Investigating the Therapeutic Potential of Crude Leech Saliva Based on Its Anticancer, Antioxidant, and Anti-Inflammatory Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Procurement of Medicinal Leeches and Collection of Crude Leech Saliva (LS)
2.2. Determination of Total Protein Content in Crude Leech Saliva (LS) by Bradford Method
2.3. Chemical Characterization of Crude Leech Saliva (LS) Content by GC-MS Analysis
2.4. Molecular Docking Analysis of Ligand–Protein Interactions
2.5. Determination of Some Cytokine Levels of Crude Leech Saliva (LS)
2.6. Determination of Antioxidant Activity of Crude Leech Saliva (LS)
2.7. Effect of Crude Leech Saliva (LS) on HUVEC and OVCAR3 Cell Lines
2.7.1. Preparation of Cell Lines
2.7.2. Cell Counting, and Treatment with Sample
2.7.3. Determination of Cell Viability Using MTT Test
2.8. Statistical Analysis
3. Results
3.1. Determination of Total Protein Content of LS
3.2. Molecular Docking Analyses
3.3. Determination of Some Cytokine Levels of LS
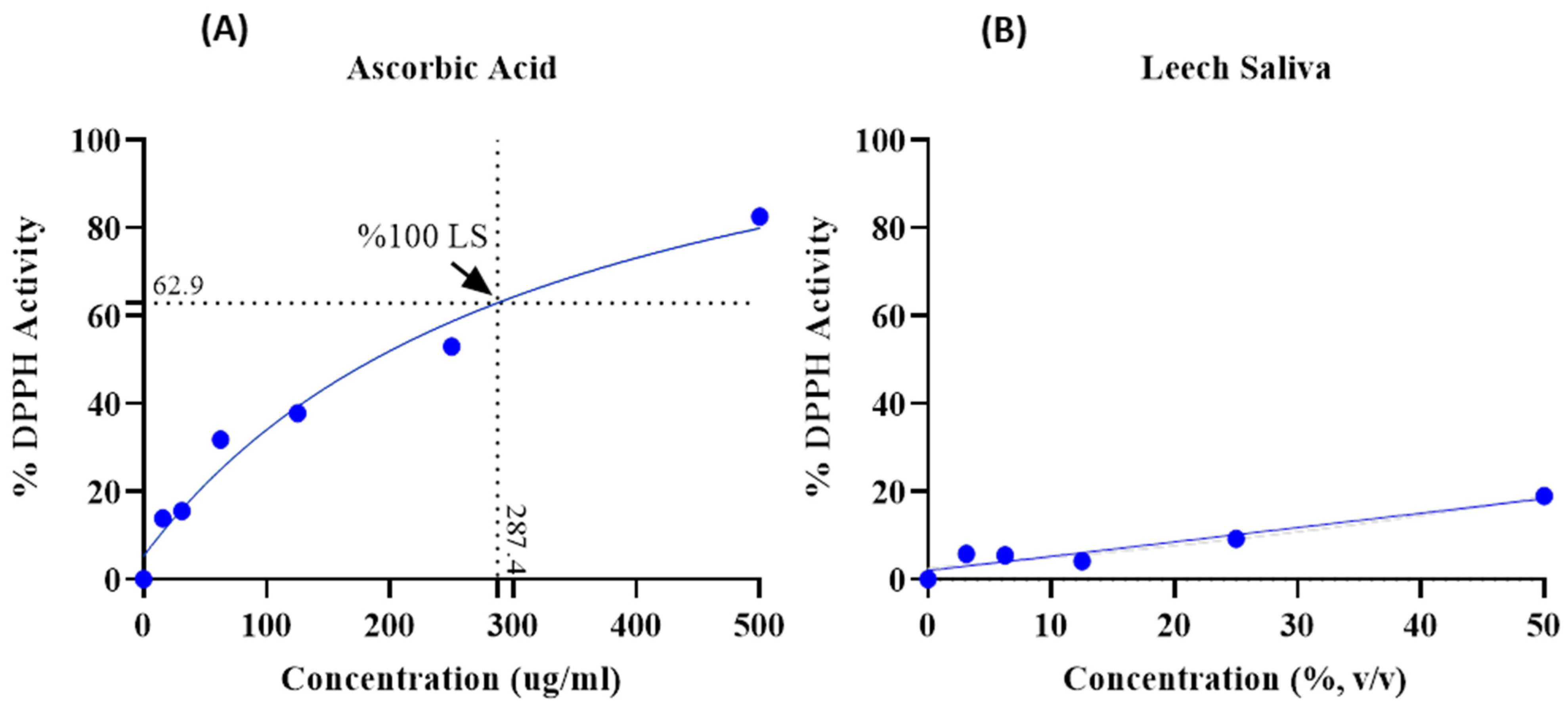
3.4. Determination of the Antioxidant Activity of LS
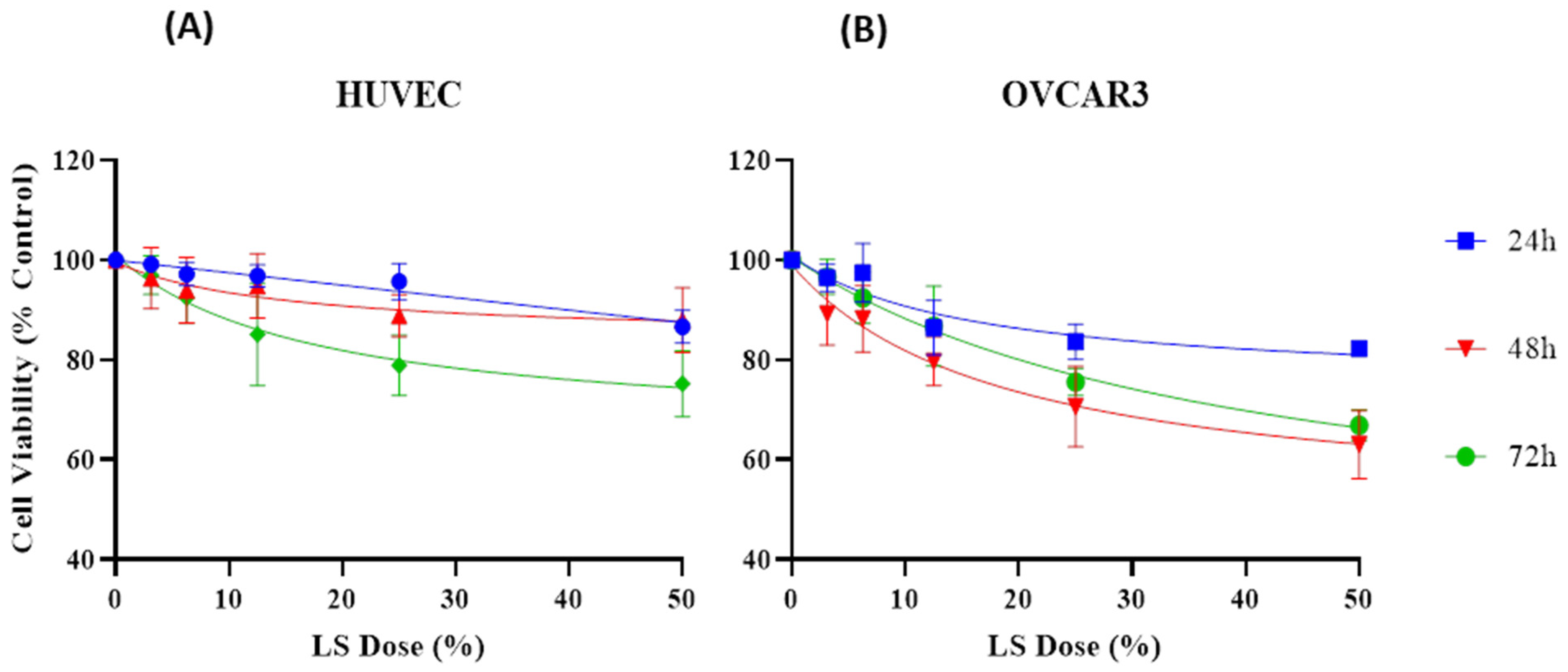
3.5. Effect of LS on Cell Viability of HUVEC and OVCAR3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Molecules | Identification Information | 3D Structure of Molecules |
|---|---|---|
| 3,8-Dimethyldecane | CAS No: 17312-55-9 Retention Time: 9.995 min Peak No: 3 Area: 10,598,479 Area %: 12.08 | 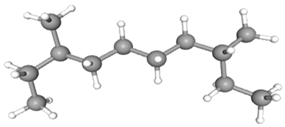 |
| 5-Methylundecane | CAS No: 1632-70-8 Retention Time: 10.906 min Peak No: 4 Area: 3,666,285 Area %: 4.18 |  |
| 2,4-Dimethylheptane | CAS No: 2213-23-2 Retention Time: 5.473 min Peak No: 2 Area: 8,151,628 Area %: 9.29 | 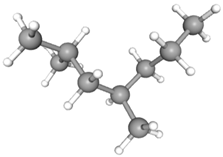 |
| Decane | CAS No: 124-18-5 Retention Time: 8.877 min Peak No: 1 Area: 6,352,537 Area %: 7.24 | 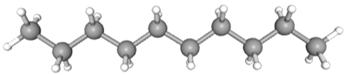 |
| 2-Methyltridecane | CAS No: 1560-96-9 Retention Time: 11.703 min Peak No: 5 Area: 3,493,446 Area %: 3.98 | 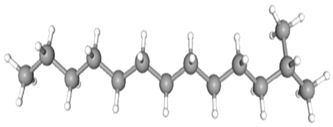 |
| Dodecane | CAS No: 112-40-3 Retention Time: 9.393 min Peak No: 2 Area: 9,127,114 Area %: 10.40 | 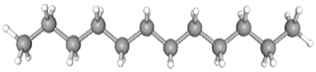 |
| Phytane | CAS No: 638-36-8 Retention Time: 20.771 min Peak No: 6 Area: 6,319,478 Area %: 7.21 | 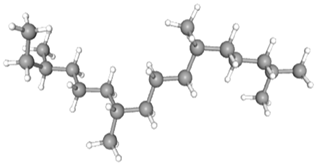 |
| Octadecane | CAS No: 593-45-3 Retention Time: 28.013 min Peak No: 7 Area: 1,799,719 Area %: 2.05 | 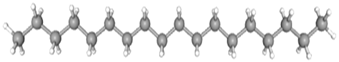 |
| Pentadecane | CAS No: 629-62-9 Retention Time: 12.778 min Peak No: 10 Area: 4,406,601 Area %: 5.02 | 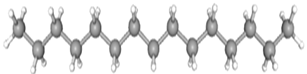 |
| Tetradecane | CAS No: 629-59-4 Retention Time: 11.187 min Peak No: 6 Area: 3,733,756 Area %: 4.25 | 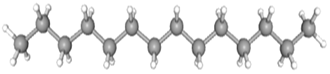 |
| 2,6-Di-tert-butyl-4-methylene-2,5-cyclohexadienone | CAS No: 2607-52-5 Retention Time: 14.682 min Peak No: 12 Area: 2,340,523 Area %: 2.67 | 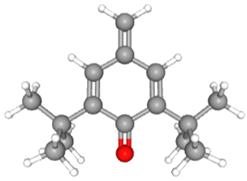 |
| 6-[(4-methoxyphenyl)methoxy]-4,4,5,7,8-pentamethyl-3H-chromen-2-one | CAS No: 39170-97-3 Retention Time: 24.063 min Peak No: 17 Area: 7,347,922 Area %: 8.36 | 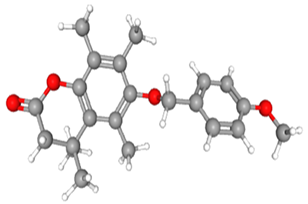 |
| 1,2-Benzisothiazol-3-amine | CAS No: 999428-58-8 Retention Time: 10.203 min Peak No: 7 Area: 1,679,551 Area %: 1.91 | 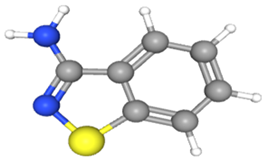 |
| Gibberellic acid | CAS No: 77-06-5 Retention Time: 25.498 min Peak No: 18 Area: 4,691,781 Area %: 5.34 |  |
References
- Whitaker, I.S.; Rao, J.; Izadi, D.; Butler, P.E. Historical article: Hirudo medicinalis: Ancient origins of, and trends in the use of medicinal leeches throughout history. Br. J. Oral Maxillofac. Surg. 2004, 42, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P. Medicinal leech therapy (Hirudotherapy): A brief overview. Complement. Ther. Clin. Pract. 2010, 16, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Galen, C. On the Natural Faculties; Brock, J., Ed.; G.P. Putnam’s Sons: New York, NY, USA; William Heinemann: London, UK, 2019. [Google Scholar]
- Sina, İ. el-Kanun fi’t-Tıbb; Atatürk Kültür, Dil ve Tarih Yüksek Kurumu: Ankara, Turkey, 2014. [Google Scholar]
- Abdualkader, A.M.; Ghawi, A.M.; Alaama, M.; Awang, M.; Merzouk, A. Leech therapeutic applications. Indian J. Pharm. Sci. 2013, 75, 127–137. [Google Scholar] [PubMed]
- Youmbi, L.M.; Makong, Y.S.D.; Mbaveng, A.T.; Tankeo, S.B.; Fotso, G.W.; Ndjakou, B.L.; Kuete, V. Cytotoxicity of the methanol extracts and compounds of Brucea antidysenterica (Simaroubaceae) towards multifactorial drug-resistant human cancer cell lines. BMC Complement. Med. Ther. 2023, 23, 1–14. [Google Scholar] [CrossRef]
- Yılmaz, S. Endometrioid over kanseri ile berrak hücreli over kanseri olan olguların prognozlarının karşılaştırılması. J. Biotechnol. Strateg. Health Res. 2024, 8, 58–65. [Google Scholar] [CrossRef]
- Ghawi, A.M.; Abdualkader, A.M. Free radical scavenging activity of the medicinal Malaysian leech saliva extract, Hirudinaria manillensis. J. Bioequiv. Bioavailab. 2012, 14, 10–14. [Google Scholar]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- Yu, Q.; Xu, C.; Song, J.; Jin, Y.; Gao, X. Mechanisms of Traditional Chinese medicine/natural medicine in HR-positive breast cancer: A comprehensive literature review. J. Ethnopharmacol. 2024, 319, 117322. [Google Scholar] [CrossRef]
- Das, B.K. An overview on hirudotherapy/leech therapy. Indian Res. J. Pharm. Sci. 2014, 1, 33–45. [Google Scholar]
- Abdualkader, A.M.; Merzouk, A.; Ghawi, A.M.; Alaama, A.M. Some biological activities of Malaysian leech saliva extract. IIUM Eng. J. 2011, 12, 1–9. [Google Scholar] [CrossRef]
- Mills, G. Mid-Value Circuit and Control System. U.S. Patent 4,633,163, 30 December 1986. [Google Scholar]
- Merzouk, A. Anticancer effects of medical Malaysian leech saliva extract (LSE). Pharm. Anal. Acta 2012, 15, 1–5. [Google Scholar] [CrossRef]
- Ammar, A.E. The Anticancer Activity of Leech Saliva Extract and the Role of Protease Activated Receptor 1 (PAR-1) in Prostate Cancer. Ph.D. Thesis, The University of British Columbia, Vancouver, BC, Canada, 2021. [Google Scholar]
- Shakouri, A.; Kahroba, H.; Hamishekar, H.; Abdolalizadeh, J. Nanoencapsulation of Hirudo medicinalis proteins in liposomes as a nanocarrier for inhibiting angiogenesis through targeting VEGFA in the breast cancer cell line (MCF-7). BioImpacts 2022, 12, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Meriaux, C.; Arafah, K.; Tasiemski, A.; Wisztorski, M.; Bruand, J.; Boidin-Wichlacz, C.; Fournier, I. Multiple changes in peptide and lipid expression associated with regeneration in the nervous system of the medicinal leech. PLoS ONE 2011, 6, e21828. [Google Scholar] [CrossRef]
- Hosseini, M.; Jadidi, A.; Derakhshan Barjoei, M.M.; Salehi, M. Applications of leech therapy in medicine: A systematic review. Front. Med. 2024, 11, 1–7. [Google Scholar] [CrossRef]
- Chhayani, K.; Daxini, P.; Patel, P. An overview on medicinal leech therapy. J. Pharm. Pharmacol. 2023, 11, 107–113. [Google Scholar]
- Alaama, M.; Kucuk, O.; Bilir, B.; Merzouk, A.; Ghawi, A.M.; Yerer, M.B.; Uddin, A.B.M.H. Development of leech extract as a therapeutic agent: A chronological review. Pharmacol. Res. Mod. Chin. Med. 2024, 10, 100355. [Google Scholar] [CrossRef]
- Zhou, J.; Song, Z.; Han, M.; Yu, B.; Lv, G.; Han, N.; Zhu, J. Evaluation of the antithrombotic activity of Zhi-Xiong Capsules, a traditional Chinese medicinal formula, via the pathway of anti-coagulation, anti-platelet activation and anti-fibrinolysis. Biomed. Pharmacother. 2018, 97, 1622–1631. [Google Scholar] [CrossRef]
- Liu, C.; Chen, G.; Chen, Y.; Dang, Y.; Nie, G.; Wu, D.; Wang, Q. Danlou Tablets inhibit atherosclerosis in apolipoprotein E-deficient mice by inducing macrophage autophagy: The role of the PI3K-Akt-mTOR pathway. Front. Pharmacol. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Liu, H.X.; Shang, J.J.; Zhou, Q.; Li, A.Y.; Wang, Y. A randomized, placebo-controlled, double-blind trial to evaluate efficacy and safety of Shen-Yuan-Dan capsules, a traditional Chinese medicine, for treatment of peri-procedure myocardial injury following percutaneous coronary intervention. Complement. Ther. Med. 2022, 69, 102841. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Singh, O.; Dwivedi, A.; Singh, A.; Rai, N. Efficacy of leech therapy in the management of osteoarthritis (Sandhivata). Anc. Sci. Life 2011, 32, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Kruger, N.J. The Bradford method for protein quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 17–24. [Google Scholar]
- Ho, S.S.H.; Yu, J.Z.; Chow, J.C.; Zielinska, B.; Watson, J.G.; Sit, E.H.L.; Fung, J.C.H. Evaluation of an in-injection port thermal desorption-gas chromatography/mass spectrometry method for analysis of non-polar organic compounds in ambient aerosol samples. J. Chromatogr. A 2008, 1200, 217–227. [Google Scholar] [CrossRef]
- Murail, S.; De Vries, S.J.; Rey, J.; Moroy, G.; Tufféry, P. SeamDock: An interactive and collaborative online docking resource to assist small compound molecular docking. Front. Mol. Biosci. 2021, 8, 716466. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Horáková, K.; Šovčíková, A.; Seemannová, Z.; Syrová, D.; Bušányová, K.; Drobná, Z.; Ferenčík, M. Detection of drug-induced, superoxide-mediated cell damage and its prevention by antioxidants. Free Radic. Biol. Med. 2001, 30, 650–664. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Koran, K.; Tekin, Ç.; Çalışkan, E.; Tekin, S.; Sandal, S.; Görgülü, A.O. Synthesis, structural and thermal characterizations and in vitro cytotoxic activities of new cyclotriphosphazene derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 1002–1011. [Google Scholar] [CrossRef]
- Koeppen, D.; Aurich, M.; Pasalar, M.; Rampp, T. Medicinal leech therapy in venous congestion and various ulcer forms: Perspectives of Western, Persian and Indian medicine. J. Tradit. Complement. Med. 2020, 10, 104–109. [Google Scholar] [CrossRef]
- Bilden, A.; Kara, Ö.; Kahraman, M.; Çaǧlayan, N.; Çiçek, M. Efficiency of medical leech on experimentally induced incisional wound healing in rats. J. Complement. Integr. Med. 2025, 1–8. [Google Scholar] [CrossRef]
- Abdullah, S.; Dar, L.; Rashid, A.; Tewari, A. Hirudotherapy/leech therapy: Applications and indications in surgery. Arch. Clin. Exp. Surg. 2012, 1, 172. [Google Scholar] [CrossRef]
- Goel, B.; Chatterjee, E.; Dey, B.; Tripathi, N.; Bhardwaj, N.; Khattri, A.; Guru, S.K.; Jain, S.K. Identification and evaluation of apoptosis-inducing activity of ipomone from Ipomoea nil: A novel, unusual bicyclo-[3.2.1] octanone containing gibberic acid diterpenoid. ACS Omeg. 2021, 6, 8253–8260. [Google Scholar] [CrossRef] [PubMed]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- López-Valverde, N.; López-Valverde, A.; Montero, J.; Rodríguez, C.; Macedo de Sousa, B.; Aragoneses, J.M. Antioxidant, anti-inflammatory and antimicrobial activity of natural products in periodontal disease: A comprehensive review. Front. Bioeng. Biotechnol. 2023, 11, 1–14. [Google Scholar] [CrossRef]
- Ayhan, H.; Sevin, S.; Karaaslan, S.; Ayaz, F. Immunomodulatory effects of medicinal leech saliva extract on in vitro activated macrophages. Immunol. Res. 2025, 73, 1–8. [Google Scholar] [CrossRef]
- Bouïs, D.; Kusumanto, Y.; Meijer, C.; Mulder, N.H.; Hospers, G.A.P. A review on pro- and anti-angiogenic factors as targets of clinical intervention. Pharmacol. Res. 2006, 53, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Sig, A.K.; Guney, M.; Uskudar Guclu, A.; Ozmen, E. Medicinal leech therapy—An overall perspective. Integr. Med. Res. 2017, 6, 337–343. [Google Scholar] [CrossRef]
- Battin, A.O.; Hobeika, N.; Zdilla, M.J. Systematic review of medicinal leech therapy in urology. Afr. J. Urol. 2023, 29, 21. [Google Scholar] [CrossRef]
- Ammar, A.; Guns, E.; Kucuk, O.; Abdualkader, A.; Alaama, M.; Uddin, A.B.M.H.; Kassem, M. Mechanism of anticancer activity of BPS-001 (lyophilized leech saliva extract). Cancer Res. 2017, 77, 107. [Google Scholar] [CrossRef]
- Shakouri, A.; Wollina, U. Time to change theory; medical leech from a molecular medicine perspective: Leech salivary proteins playing a potential role in medicine. Adv. Pharm. Bull. 2021, 11, 261. [Google Scholar] [CrossRef]
- Long, H.-Z.; Cheng, Y.; Zhou, Z.-W.; Luo, H.-Y.; Wen, D.-D.; Gao, L.-C. PI3K/AKT signal pathway: A target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, S.A.; Dzoyem, J.P.; Shai, L.J.; Eloff, J.N. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. BMC Complement. Altern. Med. 2015, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nazir, F.; Khatoon, S.; Mahajan, M.; Kumari, S.; AlAjmi, M.F.; Rehman, M.T.; Khan, M.I.R. Enhanced arsenic stress tolerance in landrace and improved rice cultivars through modulation of gibberellic acid (GA3) synthesis and antioxidant metabolism via phosphorus and silicon supplementation. Plant Stress. 2024, 13, 100511. [Google Scholar] [CrossRef]
- Wang, H.; Ke, X.; Jia, R.; Huang, L.; Liu, Z.; Zheng, Y. Gibberellic acid overproduction in Fusarium fujikuroi using regulatory modification and transcription analysis. Appl. Microbiol. Biotechnol 2023, 107, 3071–3084. [Google Scholar] [CrossRef]
- Cuevas-Cianca, S.I.; Romero-Castillo, C.; Gálvez-Romero, J.L.; Juárez, Z.N.; Hernández, L.R. Antioxidant and anti-inflammatory compounds from edible plants with anti-cancer activity and their potential use as drugs. Molecules 2023, 28, 1488. [Google Scholar] [CrossRef]

| Protein and 3D Structure | Bioactive Ligands | |
|---|---|---|
| 6-[(4-methoxyphenyl)methoxy]-4,4,5,7,8-pentamethyl-3H-chromen-2-one | Gibberellic acid | |
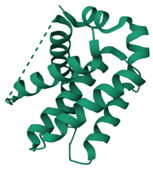 Bcl-2 | 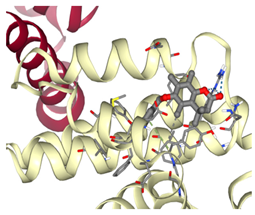 ΔG: −8.4 kcal/mol Hydrogen bonds: 3 | 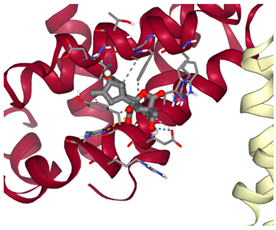 ΔG: −7.8 kcal/mol Hydrogen bonds: 2 |
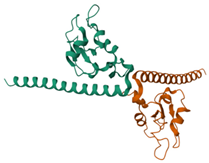 Survivin | 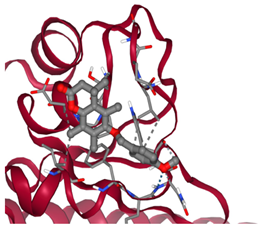 ΔG: −6.6 kcal/mol Hydrogen bonds: 1 | 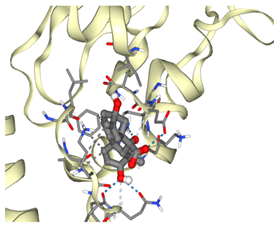 ΔG: −8.1 kcal/mol Hydrogen bonds: 4 |
| Dose (%) | 24 h (Mean ± SD) | 48 h (Mean ± SD) | 72 h (Mean ± SD) | 24 h (Mean ± SD) | 48 h (Mean ± SD) | 72 h (Mean ± SD) |
|---|---|---|---|---|---|---|
| HUVEC | HUVEC | HUVEC | OVCAR3 | OVCAR3 | OVCAR3 | |
| Control | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 3.13 | 99.16 ± 0.81 | 96.36 ± 5.58 | 96.98 ± 3.54 | 96.44 ± 2.51 | 89.25 ± 5.73 | 96.65 ± 3.20 |
| 6.25 | 97.20 ± 2.08 | 93.96 ± 6.05 | 92.43 ± 4.69 | 97.44 ± 5.35 | 88.22 ± 6.11 | 92.43 ± 4.70 |
| 12.5 | 96.87 ± 2.04 | 94.85 ± 5.93 | 85.08 ± 9.39 | 86.46 ± 5.01 | 79.72 ± 4.46 | 86.75 ± 7.32 |
| 25 | 95.68 ± 3.29 | 88.86 ± 3.85 | 78.88 ± 5.57 | 83.65 ± 3.21 | 70.64 ± 7.35 | 75.54 ± 2.52 |
| 50 | 86.62 ± 3.02 | 87.96 ± 5.91 | 75.21 ± 6.07 | 82.25 ± 1.30 | 63.02 ± 6.20 | 66.88 ± 2.80 |
| IC50 R2 | >50% 0.7582 | 15.14 0.3751 | 17.67 0.7146 | 15.84 0.7341 | 18.78 0.8228 | 39.77 0.8883 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilden, A.; Sabancılar, İ.; Yalçın Azarkan, S.; Karadağlı, K.; Kaya, S.; Kahraman, M.; Çiçek, M. Investigating the Therapeutic Potential of Crude Leech Saliva Based on Its Anticancer, Antioxidant, and Anti-Inflammatory Effects. Curr. Issues Mol. Biol. 2025, 47, 328. https://doi.org/10.3390/cimb47050328
Bilden A, Sabancılar İ, Yalçın Azarkan S, Karadağlı K, Kaya S, Kahraman M, Çiçek M. Investigating the Therapeutic Potential of Crude Leech Saliva Based on Its Anticancer, Antioxidant, and Anti-Inflammatory Effects. Current Issues in Molecular Biology. 2025; 47(5):328. https://doi.org/10.3390/cimb47050328
Chicago/Turabian StyleBilden, Alican, İlhan Sabancılar, Serap Yalçın Azarkan, Kenan Karadağlı, Seçkin Kaya, Merve Kahraman, and Muttalip Çiçek. 2025. "Investigating the Therapeutic Potential of Crude Leech Saliva Based on Its Anticancer, Antioxidant, and Anti-Inflammatory Effects" Current Issues in Molecular Biology 47, no. 5: 328. https://doi.org/10.3390/cimb47050328
APA StyleBilden, A., Sabancılar, İ., Yalçın Azarkan, S., Karadağlı, K., Kaya, S., Kahraman, M., & Çiçek, M. (2025). Investigating the Therapeutic Potential of Crude Leech Saliva Based on Its Anticancer, Antioxidant, and Anti-Inflammatory Effects. Current Issues in Molecular Biology, 47(5), 328. https://doi.org/10.3390/cimb47050328






