linking neurological disorders with reproductive endocrine dysfunction: Insights from epilepsy research. Front. Neuroendocrinol. 2023, 71, 101084. [Google Scholar] [CrossRef]
- Akman, O.; Moshe, S.L.; Galanopoulou, A.S. Sex-specific consequences of early life seizures. Neurobiol. Dis. 2014, 72 Pt B, 153–166. [Google Scholar] [CrossRef]
- Iacobas, S.; Neal-Perry, G.; Iacobas, D.A. Analyzing the Cytoskeletal Transcriptome: Sex Differences in Rat Hypothalamus. In The Cytoskeleton; Neuromethods Series; Dermietzel, R., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 79. [Google Scholar]
- Velíšková, J.; Iacobas, D.; Iacobas, S.; Sidyelyeva, G.; Chachua, T.; Velíšek, L. Oestradiol Regulates Neuropeptide Y Release and Gene Coupling with the GABAergic and Glutamatergic Synapses in the Adult Female Rat Dentate Gyrus. J. Neuroendocrinol. 2015, 27, 911–920. [Google Scholar] [CrossRef]
- Bakker, J. The Sexual Differentiation of the Human Brain: Role of Sex Hormones Versus Sex Chromosomes. Curr. Top. Behav. Neurosci. 2019, 43, 45–67. [Google Scholar] [CrossRef]
- Sex Differences in the Synaptic Genomic Fabrics of the Rat Hypothalamic Paraventricular Node. Available online: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE123721 (accessed on 1 April 2025).
- Prenatal Betamethasone Remodels the Genomic Fabrics of the Synaptic Transmission in the Rat Hypothalamic Paraventricular Nucleus. Available online: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE124613 (accessed on 1 April 2025).
- Remodeling of Synaptic Transmission Genomic Fabrics in the Hypothalamic Paraventricular Nucleus of a Rat Model of Autism. Available online: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE128091 (accessed on 1 April 2025).
- Iacobas, D.A. The Genomic Fabric Perspective on the Transcriptome Between Universal Quantifiers and Personalized Genomic Medicine. Biol. Theory 2016, 11, 123–137. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Iacobas, S.; Lee, P.R.; Cohen, J.E.; Fields, R.D. Coordinated Activity of Transcriptional Networks Responding to the Pattern of Action Potential Firing in Neurons. Genes 2019, 10, 754. [Google Scholar] [CrossRef]
- Bicknell, R.J. Sex-steroid actions on neurotransmission. Curr. Opin. Neurol. 1998, 11, 667–671. [Google Scholar] [CrossRef]
- Foster, T.C.; Kumar, A. Sex, senescence, senolytics, and cognition. Front. Aging Neurosci. 2025, 17, 1555872. [Google Scholar] [CrossRef]
- Sinclair, D.; Purves-Tyson, T.D.; Allen, K.M.; Weickert, C.S. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology 2014, 231, 1581–1599. [Google Scholar] [CrossRef]
- Iacobas, S.; Ede, N.; Iacobas, D.A. The Gene Master Regulators (GMR) Approach Provides Legitimate Targets for Personalized, Time-Sensitive Cancer Gene Therapy. Genes 2019, 10, 560. [Google Scholar] [CrossRef]
- Krishna, S.; Cheng, B.; Sharma, D.R.; Yadav, S.; Stempinski, E.S.; Mamtani, S.; Shah, E.; Deo, A.; Acherjee, T.; Thomas, T.; et al. PPAR-gamma activation enhances myelination and neurological recovery in premature rabbits with intraventricular hemorrhage. Proc. Natl. Acad. Sci. USA 2021, 118, e2103084118. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. The KEGG database. In Silico’Simulation of Biological Processes: Novartis Foundation Symposium 247; John Wiley & Sons, Ltd.: Chichester, UK, 2002; pp. 91–101; discussion 101–103, 119–128, 244–252. [Google Scholar]
- Iacobas, S.; Iacobas, D.A. Astrocyte proximity modulates the myelination gene fabric of oligodendrocytes. Neuron Glia Biol. 2010, 6, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Iacobas, S.; Thomas, N.M.; Iacobas, D.A. Plasticity of the myelination genomic fabric. Mol. Genet. Genom. 2012, 287, 237–246. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Iacobas, S.; Stout, R.F.; Spray, D.C. Cellular Environment Remodels the Genomic Fabrics of Functional Pathways in Astrocytes. Genes 2020, 11, 520. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J.; Bai, S.; Yu, H.; He, H.; Fan, C.; Hao, Y.; Guan, Y. A PIK3R2 Mutation in Familial Temporal Lobe Epilepsy as a Possible Pathogenic Variant. Front. Genet. 2021, 12, 596709. [Google Scholar] [CrossRef]
- Ge, J.; Jiao, X.; Li, H. MicroRNA-126-3P targets PIK3R2 to ameliorate autophagy and apoptosis of cortex in hypoxia-reoxygenation treated neonatal rats. Cell. Mol. Biol. 2023, 69, 210–217. [Google Scholar] [CrossRef]
- Feng, Y.; Wei, Z.H.; Liu, C.; Li, G.Y.; Qiao, X.Z.; Gan, Y.J.; Zhang, C.C.; Deng, Y.C. Genetic variations in GABA metabolism and epilepsy. Seizure 2022, 101, 22–29. [Google Scholar] [CrossRef]
- Xie, M.; Qin, H.; Liu, L.; Wu, J.; Zhao, Z.; Zhao, Y.; Fang, Y.; Yu, X.; Su, C. GABA regulates metabolic reprogramming to mediate the development of brain metastasis in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2025, 44, 61. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Yang, J.; Zhen, Y.; Ban, W.; Zhu, G. Molecular profiling of a rat model of vascular dementia: Evidences from proteomics, metabolomics and experimental validations. Brain Res. 2025, 1846, 149254. [Google Scholar] [CrossRef]
- Zheng, C.; Zhao, Y.; Hu, C.; Zhang, L.; Li, G.; Yang, C. Transcriptomic and network analysis identifies shared pathways across Alzheimer’s disease and vascular dementia. Brain Res. 2025, 1854, 149548. [Google Scholar] [CrossRef] [PubMed]
- Kilic-Berkmen, G.; Scorr, L.M.; McKay, L.; Thayani, M.; Donsante, Y.; Perlmutter, J.S.; Norris, S.A.; Wright, L.; Klein, C.; Feuerstein, J.S.; et al. Sex Differences in Dystonia. Mov. Disord. Clin. Pract. 2024, 11, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Abeledo-Machado, A.; Peña-Zanoni, M.; Bornancini, D.; Camilletti, M.A.; Faraoni, E.Y.; Marcial, A.; Rulli, S.; Alhenc-Gelas, F.; Díaz-Torga, G.S. Sex-specific Regulation of Prolactin Secretion by Pituitary Bradykinin Receptors. Endocrinology 2022, 163, bqac108. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.S.; Diaguarachchige De Silva, K.H.; Hashemi, A.; Duncan, R.E.; Grapentine, S.; Bakovic, M.; Lu, R. Transcription factor CREB3 is a potent regulator of high-fat diet-induced obesity and energy metabolism. Int. J. Obes. 2022, 46, 1446–1455. [Google Scholar] [CrossRef]
- Deluca, A.; Bascom, B.; Key Planas, D.A.; Kocher, M.A.; Torres, M.; Arbeitman, M.N. Contribution of neurons that express fruitless and Clock transcription factors to behavioral rhythms and courtship. iScience 2025, 28, 112037. [Google Scholar] [CrossRef]
- Towers, E.B.; Kilgore, M.; Bakhti-Suroosh, A.; Pidaparthi, L.; Williams, I.L.; Abel, J.M.; Lynch, W.J. Sex differences in the neuroadaptations associated with incubated cocaine-craving: A focus on the dorsomedial prefrontal cortex. Front. Behav. Neurosci. 2023, 16, 1027310. [Google Scholar] [CrossRef]
- Eskow Jaunarajs, K.L.; Scarduzio, M.; Ehrlich, M.E.; McMahon, L.L.; Standaert, D.G. Diverse Mechanisms Lead to Common Dysfunction of Striatal Cholinergic Interneurons in Distinct Genetic Mouse Models of Dystonia. J. Neurosci. 2019, 39, 7195–7205. [Google Scholar] [CrossRef]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef]
- Chen, W.; Byun, J.; Kang, H.C.; Lee, H.S.; Lee, J.Y.; Kwon, Y.J.; Cho, Y.Y. Karyoptosis as a novel type of UVB-induced regulated cell death. Free Radic. Res. 2024, 58, 796–810. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Y.; Tang, J.; Chen, K.; Wu, M.; Wu, X.; Qiu, X. A transcriptomics-based analysis of mechanisms involved in the sex-dependent effects of diazepam on zebrafish. Aquat. Toxicol. 2024, 275, 107063. [Google Scholar] [CrossRef]
- Yotova, A.Y.; Li, L.L.; O’Leary, A.; Tegeder, I.; Reif, A.; Courtney, M.J.; Slattery, D.A.; Freudenberg, F. Synaptic proteome perturbations after maternal immune activation: Identification of embryonic and adult hippocampal changes. Brain Behav. Immun. 2024, 121, 351–364. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Y.; He, F.; Chen, C.; Wu, L.W.; Yang, L.F.; Ma, Y.P.; Zhang, W.; Shi, Z.Q.; Chen, C.; et al. Novel West syndrome candidate genes in a Chinese cohort. CNS Neurosci. Ther. 2018, 24, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Ishiwata, M.; Weitemier, A.Z.; Shoji, H.; Monai, H.; Miyamoto, H.; Yamakawa, K.; Miyakawa, T.; McHugh, T.J.; Kato, T. Brain-specific heterozygous loss-of-function of ATP2A2, endoplasmic reticulum Ca2+ pump responsible for Darier’s disease, causes behavioral abnormalities and a hyper-dopaminergic state. Hum. Mol. Genet. 2021, 30, 1762–1772. [Google Scholar] [CrossRef]
- Gordon-Smith, K.; Jones, L.A.; Burge, S.M.; Munro, C.S.; Tavadia, S.; Craddock, N. The neuropsychiatric phenotype in Dari-er disease. Br. J. Dermatol. 2010, 163, 515–522. [Google Scholar] [CrossRef]
- Gray, A.L.; Hyde, T.M.; Deep-Soboslay, A.; Kleinman, J.E.; Sodhi, M.S. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol. Psychiatry 2015, 20, 1057–1068. [Google Scholar] [CrossRef]
- Kipnis, P.A.; Sullivan, B.J.; Kadam, S.D. Sex-Dependent Signaling Pathways Underlying Seizure Susceptibility and the Role of Chloride Cotransporters. Cells 2019, 8, 448. [Google Scholar] [CrossRef]
- Philibert, C.E.; Garcia-Marcos, M. Smooth operator(s): Dialing up and down neurotransmitter responses by G-protein regulators. Trends Cell Biol. 2024, 35, 330–340. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, A.J.; Munguba, H.; Levitz, J. Emerging modes of regulation of neuromodulatory G protein-coupled receptors. Trends Neurosci. 2024, 47, 635–650. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Suadicani, S.O.; Spray, D.C.; Scemes, E. A stochastic two-dimensional model of intercellular Ca2+ wave spread in glia. Biophys. J. 2006, 90, 24–41. [Google Scholar] [CrossRef]
- Zakharyan, R.; Atshemyan, S.; Boyajyan, A. Risk and protective effects of the complexin-2 gene and gene-environment interactions in schizophrenia. Recent Adv. DNA Gene Seq. 2014, 8, 30–34. [Google Scholar] [CrossRef]
- Hass, J.; Walton, E.; Kirsten, H.; Turner, J.; Wolthusen, R.; Roessner, V.; Sponheim, S.R.; Holt, D.; Gollub, R.; Calhoun, V.D.; et al. Complexin2 modulates working memory-related neural activity in patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2015, 265, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Aguzzoli Heberle, B.; Brandon, J.A.; Page, M.L.; Nations, K.A.; Dikobe, K.I.; White, B.J.; Gordon, L.A.; Fox, G.A.; Wadsworth, M.E.; Doyle, P.H.; et al. Mapping medically relevant RNA isoform diversity in the aged human frontal cortex with deep long-read RNA-seq. Nat. Biotechnol. 2025, 43, 635–646. [Google Scholar] [CrossRef]
- Liao, W.; Liu, Y.; Wang, L.; Cai, X.; Xie, H.; Yi, F.; Huang, R.; Fang, C.; Xie, P.; Zhou, J. Chronic mild stress-induced protein dysregulations correlated with susceptibility and resiliency to depression or anxiety revealed by quantitative pro-teomics of the rat prefrontal cortex. Transl. Psychiatry 2021, 11, 143. [Google Scholar] [CrossRef]
- Anderson, E.M.; Loke, S.; Wrucke, B.; Engelhardt, A.; Demis, S.; O’Reilly, K.; Hess, E.; Wickman, K.; Hearing, M.C. Suppression of pyramidal neuron G protein-gated inwardly rectifying K+ channel signaling impairs prelimbic cortical function and underlies stress-induced deficits in cognitive flexibility in male, but not female, mice. Neuropsychopharmacology 2021, 46, 2158–2169. [Google Scholar] [CrossRef]
- Chen, Q.; Kou, H.; Demy, D.L.; Liu, W.; Li, J.; Wen, Z.; Herbomel, P.; Huang, Z.; Zhang, W.; Xu, J. The different roles of V-ATPase a subunits in phagocytosis/endocytosis and autophagy. Autophagy 2024, 20, 2297–2313. [Google Scholar] [CrossRef]
- Prinslow, E.A.; Stepien, K.P.; Pan, Y.Z.; Xu, J.; Rizo, J. Multiple factors maintain assembled trans-SNARE complexes in the presence of NSF and alphaSNAP. eLife 2019, 8, e38880. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, L.; Yuan, J.; Huang, Y.; Ban, Y.; Zhang, P.; Tan, D.; Liang, M.; Li, Z.; Gong, C.; et al. GLS2 reduces the occurrence of epilepsy by affecting mitophagy function in mouse hippocampal neurons. CNS Neurosci. Ther. 2024, 30, e70036. [Google Scholar] [CrossRef]
- Gauthier, M.; Hebert, L.P.; Dugast, E.; Lardeux, V.; Letort, K.; Thiriet, N.; Belnoue, L.; Balado, E.; Solinas, M.; Belujon, P. Sex-dependent effects of stress on aIC-NAc circuit neuroplasticity: Role of the endocannabinoid system. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 138, 111335. [Google Scholar] [CrossRef]
- Sazhina, T.; Tsurugizawa, T.; Mochizuki, Y.; Saito, A.; Joji-Nishino, A.; Ouchi, K.; Yagishita, S.; Emoto, K.; Uematsu, A. Time- and sex-dependent effects of juvenile social isolation on mouse brain morphology. Neuroimage 2025, 310, 121117. [Google Scholar] [CrossRef]
- Wei, A.; Zhao, A.; Zheng, C.; Dong, N.; Cheng, X.; Duan, X.; Zhong, S.; Liu, X.; Jian, J.; Qin, Y.; et al. Sexually dimorphic dopaminergic circuits determine sex preference. Science 2025, 387, eadq7001. [Google Scholar] [CrossRef]
- Sangu, N.; Shimojima, K.; Takahashi, Y.; Ohashi, T.; Tohyama, J.; Yamamoto, T. A 7q31.33q32.1 microdeletion including LRRC4 and GRM8 is associated with severe intellectual disability and characteristics of autism. Hum. Genome Var. 2017, 4, 17001. [Google Scholar] [CrossRef]
- Friedman, D.; Kannan, K.; Faustin, A.; Shroff, S.; Thomas, C.; Heguy, A.; Serrano, J.; Snuderl, M.; Devinsky, O. Cardiac arrhythmia and neuroexcitability gene variants in resected brain tissue from patients with sudden unexpected death in epilepsy (SUDEP). NPJ Genom. Med. 2018, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, F.; Hu, D.; Zhang, Z.; Yan, Y.; Ma, Y. TMT-based proteomics profile reveals changes of the entorhinal cortex in a kainic acid model of epilepsy in mice. Neurosci. Lett. 2023, 800, 137127. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Chen, X.; Pan, X.; Deng, X.; Li, S.; Wang, Z.; Wang, J.; Liao, D.; Xu, J.; Chen, M.; et al. Single-cell transcriptome analyses reveal critical roles of RNA splicing during leukemia progression. PLoS Biol. 2023, 21, e3002088. [Google Scholar] [CrossRef] [PubMed]
- Keustermans, G.C.; Kofink, D.; Eikendal, A.; de Jager, W.; Meerding, J.; Nuboer, R.; Waltenberger, J.; Kraaijeveld, A.O.; Jukema, J.W.; Sels, J.W.; et al. Monocyte gene expression in childhood obesity is associated with obesity and complexity of atherosclerosis in adults. Sci. Rep. 2017, 7, 16826. [Google Scholar] [CrossRef]
- Cai, M.; Lin, W. The Function of NF-Kappa B During Epilepsy, a Potential Therapeutic Target. Front. Neurosci. 2022, 16, 851394. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Tuli, N.Y.; Iacobas, S.; Rasamny, J.K.; Moscatello, A.; Geliebter, J.; Tiwari, R.K. Gene master regulators of papillary and anaplastic thyroid cancers. Oncotarget 2018, 9, 2410–2424. [Google Scholar] [CrossRef]
- Benson, M.J.; Laukova, M.; Borges, K.; Velíšková, J.; Velíšek, L. Prenatal betamethasone exposure increases corticotro-pin-releasing hormone expression along with increased hippocampal slice excitability in the developing hippo-campus. Epilepsy Res. 2020, 160, 106276. [Google Scholar] [CrossRef]
- Velíšek, L. Prenatal exposure to betamethasone decreases anxiety in developing rats: Hippocampal neuropeptide Y as a target molecule. Neuropsychopharmacology 2006, 31, 2140–2149. [Google Scholar] [CrossRef]
- Schwehn, P.M.; Falter-Braun, P. Inferring protein from transcript abundances using convolutional neural networks. BioData Min. 2025, 18, 18. [Google Scholar] [CrossRef]
- Mekic, R.; Zolotovskaia, M.A.; Sorokin, M.; Mohammad, T.; Shaban, N.; Musatov, I.; Buzdin, A. Number of human protein interactions correlates with structural, but not regulatory conservation of the respective genes. Front. Genet. 2024, 15, 1472638. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, J.; Cui, X. Research progress on estrogen and estrogen receptors in the occurrence and progression of autoimmune thyroid diseases. Autoimmun. Rev. 2025, 24, 103803. [Google Scholar] [CrossRef]
- Aten, S.; Ramirez-Plascencia, O.; Blake, C.; Holder, G.; Fishbein, E.; Vieth, A.; Zarghani-Shiraz, A.; Keister, E.; Howe, S.; Appo, A.; et al. A time for sex: Circadian regulation of mammalian sexual and reproductive function. Front. Neurosci. 2025, 18, 1516767. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Iacobas, S.; Nebieridze, N.; Velíšek, L.; Velíšková, J. Estrogen Protects Neurotransmission Transcriptome During Status Epilepticus. Front. Neurosci. 2018, 12, 332. [Google Scholar] [CrossRef]
- Huang, S.; Shi, C.; Tao, D.; Yang, C.; Luo, Y. Modulating reward and aversion: Insights into addiction from the paraventricular nucleus. CNS Neurosci. Ther. 2024, 30, e70046. [Google Scholar] [CrossRef]
- Xing, M.; Li, Y.; Zhang, Y.; Zhou, J.; Ma, D.; Zhang, M.; Tang, M.; Ouyang, T.; Zhang, F.; Shi, X.; et al. Paraventricular hypothalamic RUVBL2 neurons suppress appetite by enhancing excitatory synaptic transmission in distinct neurocircuits. Nat. Commun. 2024, 15, 8939. [Google Scholar] [CrossRef]
- Griffin, H.; Hanson, J.; Phelan, K.D.; Baldini, G. MC4R Localizes at Excitatory Postsynaptic and Peri-Postsynaptic Sites of Hypothalamic Neurons in Primary Culture. Cells 2024, 13, 1235. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Iacobas, S.; Thomas, N.; Spray, D.C. Sex-dependent gene regulatory networks of the heart rhythm. Funct. Integr. Genom. 2010, 10, 73–86. [Google Scholar] [CrossRef]
- Iacobas, S.; Amuzescu, B.; Iacobas, D.A. Transcriptomic uniqueness and commonality of the ion channels and transporters in the four heart chambers. Sci. Rep. 2021, 11, 2743. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Fan, C.; Iacobas, S.; Spray, D.C.; Haddad, G.G. Transcriptomic changes in developing kidney exposed to chronic hypoxia. Biochem. Biophys. Res. Commun. 2006, 349, 329–338. [Google Scholar] [CrossRef]


| Top 5 Expressed Neurotransmission Genes | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Gene | Description | PATH | SN | BN | BY | SN | BN | BY |
| Caly | calcyon neuron-specific vesicular protein | 4 | 126 | 139 | 126 | 195 | 143 | 155 |
| Gabarapl1 | GABA(A) receptor-associated protein like 1 | 2 | 104 | 112 | 80 | 69 | 108 | 129 |
| Ap2m1 | adaptor-related protein complex 2, mu 1 subunit | 0 | 96 | 110 | 89 | 125 | 112 | 122 |
| Calm2 | calmodulin 2 | 4 | 72 | 70 | 60 | 60 | 75 | 72 |
| Hap1 | huntingtin-associated protein 1 | 2 | 63 | 63 | 67 | 73 | 72 | 73 |
| Caly | calcyon neuron-specific vesicular protein | 4 | 126 | 139 | 126 | 195 | 143 | 155 |
| Ap2m1 | adaptor-related protein complex 2, mu 1 subunit | 0 | 96 | 110 | 89 | 125 | 112 | 122 |
| Mapk3 | mitogen activated protein kinase 3 | 135 | 59 | 64 | 67 | 85 | 68 | 68 |
| Gnai2 | guanine nucleotide binding protein (G protein), alpha inhibiting activity polypeptide 2 | 12345 | 57 | 64 | 57 | 75 | 64 | 74 |
| Hap1 | huntingtin-associated protein 1 | 2 | 63 | 63 | 67 | 73 | 72 | 73 |
| Caly | calcyon neuron-specific vesicular protein | 4 | 126 | 139 | 126 | 195 | 143 | 155 |
| Gabarapl1 | GABA(A) receptor-associated protein like 1 | 2 | 104 | 112 | 80 | 69 | 108 | 129 |
| Ap2m1 | adaptor-related protein complex 2, mu 1 subunit | 0 | 96 | 110 | 89 | 125 | 112 | 122 |
| Calm2 | calmodulin 2 | 4 | 72 | 70 | 60 | 60 | 75 | 72 |
| Gnai2 | guanine nucleotide binding protein (G protein), alpha inhibiting activity polypeptide 2 | 12345 | 57 | 64 | 57 | 75 | 64 | 74 |
| Caly | calcyon neuron-specific vesicular protein | 4 | 126 | 139 | 126 | 195 | 143 | 155 |
| Ap2m1 | adaptor-related protein complex 2, mu 1 subunit | 0 | 96 | 110 | 89 | 125 | 112 | 122 |
| Gabarapl1 | GABA(A) receptor-associated protein like 1 | 2 | 104 | 112 | 80 | 69 | 108 | 129 |
| Calm2 | calmodulin 2 | 4 | 72 | 70 | 60 | 60 | 75 | 72 |
| Hap1 | huntingtin-associated protein 1 | 2 | 63 | 63 | 67 | 73 | 72 | 73 |
| Caly | calcyon neuron-specific vesicular protein | 4 | 126 | 139 | 126 | 195 | 143 | 155 |
| Ap2m1 | adaptor-related protein complex 2, mu 1 subunit | 0 | 96 | 110 | 89 | 125 | 112 | 122 |
| Gabarapl1 | GABA(A) receptor-associated protein like 1 | 2 | 104 | 112 | 80 | 69 | 108 | 129 |
| Mapk3 | mitogen activated protein kinase 3 | 135 | 59 | 64 | 67 | 85 | 68 | 68 |
| Hap1 | huntingtin-associated protein 1 | 2 | 63 | 63 | 67 | 73 | 72 | 73 |
| Caly | calcyon neuron-specific vesicular protein | 4 | 126 | 139 | 126 | 195 | 143 | 155 |
| Gabarapl1 | GABA(A) receptor-associated protein like 1 | 2 | 104 | 112 | 80 | 69 | 108 | 129 |
| Ap2m1 | adaptor-related protein complex 2, mu 1 subunit | 0 | 96 | 110 | 89 | 125 | 112 | 122 |
| Gnai2 | guanine nucleotide binding protein (G protein), alpha inhibiting activity polypeptide 2 | 12345 | 57 | 64 | 57 | 75 | 64 | 74 |
| Hap1 | huntingtin-associated protein 1 | 2 | 63 | 63 | 67 | 73 | 72 | 73 |
| Top 5 Most Controlled Neurotransmission Genes | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Gene | Description | PATH | SN | BN | BY | SN | BN | BY |
| Pik3r2 | phosphoinositide-3-kinase, regulatory subunit 2 (beta) | 3 | 3.52 | 1.04 | 0.82 | 0.83 | 0.75 | 0.73 |
| Atp6v0b | ATPase, H+ transporting, lysosomal V0 subunit B | 0 | 2.40 | −0.59 | 1.22 | 1.01 | 0.12 | −0.02 |
| Calml4 | calmodulin-like 4 | 4 | 2.15 | −0.61 | 0.65 | −0.56 | −0.02 | −0.71 |
| Ppp1ca | protein phosphatase 1, catalytic subunit, alpha isozyme | 4 | 2.13 | 0.55 | 1.12 | 1.16 | 2.00 | 1.32 |
| Atp6v1g2 | ATPase, H+ transporting, lysosomal V1 subunit G2 | 0 | 1.95 | −0.32 | 0.67 | 0.06 | 0.87 | 1.39 |
| Abat | 4-aminobutyrate aminotransferase | 2 | 0.38 | 1.52 | 1.63 | 3.58 | 1.81 | 0.33 |
| Gabbr1 | gamma-aminobutyric acid (GABA) B receptor 1 | 2 | −0.26 | 0.72 | 0.75 | 2.87 | 0.15 | 1.23 |
| Slc6a7 | solute carrier family 6 (neurotransmitter transporter), member 7 | 0 | 1.08 | −0.01 | 1.01 | 2.82 | −0.08 | 1.63 |
| Chrnb4 | cholinergic receptor, nicotinic, beta 4 (neuronal) | 3 | 0.87 | 0.09 | 0.40 | 2.75 | 0.60 | 0.11 |
| Gnal | GTP-binding protein Golf alpha subunit | 4 | 0.83 | −0.98 | −0.45 | 2.66 | −0.28 | −0.48 |
| Pik3r5 | phosphoinositide-3-kinase, regulatory subunit 5 | 3 | −0.25 | 2.42 | 1.25 | 0.94 | −1.19 | 1.17 |
| Atp2a2 | ATPase, Ca++ transporting, cardiac muscle, slow twitch 2 | 0 | 0.60 | 2.18 | 0.81 | −0.60 | 1.53 | 0.94 |
| Ppp2r5e | protein phosphatase 2, regulatory subunit B′, epsilon isoform | 4 | 0.76 | 2.04 | 2.08 | −0.44 | 0.85 | 0.47 |
| Ppp2r3c | protein phosphatase 2, regulatory subunit B″, gamma | 4 | 1.70 | 1.85 | 0.72 | −0.32 | 0.10 | 1.20 |
| Slc38a5 | solute carrier family 38, member 5 | 2 | 1.94 | 1.81 | 0.50 | −0.09 | −0.88 | 0.94 |
| Grm8 | glutamate receptor, metabotropic 8 | 1 | 0.25 | −0.24 | 1.81 | 0.47 | 2.70 | 0.70 |
| Cpne3 | copine III | 0 | 0.23 | 0.68 | 0.58 | −0.98 | 2.39 | −0.20 |
| Atp6v1h | ATPase, H+ transporting, lysosomal V1 subunit H | 0 | 0.80 | 1.55 | 0.50 | −0.24 | 2.30 | 0.57 |
| Gls | glutaminase (Gls), nuclear gene encoding mitochondrial protein | 12 | 0.04 | 1.07 | 1.27 | −1.34 | 2.19 | 0.76 |
| Gabarapl2 | GABA(A) receptor-associated protein like 2 | 2 | 0.66 | 0.62 | 1.10 | −1.40 | 2.09 | 1.43 |
| Th | tyrosine hydroxylase | 4 | −1.77 | −0.50 | 3.71 | 0.05 | −0.14 | −0.16 |
| Maoa | monoamine oxidase A | 45 | 0.59 | 0.53 | 2.62 | 1.17 | 0.93 | −0.42 |
| Mapk10 | mitogen activated protein kinase 10 | 4 | 0.97 | 0.30 | 2.35 | −1.84 | −0.04 | 0.44 |
| Scn1a | sodium channel, voltage-gated, type I, alpha | 4 | −0.30 | 0.69 | 2.29 | 1.00 | −0.67 | 0.30 |
| Gabrg1 | gamma-aminobutyric acid (GABA) A receptor, gamma 1 | 2 | 0.24 | 0.04 | 2.22 | 0.79 | 1.76 | −0.87 |
| Trpc1 | transient receptor potential cation channel, subfamily C, member 1 | 15 | 0.57 | 0.98 | 1.87 | −1.06 | 0.64 | 4.87 |
| Gng10 | guanine nucleotide binding protein (G protein), gamma 10 | 12345 | 0.56 | 1.08 | 0.11 | 0.05 | 1.38 | 2.79 |
| Gng8 | guanine nucleotide binding protein (G protein), gamma 8 | 1245 | 0.20 | 0.37 | 0.40 | 0.78 | −0.18 | 2.58 |
| Raf1 | v-raf-leukemia viral oncogene 1 | 5 | 1.05 | 1.68 | 0.68 | 0.98 | −0.64 | 2.21 |
| Atp6v0a1 | ATPase, H+ transporting, lysosomal V0 subunit A1 | 0 | −0.21 | 0.11 | 1.68 | −0.69 | 2.06 | 2.04 |
| Top 5 Least Controlled Neurotransmission Genes | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Gene | Description | PATH | SN | BN | BY | SN | BN | BY |
| Calm1 | calmodulin 1 | 4 | −2.05 | −0.87 | −1.41 | −1.27 | −0.56 | −0.30 |
| Alox15 | arachidonate 15-lipoxygenase | 5 | −2.04 | −0.40 | −1.20 | −0.03 | −1.23 | −1.47 |
| Th | tyrosine hydroxylase | 4 | −1.77 | −0.50 | 3.71 | 0.05 | −0.14 | −0.16 |
| Plcb1 | phospholipase C, beta 1 (phosphoinositide-specific) | 1345 | −1.73 | −1.61 | −1.06 | −0.27 | −1.28 | −1.58 |
| Clock | clock circadian regulator | 4 | −1.48 | −2.33 | −1.76 | −1.10 | −1.61 | −1.80 |
| Mapk10 | mitogen activated protein kinase 10 | 4 | 0.97 | 0.30 | 2.35 | −1.84 | −0.04 | 0.44 |
| Gna11 | guanine nucleotide binding protein, alpha 11 | 3 | −0.54 | −1.47 | −1.16 | −1.76 | −0.62 | −1.41 |
| Gabarapl1 | GABA(A) receptor-associated protein like 1 | 2 | −0.72 | −0.64 | −0.90 | −1.74 | −0.66 | −0.53 |
| Rims1 | regulating synaptic membrane exocytosis 1 | 0 | −1.17 | −1.73 | −1.37 | −1.69 | −1.41 | −1.67 |
| Map2k1 | mitogen activated protein kinase kinase 1 | 35 | −0.51 | 0.19 | 0.57 | −1.64 | 1.03 | −0.19 |
| Drd2 | dopamine receptor D2 | 4 | −0.63 | −2.45 | −1.42 | −0.74 | −1.59 | −1.74 |
| Slc6a12 | solute carrier family 6 (neurotransmitter transporter), member 12 | 02 | −0.58 | −2.35 | −1.35 | −0.61 | −1.29 | −1.62 |
| Clock | clock circadian regulator | 4 | −1.48 | −2.33 | −1.76 | −1.10 | −1.61 | −1.80 |
| Alox12b | arachidonate 12-lipoxygenase, 12R type | 5 | −1.16 | −2.25 | −1.39 | −0.27 | −1.53 | −1.66 |
| Gnb3 | guanine nucleotide binding protein (G protein), beta polypeptide 3 | 12345 | −0.88 | −2.24 | −1.57 | −0.96 | −1.56 | −1.81 |
| Creb3 | cAMP responsive element binding protein 3 | 34 | 0.47 | 0.61 | 0.33 | 0.41 | −2.71 | 0.13 |
| Gng5 | guanine nucleotide binding protein (G protein), gamma 5 | 12345 | 0.65 | 0.52 | 1.23 | 0.40 | −2.22 | 1.47 |
| Camk2b | calcium/calmodulin-dependent protein kinase II beta | 34 | 0.90 | −0.60 | 0.39 | −1.08 | −2.15 | −0.05 |
| Akt1 | v-akt murine thymoma viral oncogene homolog 1 | 34 | 0.46 | −0.23 | 0.29 | −0.46 | −2.15 | −0.87 |
| Slc1a3 | solute carrier family 1 (glial high affinity glutamate transporter), member 3 | 01 | −0.23 | −0.48 | −0.77 | 0.23 | −2.07 | −0.17 |
| Clock | clock circadian regulator | 4 | −1.48 | −2.33 | −1.76 | −1.10 | −1.61 | −1.80 |
| Gria2 | glutamate receptor, ionotropic, AMPA 2 | 14 | 0.28 | −0.51 | −1.64 | −1.18 | 0.77 | −0.32 |
| Htr7 | 5-hydroxytryptamine (serotonin) receptor 7, adenylate cyclase-coupled | 5 | −0.94 | −2.17 | −1.60 | −0.78 | −1.66 | −1.71 |
| Unc13b | unc-13 homolog B (C. elegans) | 0 | −1.07 | −2.22 | −1.60 | −0.24 | −1.63 | −1.78 |
| Akt2 | v-akt murine thymoma viral oncogene homolog 2 | 34 | −0.94 | −2.14 | −1.57 | −1.08 | −1.29 | −1.65 |
| Grin1 | glutamate receptor, ionotropic, N-methyl D-aspartate 1 | 1 | −0.79 | −1.87 | −1.40 | −0.45 | −1.41 | −1.87 |
| Gnaq | guanine nucleotide binding protein (G protein), q polypeptide | 1345 | −1.04 | −1.89 | −1.46 | −0.21 | −1.19 | −1.87 |
| Chrna7 | cholinergic receptor, nicotinic, alpha 7 (neuronal) | 3 | −1.38 | −2.01 | −1.57 | −0.91 | −1.36 | −1.87 |
| Cyp2c11 | cytochrome P450, subfamily 2, polypeptide 11 | 5 | 0.54 | −1.33 | −0.99 | 0.15 | −1.04 | −1.86 |
| Gnb3 | guanine nucleotide binding protein (G protein), beta polypeptide 3 | 12345 | −0.88 | −2.24 | −1.57 | −0.96 | −1.56 | −1.81 |
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene | Description | PATH | SN | BN | BY | SN | BN | BY |
| Pik3r2 | phosphoinositide-3-kinase, regulatory subunit 2 | 3 | 20 | 4 | 1 | 2 | 3 | 1 |
| Gng12 | guanine nucleotide binding protein (G protein), gamma 12 | 12345 | 8 | 2 | 8 | 5 | 1 | 8 |
| Ppp1ca | protein phosphatase 1, catalytic subunit, alpha isozyme | 4 | 7 | 9 | 4 | 2 | 6 | 4 |
| Ppp2r1a | protein phosphatase 2, regulatory subunit A, alpha | 4 | 7 | 3 | 2 | 3 | 1 | 2 |
| Calml4 | calmodulin-like 4 | 4 | 6 | 2 | 3 | 2 | 1 | 3 |
| Abat | 4-aminobutyrate aminotransferase | 2 | 3 | 4 | 5 | 26 | 6 | 5 |
| Pld1 | phospholipase D1 | 1 | 1 | 1 | 2 | 17 | 1 | 2 |
| Slc6a7 | solute carrier family 6 (neurotransmitter transporter), member 7 | 0 | 4 | 1 | 4 | 11 | 1 | 4 |
| Gabra1 | gamma-aminobutyric acid (GABA) A receptor, alpha 1 | 2 | 1 | 2 | 4 | 8 | 2 | 4 |
| Homer1 | homer homolog 1 | 1 | 1 | 2 | 13 | 8 | 2 | 13 |
| Gphn | gephyrin | 2 | 3 | 16 | 0 | 3 | 2 | 0 |
| Ap2a2 | adaptor-related protein complex 2, alpha 2 subunit | 0 | 1 | 16 | 3 | 2 | 2 | 3 |
| Creb3l1 | cAMP responsive element binding protein 3-like 1 | 34 | 1 | 14 | 4 | 3 | 1 | 4 |
| Gng10 | guanine nucleotide binding protein (G protein), gamma 10 | 12345 | 2 | 11 | 4 | 2 | 3 | 4 |
| Gabbr1 | gamma-aminobutyric acid (GABA) B receptor 1 | 2 | 1 | 10 | 5 | 5 | 2 | 5 |
| Grm8 | glutamate receptor, metabotropic 8 | 1 | 2 | 1 | 5 | 3 | 14 | 5 |
| Cpne3 | copine III | 0 | 2 | 2 | 2 | 1 | 14 | 2 |
| Atp6v1h | ATPase, H+ transporting, lysosomal V1 subunit H | 0 | 4 | 3 | 6 | 2 | 12 | 6 |
| Atp6v0e1 | ATPase, H+ transporting, lysosomal, V0 subunit e1 | 0 | 3 | 1 | 1 | 3 | 12 | 1 |
| Gabarapl2 | GABA(A) receptor-associated protein like 2 | 2 | 3 | 8 | 7 | 1 | 10 | 7 |
| Mapk10 | mitogen activated protein kinase 10 | 4 | 2 | 6 | 19 | 1 | 1 | 19 |
| Homer1 | homer homolog 1 | 1 | 1 | 2 | 13 | 8 | 2 | 13 |
| Th | tyrosine hydroxylase | 4 | 1 | 1 | 11 | 2 | 1 | 11 |
| Gnb4 | guanine nucleotide binding protein (G protein), beta polypeptide 4 | 12345 | 3 | 5 | 10 | 6 | 1 | 10 |
| Glul | glutamate-ammonia ligase | 12 | 1 | 1 | 10 | 3 | 5 | 10 |
| Mapk10 | mitogen activated protein kinase 10 | 4 | 2 | 6 | 19 | 1 | 1 | 19 |
| Homer1 | homer homolog 1 | 1 | 1 | 2 | 13 | 8 | 2 | 13 |
| Th | tyrosine hydroxylase | 4 | 1 | 1 | 11 | 2 | 1 | 11 |
| Gnb4 | guanine nucleotide binding protein (G protein), beta polypeptide 4 | 12345 | 3 | 5 | 10 | 6 | 1 | 10 |
| Glul | glutamate-ammonia ligase | 12 | 1 | 1 | 10 | 3 | 5 | 10 |
| Erg28 | ergosterol biosynthesis 28 homolog | 40 | 6 | 1 | 3 | 7 | 1 | |
| Erap1 | endoplasmic reticulum aminopeptidase 1 | 2 | 2 | 2 | 70 | 2 | 2 | |
| Tmem238 | transmembrane protein 238 | 4 | 162 | 3 | 3 | 4 | 3 | |
| Taf8 | TAF8 RNA polymerase II, TATA box binding protein (TBP)-associated factor | 1 | 1 | 3 | 5 | 38 | 3 | |
| Tmem134 | transmembrane protein 134 | 2 | 7 | 78 | 5 | 2 | 78 | |
| Tmem134 | transmembrane protein 134 | 2 | 7 | 78 | 5 | 2 | 78 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacobas, D.A.; Veliskova, J.; Chachua, T.; Chern, C.-R.; Vieira, K.; Iacobas, S.; Velíšek, L. Neurotransmission Sex Dichotomy in the Rat Hypothalamic Paraventricular Nucleus in Healthy and Infantile Spasm Model. Curr. Issues Mol. Biol. 2025, 47, 380. https://doi.org/10.3390/cimb47050380
Iacobas DA, Veliskova J, Chachua T, Chern C-R, Vieira K, Iacobas S, Velíšek L. Neurotransmission Sex Dichotomy in the Rat Hypothalamic Paraventricular Nucleus in Healthy and Infantile Spasm Model. Current Issues in Molecular Biology. 2025; 47(5):380. https://doi.org/10.3390/cimb47050380
Chicago/Turabian StyleIacobas, Dumitru Andrei, Jana Veliskova, Tamar Chachua, Chian-Ru Chern, Kayla Vieira, Sanda Iacobas, and Libor Velíšek. 2025. "Neurotransmission Sex Dichotomy in the Rat Hypothalamic Paraventricular Nucleus in Healthy and Infantile Spasm Model" Current Issues in Molecular Biology 47, no. 5: 380. https://doi.org/10.3390/cimb47050380
APA StyleIacobas, D. A., Veliskova, J., Chachua, T., Chern, C.-R., Vieira, K., Iacobas, S., & Velíšek, L. (2025). Neurotransmission Sex Dichotomy in the Rat Hypothalamic Paraventricular Nucleus in Healthy and Infantile Spasm Model. Current Issues in Molecular Biology, 47(5), 380. https://doi.org/10.3390/cimb47050380







