Mesalazine and Lactoferrin as Potential Adjuvant Therapy in Colorectal Cancer: Effects on Cell Viability and Wnt/β-Catenin Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Conditions
2.2. Compound Solutions and Cell Treatment
2.3. Cell Viability Assay
2.4. RT-qPCR
2.5. In Silico Analysis
2.6. Statistical Analysis
3. Results
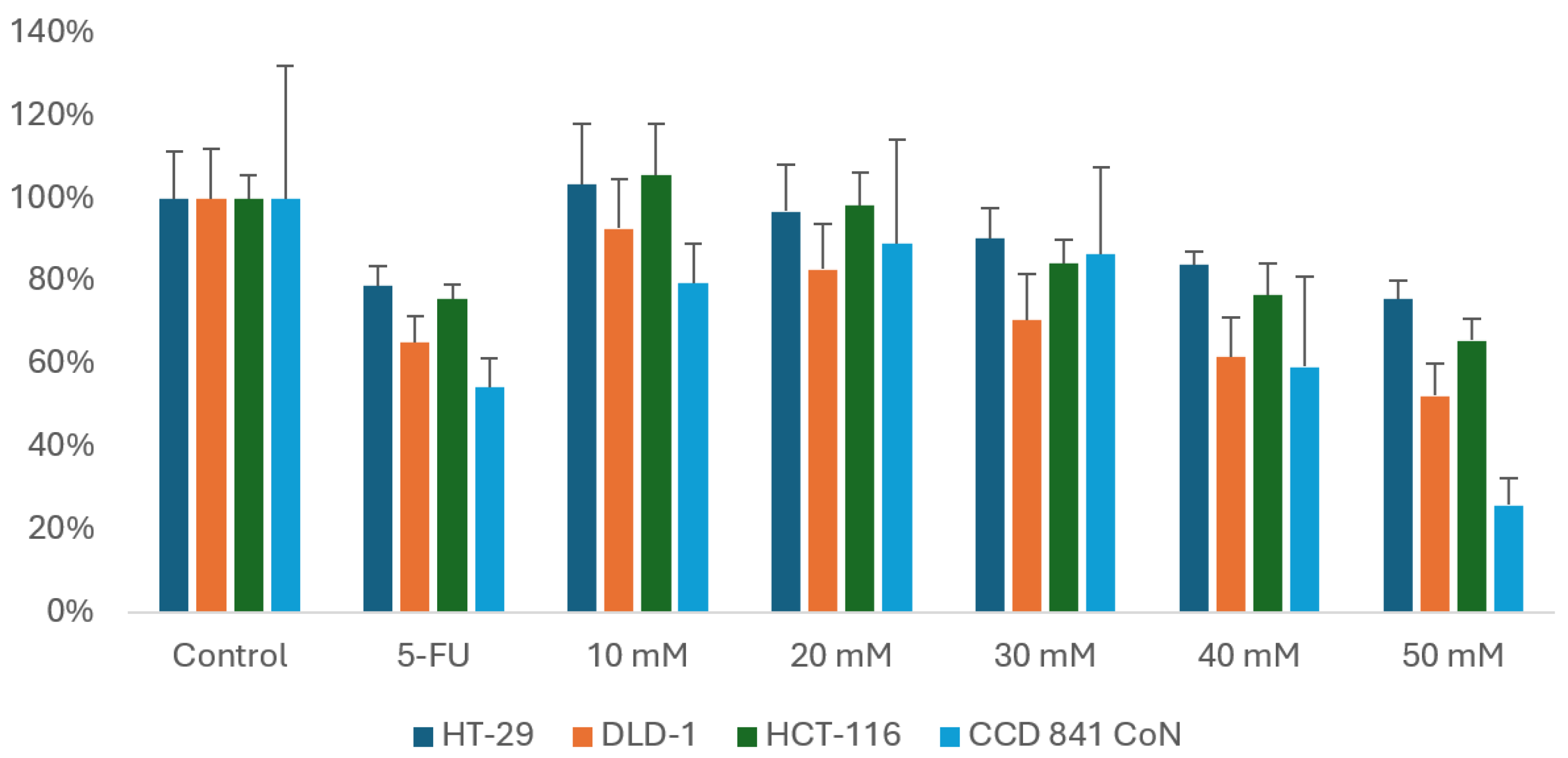
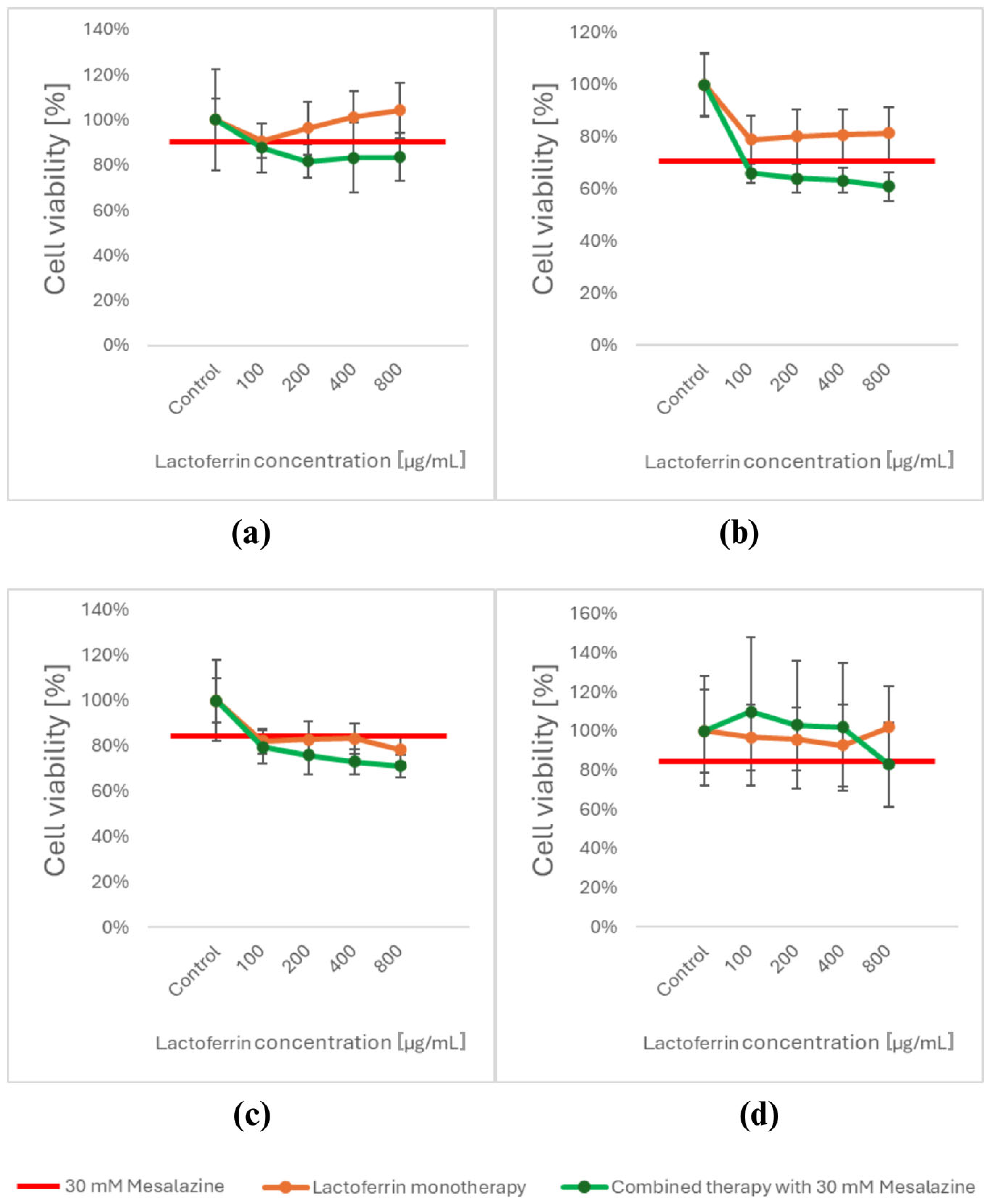
3.1. Viability Assessment Using the MTT Assay
3.2. Differential Expression of Wnt/β-Catenin Pathway Target Genes Based on Real-Time RT-qPCR
3.3. In Silico Prognosis of the Interaction of MES and LACT via ChemDIS-Mixture
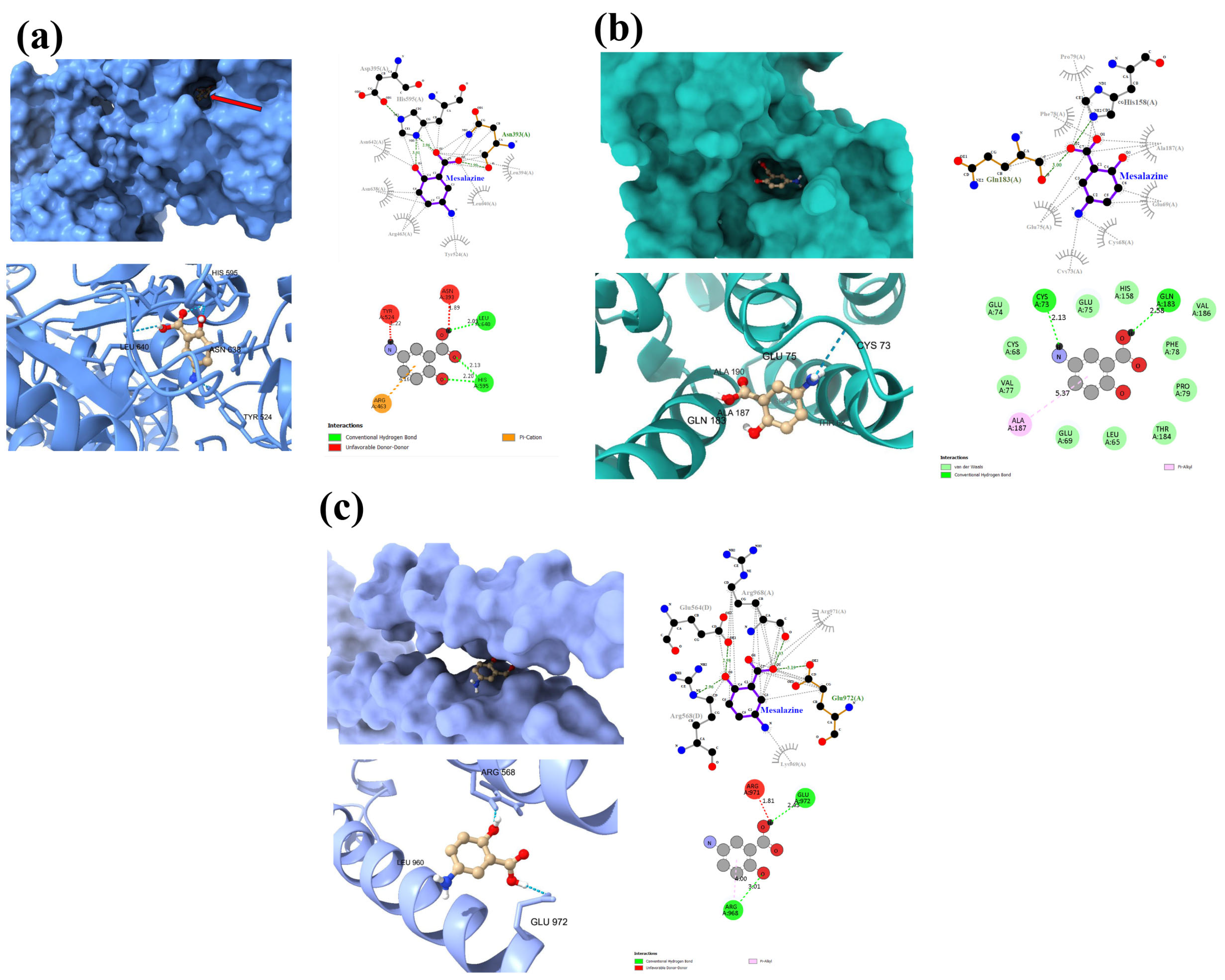
3.4. Molecular Docking of MES
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roshandel, G.; Ghasemi-Kebria, F.; Malekzadeh, R. Colorectal Cancer: Epidemiology, Risk Factors, and Prevention. Cancers 2024, 16, 1530. [Google Scholar] [CrossRef] [PubMed]
- Disoma, C.; Zhou, Y.; Li, S.; Peng, J.; Xia, Z. Wnt/β-catenin signaling in colorectal cancer: Is therapeutic targeting even possible? Biochimie 2022, 195, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, M.; Deng, K. Blocking the Wnt/β-catenin signaling pathway to treat colorectal cancer: Strategies to improve current therapies (Review). Int. J. Oncol. 2023, 62, 24. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Gan, W.J. Wnt/β-catenin signaling pathway in the development and progression of colorectal cancer. Cancer Manag. Res. 2023, 15, 435–448. [Google Scholar] [CrossRef]
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef]
- Zhu, G.X.; Gao, D.; Shao, Z.Z.; Chen, L.; Ding, W.J.; Yu, Q.F. Wnt/β-catenin signaling: Causes and treatment targets of drug resistance in colorectal cancer (Review). Mol. Med. Rep. 2021, 23, 105. [Google Scholar] [CrossRef]
- Leowattana, W.; Leowattana, P.; Leowattana, T. Systemic treatment for metastatic colorectal cancer. World J. Gastroenterol. 2023, 29, 1569–1588. [Google Scholar] [CrossRef]
- Hua, Y.; Dai, X.; Xu, Y.; Xing, G.; Liu, H.; Lu, T.; Chen, Y.; Zhang, Y. Drug repositioning: Progress and challenges in drug discovery for various diseases. Eur. J. Med. Chem. 2022, 234, 114239. [Google Scholar] [CrossRef]
- Bergman, R.; Parkes, M. Systematic review: The use of mesalazine in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2006, 23, 841–855. [Google Scholar] [CrossRef]
- Qiu, X.; Ma, J.; Wang, K.; Zhang, H. Chemopreventive Effects of 5-Aminosalicylic Acid on Inflammatory Bowel Disease-Associated Colorectal Cancer and Dysplasia: A Systematic Review with Meta-Analysis. Oncotarget. 2017, 8, 1031–1045. [Google Scholar] [CrossRef]
- Dixon, S.W.; Collard, T.J.; Mortensson, E.M.H.; Legge, D.N.; Chambers, A.C.; Greenhough, A.; Creed, T.J.; Williams, A.C. 5-Aminosalicylic acid inhibits stem cell function in human adenoma-derived cells: Implications for chemoprophylaxis in colorectal tumorigenesis. Br. J. Cancer 2021, 124, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Słoka, J.; Madej, M.; Strzalka-Mrozik, B. Molecular Mechanisms of the Antitumor Effects of Mesalazine and Its Preventive Potential in Colorectal Cancer. Molecules 2023, 28, 5081. [Google Scholar] [CrossRef] [PubMed]
- Bersuder, E.; Terciolo, C.; Lechevrel, M.; Martin, E.; Quesnelle, C.; Freund, J.-N.; Reimund, J.-M.; Gross, I. Mesalazine initiates an anti-oncogenic β-catenin/MUCDHL negative feed-back loop in colon cancer cells by cell-specific mechanisms. Biomed. Pharmacother. 2022, 146, 112543. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Di Patti, M.C.B.; Valenti, P.; Musci, G. Lactoferrin’s anti-cancer properties: Safety, selectivity, and wide range of action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef]
- Ramírez-Rico, G.; Drago-Serrano, M.E.; León-Sicairos, N.; de la Garza, M. Lactoferrin: A Nutraceutical with Activity against Colorectal Cancer. Front. Pharmacol. 2022, 13, 855852. [Google Scholar] [CrossRef]
- Superti, F. Lactoferrin from bovine milk: A protective companion for life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef]
- Eng, C.; Yoshino, T.; Ruíz-García, E.; Mostafa, N.; Cann, C.G.; O’Brian, B.; Benny, A.; Perez, R.O.; Cremolini, C. Colorectal cancer. Lancet 2024, 404, 294–310. [Google Scholar] [CrossRef]
- Shin, A.E.; Giancotti, F.G.; Rustgi, A.K. Metastatic colorectal cancer: Mechanisms and emerging therapeutics. Trends Pharmacol. Sci. 2023, 44, 222–236. [Google Scholar] [CrossRef]
- Liu, N.; Feng, G.; Zhang, X.; Hu, Q.; Sun, S.; Sun, J.; Sun, Y.; Wang, R.; Zhang, Y.; Wang, P.; et al. The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 759507. [Google Scholar] [CrossRef]
- Khare, V.; Krnjic, A.; Frick, A.; Gmainer, C.; Asboth, M.; Jimenez, K.; Lang, M.; Baumgartner, M.; Evstatiev, R.; Gasche, C. Mesalamine and azathioprine modulate junctional complexes and restore epithelial barrier function in intestinal inflammation. Sci. Rep. 2019, 9, 2842. [Google Scholar] [CrossRef]
- Cannon, A.R.; Akhtar, S.; Hammer, A.M.; Morris, N.L.; Javorski, M.J.; Li, X.; Kennedy, R.H.; Gamelli, R.L.; Choudhry, M.A. Effects of Mesalamine Treatment on Gut Barrier Integrity after Burn Injury. J. Burn. Care Res. 2016, 37, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Moastafa, T.M.; El-Sissy, A.E.-D.E.; El-Saeed, G.K.; Koura, M.S.E.-D. Study on the Therapeutic Benefit on Lactoferrin in Patients with Colorectal Cancer Receiving Chemotherapy. Int. Sch. Res. Not. 2014, 2014, 184278. [Google Scholar] [CrossRef] [PubMed]
- Słoka, J.; Strzałka-Mrozik, B.; Kubica, S.; Nowak, I.; Kruszniewska-Rajs, C. Influence of Mesalazine on Ferroptosis-Related Gene Expression in In Vitro Colorectal Cancer Culture. Biomedicines 2025, 13, 219. [Google Scholar] [CrossRef]
- Platet, N.; Hinkel, I.; Richert, L.; Murdamoothoo, D.; Moufok-Sadoun, A.; Vanier, M.; Lavalle, P.; Gaiddon, C.; Vautier, D.; Freund, J.-N.; et al. The tumor suppressor CDX2 opposes pro-metastatic biomechanical modifications of colon cancer cells through organization of the actin cytoskeleton. Cancer Lett. 2017, 386, 57–64. [Google Scholar] [CrossRef]
- Du, P.; Feng, G.; Flatow, J.; Song, J.; Holko, M.; Kibbe, W.A.; Lin, S.M. From disease ontology to disease-ontology lite: Statistical methods to adapt a general-purpose ontology for the test of gene-ontology associations. Bioinformatics 2009, 25, i63–i68. [Google Scholar] [CrossRef]
- Tung, C.W.; Wang, C.C.; Wang, S.S.; Lin, P. ChemDIS-Mixture: An online tool for analyzing potential interaction effects of chemical mixtures. Sci. Rep. 2018, 8, 10047. [Google Scholar] [CrossRef]
- Klimeck, L.; Heisser, T.; Hoffmeister, M.; Brenner, H. Colorectal cancer: A health and economic problem. Best. Pract. Res. Clin. Gastroenterol. 2023, 66, 101839. [Google Scholar] [CrossRef]
- Adebayo, A.S.; Agbaje, K.; Adesina, S.K.; Olajubutu, O. Colorectal Cancer: Disease Process, Current Treatment Options, and Future Perspectives. Pharmaceutics 2023, 15, 2620. [Google Scholar] [CrossRef]
- Schwab, M.; Reynders, V.; Loitsch, S.; Shastri, Y.M.; Steinhilber, D.; Schroder, O.; Stein, J. PPARγ is involved in mesalazine-mediated induction of apoptosis and inhibition of cell growth in colon cancer cells. Carcinogenesis 2008, 29, 1407–1414. [Google Scholar] [CrossRef]
- Stolfi, C.; Fina, D.; Caruso, R.; Caprioli, F.; Fantini, M.C.; Rizzo, A.; Sarra, M.; Pallone, F.; Monteleone, G. Mesalazine negatively regulates CDC25A protein expression and promotes accumulation of colon cancer cells in S phase. Carcinogenesis 2008, 29, 1258–1266. [Google Scholar] [CrossRef]
- Freiburghaus, C.; Janicke, B.; Lindmark-Månsson, H.; Oredsson, S.M.; Paulsson, M.A. Lactoferricin treatment decreases the rate of cell proliferation of a human colon cancer cell line. J. Dairy Sci. 2009, 92, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Yang, H.G.; Li, P.; Wang, Y.-Z.; Huang, G.-X.; Xing, L.; Wang, J.-Q.; Zheng, N. Effect of Heat Treatment on the Antitumor Activity of Lactoferrin in Human Colon Tumor (HT29) Model. J. Agric. Food Chem. 2019, 67, 140–147. [Google Scholar] [CrossRef] [PubMed]
- León-Flores, D.B.; Siañez-Estada, L.I.; Iglesias-Figueroa, B.F.; Siqueiros-Cendón, T.S.; Espinoza-Sánchez, E.A.; Varela-Ramírez, A.; Aguilera, R.J.; Rascón-Cruz, Q. Anticancer potential of lactoferrin: Effects, drug synergy and molecular interactions. BioMetals 2025, 38, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Bos, C.L.; Diks, S.H.; Hardwick, J.C.H.; Walburg, K.V.; Peppelenbosch, M.P.; Richel, D.J. Protein phosphatase 2A is required for mesalazine-dependent inhibition of Wnt/β-catenin pathway activity. Carcinogenesis 2006, 27, 2371–2382. [Google Scholar] [CrossRef]
- Parenti, S.; Montorsi, L.; Fantini, S.; Mammoli, F.; Gemelli, C.; Atene, C.G.; Losi, L.; Frassineti, C.; Calabretta, B.; Tagliafico, E.; et al. KLF4 mediates the effect of 5-ASA on the b-catenin pathway in colon cancer cells. Cancer Prev. Res. 2018, 11, 503–510. [Google Scholar] [CrossRef]
- Khare, V.; Lyakhovich, A.; Dammann, K.; Lang, M.; Borgmann, M.; Tichy, B.; Pospisilova, S.; Luciani, G.; Campregher, C.; Evstatiev, R.; et al. Mesalamine modulates intercellular adhesion through inhibition of p-21 activated kinase-1. Biochem. Pharmacol. 2013, 85, 234–244. [Google Scholar] [CrossRef]
- Munding, J.; Ziebarth, W.; Pox, C.P.; Ladigan, S.; Reiser, M.; Hüppe, D.; Brand, L.; Schmiegel, W.; Tannapfel, A.; Reinacher-Schick, A.C. The influence of 5-aminosalicylic acid on the progression of colorectal adenomas via the ß-catenin signaling pathway. Carcinogenesis 2012, 33, 637–643. [Google Scholar] [CrossRef]
- Monteleone, G.; Franchi, L.; Fina, D.; Caruso, R.; Vavassori, P.; Monteleone, I.; Calabrese, E.; Naccari, G.C.; Bellinvia, S.; Testi, R.; et al. Silencing of SH-PTP2 defines a crucial role in the inactivation of epidermal growth factor receptor by 5-aminosalicylic acid in colon cancer cells. Cell Death Differ. 2006, 13, 202–211. [Google Scholar] [CrossRef]
- Rousseaux, C.; Lefebvre, B.; Dubuquoy, L.; Lefebvre, P.; Romano, O.; Auwerx, J.; Metzger, D.; Wahli, W.; Desvergne, B.; Naccari, G.C.; et al. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-γ. J. Exp. Med. 2005, 201, 1205–1215. [Google Scholar] [CrossRef]
- Wang, M.; Chen, T.; Zhang, J.; Wen, X. PP2A drives the stemness in colorectal cancer cells by decreasing the Hippo signaling pathway. Cell Mol. Biol. 2023, 69, 114–120. [Google Scholar] [CrossRef]
- Furmanski, P.; Li, Z.P.; Fortuna, M.B.; Swamy, C.V.B.; Ramachandra Das, M. Multiple molecular forms of human lactoferrin. Identification of a Class of Lactoferrins That Possess Ribonuclease Activity and Lack Iron-Binding Capacity. J. Exp. Med. 1989, 170, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lönnerdal, B. Bovine Lactoferrin and Lactoferricin Exert Antitumor Activities on Human Colorectal Cancer Cells (HT-29) by Activating Various Signaling Pathways. Biochem. Cell Biol. 2017, 95, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Akasu, T.; Saito, D.; Alexander, D.B.; Iigo, M.; Kakizoe, T.; Tsuda, H. Effect of orally administered bovine lactoferrin on the growth of adenomatous colorectal polyps in a randomized, placebo-controlled clinical trial. Cancer Prev. Res. 2009, 2, 975–983. [Google Scholar] [CrossRef]
- Din, F.V.N.; Dunlop, M.G.; Stark, L.A. Evidence for colorectal cancer cell specificity of aspirin effects on NF kappa B signalling and apoptosis. Br. J. Cancer 2004, 91, 381–388. [Google Scholar] [CrossRef]
- Pyo, J.S.; Min, K.W.; Oh, I.H.; Lim, D.H.; Son, B.K. Clinicopathological significance and the associated signaling pathway of p21-activated kinase 1 (PAK1) in colorectal cancer. Pathol. Res. Pract. 2023, 251, 154820. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Z. TNF-α promotes colon cancer cell migration and invasion by upregulating TROP-2. Oncol. Lett. 2018, 15, 3820–3827. [Google Scholar] [CrossRef]
- Chen, J.; Elfiky, A.; Han, M.; Chen, C.; Saif, M.W. The role of src in colon cancer and its therapeutic implications. Clin. Color. Cancer 2014, 13, 5–13. [Google Scholar] [CrossRef]
- Ha, S.Y.; Kim, J.Y.; Choi, J.H. Transcriptional regulation of genetic variants in the SLC40A1 promoter. Korean J. Physiol. Pharmacol. 2024, 28, 113–120. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer. 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Liu, X.; Tuerxun, H.; Li, Y.; Li, Y.; He, Y.; Zhao, Y. Ferroptosis: Reviewing CRC with the Third Eye. J. Inflamm. Res. 2022, 15, 6801–6812. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Ding, J.; Chen, Y. Iron metabolism in cancer. Int. J. Mol. Sci. 2019, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Hang, Y.; Tsai, H.I.I.; Wang, D.; Zhu, H. Iron metabolism: Backfire of cancer cell stemness and therapeutic modalities. Cancer Cell Int. 2024, 24, 157. [Google Scholar] [CrossRef] [PubMed]
- Chieppa, M.; Kashyrina, M.; Miraglia, A.; Vardanyan, D. Enhanced CRC Growth in Iron-Rich Environment, Facts and Speculations. Int. J. Mol. Sci. 2024, 25, 12389. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Abdelnour, S.A.; Kamal, M.; Khafaga, A.F.; Shakoori, A.M. Lactoferrin: Antimicrobial impacts, genomic guardian, therapeutic uses and clinical significance for humans and animals. Biomed. Pharmacother. 2023, 164, 114967. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, D.; Chen, X.; Li, X.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Chen, X.; Tang, D.; Liu, J. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy 2023, 19, 1982–1996. [Google Scholar] [CrossRef]
- Xia, W.; Lv, Y.; Zou, Y.; Kang, Z.; Li, Z.; Tian, J.; Zhou, H.; Su, W.; Zhong, J. The role of ferroptosis in colorectal cancer and its potential synergy with immunotherapy. Front. Immunol. 2025, 15, 1526749. [Google Scholar] [CrossRef]
- Bębenek, E.; Pęcak, P.; Kadela-Tomanek, M.; Orzechowska, B.; Chrobak, E. Derivatives of Betulin and Betulinic Acid Containing a Phosphonate Group—In Silico Studies and Preliminary In Vitro Assessment of Antiviral Activity. Appl. Sci. 2024, 14, 1452. [Google Scholar] [CrossRef]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef]
- Li, R.; Huang, X.; Yang, L.; Liang, X.; Huang, W.; Lai, K.P.; Zhou, L. Integrated Analysis Reveals the Targets and Mechanisms in Immunosuppressive Effect of Mesalazine on Ulcerative Colitis. Front. Nutr. 2022, 19, 9. [Google Scholar] [CrossRef]
- Li, P.; Zheng, X.; Shou, K.; Niu, Y.; Jian, C.; Zhao, Y.; Yi, W.; Hu, X.; Yu, A. The Iron Chelator Dp44mT Suppresses Osteosarcoma’s Proliferation, Invasion and Migration: In Vitro and in Vivo. Am. J. Transl. Res. 2016, 8, 5370. [Google Scholar]
- Lui, G.Y.L.; Kovacevic, Z.; Richardson, V.; Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Targeting Cancer by Binding Iron: Dissecting Cellular Signaling Pathways. Oncotarget 2015, 7, 18748–18779. [Google Scholar] [CrossRef]

| MES | ||||
| Protein | Gene Symbol | Gene ID | Gene Name | Score |
| ENSP00000312304 | TPMTD | 7172 | thiopurine S-methyltransferase | 0.981 |
| ENSP00000363512 | ALOX5 | 240 | arachidonate 5-lipoxygenase | 0.981 |
| ENSP00000287820 | PPARG | 5468 | peroxisome proliferator activated receptor gamma | 0.968 |
| ENSP00000225275 | MPO | 4353 | myeloperoxidase | 0.913 |
| ENSP00000388001 | OAS1 | 4938 | 2′-5′-oligoadenylate synthetase 1 | 0.864 |
| ENSP00000228928 | OAS3 | 4940 | 2′-5′-oligoadenylate synthetase 3 | 0.861 |
| ENSP00000342278 | OAS2 | 4939 | 2′-5′-oligoadenylate synthetase 2 | 0.861 |
| ENSP00000278568 | PAK1 | 5058 | p21 (RAC1) activated kinase 1 | 0.820 |
| ENSP00000356438 | PTGS2 | 5743 | prostaglandin-endoperoxide synthase 2 | 0.819 |
| ENSP00000275493 | mENA | 1956 | epidermal growth factor receptor | 0.800 |
| ENSP00000276431 | DR5 | 8795 | TNF receptor superfamily member 10b | 0.800 |
| ENSP00000350941 | SRC | 6714 | SRC proto-oncogene, non-receptor tyrosine kinase | 0.800 |
| ENSP00000354612 | PTGS1 | 5742 | prostaglandin-endoperoxide synthase 1 | 0.800 |
| ENSP00000370989 | CD274 | 29126 | CD274 molecule | 0.800 |
| ENSP00000373691 | DUOX2 | 50506 | dual oxidase 2 | 0.800 |
| ENSP00000430684 | IKBKB | 3551 | inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta | 0.800 |
| ENSP00000303706 | CDC25A | 993 | cell division cycle 25A | 0.800 |
| LACT | ||||
| Protein | Gene Symbol | Gene ID | Gene Name | Score |
| ENSP00000231751 | LF | 4057 | lactotransferrin | 0.177 |
| ENSP00000261024 | SLC40A1 | 30061 | solute carrier family 40 member 1 | 0.156 |
| ENSP00000327758 | CSX1 | 1482 | NK2 homeobox 5 | 0.220 |
| ENSP00000336764 | OPRL1 | 4987 | opioid related nociceptin receptor 1 | 0.228 |
| ID | Description | Gene Ratio | Adj. p MES | Gene Ratio | Adj. p LACT | Adj. p Joint |
|---|---|---|---|---|---|---|
| GO:0007193 | adenylate cyclase-inhibiting G-protein coupled receptor signaling pathway | 8/169 | 6.48 × 10−7 | 1/4 | 0.01589 | 1.03 × 10−8 |
| GO:0045071 | negative regulation of viral genome replication | 5/169 | 0.00042 | 1/4 | 0.01398 | 5.88 × 10−6 |
| GO:0043066 | negative regulation of apoptotic process | 11/169 | 0.01080 | 3/4 | 0.00519 | 0.00006 |
| GO:0051092 | positive regulation of NF-κB transcription factor activity | 7/169 | 0.00207 | 1/4 | 0.03191 | 0.00007 |
| GO:0045454 | cell redox homeostasis | 4/169 | 0.01428 | 1/4 | 0.02089 | 0.00030 |
| GO:0043123 | positive regulation of I-κB kinase/NF-κB signaling | 6/169 | 0.01156 | 1/4 | 0.03764 | 0.00044 |
| GO:0006959 | humoral immune response | 3/169 | 0.02928 | 1/4 | 0.01759 | 0.00052 |
| GO:0045669 | positive regulation of osteoblast differentiation | 3/169 | 0.03114 | 1/4 | 0.01760 | 0.00055 |
| GO:0045944 | positive regulation of transcription from RNA polymerase II promoter | 16/169 | 0.04039 | 2/4 | 0.02089 | 0.00084 |
| GO:0005506 | iron ion binding | 5/168 | 0.01795 | 1/4 | 0.04900 | 0.00088 |
| GO:0001503 | ossification | 3/169 | 0.04643 | 1/4 | 0.02089 | 0.00097 |
| GO:0090575 | RNA polymerase II transcription factor complex | 2/175 | 0.04601 | 1/4 | 0.02134 | 0.00098 |
| GO:0001934 | positive regulation of protein phosphorylation | 4/169 | 0.04414 | 1/4 | 0.03058 | 0.00135 |
| GO:0016323 | basolateral plasma membrane | 5/175 | 0.03440 | 1/4 | 0.04018 | 0.00138 |
| Protein | ΔG [kcal/mol] |
|---|---|
| LACT | −6.7 |
| CCND1 | −5.4 |
| MYC | −5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słoka, J.; Madej, M.; Nowak, I.; Strzałka-Mrozik, B. Mesalazine and Lactoferrin as Potential Adjuvant Therapy in Colorectal Cancer: Effects on Cell Viability and Wnt/β-Catenin Pathway. Curr. Issues Mol. Biol. 2025, 47, 327. https://doi.org/10.3390/cimb47050327
Słoka J, Madej M, Nowak I, Strzałka-Mrozik B. Mesalazine and Lactoferrin as Potential Adjuvant Therapy in Colorectal Cancer: Effects on Cell Viability and Wnt/β-Catenin Pathway. Current Issues in Molecular Biology. 2025; 47(5):327. https://doi.org/10.3390/cimb47050327
Chicago/Turabian StyleSłoka, Joanna, Marcel Madej, Ilona Nowak, and Barbara Strzałka-Mrozik. 2025. "Mesalazine and Lactoferrin as Potential Adjuvant Therapy in Colorectal Cancer: Effects on Cell Viability and Wnt/β-Catenin Pathway" Current Issues in Molecular Biology 47, no. 5: 327. https://doi.org/10.3390/cimb47050327
APA StyleSłoka, J., Madej, M., Nowak, I., & Strzałka-Mrozik, B. (2025). Mesalazine and Lactoferrin as Potential Adjuvant Therapy in Colorectal Cancer: Effects on Cell Viability and Wnt/β-Catenin Pathway. Current Issues in Molecular Biology, 47(5), 327. https://doi.org/10.3390/cimb47050327






