Computational Modelling of Tunicamycin C Interaction with Potential Protein Targets: Perspectives from Inverse Docking with Molecular Dynamic Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Target Proteins
2.2. Computational Modelling Studies
2.2.1. Computer Hardware Used for Computational Modelling Studies
2.2.2. Preparation of TK1, PKAc, and Tunicamycin C for Induced Fit Docking and MD Simulation
2.2.3. Docking Tunicamycin C into TK1 and PKAc Using Induced Fit Ligand Docking
2.2.4. Molecular Dynamics Simulation Studies
2.2.5. Post-Dynamic Analysis
2.2.6. Implicit Physiological Condition MM/PBSA Binding Free Energy Calculation
3. Results
3.1. Screening Results
Screening Potential Tunicamycin C Protein Targets
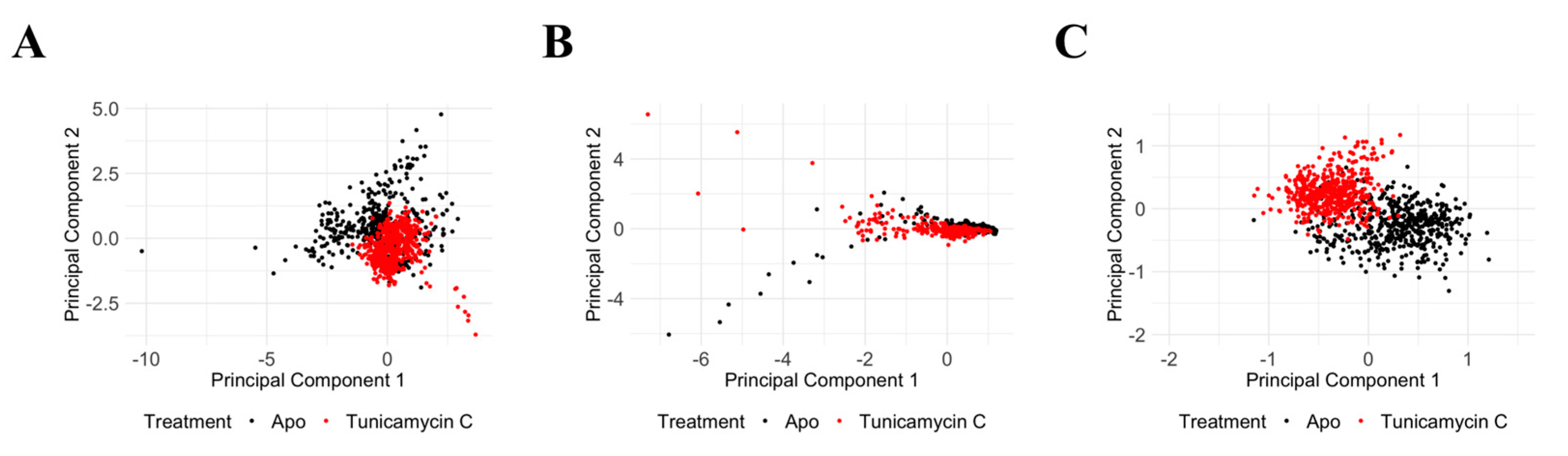
3.2. Molecular Dynamics Simulation and Post-Dynamic Analysis
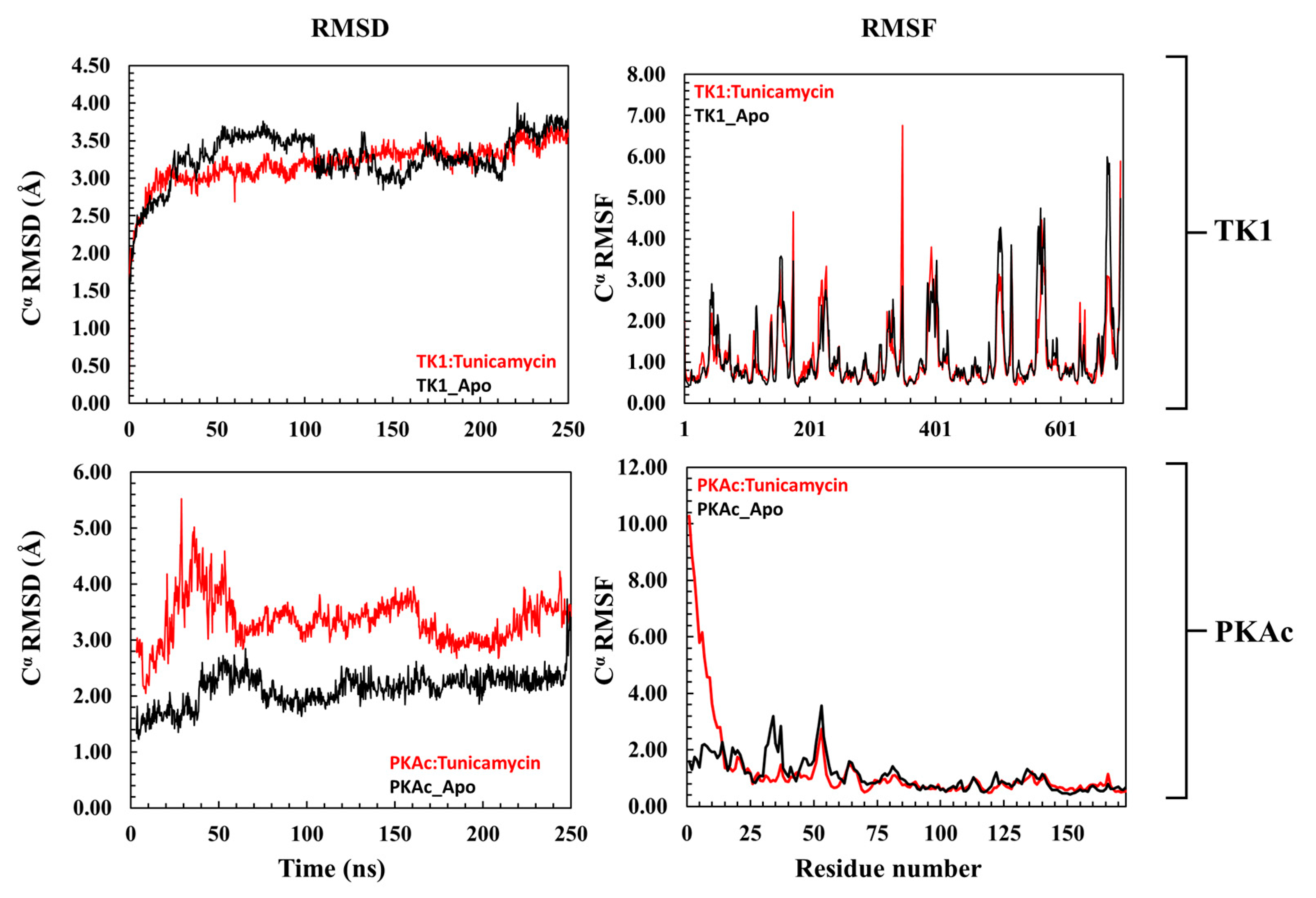
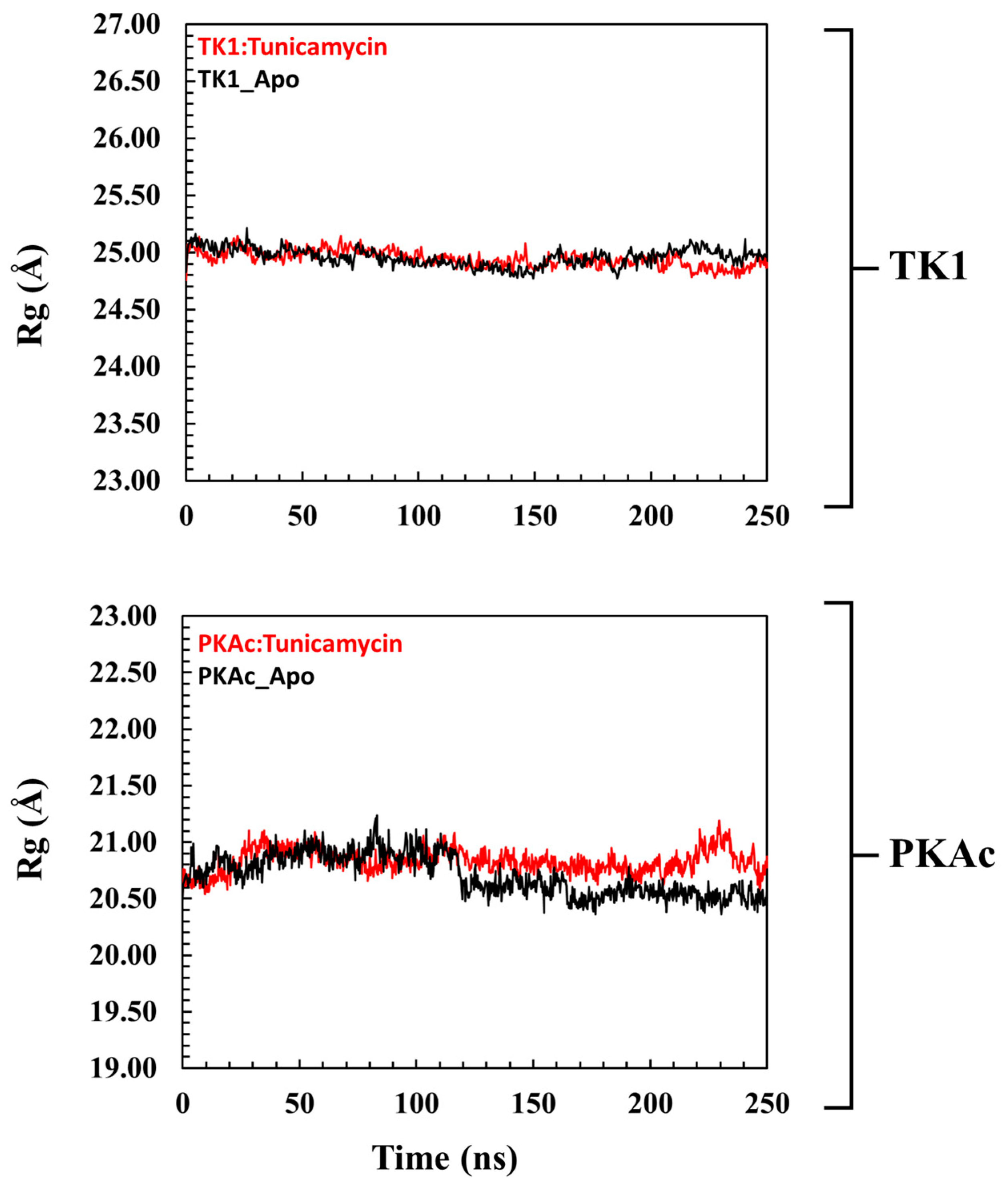
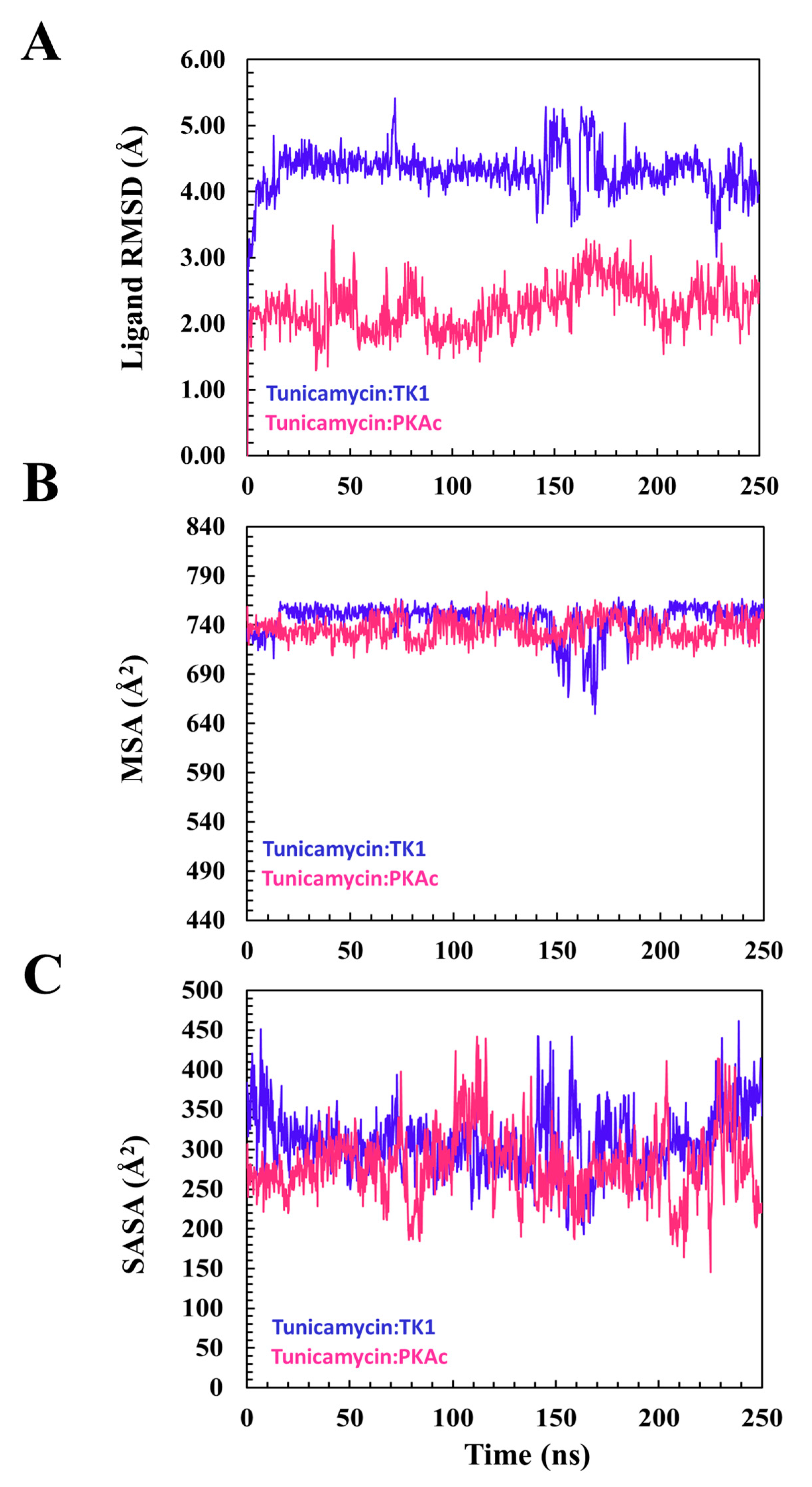
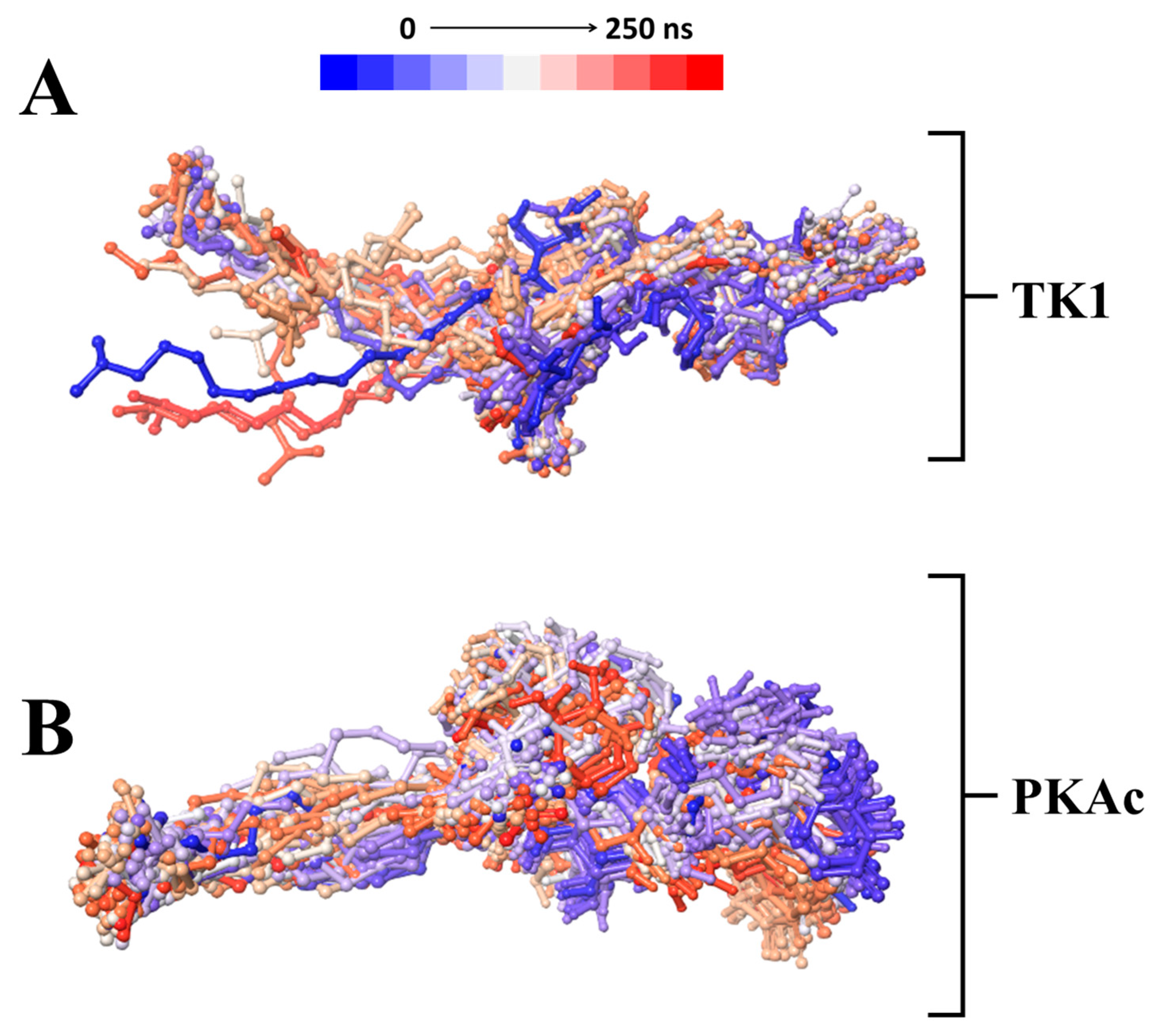
3.2.1. Comparative Evaluation of Structural Drift from Their Centre of Mass
3.2.2. Estimation of Relative Tunicamycin C Binding Dynamics for TK1 and PKAc
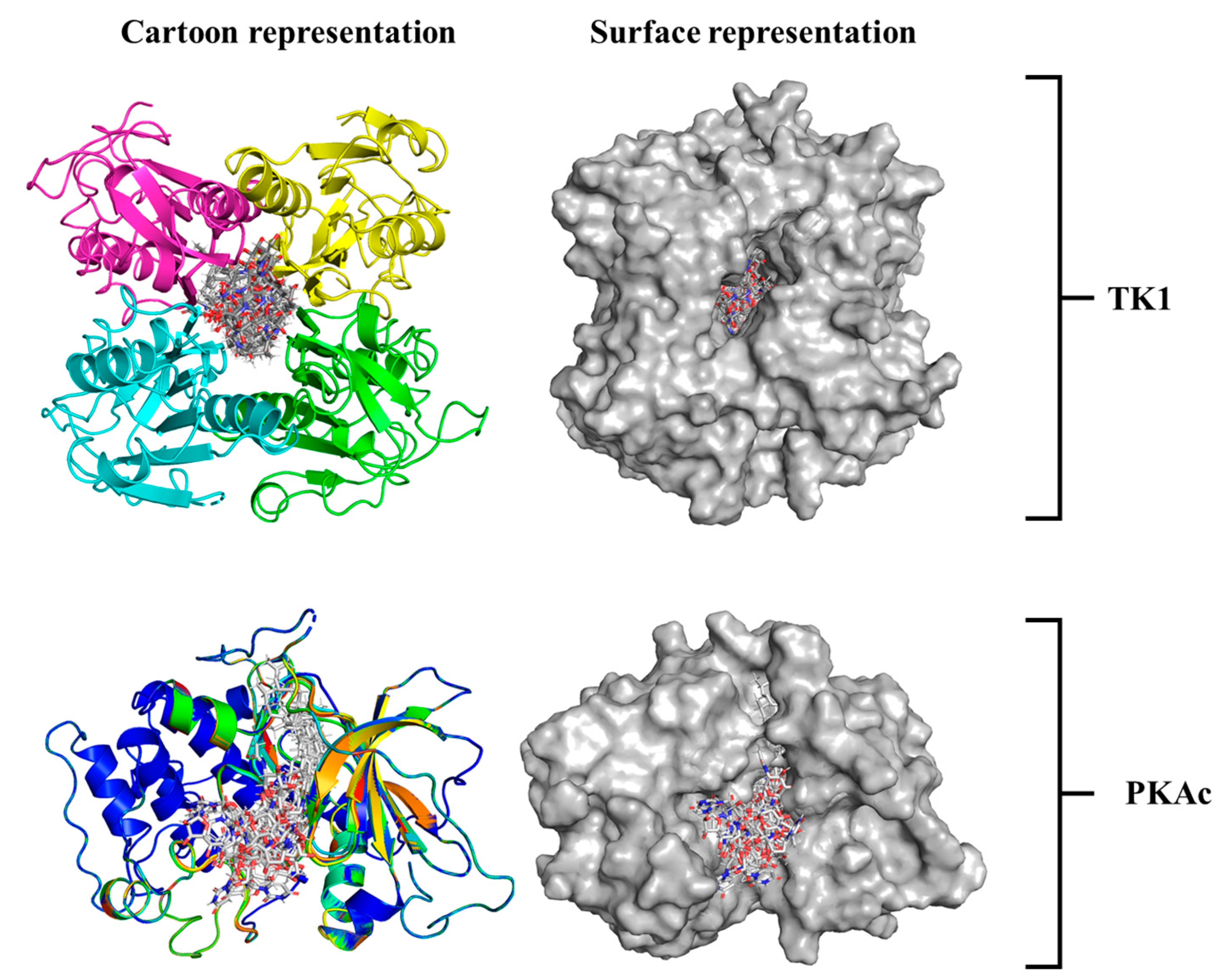
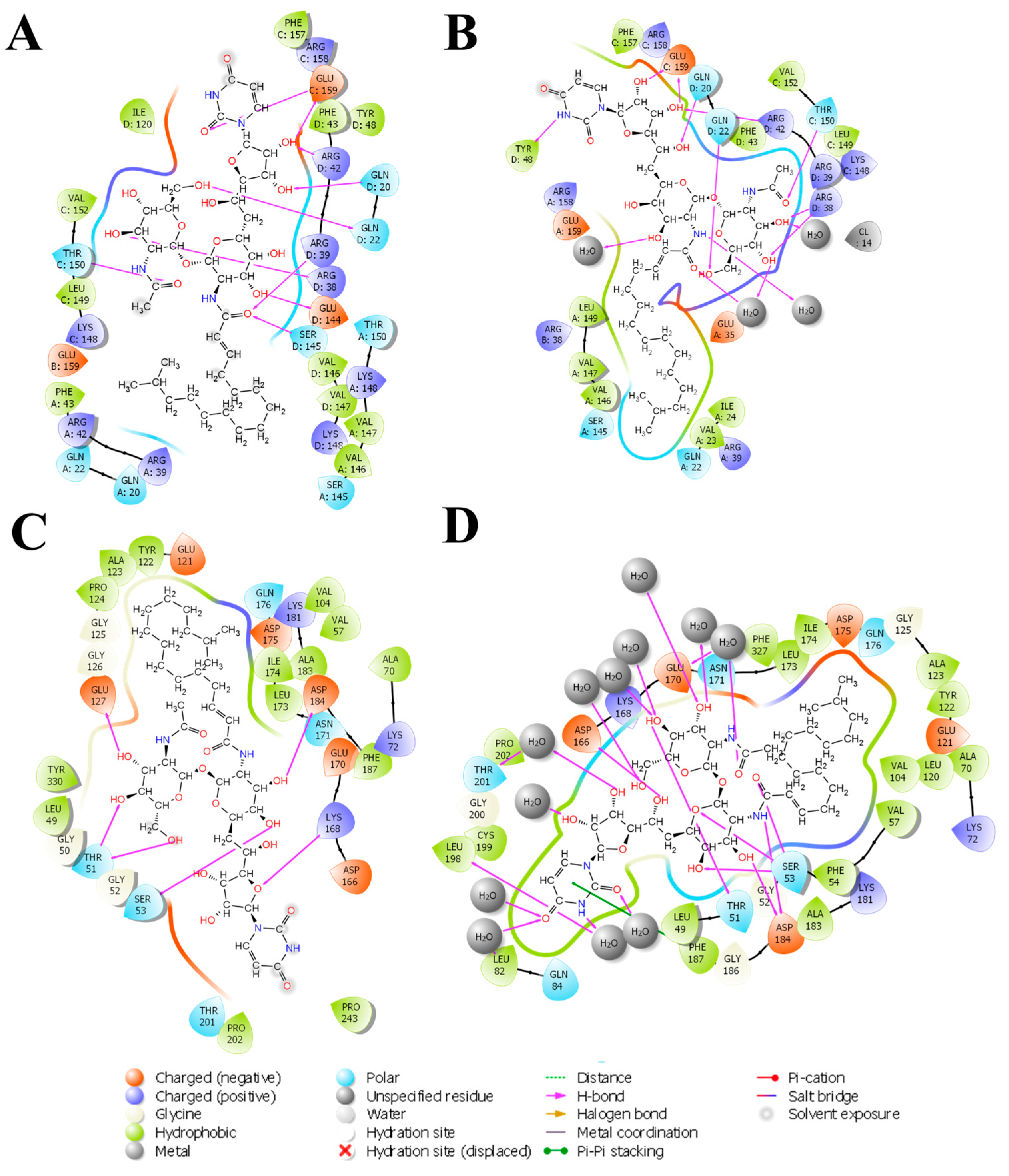
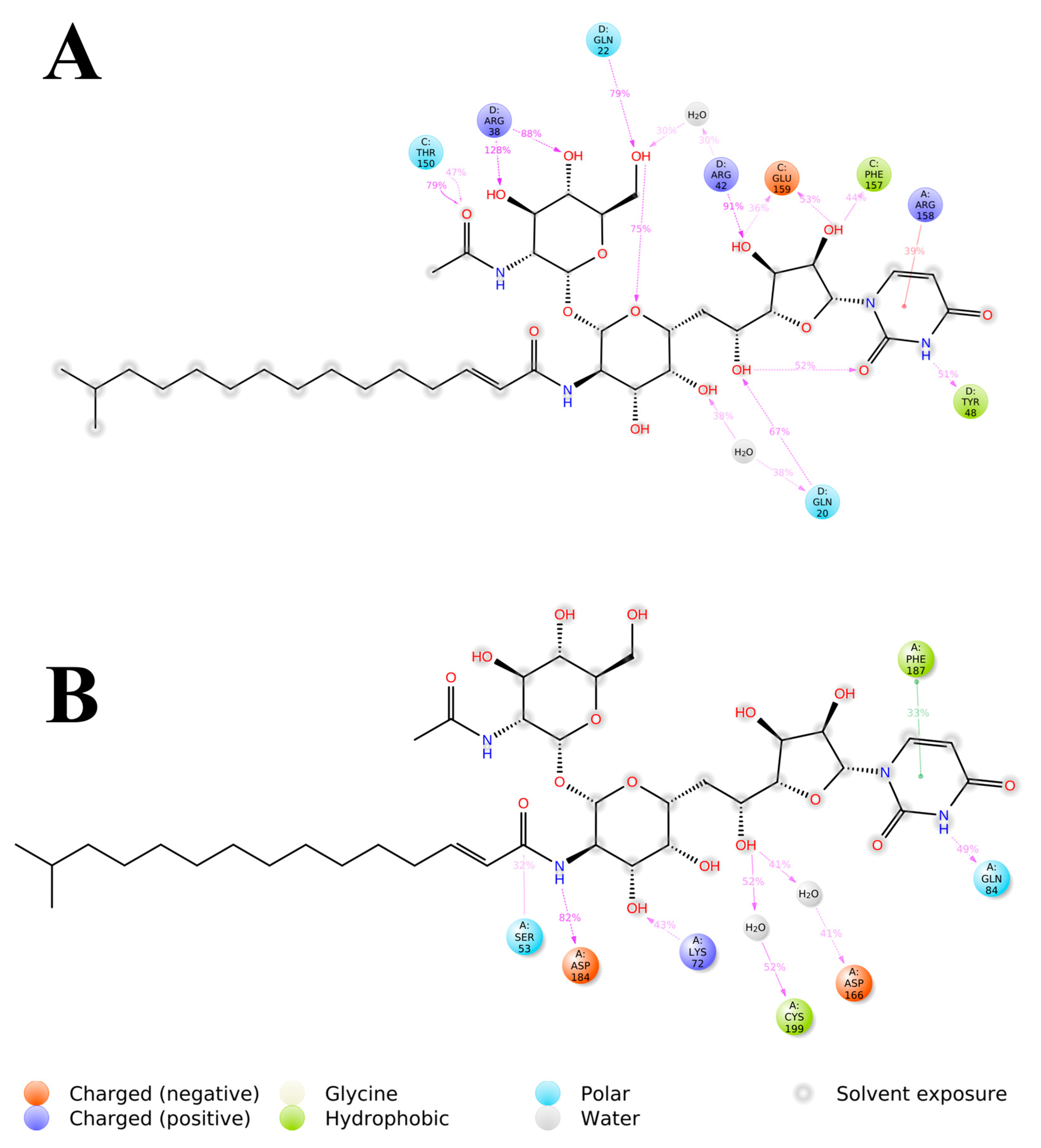
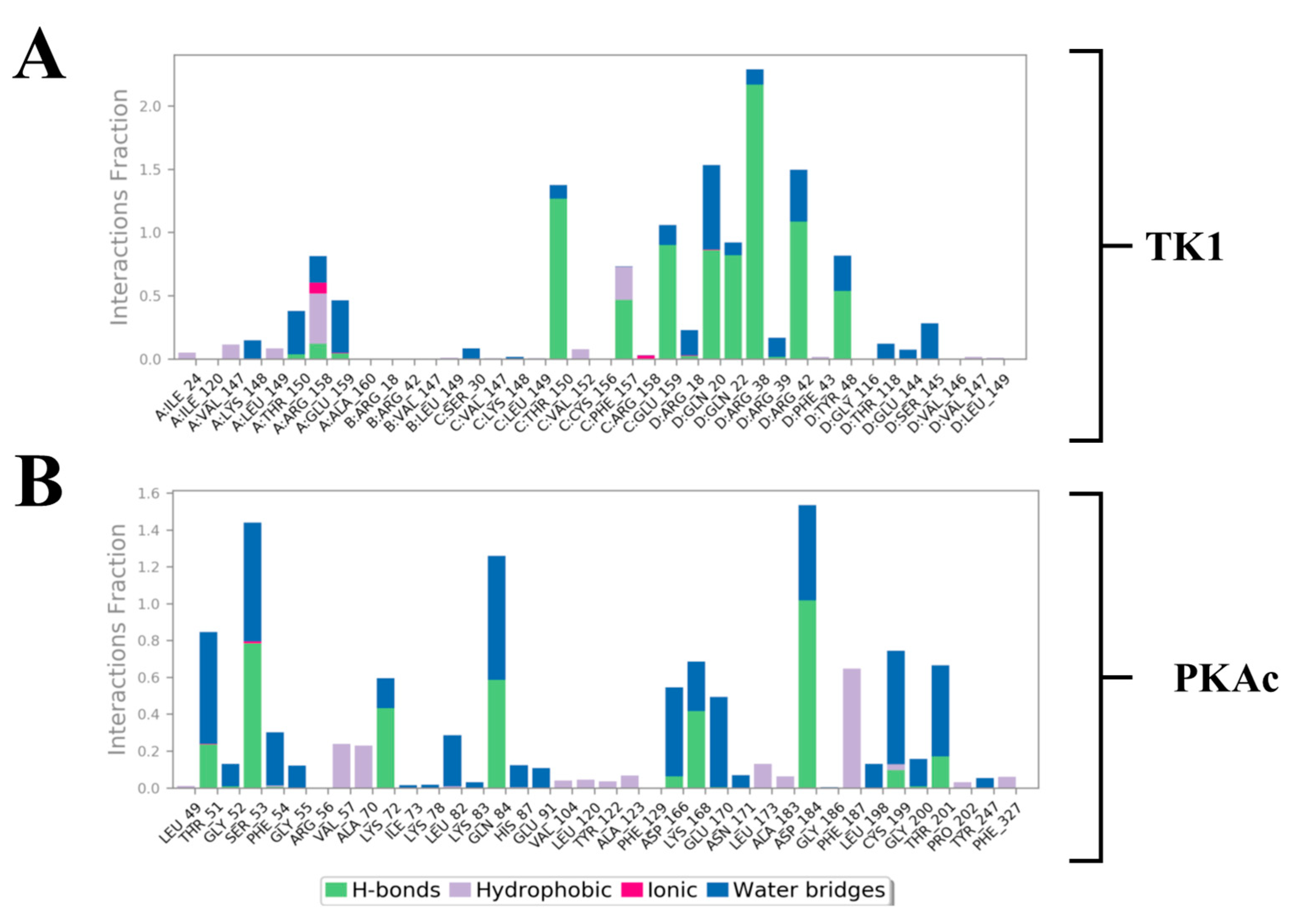
3.3. System-Based Visualisation of Ligand Interaction
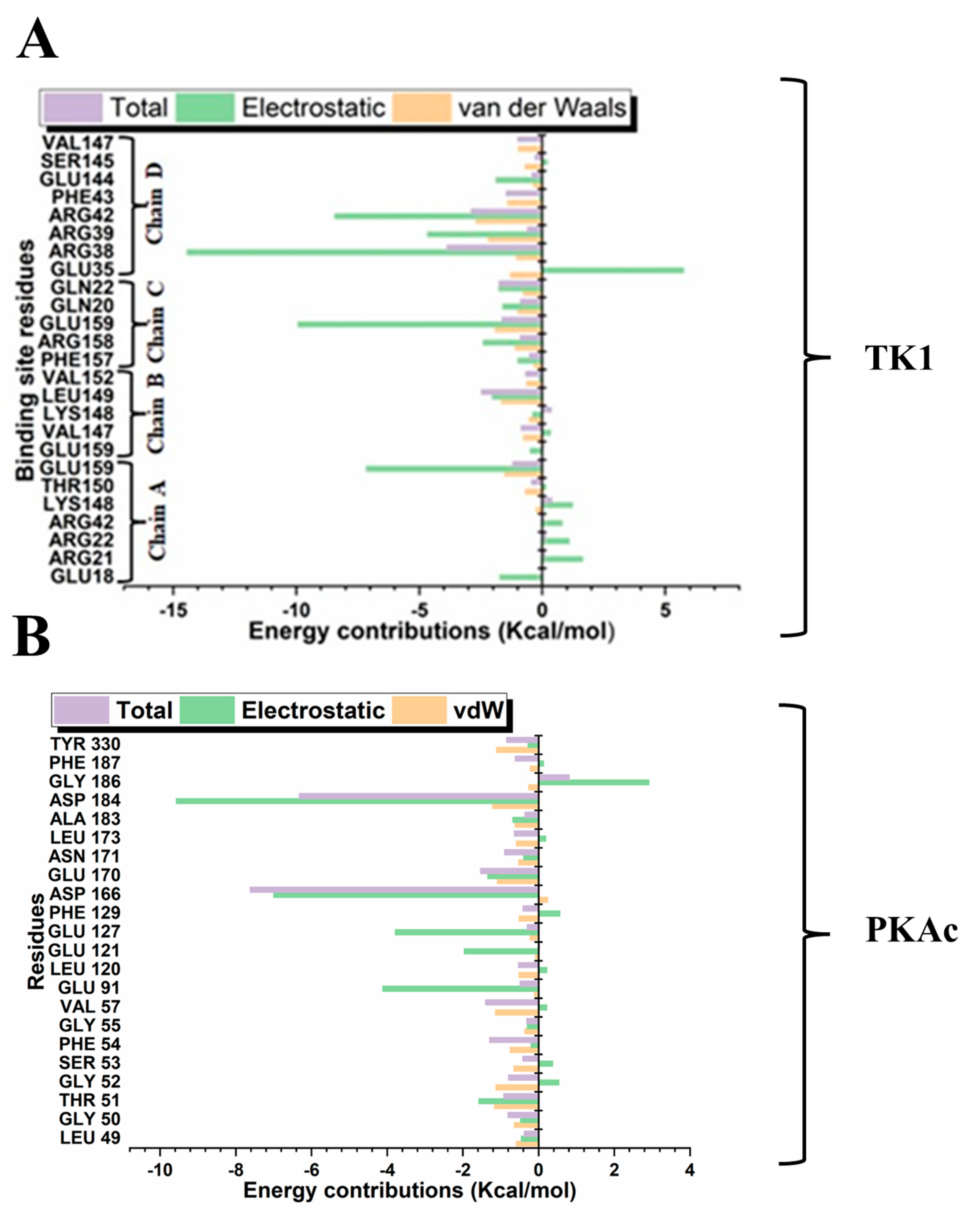
3.4. Quantitative Estimation of Implicit Interaction Contributors
3.5. Quantification Evaluation of Total Binding Free Energy of TK1 and PKAc
3.6. Molecular Dynamics Data Analysis of PKAc and TK1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandrasekar, D.; Guerrier, C.; Alisson-Silva, F.; Dhar, C.; Caval, T.; Schwarz, F.; Hommes, D.W. Warning signs from the crypt: Aberrant protein glycosylation marks opportunities for early colorectal cancer detection. Clin. Transl. Gastroenterol. 2023, 14, e00592. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Bedard, P.L.; Hyman, D.M.; Davids, M.S.; Siu, L.L. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet 2020, 395, 1078–1088. [Google Scholar] [CrossRef]
- Shin, A.E.; Giancotti, F.G.; Rustgi, A.K. Metastatic colorectal cancer: Mechanisms and emerging therapeutics. Trends Pharmacol. Sci. 2023, 44, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Xu, M.; Yang, Z.; Yue, S.; Zhou, W.; Gui, C.; Zhang, H.; Li, S.; Wang, P.G.; et al. Advances in glycopeptide enrichment methods for the analysis of protein glycosylation over the past decade. J. Sep. Sci. 2022, 45, 3169–3186. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J.; Elliott, D.J. Hallmarks of glycosylation in cancer. Oncotarget 2016, 7, 35478–35489. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef]
- Vajaria, B.N.; Patel, P.S. Glycosylation: A hallmark of cancer? Glycoconj. J. 2017, 34, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ponce, C.; Geribaldi-Doldán, N.; Sánchez-Gomar, I.; Quiroz, R.N.; Ibarra, L.A.; Escorcia, L.G.; Fernández-Cisnal, R.; Martinez, G.A.; García-Cózar, F.; Quiroz, E.N. The Role of Glycosyltransferases in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 5822. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Jin, L.; Sun, W.; Wang, S.; Liu, N. Proteomics of post-translational modifications in colorectal cancer: Discovery of new biomarkers. Biochim. Biophys. Acta—Rev. Cancer 2022, 1877, 188735. [Google Scholar] [CrossRef]
- Very, N.; Lefebvre, T.; El Yazidi-Belkoura, I. Drug resistance related to aberrant glycosylation in colorectal cancer. Oncotarget 2018, 9, 1380–1402. [Google Scholar] [CrossRef] [PubMed]
- Mereiter, S.; Balmaña, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef]
- Costa, A.F.; Campos, D.; Reis, C.A.; Gomes, C. Targeting Glycosylation: A New Road for Cancer Drug Discovery. Trends Cancer 2020, 6, 757–766. [Google Scholar] [CrossRef]
- Keenan, R.W.; Hamill, R.L.; Occolowitz, J.L.; Elbein, A.D. Biological activities of isolated tunicamycin and streptovirudin fractions. Biochemistry 1981, 20, 2968–2973. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ichikawa, S. Tunicamycin: Chemical synthesis and biosynthesis. J. Antibiot. 2019, 72, 924–933. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Li, H.; Huang, S.; Zhang, S.; Wang, F.; Shi, Y. Tunicamycin enhances the antitumor activity of trastuzumab on breast cancer in vitro and in vivo. Oncotarget 2015, 6, 38912–38925. [Google Scholar] [CrossRef]
- de Freitas Junior, J.C.; Morgado-Díaz, J.A. The role of N-glycans in colorectal cancer progression: Potential biomarkers and therapeutic applications. Oncotarget 2016, 7, 19395–19413. [Google Scholar] [CrossRef]
- Ahmmed, B.; Khan, M.N.; Nisar, M.A.; Kampo, S.; Zheng, Q.; Li, Y.; Yan, Q. Tunicamycin enhances the suppressive effects of cisplatin on lung cancer growth through PTX3 glycosylation via AKT/NF-κB signaling pathway. Int. J. Oncol. 2019, 54, 431–442. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Junior, J.C.; Silva Bdu, R.; de Souza, W.F.; de Araújo, W.M.; Abdelhay, E.S.; Morgado-Díaz, J.A. Inhibition of N-linked glycosylation by tunicamycin induces E-cadherin-mediated cell-cell adhesion and inhibits cell proliferation in undifferentiated human colon cancer cells. Cancer Chemother. Pharmacol. 2011, 68, 227–238. [Google Scholar] [CrossRef]
- Tsitsipatis, D.; Jayavelu, A.K.; Müller, J.P.; Bauer, R.; Schmidt-Arras, D.; Mahboobi, S.; Schnöder, T.M.; Heidel, F.; Böhmer, F.D. Synergistic killing of FLT3ITD-positive AML cells by combined inhibition of tyrosine-kinase activity and N-glycosylation. Oncotarget 2017, 8, 26613–26624. [Google Scholar] [CrossRef]
- Banerjee, D.K.; Seijo Lebrón, A.; Baksi, K. Glycotherapy: A New Paradigm in Breast Cancer Research. Biomolecules 2022, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Lengauer, T.; Rarey, M. Computational methods for biomolecular docking. Curr. Opin. Struct. Biol. 1996, 6, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Welin, M.; Kosinska, U.; Mikkelsen, N.E.; Carnrot, C.; Zhu, C.; Wang, L.; Eriksson, S.; Munch-Petersen, B.; Eklund, H. Structures of thymidine kinase 1 of human and mycoplasmic origin. Proc. Natl. Acad. Sci. USA 2004, 101, 17970–17975. [Google Scholar] [CrossRef]
- Arter, C.; Trask, L.; Ward, S.; Yeoh, S.; Bayliss, R. Structural features of the protein kinase domain and targeted binding by small-molecule inhibitors. J. Biol. Chem. 2022, 298, 102247. [Google Scholar] [CrossRef]
- Tang, Y.; Horikoshi, M.; Li, W. ggfortify: Unified interface to visualize statistical results of popular R packages. R J. 2016, 8, 474–485. [Google Scholar] [CrossRef]
- The R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Astani, E.K.; Malek Zadeh, S.; Hsu, N.-S.; Lin, K.-H.; Sardari, S.; Li, T.-L. Intermolecular Interactions of Nucleoside Antibiotic Tunicamycin with On-Target MraYCB-TUN and Off-Target DPAGT1-TUN in the Active Sites Delineated by Quantum Mechanics/Molecular Mechanics Calculations. ACS Omega 2022, 7, 32970–32987. [Google Scholar] [CrossRef]
- Toullec, D.; Pianetti, P.; Coste, H.; Bellevergue, P.; Grand-Perret, T.; Ajakane, M.; Baudet, V.; Boissin, P.; Boursier, E.; Loriolle, F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 1991, 266, 15771–15781. [Google Scholar] [CrossRef]
- Hers, I.; Tavaré, J.M.; Denton, R.M. The protein kinase C inhibitors bisindolylmaleimide I (GF 109203x) and IX (Ro 31-8220) are potent inhibitors of glycogen synthase kinase-3 activity. FEBS Lett. 1999, 460, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.R.; Komander, D.; Alessi, D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Shang, Y.; Ni, J.; Wang, W.; Wang, C.; Li, G.; Chen, S.-Z. Thymidine Kinase 1 mediates the synergistic antitumor activity of ubenimex and celecoxib via regulation of cell cycle in colorectal cancer. J. Pharmacol. Exp. Ther. 2022, 382, 188–198. [Google Scholar] [CrossRef]
- Mering, C.v.; Huynen, M.; Jaeggi, D.; Schmidt, S.; Bork, P.; Snel, B. STRING: A database of predicted functional associations between proteins. Nucleic Acids Res. 2003, 31, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, I.A.; Olotu, F.A.; Agoni, C.; Soliman, M.E.S. Deciphering the ‘Elixir of Life’: Dynamic Perspectives into the Allosteric Modulation of Mitochondrial ATP Synthase by J147, a Novel Drug in the Treatment of Alzheimer’s Disease. Chem. Biodivers. 2019, 16, e1900085. [Google Scholar] [CrossRef]
- Emmanuel, I.A.; Olotu, F.A.; Agoni, C.; Soliman, M.E.S. In Silico Repurposing of J147 for Neonatal Encephalopathy Treatment: Exploring Molecular Mechanisms of Mutant Mitochondrial ATP Synthase. Curr. Pharm. Biotechnol. 2020, 21, 1551–1566. [Google Scholar] [CrossRef]
- Am Busch, M.S.; Lopes, A.; Amara, N.; Bathelt, C.; Simonson, T. Testing the Coulomb/Accessible Surface Area solvent model for protein stability, ligand binding, and protein design. BMC Bioinform. 2008, 9, 148. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex roles of cAMP–PKA–CREB signaling in cancer. Exp. Hematol. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef]
- Fujishita, T.; Kojima, Y.; Kajino-Sakamoto, R.; Mishiro-Sato, E.; Shimizu, Y.; Hosoda, W.; Yamaguchi, R.; Taketo, M.M.; Aoki, M. The cAMP/PKA/CREB and TGFβ/SMAD4 pathways regulate stemness and metastatic potential in colorectal cancer cells. Cancer Res. 2022, 82, 4179–4190. [Google Scholar] [CrossRef]
- Hou, J.-Y.; Gao, L.-J.; Shen, J.; Zhou, L.; Shi, J.-Y.; Sun, T.; Hao, S.-L.; Wang, D.-P.; Cao, J.-M. Crotonylation of PRKACA enhances PKA activity and promotes colorectal cancer development via the PKA-FAK-AKT pathway. Genes Dis. 2023, 10, 332–335. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Liu, J.; Chen, M.; Li, W.; Chen, Y.; Xu, M. The diagnostic value of extracellular protein kinase A (ECPKA) in serum for gastric and colorectal cancer. Transl. Cancer Res. 2020, 9, 3870–3878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kim, K.-M. Multifactorial regulation of G protein-coupled receptor endocytosis. Biomol. Ther. 2017, 25, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H. Protein glycosylation alterations in hepatocellular carcinoma: Function and clinical implications. Oncogene 2023, 42, 1970–1979. [Google Scholar] [CrossRef]
- Weagel, E.G.; Burrup, W.; Kovtun, R.; Velazquez, E.J.; Felsted, A.M.; Townsend, M.H.; Ence, Z.E.; Suh, E.; Piccolo, S.R.; Weber, K.S.; et al. Membrane expression of thymidine kinase 1 and potential clinical relevance in lung, breast, and colorectal malignancies. Cancer Cell Int. 2018, 18, 135. [Google Scholar] [CrossRef]
- Bitter, E.E.; Townsend, M.H.; Erickson, R.; Allen, C.; O’Neill, K.L. Thymidine kinase 1 through the ages: A comprehensive review. Cell Biosci. 2020, 10, 138. [Google Scholar] [CrossRef]
- Hannigan, B.M.; Barnett, Y.A.; Armstrong, D.B.; McKelvey-Martin, V.J.; McKenna, P.G. Thymidine kinases: The enzymes and their clinical usefulness. Cancer Biother. 1993, 8, 189–197. [Google Scholar] [CrossRef]
- Xu, D.; Michie, S.A.; Zheng, M.; Takeda, S.; Wu, M.; Peltz, G. Humanized thymidine kinase-NOG mice can be used to identify drugs that cause animal-specific hepatotoxicity: A case study with furosemide. J. Pharmacol. Exp. Ther. 2015, 354, 73–78. [Google Scholar] [CrossRef] [PubMed]
- He, E.; Xu, X.H.; Guan, H.; Chen, Y.; Chen, Z.H.; Pan, Z.L.; Tang, L.L.; Hu, G.Z.; Li, Y.; Zhang, M.; et al. Thymidine kinase 1 is a potential marker for prognosis and monitoring the response to treatment of patients with breast, lung, and esophageal cancer and non-Hodgkin’s lymphoma. Nucleosides Nucleotides Nucleic Acids 2010, 29, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Y.; Wang, H.; Pike, A.C.; Cochrane, S.A.; Hamedzadeh, S.; Wyszyński, F.J.; Bushell, S.R.; Royer, S.F.; Widdick, D.A.; Sajid, A.; et al. Structures of DPAGT1 explain glycosylation disease mechanisms and advance TB antibiotic design. Cell 2018, 175, 1045–1058.e16. [Google Scholar] [CrossRef]
- Chang, J.Y.; Korolev, V.V. Specific toxicity of tunicamycin in induction of programmed cell death of sympathetic neurons. Exp. Neurol. 1996, 137, 201–211. [Google Scholar] [CrossRef]
- Banerjee, S.; Ansari, A.A.; Upadhyay, S.P.; Mettman, D.J.; Hibdon, J.R.; Quadir, M.; Ghosh, P.; Kambhampati, A.; Banerjee, S.K. Benefits and Pitfalls of a Glycosylation Inhibitor Tunicamycin in the Therapeutic Implication of Cancers. Cells 2024, 13, 395. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, A.; Kim, H.-D.; Chang, Y.-C.; Kim, C.-H. Revisited metabolic control and reprogramming cancers by means of the warburg effect in tumor cells. Int. J. Mol. Sci. 2022, 23, 10037. [Google Scholar] [CrossRef]
- Ho, W.-L.; Hsu, W.-M.; Huang, M.-C.; Kadomatsu, K.; Nakagawara, A. Protein glycosylation in cancers and its potential therapeutic applications in neuroblastoma. J. Hematol. Oncol. 2016, 9, 100. [Google Scholar] [CrossRef]
- Lee, B.-H.; Eskandari, R.; Jones, K.; Reddy, K.R.; Quezada-Calvillo, R.; Nichols, B.L.; Rose, D.R.; Hamaker, B.R.; Pinto, B.M. Modulation of starch digestion for slow glucose release through “toggling” of activities of mucosal α-glucosidases. J. Biol. Chem. 2012, 287, 31929–31938. [Google Scholar] [CrossRef]
- Ankenbauer, K.E.; Rao, T.C.; Mattheyses, A.L.; Bellis, S.L. Sialylation of EGFR by ST6GAL1 induces receptor activation and modulates trafficking dynamics. J. Biol. Chem. 2023, 299, 105217. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Weng, C.-L.; Lin, K.-I. O-GlcNAcylation and its role in the immune system. J. Biomed. Sci. 2020, 27, 57. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Rathinavel, A.K.; Radhakrishnan, P. Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim. Biophys. Acta (BBA) Rev. Cancer 2021, 1875, 188464. [Google Scholar] [CrossRef]
- Guterres, H.; Im, W. Improving Protein-Ligand Docking Results with High-Throughput Molecular Dynamics Simulations. J. Chem. Inf. Model. 2020, 60, 2189–2198. [Google Scholar] [CrossRef]
- Fusani, L.; Palmer, D.S.; Somers, D.O.; Wall, I.D. Exploring Ligand Stability in Protein Crystal Structures Using Binding Pose Metadynamics. J. Chem. Inf. Model. 2020, 60, 1528–1539. [Google Scholar] [CrossRef]
- An, X.; Lu, S.; Song, K.; Shen, Q.; Huang, M.; Yao, X.; Liu, H.; Zhang, J. Are the Apo Proteins Suitable for the Rational Discovery of Allosteric Drugs? J. Chem. Inf. Model. 2019, 59, 597–604. [Google Scholar] [CrossRef] [PubMed]

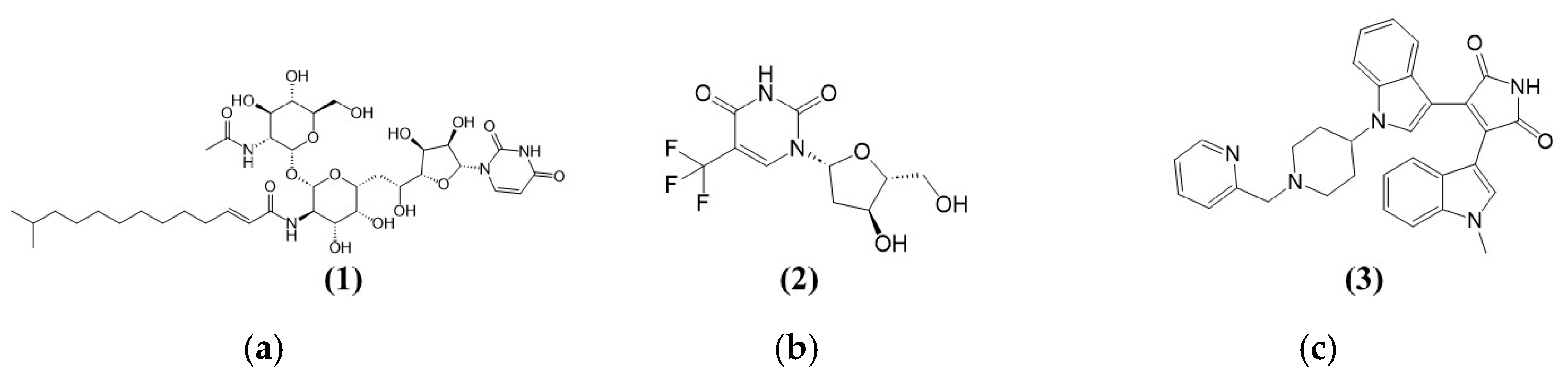

| Target | Common Name | Uniprot ID | Known Actives | HTDocking Score |
|---|---|---|---|---|
| Tunicamycin C targets | ||||
| Cytidine deaminase | CDA | P32320 | 0/2 | 6.8 |
| Protein farnesyltransferase | FNTA FNTB | P49354 P49356 | 2/0 | 7.7 |
| Apoptosis regulator Bcl-X | BCL2L1 | Q07817 | 1/0 | 8.2 |
| Apoptosis regulator Bcl-2 | BCL2 | P10415 | 1/0 | 8.2 |
| Thymidine kinase, cytosolic | TK1 | P04183 | 0/13 | 7.4 |
| Protein Kinase A catalytic subunit | PKAc | P17612 | 0/1 | 6.8 |
| Trifluorothymidine target validation | ||||
| Thymidine kinase, cytosolic | TK1 | P04183 | 0/18 | 6.5 |
| Bisindolylmaleimide I (GF109203X) | ||||
| Protein kinase C beta | PKCb | P05771 | 233/135 |
| #Term ID | Term Description | Gene Count | False Discovery Rate | Matching Proteins in Your Network (Labels) |
|---|---|---|---|---|
| hsa04014 | Ras signalling pathway | 11 | 7.94 × 10−7 | FLT3, FGF2, PKAc, PLA2G5, BCL2L1, PLA2G2A, PLA2G10, PRKCA, FGF1, PKCb, AKT3 |
| hsa05205 | Proteoglycans in cancer | 10 | 1.75 × 10−6 | MMP2, FGF2, PDCD4, PKAc, ROCK2, MMP9, HPSE, PKAc, PKCb, AKT3 |
| hsa05200 | Pathways in cancer | 13 | 2.49 × 10−5 | MMP2, FLT3, FGF2, PKAc, ROCK2, HSP90AA1, MMP9, BCL2L1, BCL2, PKAc, FGF1, PKCb, AKT3 |
| hsa01521 | EGFR tyrosine kinase inhibitor resistance | 6 | 5.42 × 10−5 | FGF2, BCL2L1, BCL2, PKAc, PKCb, AKT3 |
| hsa05206 | MicroRNAs in cancer | 6 | 0.0014 | PDCD4, PKCE, MMP9, BCL2, PKAc, PKCB |
| hsa04151 | PI3K-Akt signalling pathway | 8 | 0.0027 | FLT3, FGF2, HSP90AA1, BCL2L1, BCL2, PKAc, FGF1, AKT3 |
| hsa05230 | Central carbon metabolism in cancer | 4 | 0.0033 | FLT3, HK2, HK1, AKT3 |
| hsa04010 | MAPK signalling pathway | 7 | 0.0037 | FLT3, FGF2, PKAc, PKAc, FGF1, PRKCB, AKT3 |
| hsa04370 | VEGF signalling pathway | 3 | 0.0146 | PKAc, PKCB, AKT3 |
| hsa04310 | Wnt signalling pathway | 4 | 0.0267 | PRKACA, ROCK2, PKAc, PKCB |
| hsa04630 | JAK-STAT signalling pathway | 4 | 0.0284 | PTPN2, BCL2L1, BCL2, AKT3 |
| KEGG pathways implicated in glycosylation | ||||
| hsa00052 | Galactose metabolism | 6 | 7.94 × 10−7 | SI, HK2, GAA, B4GALT1, MGAM, HK1 |
| hsa00500 | Starch and sucrose metabolism | 7 | 4.60 × 10−8 | PYGM, SI, HK2, GAA, MGAM, AMY2A, HK1 |
| hsa00514 | Other types of O-glycan biosynthesis | 3 | 0.0082 | ST6GAL1, OGT, B4GALT1 |
| Protein Complex | Energy Components (kcal/mol) | ||||
|---|---|---|---|---|---|
| ∆Evdw | ∆Eele | ∆Ggas | ∆Gsol | ∆Gbind | |
| TK1_Tunicamycin C | −69.13 ± 4.25 | −70.08 ± 15.05 | −139.20 ± 14.67 | 78.99 ± 9.96 | −60.21 ± 9.46 |
| PKAc_Tunicamycin C | −37.05 ± 8.81 | −110.32 ± 17.46 | −147.37 ± 16.75 | 106.78 ± 9.54 | −40.59 ± 9.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naidoo, V.; Achilonu, I.; Mirza, S.; Hull, R.; Kandhavelu, J.; Soobben, M.; Penny, C. Computational Modelling of Tunicamycin C Interaction with Potential Protein Targets: Perspectives from Inverse Docking with Molecular Dynamic Simulation. Curr. Issues Mol. Biol. 2025, 47, 339. https://doi.org/10.3390/cimb47050339
Naidoo V, Achilonu I, Mirza S, Hull R, Kandhavelu J, Soobben M, Penny C. Computational Modelling of Tunicamycin C Interaction with Potential Protein Targets: Perspectives from Inverse Docking with Molecular Dynamic Simulation. Current Issues in Molecular Biology. 2025; 47(5):339. https://doi.org/10.3390/cimb47050339
Chicago/Turabian StyleNaidoo, Vivash, Ikechukwu Achilonu, Sheefa Mirza, Rodney Hull, Jeyalakshmi Kandhavelu, Marushka Soobben, and Clement Penny. 2025. "Computational Modelling of Tunicamycin C Interaction with Potential Protein Targets: Perspectives from Inverse Docking with Molecular Dynamic Simulation" Current Issues in Molecular Biology 47, no. 5: 339. https://doi.org/10.3390/cimb47050339
APA StyleNaidoo, V., Achilonu, I., Mirza, S., Hull, R., Kandhavelu, J., Soobben, M., & Penny, C. (2025). Computational Modelling of Tunicamycin C Interaction with Potential Protein Targets: Perspectives from Inverse Docking with Molecular Dynamic Simulation. Current Issues in Molecular Biology, 47(5), 339. https://doi.org/10.3390/cimb47050339


_Kim.png)



