The Role of BCL-2 Expression in Patients with Myelodysplastic Neoplasms
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
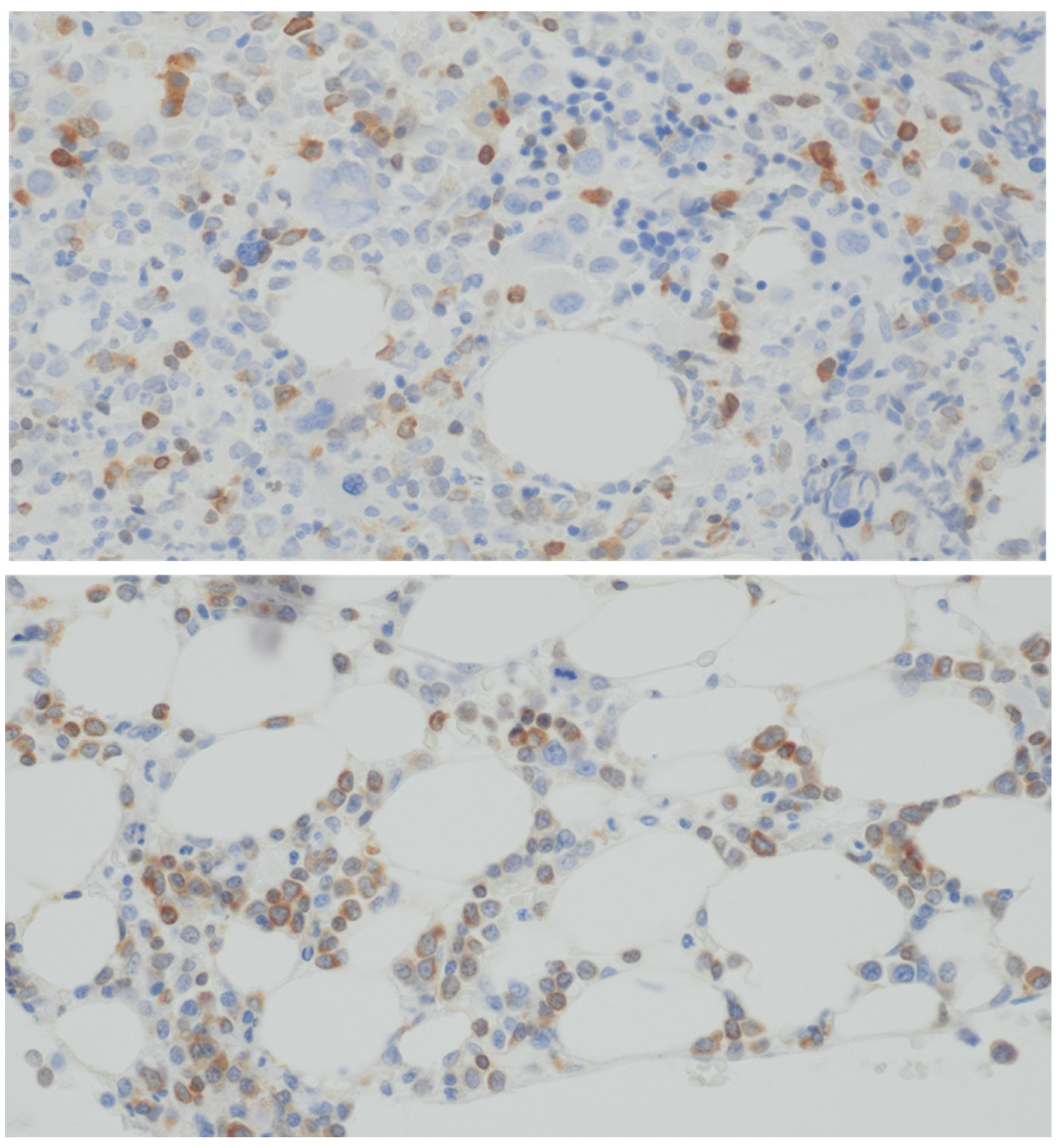
2.2. Staining Technique Evaluation
2.3. Statistical Analysis
3. Results
3.1. BCL-2 Expression and Survival
3.2. BCL-2 Expression and Cytogenetic Risk
3.3. BCL-2 Expression and IPSS-R
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Hu, F.; Gale, R.P.; Sekeres, M.A.; Liang, Y. Myelodysplastic Syndromes. Nat. Rev. Dis. Prim. 2022, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X. Epidemiology of Myelodysplastic Syndromes: Why Characterizing the Beast Is a Prerequisite to Taming It. Blood Rev. 2019, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Vardinam, J.W. Mechanisms of Disease. Myelodysplastic Syndromes. N. Engl. J. Med. 2009, 361, 1872–1885. [Google Scholar] [CrossRef] [PubMed]
- Neukirchen, J.; Schoonen, W.M.; Strupp, C.; Gattermann, N.; Aul, C.; Haas, R.; Germing, U. Incidence and Prevalence of Myelodysplastic Syndromes: Data from the Düsseldorf MDS-Registry. Leuk. Res. 2011, 35, 1591–1596. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Detappe, A.; Anderson, K.C.; Steensma, D.P. The Bone-Marrow Niche in MDS and MGUS: Implications for AML and MM. Nat. Rev. Clin. Oncol. 2018, 15, 219–233. [Google Scholar] [CrossRef]
- Graf, J.R.; Forster, S.; Bruehl, F.K.; Banz, Y.; Hallal, M.; Brodard, J.; Bacher, V.U.; Allam, R.; Schürch, C.M.; Bonadies, N. Diagnostic and Prognostic Implications of Caspase-1 and Pd-l1 Co-expression Patterns in Myelodysplastic Syndromes. Cancers 2021, 13, 5712. [Google Scholar] [CrossRef]
- Kliszek, P.; Juszczyński, P. Deregulacja Rodziny Białek BCL2 w Chłoniakach B-Komórkowych—Implikacje Molekularne, Patogenetyczne, Kliniczne i Terapeutyczne Deregulation of BCL2 Family Proteins in B-Cell Lymphomas—Molecular, Pathogenetic, Clinical and Therapeutic Implications. Hematologia 2012, 3, 288–301. [Google Scholar]
- Campbell, K.J.; Tait, S.W.G. Targeting BCL-2 Regulated Apoptosis in Cancer. Open Biol. 2018, 8, 180002. [Google Scholar] [CrossRef]
- Davis, R.E.; Greenberg, P.L. Bcl-2 Expression by Myeloid Precursors in Myelodysplastic Syndromes: Relation to Disease Progression. Leuk. Res. 1998, 22, 767–777. [Google Scholar] [CrossRef]
- Mittelman, M.; Oster, H.S.; Hoffman, M.; Neumann, D. The Lower Risk MDS Patient at Risk of Rapid Progression. Leuk. Res. 2010, 34, 1551–1555. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Borate, U.; Pollyea, D.A.; Brunner, A.M.; Roncolato, F.; Garcia, J.S.; Filshie, R.; Odenike, O.; Watson, A.M.; Krishnadasan, R.; et al. A Phase 1b Study of Venetoclax and Azacitidine Combination in Patients with Relapsed or Refractory Myelodysplastic Syndromes. Am. J. Hematol. 2023, 98, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.S.; Kim, H.T.; Murdock, H.M.; Cutler, C.S.; Brock, J.; Gooptu, M.; Ho, V.T.; Koreth, J.; Nikiforow, S.; Romee, R.; et al. Adding Venetoclax to Fludarabine/Busulfan RIC Transplant for High-Risk MDS and AML Is Feasible, Safe, and Active. Blood Adv. 2021, 5, 5536–5545. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Garcia, J.S.; Borate, U.; Fong, C.Y.; Baer, M.R.; Nolte, F.; Peterlin, P.; Jurcic, J.G.; Garcia-Manero, G.; Hong, W.-J.; et al. A Phase 1b Study Evaluating the Safety and Efficacy of Venetoclax in Combination with Azacitidine in Treatment-Naïve Patients with Higher-Risk Myelodysplastic Syndrome. Blood 2019, 134, 568. [Google Scholar] [CrossRef]
- Fenaux, P.; Haase, D.; Santini, V.; Sanz, G.F.; Platzbecker, U.; Mey, U. Myelodysplastic Syndromes: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†☆. Ann. Oncol. 2021, 32, 142–156. [Google Scholar] [CrossRef]
- Greenberg, P.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Boudard, D.; Vasselon, C.; Berthéas, M.F.; Jaubert, J.; Mounier, C.; Reynaud, J.; Viallet, A.; Chautard, S.; Guyotat, D.; Campos, L. Expression and Prognostic Significance of Bcl-2 Family Proteins in Myelodysplastic Syndromes. Am. J. Hematol. 2002, 70, 115–125. [Google Scholar] [CrossRef]
- Kurotaki, H.; Tsushima, Y.; Nagai, K.; Yagihashi, S. Apoptosis, Bcl-2 Expression and P53 Accumulation in Myelodysplastic Syndrome, Myelodysplastic-Syndrome-Derived Acute Myelogenous Leukemia and de Novo Acute Myelogenous Leukemia. Acta Haematol. 1999, 102, 115–123. [Google Scholar] [CrossRef]
- Parker, J.E.; Mufti, G.J.; Rasool, F.; Mijovic, A.; Devereux, S.; Pagliuca, A. The Role of Apoptosis, Proliferation, and the Bcl-2-Related Proteins in the Myelodysplastic Syndromes and Acute Myeloid Leukemia Secondary to MDS. Blood 2000, 96, 3932–3938. [Google Scholar] [CrossRef]
- Dror, Y. The Role of Mitochondrial-Mediated Apoptosis in a Myelodysplastic Syndrome Secondary to Congenital Deletion of the Short Arm of Chromosome 4. Exp. Hematol. 2003, 31, 211–217. [Google Scholar] [CrossRef]
- Bazinet, A.; Darbaniyan, F.; Jabbour, E.; Montalban-Bravo, G.; Ohanian, M.; Chien, K.; Kadia, T.; Takahashi, K.; Masarova, L.; Short, N.; et al. Azacitidine plus Venetoclax in Patients with High-Risk Myelodysplastic Syndromes or Chronic Myelomonocytic Leukaemia: Phase 1 Results of a Single-Centre, Dose-Escalation, Dose-Expansion, Phase 1–2 Study. Lancet Haematol. 2022, 9, e756–e765. [Google Scholar] [CrossRef]
- Du, Y.; Li, C.; Yan, J. The Efficacy and Safety of Venetoclax and Azacytidine Combination Treatment in Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome: Systematic Review and Meta-Analysis. Hematology 2023, 28, 2198098. [Google Scholar] [CrossRef] [PubMed]
- Ganan-Gomez, I.; Yang, H.; Ma, F.; Montalban-Bravo, G.; Thongon, N.; Marchica, V.; Richard-Carpentier, G.; Chien, K.; Manyam, G.; Wang, F.; et al. Stem cell architecture drives myelodysplastic syndrome progression and predicts response to venetoclax-based therapy. Nat. Med. 2022, 28, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Blum, S.; Tsilimidos, G.; Bresser, H.; Lübbert, M. Role of BCL-2 inhibition in myelodysplastic syndromes. Int. J. Cancer 2023, 152, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.R.; Chakraborty, S.; Shastri, A. Mechanisms of resistance to hypomethylating agents and BCL-2 inhibitors. Best. Pract. Res. Clin. Haematol. 2023, 36, 101521. [Google Scholar] [CrossRef]
- Kuszczak, B.; Wróbel, T.; Wicherska-Pawłowska, K.; Rybka, J. The Role of BCL-2 and PD-1/PD-L1 Pathway in Pathogenesis of Myelodysplastic Syndromes. Int. J. Mol. Sci. 2023, 24, 4708. [Google Scholar] [CrossRef]
- Kok, C.H.; Yeung, D.T.; Hiwase, D.K. Special Issue “Advances in Molecular Pathogenesis and Targeted Therapies for Myeloid Neoplasms”. Int. J. Mol. Sci. 2024, 25, 2056. [Google Scholar] [CrossRef]
- Marchica, V.; Mazzetti, C.; Galli, S. BCL-2 family proteins in myelodysplastic syndromes: From pathogenesis to targeted therapy. Semin. Hematol. 2022, 59, 66–72. [Google Scholar]
- Zeidan, A.M.; Pollyea, D.A.; Garcia, J.S.; Brunner, A.; Roncolato, F.; Borate, U.; Filshie, R.; Odenike, O.; Watson, A.M.; Krishnadasan, R.; et al. A phase 1b study of venetoclax and azacitidine in patients with relapsed/refractory myelodysplastic syndrome. Blood 2019, 134 (Suppl. 1), 565. [Google Scholar] [CrossRef]
- Kusumoto, S.; Tanaka, T.; Ueda, R. Novel treatment strategies of targeting BCL-2, BCL-XL and MCL-1. Jpn. J. Clin. Oncol. 2022, 52, 521–529. [Google Scholar]


| Characteristic | |
|---|---|
| Age range, median [years] | 23–91, 66 |
| Hemoglobin, median [g/dL] | 9.3 (5.0–12.2) |
| Leukocytes × 103/µm, median | 2,76 (0.92–29.22) |
| Neutrofiles × 103/µm, median | 1.28 (0.08–24.1) |
| Platelets × 103/µm, median | 81 (1.0–731) |
| WHO a 2022 classification | Number of patients |
| MDS-LB b,c | 24 |
| MDS-5q- b,d | 1 |
| MDS-IB1 b,e | 11 |
| MDS-IB2 b,f | 33 |
| MDS-F b,g | 6 |
| Hypoplastic MDS b | 1 |
| IPSS-R h risk group | Number of patients |
| Low and very low | 16 |
| Intermediate | 17 |
| High | 22 |
| Very high | 21 |
| First line treatment | Number of patients |
| Azacitidine | 48 |
| Intensive chemotherapy | 6 |
| Lenalidomide | 5 |
| No treatment | 15 |
| Other therapies | 2 |
| Response after 1 line treatment | Number of patients |
| CR i | 19 |
| PR j | 4 |
| NR k | 29 |
| Assessment not performed | 24 |
| IPSS-R Risk Group | Median H-Score | Min H-Score | Max H-Score | N |
|---|---|---|---|---|
| Very Low | 0 | 0 | 0 | 0 |
| Low | 0 | 0 | 0 | 0 |
| Intermediate | 0 | 0 | 0 | 0 |
| High | 5 | 0 | 50 | 3 |
| Very High | 15 | 5 | 140 | 8 |
| MDS type (WHO 2022) | Median H-score | Min H-score | Max H-score | N |
| Hypoplastic MDS | 0 | 0 | 0 | 0 |
| MDS-LB | 37.5 | 25 | 55 | 4 |
| MDS-IB-1 | 20 | 0 | 45 | 6 |
| MDS-IB-2 | 32.5 | 0 | 140 | 14 |
| MDS-5q | 0 | 0 | 0 | 0 |
| MDS-F | 135 | 135 | 135 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuszczak, B.; Zduniak, K.; Jendzierowska, A.; Wróbel, T.; Ziółkowski, P.; Rybka, J. The Role of BCL-2 Expression in Patients with Myelodysplastic Neoplasms. Curr. Issues Mol. Biol. 2025, 47, 346. https://doi.org/10.3390/cimb47050346
Kuszczak B, Zduniak K, Jendzierowska A, Wróbel T, Ziółkowski P, Rybka J. The Role of BCL-2 Expression in Patients with Myelodysplastic Neoplasms. Current Issues in Molecular Biology. 2025; 47(5):346. https://doi.org/10.3390/cimb47050346
Chicago/Turabian StyleKuszczak, Bartłomiej, Krzysztof Zduniak, Angela Jendzierowska, Tomasz Wróbel, Piotr Ziółkowski, and Justyna Rybka. 2025. "The Role of BCL-2 Expression in Patients with Myelodysplastic Neoplasms" Current Issues in Molecular Biology 47, no. 5: 346. https://doi.org/10.3390/cimb47050346
APA StyleKuszczak, B., Zduniak, K., Jendzierowska, A., Wróbel, T., Ziółkowski, P., & Rybka, J. (2025). The Role of BCL-2 Expression in Patients with Myelodysplastic Neoplasms. Current Issues in Molecular Biology, 47(5), 346. https://doi.org/10.3390/cimb47050346





