K-Homology Splicing Regulatory Protein (KSRP) Augments Survival and Proliferation of Human Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Transfection of Human Melanoma Cells
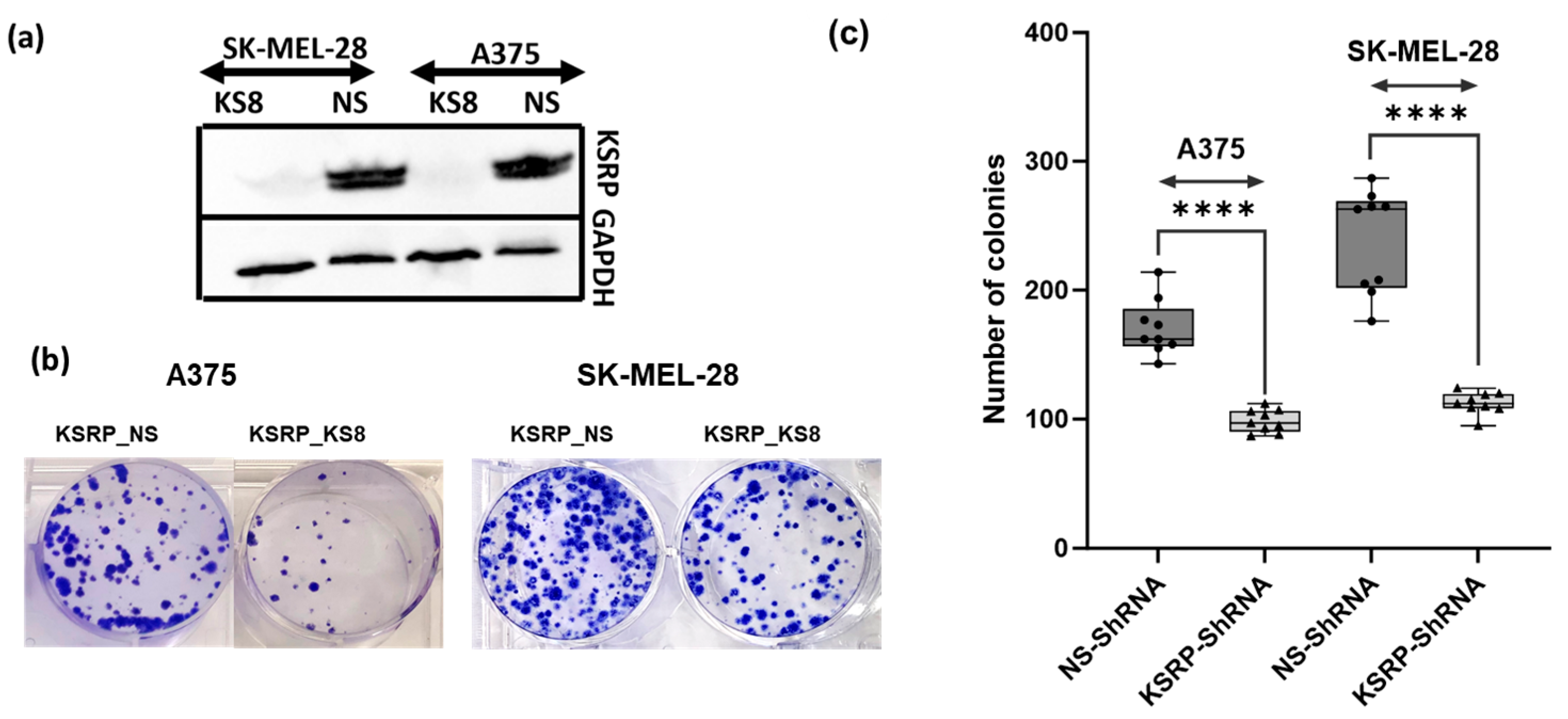
2.2. Preparation of Cell Lysates and Western Blot Analysis
2.3. Clonogenic Assay
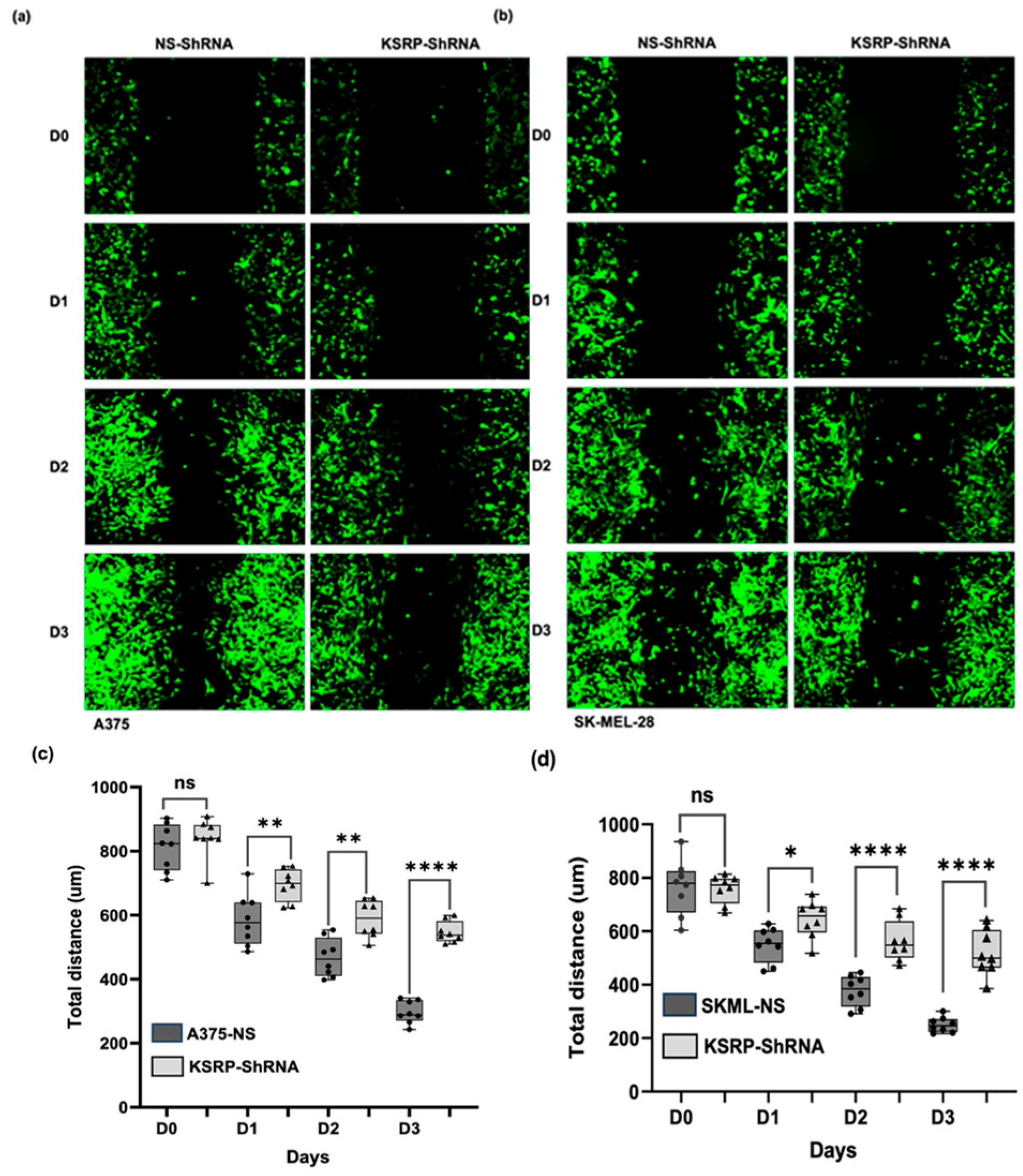
2.4. Cell Migration Assay
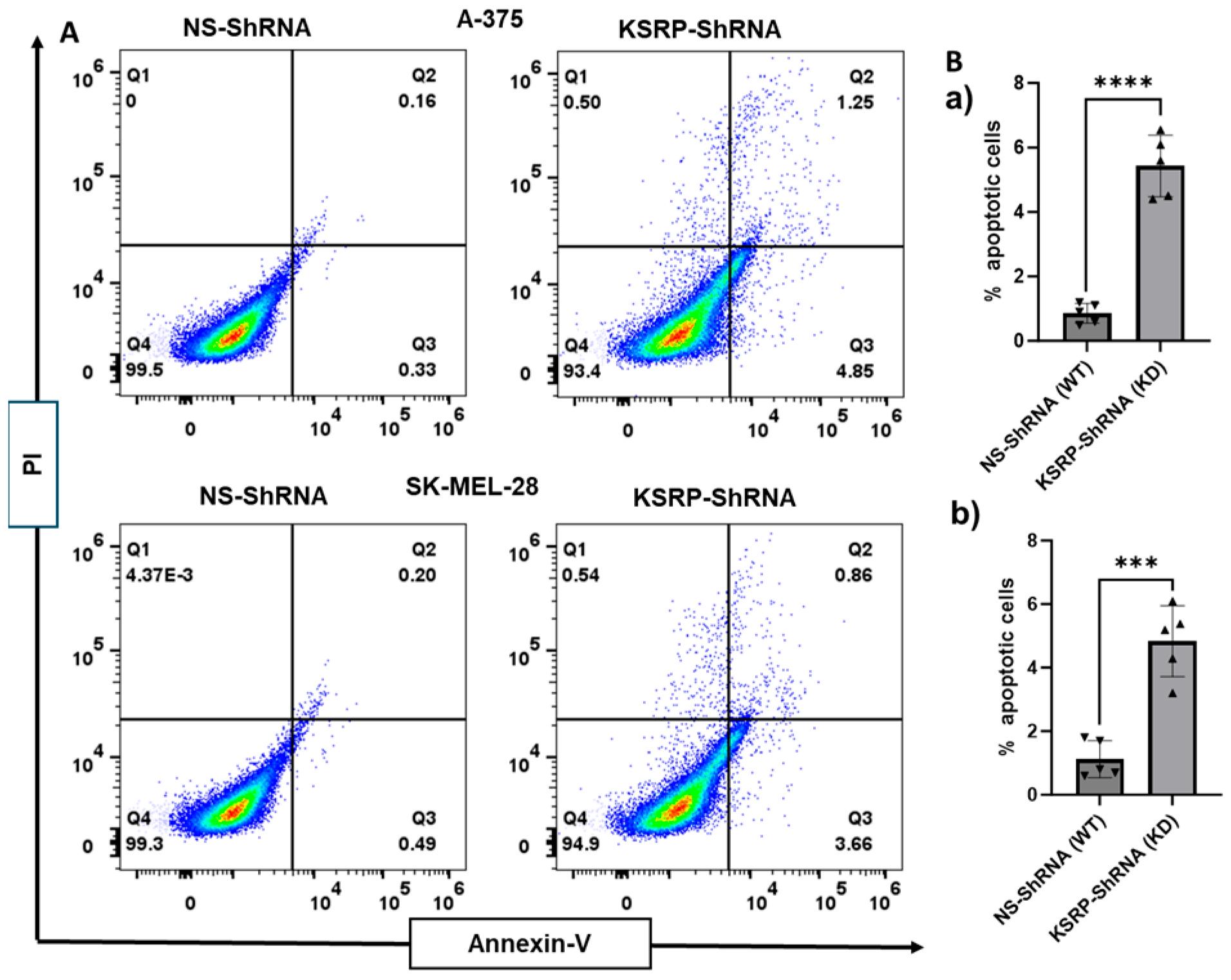
2.5. Apoptosis Assay
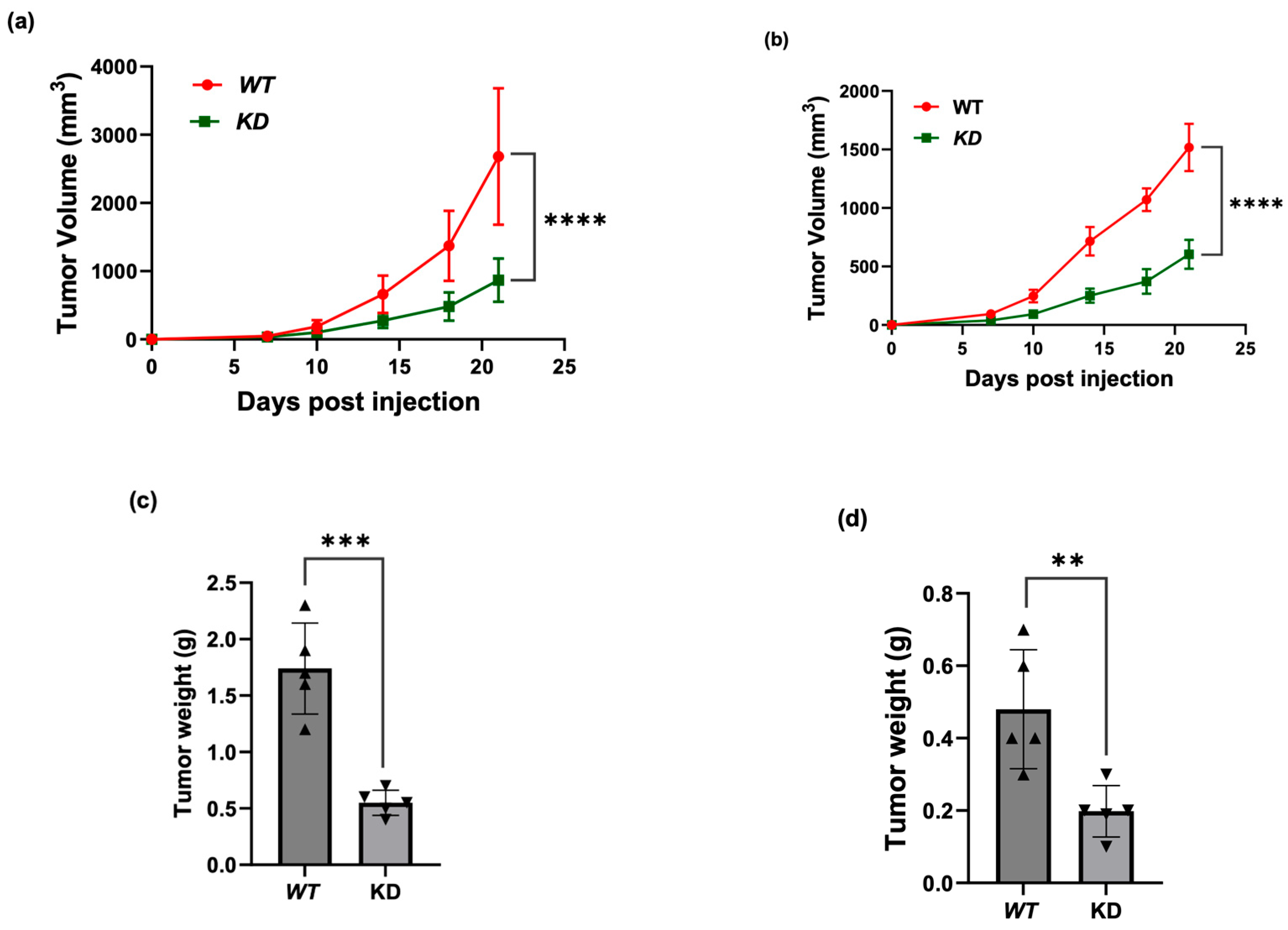
2.6. Tumor Growth in Nude Mice
2.7. RNA Extraction and Quantitative Real-Time PCR
2.8. Statistical Analysis
3. Results
3.1. KSRP Silencing Inhibits Colony Formation and Proliferation of Melanoma Cells
3.2. KSRP Silencing Inhibits Migration of Melanoma Cells
3.3. KSRP Silencing Increases Apoptosis of Melanoma Cells
3.4. KSRP Silencing Inhibits Melanoma Tumor Growth in Nude Mice
3.5. KSRP Silencing Alters Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Giubellino, A. The Current State of Treatment and Future Directions in Cutaneous Malignant Melanoma. Biomedicines 2022, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chou, C.F.; Liu, S.; Crossman, D.; Yusuf, N.; Wu, Y.; Chen, C.Y. KSRP modulates melanoma growth and efficacy of vemurafenib. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Gu, X.L.; Wang, C.; Wang, H.; Ni, Q.C.; Zhang, C.H.; Yu, X.F.; Yang, L.Y.; He, Z.X.; Mao, G.X.; et al. The far-upstream element-binding protein 2 is correlated with proliferation and doxorubicin resistance in human breast cancer cell lines. Tumor Biol. 2016, 37, 9755–9769. [Google Scholar] [CrossRef]
- Pruksakorn, D.; Teeyakasem, P.; Klangjorhor, J.; Chaiyawat, P.; Settakorn, J.; Diskul-Na-Ayudthaya, P.; Chokchaichamnankit, D.; Pothacharoen, P.; Srisomsap, C. Overexpression of KH-type splicing regulatory protein regulates proliferation, migration, and implantation ability of osteosarcoma. Int. J. Oncol. 2016, 49, 903–912. [Google Scholar] [CrossRef]
- Tong, L.; Luo, Y.; Wei, T.; Guo, L.; Wang, H.; Zhu, W.; Zhang, J. KH-type splicing regulatory protein (KHSRP) contributes to tumorigenesis by promoting miR-26a maturation in small cell lung cancer. Mol. Cell. Biochem. 2016, 422, 61–74. [Google Scholar] [CrossRef]
- Bikkavilli, R.K.; Zerayesus, S.A.; Van Scoyk, M.; Wilson, L.; Wu, P.Y.; Baskaran, A.; Tang, K.; Raheem, S.; Samuelson, B.A.; Reddy, N.M.; et al. K-homology splicing regulatory protein (KSRP) promotes post-transcriptional destabilization of Spry4 transcripts in non-small cell lung cancer. J. Biol. Chem. 2017, 292, 7423–7434. [Google Scholar] [CrossRef]
- Yang, J.; Fan, J.; Li, Y.; Li, F.; Chen, P.; Fan, Y.; Xia, X.; Wong, S.T. Genome-wide RNAi screening identifies genes inhibiting the migration of glioblastoma cells. PLoS ONE 2013, 8, e61915. [Google Scholar] [CrossRef]
- Walerych, D.; Lisek, K.; Sommaggio, R.; Piazza, S.; Ciani, Y.; Dalla, E.; Rajkowska, K.; Gaweda-Walerych, K.; Ingallina, E.; Tonelli, C.; et al. Proteasome machinery is instrumental in a common gain-of-function program of the p53 missense mutants in cancer. Nat. Cell Biol. 2016, 18, 897–909. [Google Scholar] [CrossRef]
- Min, H.; Turck, C.W.; Nikolic, J.M.; Black, D.L. A new regulatory protein, KSRP, mediates exon inclusion through an intronic splicing enhancer. Genes Dev. 1997, 11, 1023–1036. [Google Scholar] [CrossRef]
- Gherzi, R.; Lee, K.Y.; Briata, P.; Wegmüller, D.; Moroni, C.; Karin, M.; Chen, C.Y. A KH domain RNA binding protein, KSRP, promotes ARE-directed mRNA turnover by recruiting the degradation machinery. Mol. Cell 2004, 14, 571–583. [Google Scholar] [CrossRef]
- Trabucchi, M.; Briata, P.; Garcia-Mayoral, M.; Haase, A.D.; Filipowicz, W.; Ramos, A.; Gherzi, R.; Rosenfeld, M.G. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature 2009, 459, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Dhamija, S.; Kuehne, N.; Winzen, R.; Doerrie, A.; Dittrich-Breiholz, O.; Thakur, B.K.; Kracht, M.; Holtmann, H. Interleukin-1 activates synthesis of interleukin-6 by interfering with a KH-type splicing regulatory protein (KSRP)-dependent translational silencing mechanism. J. Biol. Chem. 2011, 286, 33279–33288. [Google Scholar] [CrossRef] [PubMed]
- Leavenworth, J.D.; Yusuf, N.; Hassan, Q. K-Homology Type Splicing Regulatory Protein: Mechanism of Action in Cancer and Immune Disorders. Crit. Rev. Eukaryot. Gene Expr. 2024, 34, 75–87. [Google Scholar] [CrossRef]
- Hollingworth, D.; Candel, A.M.; Nicastro, G.; Martin, S.R.; Briata, P.; Gherzi, R.; Ramos, A. KH domains with impaired nucleic acid binding as a tool for functional analysis. Nucleic Acids Res. 2012, 40, 6873–6886. [Google Scholar] [CrossRef]
- Musunuru, K.; Darnell, R.B. Determination and augmentation of RNA sequence specificity of the Nova K-homology domains. Nucleic Acids Res. 2004, 32, 4852–4861. [Google Scholar] [CrossRef]
- García-Mayoral, M.F.; Díaz-Moreno, I.; Hollingworth, D.; Ramos, A. The sequence selectivity of KSRP explains its flexibility in the recognition of the RNA targets. Nucleic Acids Res. 2008, 36, 5290–5296. [Google Scholar] [CrossRef]
- García-Mayoral, M.F.; Díaz-Moreno, I.; Hollingworth, D.; Ramos, A. The structure of the C-terminal KH domains of KSRP reveals a noncanonical motif important for mRNA degradation. Structure 2007, 15, 485–498. [Google Scholar] [CrossRef]
- Michlewski, G.; Cáceres, J.F. Antagonistic role of hnRNP A1 and KSRP in the regulation of let-7a biogenesis. Nat. Struct. Mol. Biol. 2010, 17, 1011–1018. [Google Scholar] [CrossRef]
- Shyu, A.B.; Wilkinson, M.F. The double lives of shuttling mRNA binding proteins. Cell 2000, 102, 135–138. [Google Scholar] [CrossRef]
- Zhang, T.; Kruys, V.; Huez, G.; Gueydan, C. AU-rich element-mediated translational control: Complexity and multiple activities of trans-activating factors. Biochem. Soc. Trans. 2002, 30 Pt 6, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Trabucchi, M.; Briata, P.; Filipowicz, W.; Ramos, A.; Gherzi, R.; Rosenfeld, M.G. KSRP promotes the maturation of a group of miRNA precursors. Adv. Exp. Med. Biol. 2010, 700, 36–42. [Google Scholar] [PubMed]
- Briata, P.; Forcales, S.V.; Ponassi, M.; Corte, G.; Chen, C.Y.; Karin, M.; Puri, P.L.; Gherzi, R. p38-dependent phosphorylation of the mRNA decay-promoting factor KSRP controls the stability of select myogenic transcripts. Mol. Cell 2005, 20, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Elton, T.S.; Selemon, H.; Elton, S.M.; Parinandi, N.L. Regulation of the MIR155 host gene in physiological and pathological processes. Gene 2013, 532, 1–12. [Google Scholar] [CrossRef]
- Ruggiero, T.; Trabucchi, M.; De Santa, F.; Zupo, S.; Harfe, B.D.; McManus, M.T.; Rosenfeld, M.G.; Briata, P.; Gherzi, R. LPS induces KH-type splicing regulatory protein-dependent processing of microRNA-155 precursors in macrophages. FASEB J. 2009, 23, 2898–2908. [Google Scholar] [CrossRef]
- Malz, M.; Weber, A.; Singer, S.; Riehmer, V.; Bissinger, M.; Riener, M.O.; Longerich, T.; Soll, C.; Vogel, A.; Angel, P.; et al. Overexpression of far upstream element binding proteins: A mechanism regulating proliferation and migration in liver cancer cells. Hepatology 2009, 50, 1130–1139. [Google Scholar] [CrossRef]
- Zubaidah, R.M.; Tan, G.S.; Tan, S.B.; Lim, S.G.; Lin, Q.; Chung, M.C. 2-D DIGE profiling of hepatocellular carcinoma tissues identified isoforms of far upstream binding protein (FUBP) as novel candidates in liver carcinogenesis. Proteomics 2008, 8, 5086–5096. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Chien, M.H.; Lee, W.J.; Yang, Y.C.; Li, Y.L.; Chen, B.R.; Cheng, T.Y.; Yang, P.W.; Wang, M.Y.; Jan, Y.H.; Lin, Y.K.; et al. KSRP suppresses cell invasion and metastasis through miR-23a-mediated EGR3 mRNA degradation in non-small cell lung cancer. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 1013–1024. [Google Scholar] [CrossRef]
- Cho, Y.J.; Liang, P. Killin is a p53-regulated nuclear inhibitor of DNA synthesis. Proc. Natl. Acad. Sci. USA 2008, 105, 5396–5401. [Google Scholar] [CrossRef]
- Pal, H.C.; Baxter, R.D.; Hunt, K.M.; Agarwal, J.; Elmets, C.A.; Athar, M.; Afaq, F. Fisetin, a phytochemical, potentiates sorafenib-induced apoptosis and abrogates tumor growth in athymic nude mice implanted with BRAF-mutated melanoma cells. Oncotarget 2015, 6, 28296–28311. [Google Scholar] [CrossRef] [PubMed]
- Pal, H.C.; Diamond, A.C.; Strickland, L.R.; Kappes, J.C.; Katiyar, S.K.; Elmets, C.A.; Athar, M.; Afaq, F. Fisetin, a dietary flavonoid, augments the anti-invasive and anti-metastatic potential of sorafenib in melanoma. Oncotarget 2016, 7, 1227–1241. [Google Scholar] [CrossRef]
- Sándor, N.; Schilling-Tóth, B.; Kis, E.; Fodor, L.; Mucsányi, F.; Sáfrány, G.; Hegyesi, H. TP53inp1 Gene Is Implicated in Early Radiation Response in Human Fibroblast Cells. Int. J. Mol. Sci. 2015, 16, 25450–25465. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Xu, T.; Bao, L.; Hu, X.; Jin, T.; Chen, J.; Chen, J.; Qian, Y.; Lu, X.; Li, L.; et al. CREB3L1 promotes tumor growth and metastasis of anaplastic thyroid carcinoma by remodeling the tumor microenvironment. Mol. Cancer 2022, 21, 190. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Wang, W.; Gao, W.; Li, S.; Lu, P.; Chen, J.; Ding, X.; Ma, P.; Yuan, H.; Lun, Y.; et al. Calcitriol alleviates noise-induced hearing loss by regulating the ATF3/DUSP1 signalling pathway. Ecotoxicol. Environ. Saf. 2024, 284, 116906. [Google Scholar] [CrossRef]
- Song, S.; He, X.; Wang, J.; Wang, R.; Wang, L.; Zhao, W.; Wang, Y.; Zhang, Y.; Yu, Z.; Miao, D.; et al. ELF3-AS1 contributes to gastric cancer progression by binding to hnRNPK and induces thrombocytosis in peripheral blood. Cancer Sci. 2021, 112, 4553–4569. [Google Scholar] [CrossRef]
- Abdel-Maksoud, M.A.; Ullah, S.; Nadeem, A.; Shaikh, A.; Zia, M.K.; Zakri, A.M.; Almanaa, T.N.; Alfuraydi, A.A.; Mubarak, A.; Hameed, Y. Unlocking the diagnostic, prognostic roles, and immune implications of BAX gene expression in pan-cancer analysis. Am. J. Transl. Res. 2024, 16, 63–74. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, S. BAP1 mutations inhibit the NF-κB signaling pathway to induce an immunosuppressive microenvironment in uveal melanoma. Mol. Med. 2023, 29, 126. [Google Scholar] [CrossRef]
- Chen, G.G.; Lai, P.B.; Chan, P.K.; Chak, E.C.; Yip, J.H.; Ho, R.L.; Leung, B.C.; Lau, W.Y. Decreased expression of Bid in human hepatocellular carcinoma is related to hepatitis B virus X protein. Eur. J. Cancer 2001, 37, 1695–1702. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, H.; Lu, J. PTEN and hTERT gene expression and the correlation with human hepatocellular carcinoma. Pathol. Res. Pract. 2015, 211, 316–319. [Google Scholar] [CrossRef]
- Fujita, Y.; Masuda, K.; Hamada, J.; Shoda, K.; Naruto, T.; Hamada, S.; Miyakami, Y.; Kohmoto, T.; Watanabe, M.; Takahashi, R.; et al. KH-type splicing regulatory protein is involved in esophageal squamous cell carcinoma progression. Oncotarget 2017, 8, 101130–101145. [Google Scholar] [CrossRef] [PubMed]
- Santarosa, M.; Del Col, L.; Viel, A.; Bivi, N.; D’Ambrosio, C.; Scaloni, A.; Tell, G.; Maestro, R. BRCA1 modulates the expression of hnRNPA2B1 and KHSRP. Cell Cycle 2010, 9, 4666–4673. [Google Scholar] [CrossRef] [PubMed]
- Taniuchi, K.; Ogasawara, M. KHSRP-bound small nucleolar RNAs associate with promotion of cell invasiveness and metastasis of pancreatic cancer. Oncotarget 2020, 11, 131–147. [Google Scholar] [CrossRef]
- Bae, J.A.; Bae, W.K.; Kim, S.J.; Ko, Y.S.; Kim, K.Y.; Park, S.Y.; Yu, Y.H.; Kim, E.A.; Chung, I.J.; Kim, H.; et al. A new KSRP-binding compound suppresses distant metastasis of colorectal cancer by targeting the oncogenic KITENIN complex. Mol. Cancer 2021, 20, 78. [Google Scholar] [CrossRef]
- Zaffaroni, N.; Beretta, G.L. The Therapeutic Potential of Pyroptosis in Melanoma. Int. J. Mol. Sci. 2023, 24, 1285. [Google Scholar] [CrossRef]
- Seillier, M.; Peuget, S.; Gayet, O.; Gauthier, C.; N’Guessan, P.; Monte, M.; Carrier, A.; Iovanna, J.L.; Dusetti, N.J. TP53INP1, a tumor suppressor, interacts with LC3 and ATG8-family proteins through the LC3-interacting region (LIR) and promotes autophagy-dependent cell death. Cell Death Differ. 2012, 19, 1525–1535. [Google Scholar] [CrossRef]
- Zu, T.; Wang, D.; Xu, S.; Lee, C.A.A.; Zhen, E.; Yoon, C.H.; Abarzua, P.; Wang, S.; Frank, N.Y.; Wu, X.; et al. ATF-3 expression inhibits melanoma growth by downregulating ERK and AKT pathways. Lab. Investig. 2021, 101, 636–647. [Google Scholar] [CrossRef]
- Fecker, L.F.; Geilen, C.C.; Tchernev, G.; Trefzer, U.; Assaf, C.; Kurbanov, B.M.; Schwarz, C.; Daniel, P.T.; Eberle, J. Loss of proapoptotic Bcl-2-related multidomain proteins in primary melanomas is associated with poor prognosis. J. Investig. Dermatol. 2006, 126, 1366–1371. [Google Scholar] [CrossRef][Green Version]
- Eskes, R.; Desagher, S.; Antonsson, B.; Martinou, J.C. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell Biol. 2000, 20, 929–935. [Google Scholar] [CrossRef]
- Kuwana, T.; Mackey, M.R.; Perkins, G.; Ellisman, M.H.; Latterich, M.; Schneiter, R.; Green, D.R.; Newmeyer, D.D. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 2002, 111, 331–342. [Google Scholar] [CrossRef]
- Letai, A.; Bassik, M.C.; Walensky, L.D.; Sorcinelli, M.D.; Weiler, S.; Korsmeyer, S.J. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2002, 2, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, T.; Bouchier-Hayes, L.; Chipuk, J.E.; Bonzon, C.; Sullivan, B.A.; Green, D.R.; Newmeyer, D.D. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell 2005, 17, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Anvekar, R.A.; Asciolla, J.J.; Lopez-Rivera, E.; Floros, K.V.; Izadmehr, S.; Elkholi, R.; Belbin, G.; Sikora, A.G.; Chipuk, J.E. Sensitization to the mitochondrial pathway of apoptosis augments melanoma tumor cell responses to conventional chemotherapeutic regimens. Cell Death Dis. 2012, 3, e420. [Google Scholar] [CrossRef] [PubMed]
- Aung, P.P.; Aung, P.P.; Nagarajan, P.; Tetzlaff, M.T.; Curry, J.L.; Tang, G.; Abdullaev, Z.; Pack, S.D.; Ivan, D.; Prieto, V.G.; et al. Melanoma With Loss of BAP1 Expression in Patients With No Family History of BAP1-Associated Cancer Susceptibility Syndrome: A Case Series. Am. J. Dermatopathol. 2019, 41, 167–179. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Y.; Xiao, X.; Zhang, X.; Wan, J.; Zheng, T.; Chen, H.; Liu, T.; Tang, X. Pan-cancer analysis of CREB3L1 as biomarker in the prediction of prognosis and immunotherapeutic efficacy. Front. Genet. 2022, 13, 938510. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, C. Suppression of malignant melanoma by knocking down growth differentiation factor-15 via inhibiting PTEN/PI3K/AKT signaling pathway. J. Cancer 2024, 15, 1115–1123. [Google Scholar] [CrossRef]
- Ünal, B.; Alan, S.; Başsorgun, C.İ.; Karakaş, A.A.; Elpek, G.Ö.; Çiftçioğlu, M.A. The divergent roles of growth differentiation factor-15 (GDF-15) in benign and malignant skin pathologies. Arch. Dermatol. Res. 2015, 307, 551–557. [Google Scholar] [CrossRef]
- Dong, Y.; Richards, J.A.; Gupta, R.; Aung, P.P.; Emley, A.; Kluger, Y.; Dogra, S.K.; Mahalingam, M.; Wajapeyee, N. PTEN functions as a melanoma tumor suppressor by promoting host immune response. Oncogene 2014, 33, 4632–4642. [Google Scholar] [CrossRef]
- Qu, H.; Zhao, H.; Zhang, X.; Liu, Y.; Li, F.; Sun, L.; Song, Z. Integrated Analysis of the ETS Family in Melanoma Reveals a Regulatory Role of ETV7 in the Immune Microenvironment. Front. Immunol. 2020, 11, 612784. [Google Scholar] [CrossRef]

| Gene | Primer Sequence | References |
|---|---|---|
| GAPDH | 5′-CGACCACTTTGTCAAGCTCA-3′ 5′-AGGGGTCTACATGGCAACTG-3′ | [33] |
| Caspase1 | 5′-CTTGGAGACATCCCACAATG-3′ 5′-CTGCCCACAGACATTCATAC-3′ | [3] |
| Tp53INP1 | 5′-TCAGCAGAAGAAGAAGAAGAAGAG-3′ 5′-AGCAGGAATCACTTGTATCAGC-3′ | [33] |
| GDF15 | 5′-TCACGCCAGAAGTGCGGCTG-3′ 5′-CGTCCCACGACCTTGACGCC-3′ | [33] |
| CREB3L1 | 5′-GGAGAATGCCAACAGGACC-3′ 5′-GCACCAGAACAAAGCACAAG-3′ | [34] |
| ATF3 | 5′-AGTCAGTTACCGTCAACAACAGA-3′ 5′-CTCAGCATTCACACTCTCCAGTT-3′ | [35] |
| ELF3 | 5′-CCACCACCATCTTTCCGAGT-3′ 5′-TACGGGTGACAGGCTACAAA-3′ | [36] |
| BAX | 5′-TTTGCTTCAGGGTTTCATCC-3′ 5′-CAGTTGAAGTTGCCGTCAGA-3′ | [37] |
| Bap1 | 5′-AGGAGCTGCTGGCACTGCTGA-3′ 5′-TTGTGGAGCCGGCCGATGCT-3′ | [38] |
| Bid | 5′-ATGGACTGTGAGGTCAACAACGG-3′ 5′-CACGTAGGTGCGTAGGTTCTGGTTA-3′ | [39] |
| PTEN | 5′-AGTTTGTGGTCTGCCAGCTA-3′ 5′-TCAGAGTCAGTGGTGTCAGA-3′ | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, H.; Sherwani, M.A.; Seo, J.V.; Ahmad, A.; Tasnim, S.; Hassan, Q.; Yusuf, N. K-Homology Splicing Regulatory Protein (KSRP) Augments Survival and Proliferation of Human Melanoma Cells. Curr. Issues Mol. Biol. 2025, 47, 356. https://doi.org/10.3390/cimb47050356
Rashid H, Sherwani MA, Seo JV, Ahmad A, Tasnim S, Hassan Q, Yusuf N. K-Homology Splicing Regulatory Protein (KSRP) Augments Survival and Proliferation of Human Melanoma Cells. Current Issues in Molecular Biology. 2025; 47(5):356. https://doi.org/10.3390/cimb47050356
Chicago/Turabian StyleRashid, Harunur, Mohammad Asif Sherwani, Jung Vin Seo, Azeem Ahmad, Sumaiya Tasnim, Quamarul Hassan, and Nabiha Yusuf. 2025. "K-Homology Splicing Regulatory Protein (KSRP) Augments Survival and Proliferation of Human Melanoma Cells" Current Issues in Molecular Biology 47, no. 5: 356. https://doi.org/10.3390/cimb47050356
APA StyleRashid, H., Sherwani, M. A., Seo, J. V., Ahmad, A., Tasnim, S., Hassan, Q., & Yusuf, N. (2025). K-Homology Splicing Regulatory Protein (KSRP) Augments Survival and Proliferation of Human Melanoma Cells. Current Issues in Molecular Biology, 47(5), 356. https://doi.org/10.3390/cimb47050356







