The Mechanisms of Chronic Inflammation in Obesity and Potential Therapeutic Strategies: A Narrative Review
Abstract
:1. Introduction
2. Pathophysiology of LGCI
3. Mechanisms of Chronic Inflammation in Obesity
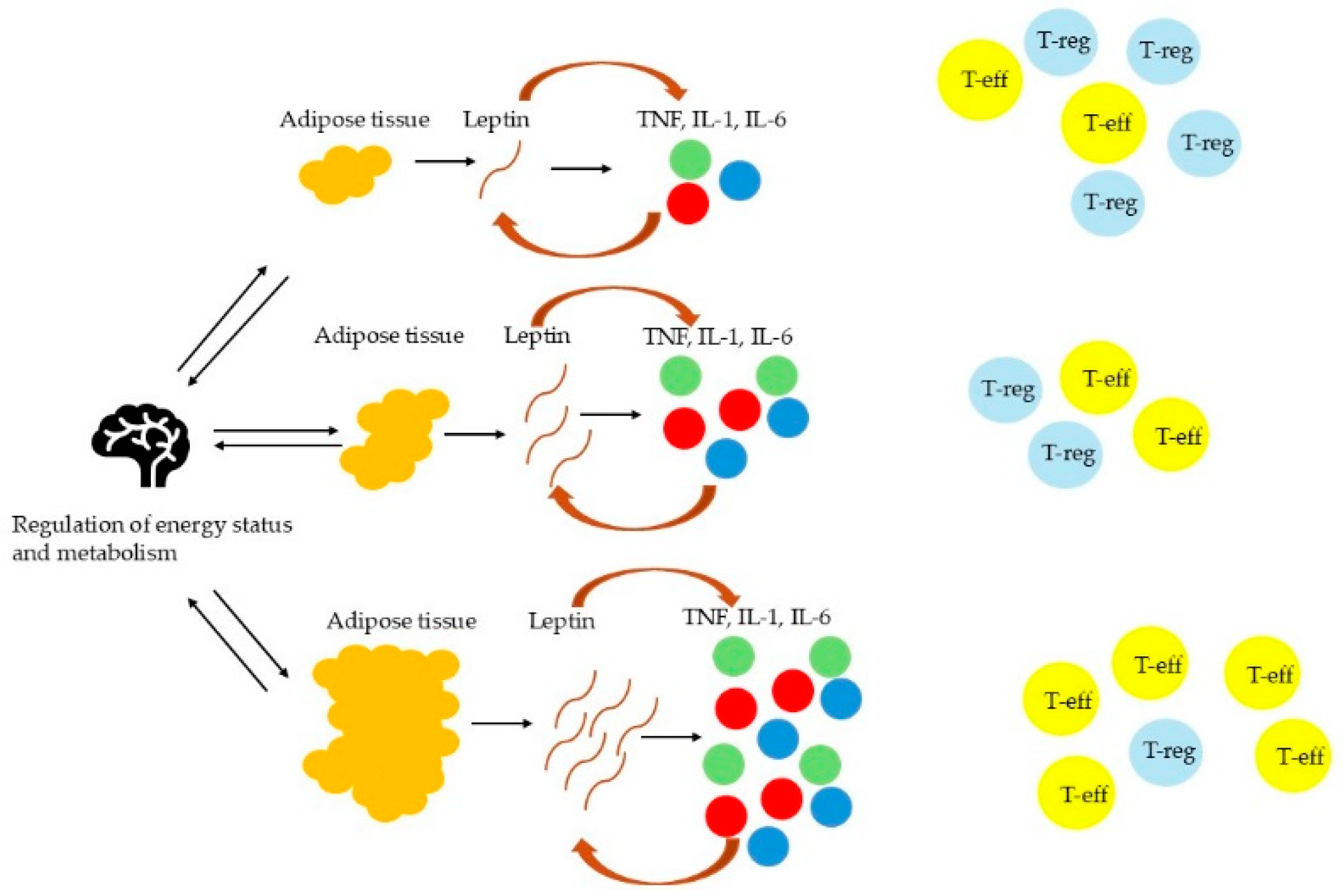
3.1. AT Inflammation
3.2. Inflammatory Signaling Pathways
3.3. Role of FFAs
3.4. Systemic Effects of Inflammation
4. Potential Therapeutic Strategies
4.1. Pharmaceutical Interventions
4.2. Nutritional Anti-Inflammatory Interventions
4.3. Lifestyle Modifications
5. Future Directions and Current Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AT | Adipose tissue |
| ATM | Adipose tissue macrophages |
| BMI | Body mass index |
| CRP | C-reactive protein |
| DII | Dietary Inflammatory Index |
| ER | Endoplasmic reticulum |
| FFA | Free fatty acids |
| GLP-1 RAs | GLP-1 receptor agonists |
| HPA | Hypothalamic-pituitary-adrenal |
| hs-CRP | High sensitivity CRP |
| IKKβ | IκB kinase |
| IL | Interleukins |
| IRS-1 | Insulin receptor substrate-1 |
| JNK | c-Jun N-terminal kinase |
| LGCI | Low-grade chronic inflammation |
| MCP | Monocyte chemotactic protein |
| NF-κB | Nuclear factor-kappa B |
| PUFAs | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| SFAs | Saturated fatty acids |
| T2D | Type 2 diabetes |
| T-eff | Effector T cell |
| Th | T helper |
| TLR4 | Toll-like receptor 4 |
| TNF | Tumor necrosis factor |
| T-reg | Regulatory T cell |
References
- De Lorenzo, A.; Romano, L.; Di Renzo, L.; Di Lorenzo, N.; Cenname, G.; Gualtieri, P. Obesity: A Preventable, Treatable, but Relapsing Disease. Nutrition 2019, 71, 110615. [Google Scholar] [CrossRef] [PubMed]
- World Obesity Federation. World Obesity Atlas. 2025. Available online: https://data.worldobesity.org/publications/?cat=23 (accessed on 14 March 2025).
- Belančić, A.; Klobučar Majanović, S.; Štimac, D. The Escalating Global Burden of Obesity Following the COVID-19 Times—Are We Ready? Clin. Obes. 2020, 10, e12410. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Belančić, A.; Kenđel Jovanović, G.; Klobučar Majanović, S. Obesity-Related Low-Grade Chronic Inflammation: Implementation of the Dietary Inflammatory Index in Clinical Practice Is the Milestone? Med. Flum. 2018, 54, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Štimac, D.; Klobučar Majanović, S.A.; Baretić, M.; Bekavac Bešlin, M.I.; Belančić, A.; Crnčević Orlić, Ž.E.; Đorđević, V.; Marčinko, D.; Miličić, D.; Mirošević, G.; et al. Croatian guidelines for the treatment of adults with obesity. Acta Medica Croatica 2022, 76, 19–32. Available online: https://hrcak.srce.hr/285231 (accessed on 15 March 2025).
- Štimac, D.; Klobučar Majanović, S.; Belančić, A. Endoscopic Treatment of Obesity: From Past to Future. Dig. Dis. 2020, 38, 150–162. [Google Scholar] [CrossRef]
- Belančić, A.; Kresović, A.; Troskot Dijan, M. Glucagon-like Peptide-1 Receptor Agonists in the Era of COVID-19: Friend or Foe? Clin. Obes. 2021, 11, e12439. [Google Scholar] [CrossRef]
- Belančić, A. Gut Microbiome Dysbiosis and Endotoxemia—Additional Pathophysiological Explanation for Increased COVID-19 Severity in Obesity. Obes. Med. 2020, 20, 100302. [Google Scholar] [CrossRef]
- Belančić, A.; Kresović, A.; Rački, V. Potential Pathophysiological Mechanisms Leading to Increased COVID-19 Susceptibility and Severity in Obesity. Obes. Med. 2020, 19, 100259. [Google Scholar] [CrossRef]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hébert, J.R.; Hodge, A.; Courten, B. The Dietary Inflammatory Index, Obesity, Type 2 Diabetes, and Cardiovascular Risk Factors and Diseases. Obes. Rev. 2021, 23, e13349. [Google Scholar] [CrossRef]
- Hintikka, J.E.; Ahtiainen, J.P.; Permi, P.; Jalkanen, S.; Lehtonen, M.; Pekkala, S. Aerobic Exercise Training and Gut Microbiome-Associated Metabolic Shifts in Women with Overweight: A Multi-Omic Study. Sci. Rep. 2023, 13, 11228. [Google Scholar] [CrossRef] [PubMed]
- Beals, J.W.; Kayser, B.D.; Smith, G.I.; Schweitzer, G.G.; Kirbach, K.; Kearney, M.L.; Yoshino, J.; Rahman, G.; Knight, R.; Patterson, B.W.; et al. Dietary Weight Loss-Induced Improvements in Metabolic Function Are Enhanced by Exercise in People with Obesity and Prediabetes. Nat. Metab. 2023, 5, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.P.; Wilkens, L.R.; Shvetsov, Y.B.; Maskarinec, G.; Park, S.-Y.; Shepherd, J.A.; Boushey, C.J.; Hebert, J.R.; Wirth, M.D.; Ernst, T.; et al. Associations of the Dietary Inflammatory Index with Total Adiposity and Ectopic Fat through the Gut Microbiota, LPS, and C-Reactive Protein in the Multiethnic Cohort–Adiposity Phenotype Study. Am. J. Clin. Nutr. 2021, 115, 1344–1356. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Valentić, S.; Šestan, M.; Turk Wensveen, T.; Polić, B. The “Big Bang” in Obese Fat: Events Initiating Obesity-Induced Adipose Tissue Inflammation. Eur. J. Immunol. 2015, 45, 2446–2456. [Google Scholar] [CrossRef]
- Santillana, N.; Astudillo-Guerrero, C.; D’Espessailles, A.; Cruz, G. White Adipose Tissue Dysfunction: Pathophysiology and Emergent Measurements. Nutrients 2023, 15, 1722. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, W. Weight Cycling Based on Altered Immune Microenvironment as a Result of Metaflammation. Nutr. Metab. 2023, 20, 13. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Li, Z. Adipokines in Glucose and Lipid Metabolism. Adipocyte 2023, 12, 2202976. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling Pathways in Obesity: Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef]
- Kiran, S.; Kumar, V.; Kumar, S.; Price, R.L.; Singh, U.P. Adipocyte, Immune Cells, and MiRNA Crosstalk: A Novel Regulator of Metabolic Dysfunction and Obesity. Cells 2021, 10, 1004. [Google Scholar] [CrossRef]
- Sears, B.; Ricordi, C. Anti-Inflammatory Nutrition as a Pharmacological Approach to Treat Obesity. J. Obes. 2011, 2011, 431985. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Scherer, P.E. Adipose Tissue, Inflammation, and Cardiovascular Disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Tanti, J.-F.; Ceppo, F.; Jager, J.; Berthou, F. Implication of Inflammatory Signaling Pathways in Obesity-Induced Insulin Resistance. Front. Endocrinol. 2013, 3, 181. [Google Scholar] [CrossRef]
- Volpe, C.; Nogueira-Machado, J. The Dual Role of Free Fatty Acid Signaling in Inflammation and Therapeutics. Recent. Pat. Endocr. Metab. Immune Drug Discov. 2013, 7, 189–197. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Adipose Tissue Inflammation Induces B Cell Inflammation and Decreases B Cell Function in Aging. Front. Immunol. 2017, 8, 1003. [Google Scholar] [CrossRef]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory Cytokines Il-6 and TNF-α and the Development of Inflammation in Obese Subjects. Eur. J. Med. Res. 2010, 15 (Suppl. 2), 120–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Nikolajczyk, B.S. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front. Immunol. 2019, 10, 1587. [Google Scholar] [CrossRef]
- Luan, D.; Dadpey, B.; Zaid, J.; Bridge-Comer, P.E.; DeLuca, J.H.; Xia, W.; Castle, J.; Reilly, S.M. Adipocyte-Secreted IL-6 Sensitizes Macrophages to IL-4 Signaling. Diabetes 2022, 72, 367–374. [Google Scholar] [CrossRef]
- Solinas, G.; Becattini, B. JNK at the Crossroad of Obesity, Insulin Resistance, and Cell Stress Response. Mol. Metab. 2017, 6, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A Central Role for JNK in Obesity and Insulin Resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lu, S.; Ou, B.; Liu, Q.; Dai, J.; Ji, C.; Zhou, H.; Huang, H.; Ma, Y. The Role of JNk Signaling Pathway in Obesity-Driven Insulin Resistance. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Vilariño-García, T.; Fernández-Riejos, P.; Martín-González, J.; Segura-Egea, J.J.; Sánchez-Margalet, V. Role of Leptin as a Link between Metabolism and the Immune System. Cytokine Growth Factor. Rev. 2017, 35, 71–84. [Google Scholar] [CrossRef]
- Fantuzzi, G.; Faggioni, R. Leptin in the Regulation of Immunity, Inflammation, and Hematopoiesis. J. Leukoc. Biol. 2000, 68, 437–446. [Google Scholar] [CrossRef]
- Zarkesh-Esfahani, H.; Pockley, G.; Metcalfe, R.A.; Bidlingmaier, M.; Wu, Z.; Ajami, A.; Weetman, A.P.; Strasburger, C.J.; Ross, R.J. High-Dose Leptin Activates Human Leukocytes via Receptor Expression on Monocytes. J. Immunol. 2001, 167, 4593–4599. [Google Scholar] [CrossRef]
- Martín-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Sánchez-Margalet, V. Human Leptin Enhances Activation and Proliferation of Human Circulating T Lymphocytes. Cell Immunol. 2000, 199, 15–24. [Google Scholar] [CrossRef]
- Sanchez-Margalet, V.; Martin-Romero, C. Human Leptin Signaling in Human Peripheral Blood Mononuclear Cells: Activation of the JAK-STAT Pathway. Cell Immunol. 2001, 211, 30–36. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef]
- Matarese, G.; Procaccini, C.; De Rosa, V.; Horvath, T.L.; La Cava, A. Regulatory T Cells in Obesity: The Leptin Connection. Trends Mol. Med. 2010, 16, 247–256. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, W.; Li, D.; Guo, Y.; Ding, H. Overactivation of NF-ΚB Impairs Insulin Sensitivity and Mediates Palmitate-Induced Insulin Resistance in C2C12 Skeletal Muscle Cells. Endocrine 2009, 37, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, H.; Haugen, F.; Zadelaar, S.; Kleemann, R.; Kooistra, T.; Drevon, C.A.; Blomhoff, R. Diet-Induced Obesity Increases NF-ΚB Signaling in Reporter Mice. Genes. Nutr. 2009, 4, 215–222. [Google Scholar] [CrossRef]
- Nieto-Vazquez, I.; Fernández-Veledo, S.; Krämer, D.K.; Vila-Bedmar, R.; Garcia-Guerra, L.; Lorenzo, M. Insulin Resistance Associated to Obesity: The Link TNF-Alpha. Arch. Physiol. Biochem. 2008, 114, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Urso, C.J.; Jadeja, V. Saturated Fatty Acids in Obesity-Associated Inflammation. J. Inflamm. Res. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Boden, G. Obesity and Free Fatty Acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.; Zhang, M.J.; Tang, Y.; Rane, M.; Bhatnagar, A.; Spite, M. Increased Saturated Fatty Acids in Obesity Alter Resolution of Inflammation in Part by Stimulating Prostaglandin Production. J. Immunol. 2013, 191, 1383–1392. [Google Scholar] [CrossRef]
- Stelzner, K.; Herbert, D.; Popkova, Y.; Lorz, A.; Schiller, J.; Gericke, M.; Klöting, N.; Blüher, M.; Franz, S.; Simon, J.C.; et al. Free Fatty Acids Sensitize Dendritic Cells to Amplify TH1/TH17-Immune Responses. Eur. J. Immunol. 2016, 46, 2043–2053. [Google Scholar] [CrossRef]
- Frommer, K.W.; Hasseli, R.; Schäffler, A.; Lange, U.; Rehart, S.; Steinmeyer, J.; Rickert, M.; Sarter, K.; Zaiss, M.M.; Culmsee, C.; et al. Free Fatty Acids in Bone Pathophysiology of Rheumatic Diseases. Front. Immunol. 2019, 10, 2757. [Google Scholar] [CrossRef]
- Silva Figueiredo, P.; Carla Inada, A.; Marcelino, G.; Maiara Lopes Cardozo, C.; de Cássia Freitas, K.; de Cássia Avellaneda Guimarães, R.; Pereira de Castro, A.; Aragão do Nascimento, V.; Aiko Hiane, P. Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients 2017, 9, 1158. [Google Scholar] [CrossRef]
- Benzler, J.; Ganjam, G.K.; Pretz, D.; Oelkrug, R.; Koch, C.E.; Legler, K.; Stöhr, S.; Culmsee, C.; Williams, L.M.; Tups, A. Central Inhibition of IKKβ/NF-ΚB Signaling Attenuates High-Fat Diet–Induced Obesity and Glucose Intolerance. Diabetes 2015, 64, 2015–2027. [Google Scholar] [CrossRef]
- Khafagy, R.; Dash, S. Obesity and Cardiovascular Disease: The Emerging Role of Inflammation. Front. Cardiovasc. Med. 2021, 8, 768119. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, H.; Simental-Mendía, L.E.; Rodríguez-Ramírez, G.; Reyes-Romero, M.A. Obesity and Inflammation: Epidemiology, Risk Factors, and Markers of Inflammation. Int. J. Endocrinol. 2013, 2013, 678159. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, L.K.; Wallace, J.M.W.; Livingstone, M.B.E. Obesity and Inflammation: The Effects of Weight Loss. Nutr. Res. Rev. 2008, 21, 117–133. [Google Scholar] [CrossRef]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef]
- Salas-Venegas, V.; Flores-Torres, R.P.; Rodríguez-Cortés, Y.M.; Rodríguez-Retana, D.; Ramírez-Carreto, R.J.; Concepción-Carrillo, L.E.; Pérez-Flores, L.J.; Alarcón-Aguilar, A.; López-Díazguerrero, N.E.; Gómez-González, B.; et al. The Obese Brain: Mechanisms of Systemic and Local Inflammation, and Interventions to Reverse the Cognitive Deficit. Front. Integr. Neurosci. 2022, 16, 798995. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and Inflammation: The Linking Mechanism and the Complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.-E.; Caruntu, C.; Benea, S.N. The Interplay between Obesity and Inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Jensen, T.L.; Brønden, A.; Karstoft, K.; Sonne, D.; Christensen, M. The Body Weight Reducing Effects of Tirzepatide in People with and without Type 2 Diabetes: A Review on Efficacy and Adverse Effects. Patient Prefer. Adherence 2024, 18, 373–382. [Google Scholar] [CrossRef]
- Melson, E.; Miras, A.D.; Papamargaritis, D. Future Therapies for Obesity. Clin. Med. 2023, 23, 337–346. [Google Scholar] [CrossRef]
- Roomy, M.A.; Hussain, K.; Behbehani, H.M.; Abu-Farha, J.; Al-Harris, R.; Ambi, A.M.; Abdalla, M.A.; Al-Mulla, F.; Abu-Farha, M.; Abubaker, J. Therapeutic Advances in Obesity Management: An Overview of the Therapeutic Interventions. Front. Endocrinol. 2024, 15, 1364503. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.Y.D.; Cox, A.; McNeil, S.; Sumithran, P. Obesity Medications: A Narrative Review of Current and Emerging Agents. Osteoarthr. Cartil. Open 2024, 6, 100472. [Google Scholar] [CrossRef]
- Kim, S.H.; Abbasi, F.; Lamendola, C.; Liu, A.; Ariel, D.; Schaaf, P.; Grove, K.; Tomasso, V.; Ochoa, H.; Liu, Y.V.; et al. Benefits of Liraglutide Treatment in Overweight and Obese Older Individuals with Prediabetes. Diabetes Care 2013, 36, 3276–3282. [Google Scholar] [CrossRef]
- Gusmão-Nascimento, J.W.; Nunes Cruz, D.M.; Almeida Gama, L.; Luz Alves, W.D.; Machado, M.P.R.; Corá, L.A.; Américo, M.F. Liraglutide Modulates Morpho-Functional and Inflammatory Gastrointestinal Responses in Rats. Eur. J. Clin. Investig. 2023, 54, e14112. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zou, M.; Pan, Q.; Song, Y.; Li, M.; Zhang, X.; Zhou, Y.; Wang, X.; Guo, L. Effects of Liraglutide or Lifestyle Interventions Combined with Other Antidiabetic Drugs on Abdominal Fat Distribution in People with Obesity and Type 2 Diabetes Mellitus Evaluated by the Energy Spectrum Ct: A Prospective Randomized Controlled Study. Front. Endocrinol. 2022, 13, 951570. [Google Scholar] [CrossRef]
- Obesity Alters Response to Anti-Inflammatory Treatment. Available online: https://www.nih.gov/news-events/nih-research-matters/obesity-alters-response-anti-inflammatory-treatment (accessed on 15 March 2025).
- Brandfon, S.; Eylon, A.; Khanna, D.; Parmar, M.S.; Brandfon, S.; Eylon, A.; Khanna, D.; Parmar, M.S. Advances in Anti-Obesity Pharmacotherapy: Current Treatments, Emerging Therapies, and Challenges. Cureus 2023, 15, e46623. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.-A. A Review of Current Guidelines for the Treatment of Obesity. Am. J. Manag. Care 2022, 28, S288–S296. [Google Scholar]
- Valladares, A.C.; Astudillo, M.A.; Drinnon, A.R.; Dowlatshahi, S.; Kansara, A.; Shakil, J.; Patham, B. Medical Management of Obesity: Current Trends and Future Perspectives. Methodist. Debakey Cardiovasc. J. 2025, 21, 62–73. [Google Scholar] [CrossRef]
- Melson, E.; Ashraf, U.; Papamargaritis, D.; Davies, M.J. What Is the Pipeline for Future Medications for Obesity? Int. J. Obes. 2024, 49, 1–19. [Google Scholar] [CrossRef]
- Diet Review: Anti-Inflammatory Diet. Available online: https://nutritionsource.hsph.harvard.edu/healthy-weight/diet-reviews/anti-inflammatory-diet/ (accessed on 15 March 2025).
- Florkowski, M.; Abiona, E.; Frank, K.M.; Brichacek, A.L. Obesity-Associated Inflammation Countered by a Mediterranean Diet: The Role of Gut-Derived Metabolites. Front. Nutr. 2024, 11, 1392666. [Google Scholar] [CrossRef]
- Kenđel Jovanović, G.; Mrakovcic-Sutic, I.; Pavičić Žeželj, S.; Šuša, B.; Rahelić, D.; Klobučar Majanović, S. The Efficacy of an Energy-Restricted Anti-Inflammatory Diet for the Management of Obesity in Younger Adults. Nutrients 2020, 12, 3583. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, G.R.M.; Yousefabadi, H.A.; Niyazi, A.; Rahimi, N.M.; Alikhajeh, Y. Effects of Lifestyle Intervention on Inflammatory Markers and Waist Circumference in Overweight/Obese Adults with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biol. Res. Nurs. 2021, 24, 94–105. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Ambrosius, W.; Messier, S.P.; Miller, G.D.; Penninx, B.W.; Loeser, R.F.; Palla, S.; Bleecker, E.; Pahor, M. Diet-Induced Weight Loss, Exercise, and Chronic Inflammation in Older, Obese Adults: A Randomized Controlled Clinical Trial. Am. J. Clin. Nutr. 2004, 79, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Kantor, E.D.; Lampe, J.W.; Kratz, M.; White, E. Lifestyle Factors and Inflammation: Associations by Body Mass Index. PLoS ONE 2013, 8, e67833. [Google Scholar] [CrossRef] [PubMed]
- Margină, D.; Ungurianu, A.; Purdel, C.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Tekos, F.; Mesnage, R.; Kouretas, D.; Tsatsakis, A. Chronic Inflammation in the Context of Everyday Life: Dietary Changes as Mitigating Factors. Int. J. Environ. Res. Public Health 2020, 17, 4135. [Google Scholar] [CrossRef]
- Do Pro-Inflammatory Diets Harm Our Health? And Can Anti-Inflammatory Diets Help? Available online: https://www.health.harvard.edu/blog/do-pro-inflammatory-diets-harm-our-health-and-can-anti-inflammatory-diets-help-2020122321624 (accessed on 15 March 2025).
- Anti-Inflammation Lifestyle—Brigham and Women’s Hospital. Available online: https://www.brighamandwomens.org/patients-and-families/meals-and-nutrition/bwh-nutrition-and-wellness-hub/special-topics/anti-inflammatory-lifestyle (accessed on 15 March 2025).
- Xia, Y.; Jin, J.; Sun, Y.; Kong, X.; Shen, Z.; Yan, R.; Huang, R.; Liu, X.; Xia, W.; Ma, J.; et al. Tirzepatide’s Role in Targeting Adipose Tissue Macrophages to Reduce Obesity-Related Inflammation and Improve Insulin Resistance. Int. Immunopharmacol. 2024, 143, 113499. [Google Scholar] [CrossRef]
| Cytokine | Cellular Source | Metabolic Impact |
|---|---|---|
| TNF-α | M1 macrophages | Serine phosphorylation of IRS-1 → insulin resistance [24,29] |
| IL-6 | Adipocytes, macrophages | Hepatic CRP production, STAT3-mediated inflammation [29,31] |
| IL-1β | NLRP3 inflammasome (macrophages) | β-cell dysfunction, endothelial activation [28] |
| Mediator | Source | Target Issue | Metabolic Effect |
|---|---|---|---|
| TNF-α | Adipose macrophages | Liver | Increasing glucogenesis, decreasing glycogen synthesis |
| IL-6 | Hypertrophic adipocytes | Muscle | Decreasing insulin receptor tyrosine phosphorylation |
| FFAs | Lipolysis | Endothelium | Increasing ROS production, decreasing nitric oxide bioavailability |
| Leptin | Adipocytes | Hypothalamus | Leptin resistance leading to hyperphagia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkrinia, E.M.M.; Belančić, A. The Mechanisms of Chronic Inflammation in Obesity and Potential Therapeutic Strategies: A Narrative Review. Curr. Issues Mol. Biol. 2025, 47, 357. https://doi.org/10.3390/cimb47050357
Gkrinia EMM, Belančić A. The Mechanisms of Chronic Inflammation in Obesity and Potential Therapeutic Strategies: A Narrative Review. Current Issues in Molecular Biology. 2025; 47(5):357. https://doi.org/10.3390/cimb47050357
Chicago/Turabian StyleGkrinia, Elvira Meni Maria, and Andrej Belančić. 2025. "The Mechanisms of Chronic Inflammation in Obesity and Potential Therapeutic Strategies: A Narrative Review" Current Issues in Molecular Biology 47, no. 5: 357. https://doi.org/10.3390/cimb47050357
APA StyleGkrinia, E. M. M., & Belančić, A. (2025). The Mechanisms of Chronic Inflammation in Obesity and Potential Therapeutic Strategies: A Narrative Review. Current Issues in Molecular Biology, 47(5), 357. https://doi.org/10.3390/cimb47050357







