Key Sweet Potato Viruses in Fujian Province and Their Distribution, Harmfulness, and Implications in China
Abstract
1. Introduction
2. Materials and Methods
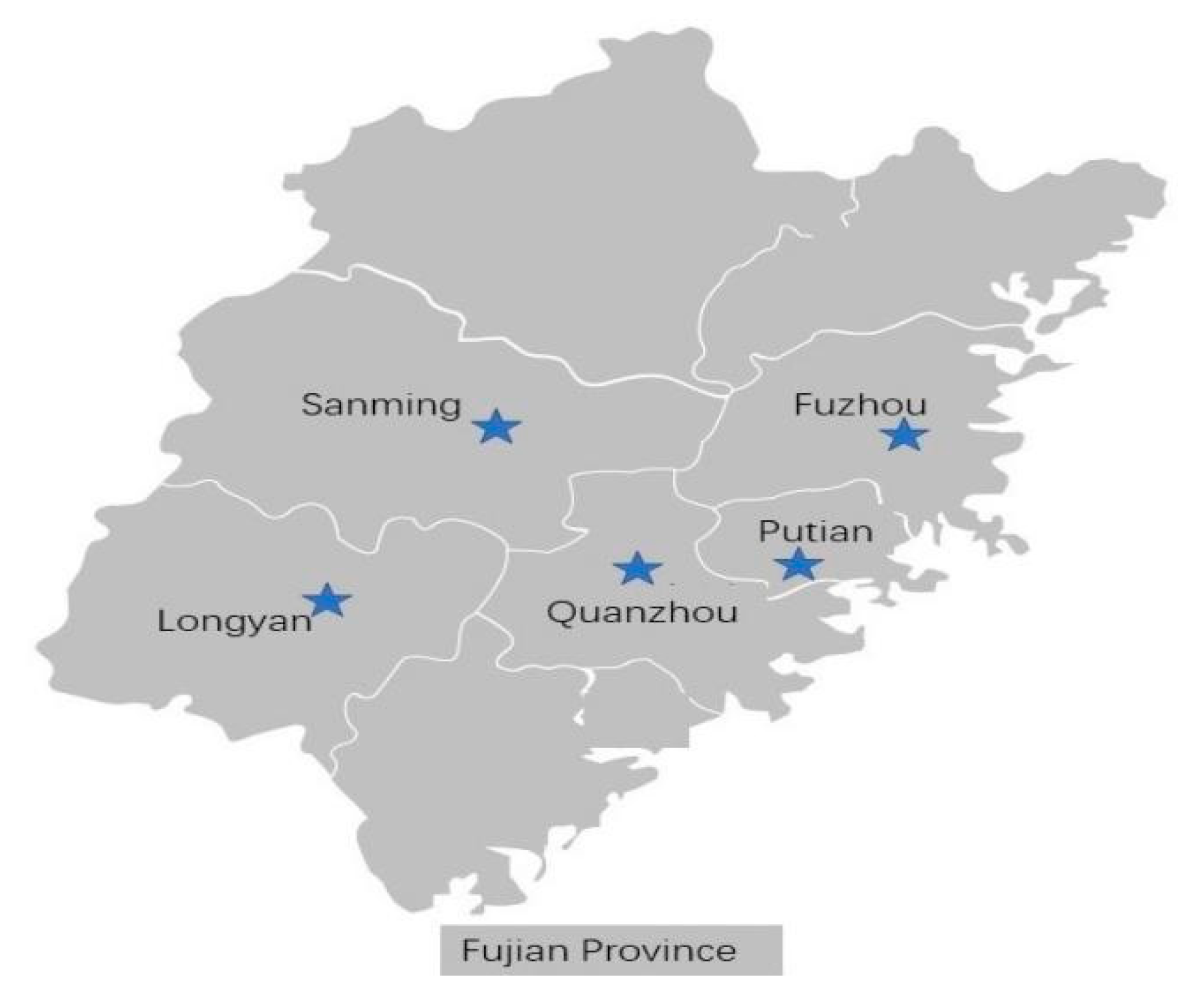
2.1. Sweet Potato Sampling
2.2. Reagents
2.3. PCR Analysis
2.3.1. Primer Design
2.3.2. RNA and DNA Extraction
2.3.3. PCR and RT-PCR Protocols
2.4. Identification of PCR Products
2.5. Sequence Analysis
2.6. qRT-PCR to Detect RNase3 Expression
2.7. qRT-PCR Conditions
3. Results
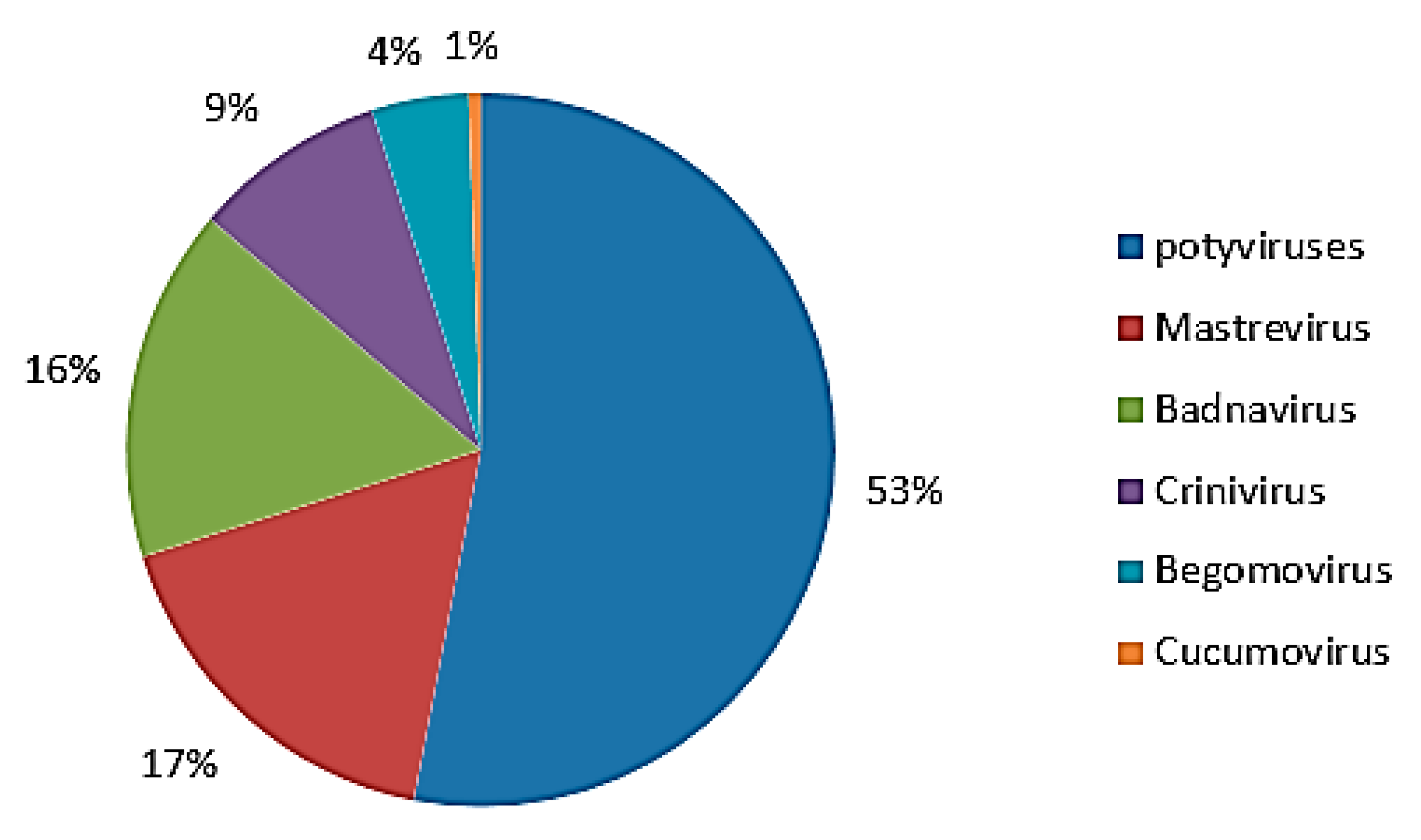
3.1. Detection of 11 Viruses in Fujian Sweet Potatoes
3.1.1. SPFMV (Sweet Potato Feathery Mottle Virus)
3.1.2. SPCSV (Sweet Potato Chlorotic Stunt Virus)
3.1.3. SPV2 (Sweet Potato Virus 2)
3.1.4. SPVC (Sweet Potato Virus C)
3.1.5. SPVG (Sweet Potato Virus G)
3.1.6. SPLV (Sweet Potato Latent Virus)
3.1.7. CMV (Cucumber Mosaic Virus)
3.1.8. SPLCV (Sweet Potato Leaf Curl Virus)
3.1.9. SPVMV (Sweet Potato Vein Mosaic Virus)
3.2. Regional Variations in Sweet Potato Virus Infections in Fujian Province
3.3. Variations in Sweet Potato Virus Incidence Across Growth Stages
3.4. SPVD Detection Across Sweet Potato Growth Stages
3.5. Virus Variations Among Sweet Potato Varieties
3.6. Occurrence of Viral Diseases in Different Regions
3.7. Complex Infections of Sweet Potato Viral Diseases
3.8. SPVD Detection in Sweet Potato Varieties
3.9. Occurrence of Viral Diseases in Different Sweet Potato Parts
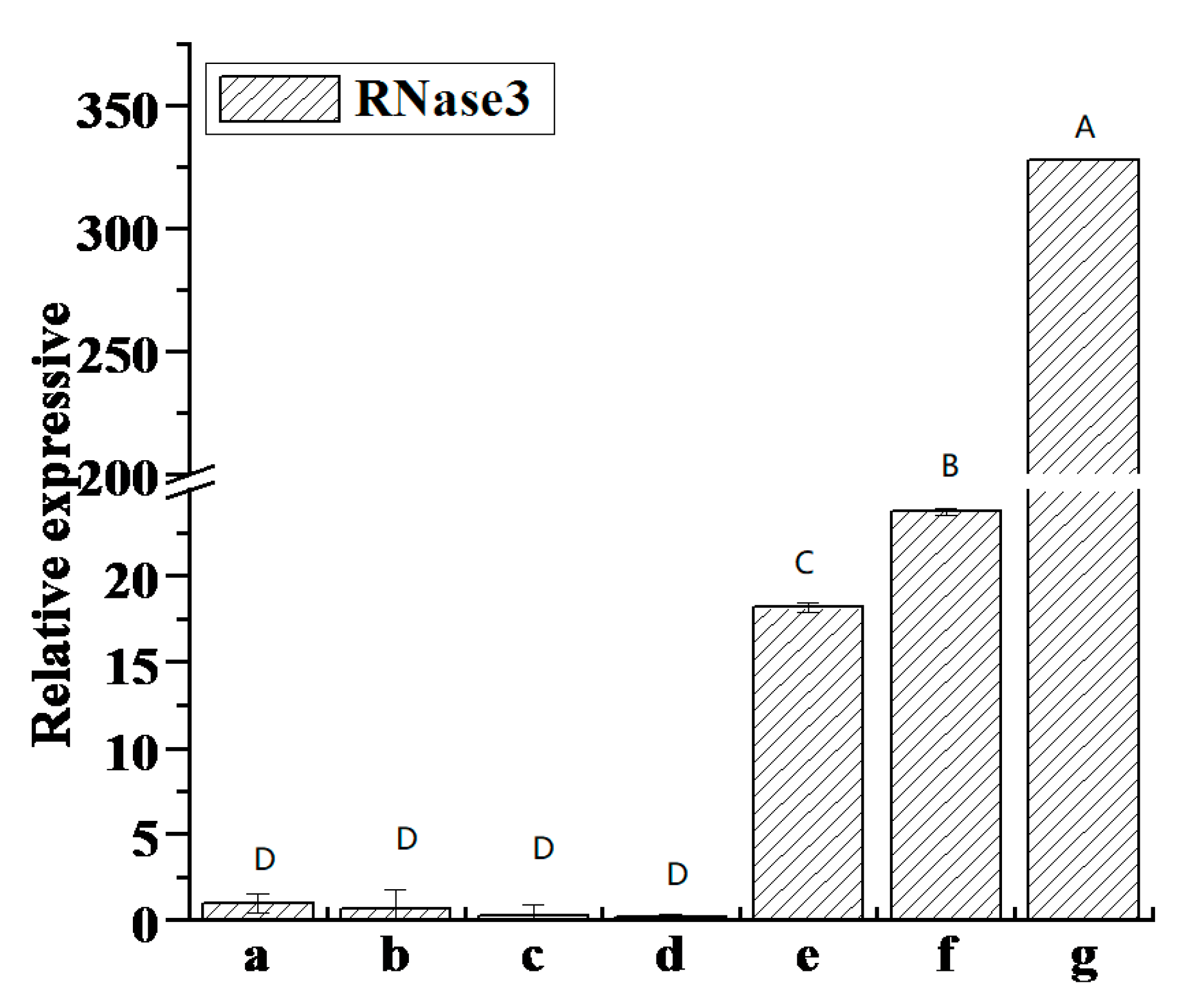
3.10. Correlation Between RNase3 Gene Expression and SPVD Pathogenesis
4. Discussion
4.1. Viral Diseases Threaten Sweet Potato Production
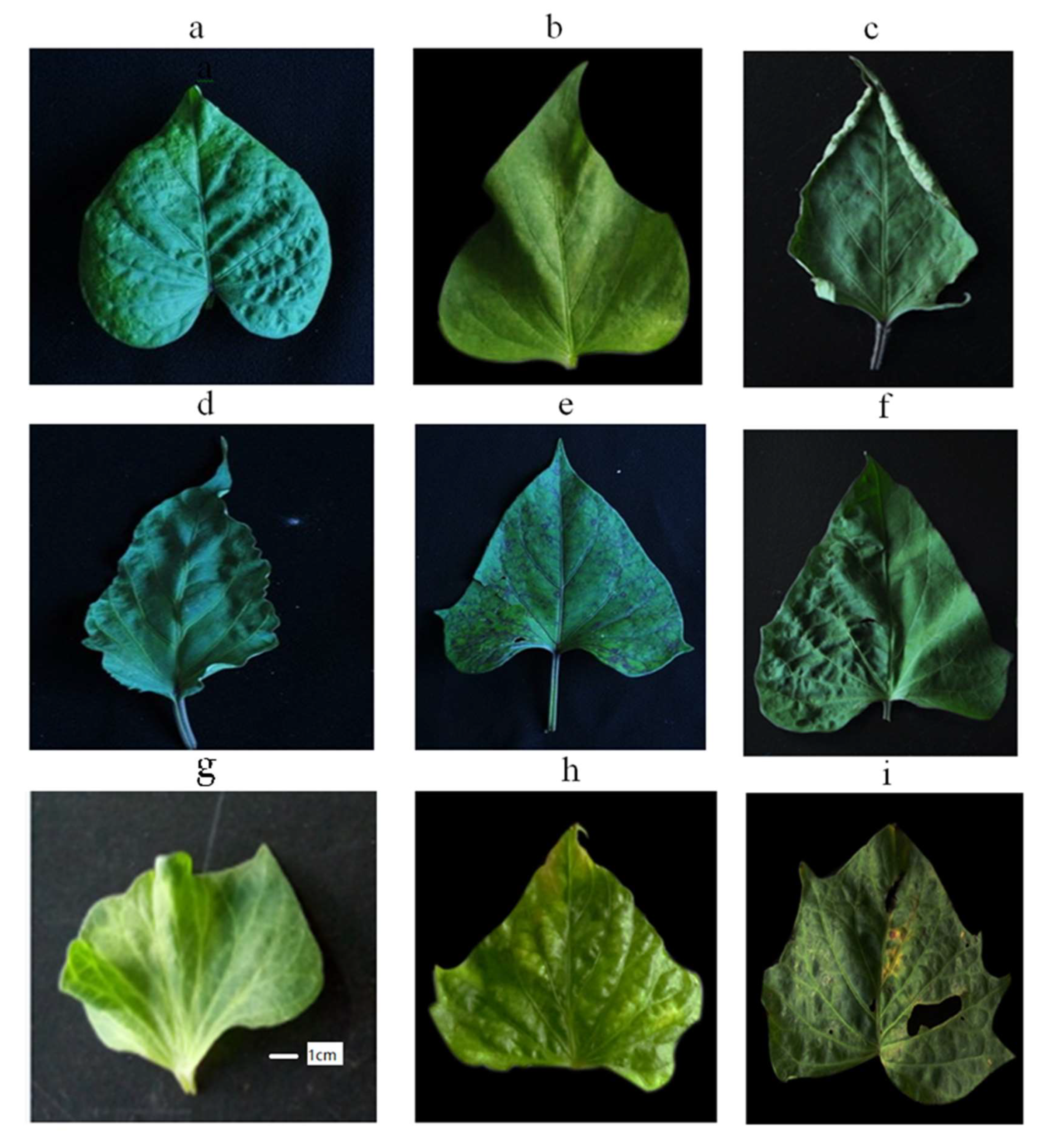
4.2. Symptoms of Sweet Potato Viral Diseases
4.3. Prevention and Control of Sweet Potato Viral Diseases
4.4. The Relationship Between the Expression of the RNase3 Gene and SPVD
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- David, M.; Kante, M.; Fuentes, S.; Eyzaguirre, R.; Diaz, F.; De, B.B.; Mwanga, R.; Kreuze, J.; Grüneberg, W.J. Early-Stage Phenotyping of Sweet Potato Virus Disease Caused by Sweet Potato Chlorotic Stunt Virus and Sweet Potato Virus C to Support Breeding. Plant Dis. 2023, 107, 2061–2069. [Google Scholar] [PubMed]
- Adero, J.; Wokorach, G.; Stomeo, F.; Yao, N.; Machuka, E.; Njuguna, J.; Byarugaba, D.K.; Kreuze, J.; Yencho, G.C.; Otema, M.A.; et al. Next Generation Sequencing and Genetic Analyses Reveal Factors Driving Evolution of Sweetpotato Viruses in Uganda. Pathogens 2024, 13, 833. [Google Scholar] [CrossRef]
- Untiveros, M.; Fuentes, S.; Salazar, L.F. Synergistic Interaction of Sweet potato chlorotic stunt virus (Crinivirus) with Carla-, Cucumo-, Ipomo-, and Potyviruses Infecting Sweet Potato. Plant Dis. 2007, 9, 1669–1676. [Google Scholar]
- Tibiri, E.B.; Pita, J.S.; Tiendrébéogo, F.; Bangratz, M.; Néya, J.B.; Brugidou, C.; Somé, K.; Barro, N. Characterization of virus species associated with sweetpotato virus diseases in Burkina Faso. Plant Pathol. 2020, 69, 1003–1017. [Google Scholar]
- Fiallo-Olivé, E.; García-Merenciano, A.C.; Navas-Castillo, J. Sweet Potato Symptomless Virus 1: First Detection in Europe and Generation of an Infectious Clone. Microorganisms 2022, 10, 1736. [Google Scholar] [CrossRef]
- Ho, P.T.; Byun, H.S.; Vo, T.T.B.; Lal, A.; Lee, S.; Kil, E.J. Construction of an Agroinfectious Clone of a Korean Isolate of Sweet Potato Symptomless Virus 1 and Comparison of Its Infectivity According to Agrobacterium tumefaciens Strains in Nicotiana benthamiana. Plant Pathol. J. 2023, 39, 255–264. [Google Scholar] [PubMed]
- Wanjala, B.W.; Ateka, E.M.; Miano, D.W.; Low, J.W.; Kreuze, J.F. Storage Root Yield of Sweetpotato as Influenced by Sweetpotato leaf curl virus and Its Interaction With Sweetpotato feathery mottle virus and Sweetpotato chlorotic stunt virus in Kenya. Plant Dis. 2020, 104, 1477–1486. [Google Scholar] [CrossRef]
- Qiao, Q.; Zhang, Z.; Zhang, D.; Qin, Y.; Tian, Y.; Wang, Y. Serological and molecular detection of sweet potato virus species in China. Acta Phytopathol. 2012, 42, 10–16. [Google Scholar]
- Villalba, A.; Martínez-Ispizua, E.; Morard, M.; Crespo-Sempere, A.; Albiach-Marti, M.R.; Calatayud, A.; Penella, C. Optimizing sweet potato production: Insights into the interplay of plant sanitation, virus influence, and cooking techniques for enhanced crop quality and food security. Front. Plant Sci. 2024, 18, 1357611. [Google Scholar] [CrossRef]
- Wainaina, J.M.; Ateka, E.; Makori, T.; Kehoe, M.A.; Boykin, L.M. Phylogenomic relationship and evolutionary insights of sweet potato viruses from the western highlands of Kenya. PeerJ 2018, 6, e5254. [Google Scholar]
- Ferro, C.G.; Zerbini, F.M.; Navas-Castillo, J.; Fiallo-Olivé, E. Revealing the Complexity of Sweepovirus-Deltasatellite-Plant Host Interactions: Expanded Natural and Experimental Helper Virus Range and Effect Dependence on Virus-Host Combination. Microorganisms 2021, 9, 1018. [Google Scholar] [CrossRef] [PubMed]
- Ogero, K.O.; Kreuze, J.F.; McEwan, M.A.; Luambano, N.D.; Bachwenkizi, H.; Garrett, K.A.; Andersen, K.F.; Thomas-Sharma, S.; van der Vlugt, R.A.A. Efficiency of insect-proof net tunnels in reducing virus-related seed degeneration in sweet potato. Plant Pathol. 2019, 68, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Loebenstein, G. Control of sweet potato virus diseases. Adv Virus Res. 2015, 91, 33–45. [Google Scholar]
- Wokorach, G.; Edema, H.; Echodu, R. Sweetpotato seed exchange systems and knowledge on sweetpotato viral diseases among local farmers in Acholi Sub Region-Northern Uganda. Afr. J. Agric. Res. 2018, 13, 45. [Google Scholar]
- Kreuze, J.F.; Ramírez, D.A.; Fuentes, S.; Loayza, H.; Ninanya, J.; Rinza, J.; David, M.; Gamboa, S.; De, B.B.; Diaz, F.; et al. High-throughput characterization and phenotyping of resistance and tolerance to virus infection in sweetpotato. Virus Res. 2024, 339, 199276. [Google Scholar] [CrossRef]
- Cillo, F.; Mascia, T.; Fanelli, V.; De Stradis, A.; Finetti Sialer, M.M.; Gallitelli, D. Characterization of synergy between cucumber mosaic virus and potato virus Y in tomato. J. Plant Pathol. 2006, 88 (Suppl. S3), S18–S19. [Google Scholar]
- Rajagopalbabu, S.; Alvarez, J.M. Effect of mixed viral infections (potato virus Y-potato leafroll virus) on biology and preference of vectors Myzus persicae and Macrosiphum euphorbiae (Hemiptera: Aphididae). J. Econ. Entomol. 2007, 100, 646–655. [Google Scholar]
- Untiveros, M.; Quispe, D.; Kreuze, J. Analysis of complete genomic sequences of isolates of the Sweet potato feathery mottle virus strains C and EA: Molecular evidence for two distinct potyvirus species and two P1 protein domains. Arch. Virol. 2010, 155, 2059–2063. [Google Scholar] [CrossRef]
- Gibson, R.W.; Kaitisha, G.C.; Randrianaivoarivony, J.M.; Vetten, H.J. Identification of the East African Strain of Sweet Potato Chlorotic Stunt Virus as a Major Component of Sweet Potato Virus Disease in Southern Africa. Plant Dis. 1998, 82, 1063. [Google Scholar] [CrossRef]
- Tairo, F.; Mukasa, S.B.; Jones, R.A.; Kullaya, A.; Rubaihayo, P.R.; Valkonen, J.P. Unravelling the genetic diversity of the three main viruses involved in Sweet Potato Virus Disease (SPVD), and its practical implications. Mol. Plant Pathol. 2005, 6, 199–211. [Google Scholar] [CrossRef]
- Miano, D.W.; Labonte, D.R.; Clark, C.A. Identification of molecular markers associated with sweet potato resistance to sweet potato virus disease in Kenya. Euphytica 2007, 160, 15–24. [Google Scholar]
- Zhang, K.; Lu, H.; Wan, C.; Tang, D.; Zhao, Y.; Luo, K.; Li, S.; Wang, J. The Spread and Transmission of Sweet Potato Virus Disease (SPVD) and Its Effect on the Gene Expression Profile in Sweet Potato. Plants 2020, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, R.; David, M.; Fuentes, S.; Kreuze, J.; Fei, Z. Transcriptome analysis provides insights into the responses of sweet potato to sweet potato virus disease (SPVD). Virus Res. 2021, 295, 198293. [Google Scholar]
- Mukasa, S.B.; Rubaihayo, P.R.; Valkonen, J.P.T. Interactions between a crinivirus, an ipomovirus and a potyvirus in coinfected sweetpotato plants. Plant Pathol. 2006, 55, 458–467. [Google Scholar]
- IsHak, J.A.; Kreuze, J.F.; Johansson, A.; Mukasa, S.B.; Tairo, F.; Abo El-Abbas, F.M.; Valkonen, J.P. Some molecular characteristics of three viruses from SPVD-affected sweet potato plants in Egypt. Arch. Virol. 2003, 148, 2449–2460. [Google Scholar]
- Yu, Y.; Pan, Z.; Wang, X.; Bian, X.; Wang, W.; Liang, Q.; Kou, M.; Ji, H.; Li, Y.; Ma, D.; et al. Targeting of SPCSV-RNase3 via CRISPR-Cas13 confers resistance against sweet potato virus disease. Mol. Plant Pathol. 2022, 23, 104–117. [Google Scholar]
- Cuellar, W.J.; Tairo, F.; Kreuze, J.F.; Valkonen, J.P.T. Analysis of gene content in sweet potato chlorotic stunt virus RNA1 reveals the presence of the p22 RNA silencing suppressor in only a few isolates: Implications for viral evolution and synergism. J. Gen. Virol. 2008, 89, 573–582. [Google Scholar] [PubMed]
- Kreuze, J.F.; Savenkov, E.I.; Cuellar, W.; Li, X.; Valkonen, J.P. Viral class 1 RNase III involved in suppression of RNA silencing. J. Virol. 2005, 79, 7227–7238. [Google Scholar]
- Cuellar, W.J.; Kreuze, J.F.; Rajamäki, M.L.; Cruzado, K.R.; Untiveros, M.; Valkonen, J.P. Elimination of antiviral defense by viral RNase III. Proc. Natl. Acad. Sci. USA 2009, 106, 10354–10358. [Google Scholar]
- Li, F.; Zuo, R.; Abad, J.; Xu, D.; Bao, G.; Li, R. Simultaneous detection and differentiation of four closely related sweet potato potyviruses by a multiplex one-step RT-PCR. J. Virol. Methods 2012, 186, 161–166. [Google Scholar]
- Opiyo, S.A.; Ateka, E.M.; Owuor, P.O.; Manguro LO, A.; Miano, D.W. Development of a multiplex PCR technique for simultaneous detection of sweet potato feathery mottle virus and sweet potato chlorotic stunt virus. J. Plant Pathol. 2010, 92, 363–366. [Google Scholar]
- Qiao, Z.; Qin, Y.; Qiao, Q.; Zhang, D.; Tian, Y.; Gao, J.; Zhang, Z. Sequencing of Sweet Potato Leaf Curl Virus Genome and Expression of Coat Protein Gene in E. coli. J. Henan Agric. Sci. 2012, 41, 86–89. [Google Scholar]
- Kashif, M.; Pietilä, S.; Artola, K.; Jones, R.A.C.; Tugume, A.K.; Mäkinen, V.; Valkonen, J.P.T. Detection of Viruses in Sweetpotato from Honduras and Guatemala Augmented by Deep-Sequencing of Small-RNAs. Plant Dis. 2012, 96, 1430–1437. [Google Scholar]
- Park, S.-C.; Kim, Y.-H.; Ji, C.Y.; Park, S.; Jeong, J.C.; Lee, H.-S.; Kwak, S.-S. Stable internal reference genes for the normalization of real-time PCR in different sweetpotato cultivars subjected to abiotic stress conditions. PLoS ONE 2012, 7, e51502. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, H.; Zhang, A. Guilan Research progress on sweet potato virus disease. Henan Agric. Sci. 2000, 9, 19–22. [Google Scholar]
- Morales, F.J. Tropical Whitefly IPM Project. Adv. Virus Res. 2007, 69, 249–311. [Google Scholar]
- Miano, D.W.; LaBonte, D.R.; Clark, C.A.; Valverde, R.A.; Hoy, M.W.; Hurtt, S.; Li, R. First Report of a Begomovirus Infecting Sweetpotato in Kenya. Plant Dis. 2006, 90, 832. [Google Scholar] [CrossRef]
- Gu, Y.; Tang, H.; Zhang, Y. Cloning and Sequence Analysis of Sweet Potato G Virus Coat Protein Gene. Chin. J. Agron. 2006, 22, 50–55. [Google Scholar]
- Tugume, A.K.; Amayo, R.; Weinheimer, I.; Mukasa, S.B.; Rubaihayo, P.R.; Valkonen, J.P. Genetic variability and evolutionary implications of RNA silencing suppressor genes in RNA1 of sweet potato chlorotic stunt virus isolates infecting sweetpotato and related wild species. PLoS ONE 2013, 8, e81479. [Google Scholar]
- Clark, C.A.; Davis, J.A.; Abad, J.A.; Cuellar, W.J.; Fuentes, S.; Kreuze, J.F.; Gibson, R.W.; Mukasa, S.B.; Tugume, A.K.; Tairo, F.D.; et al. Sweetpotato Viruses: 15 Years of Progress on Understanding and Managing Complex Diseases. Plant Dis. 2012, 96, 168–185. [Google Scholar] [CrossRef]
- Feng, Z.; Yan, Z. Discussion on production technology system of virus-free sweet potato. Seed World 2001, 6, 24–25. [Google Scholar]
- Zhang, Z.; Qiao, Q.; Qin, Y.; Zhang, D.; Tian, Y. First evidence for occurrence of Sweet potato virus disease (SPVD) caused by dual infection of Sweet potato feathery mottle virus and Sweet potato chlorotic stunt virus in China. Acta Phytopathol. Sin. 2012, 42, 328–333. [Google Scholar]
- Weinheimer, I.; Boonrod, K.; Moser, M.; Wassenegger, M.; Krczal, G.; Butcher, S.J.; Valkonen, J.P.T. Binding and processing of small dsRNA molecules by the class 1 RNase III protein encoded by sweet potato chlorotic stunt virus. J. Gen. Virol. 2014, 95, 486–495. [Google Scholar] [PubMed]
- Kreuze, J.F.; Klein, I.S.; Lazaro, M.U.; Chuquiyuri, W.J.; Morgan, G.L.; Mejía, P.G.; Ghislain, M.; Valkonen, J.P. RNA silencing-mediated resistance to a crinivirus (Closteroviridae) in cultivated sweet potato (Ipomoea batatas L.) and development of sweet potato virus disease following co-infection with a potyvirus. Mol. Plant Pathol. 2008, 9, 589–598. [Google Scholar]
| Virus Species | Fuzhou Area | Quanzhou Area | Putian Area | Longyan Area | Sanming Area | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infection Number | Detection Rate | Infection Number | Detection Rate | Infection Number | Detection Rate | Infection Number | Detection Rate | Infection Number | Detection Rate | Infection Number | Detection Rate | |
| SPFMV | 26 | 60 | 10 | 91 | 2 | 50 | 0 | 0 | 0 | 0 | 38 | 60 |
| SPCSV | 11 | 26 | 4 | 36 | 2 | 50 | 0 | 0 | 0 | 0 | 16 | 25 |
| SPVC | 13 | 30 | 3 | 27 | 3 | 75 | 0 | 0 | 0 | 0 | 19 | 30 |
| SPVG | 3 | 7 | 3 | 27 | 1 | 25 | 0 | 0 | 0 | 0 | 7 | 11 |
| SPV2 | 19 | 44 | 2 | 18 | 4 | 100 | 2 | 100 | 1 | 33 | 28 | 44 |
| SPLV | 1 | 2 | 1 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 |
| SPVMV | 0 | 0 | 2 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 |
| SPCFV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SPMMV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CMV | 0 | 0 | 1 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| SPLCV | 7 | 16 | 0 | 0 | 1 | 25 | 0 | 0 | 0 | 0 | 8 | 16 |
| SPPV | 20 | 47 | 4 | 36 | 1 | 25 | 2 | 100 | 2 | 67 | 29 | 58 |
| SPSMV-1 | 21 | 49 | 5 | 45 | 0 | 0 | 2 | 100 | 3 | 100 | 31 | 62 |
| TLCV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Virus Species | Period | |||
|---|---|---|---|---|
| Sweet Potato Seedling Stage | Pre Growth Stage | Peak of Growth | Anaphase | |
| SPFMV | 79 | 63 | 44 | 100 |
| SPCSV | 43 | 50 | 0 | 15 |
| SPVC | 36 | 44 | 11 | 23 |
| SPVG | 21 | 6 | 11 | 8 |
| SPV2 | 64 | 88 | 56 | 0 |
| SPLV | 14 | 0 | 0 | 0 |
| SPVMV | 7 | 0 | 11 | 0 |
| CMV | 7 | 0 | 0 | 0 |
| SPLCV | 14 | 14 | 38 | 15 |
| SPPV | 64 | 57 | 50 | 70 |
| SPSMV-1 | 71 | 43 | 75 | 70 |
| Virus Species | Period | |||
|---|---|---|---|---|
| Seedling Stage | Pre Growth Stage | Peak of Growth | Anaphase | |
| SPFMV | 79 | 63 | 44 | 100 |
| SPCSV | 43 | 50 | 0 | 15 |
| SPVD | 27 | 64 | 0 | 15 |
| Variety Name | Virus Name | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SPFMV | SPCSV | SPVC | SPVG | SPV2 | SPLV | SPVMV | CMV | SPLCV | SPPV | SPSMV-1 | |
| LY1 | + | + | + | ||||||||
| LY2 | + | + | + | ||||||||
| SM1 | + | + | + | ||||||||
| SM2 | + | + | |||||||||
| SM3 | + | ||||||||||
| Shenglibaihao | + | ||||||||||
| Jin763 | |||||||||||
| Jin11 | + | ||||||||||
| Jin208 | |||||||||||
| FZ1 | + | + | |||||||||
| Rong910 | + | ||||||||||
| 11-500 | + | ||||||||||
| QZJJC1 | + | + | + | + | |||||||
| QZJJC4 | + | + | + | ||||||||
| FZ2 | + | + | + | ||||||||
| FZ3 | + | + | + | + | |||||||
| Guang08-6 | + | + | + | + | |||||||
| Zhan271 | + | + | + | + | + | ||||||
| Zhan11 | + | + | + | + | + | ||||||
| Guagn79 | + | + | + | + | + | ||||||
| Fu18 | + | + | + | ||||||||
| Quan32 | + | + | + | + | + | ||||||
| Puhang1hao | + | + | + | ||||||||
| Quan12 | + | + | + | + | + | + | |||||
| 13286 | + | + | + | + | + | ||||||
| Mianshu8hao | + | + | + | + | + | + | |||||
| Jin2-1 | + | + | + | + | |||||||
| Guang79-44 | + | + | |||||||||
| Guang87-57 | + | + | + | + | + | ||||||
| Jin3 | + | + | + | + | + | ||||||
| Guagn87-72 | + | + | + | + | + | ||||||
| Jin57 | + | + | + | + | + | + | |||||
| FZ4 | + | + | + | ||||||||
| Pu3hong | + | ||||||||||
| Jin15 | + | + | + | + | + | ||||||
| Longjinshu1hao | + | + | + | + | + | ||||||
| Funingzi3hao | + | + | |||||||||
| Long710 | + | + | + | + | + | ||||||
| Puzi5hao | + | + | |||||||||
| Jin3-97 | + | + | |||||||||
| Jin3-82 | + | + | + | + | + | + | |||||
| Virus Type | Fuzhou | Quanzhou | Putian | Longyan | Sanming | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infection Number | Detection Rate | Infection Number | Detection Rate | Infection Number | Detection Rate | Infection Number | Detection Rate | Infection Number | Detection Rate | Infection Number | Detection Rate | |
| SPFMV | 26 | 60 | 10 | 91 | 2 | 50 | 0 | 0 | 0 | 0 | 38 | 60 |
| SPCSV | 11 | 26 | 4 | 36 | 2 | 50 | 0 | 0 | 0 | 0 | 16 | 25 |
| SPVD | 7 | 21 | 3 | 27 | 2 | 50 | 0 | 0 | 0 | 0 | 12 | 19 |
| Virus Species | Leaf | Tuber | ||||||
|---|---|---|---|---|---|---|---|---|
| Guang79 | Fu18 | Zhan271 | Quan12 | Guang79 | Fu18 | Zhan271 | Quan12 | |
| SPFMV | + | + | + | + | + | + | + | + |
| SPCSV | + | + | ||||||
| SPVC | + | + | + | + | ||||
| SPVG | + | + | + | |||||
| SPV2 | ||||||||
| SPLV | ||||||||
| SPMMV | ||||||||
| CMV | + | |||||||
| SPLCV | + | + | ||||||
| SPPV | + | + | + | |||||
| SPSMV-1 | + | + | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, W.; Chen, S.-P.; Yang, Z.; Chen, X. Key Sweet Potato Viruses in Fujian Province and Their Distribution, Harmfulness, and Implications in China. Curr. Issues Mol. Biol. 2025, 47, 242. https://doi.org/10.3390/cimb47040242
Zou W, Chen S-P, Yang Z, Chen X. Key Sweet Potato Viruses in Fujian Province and Their Distribution, Harmfulness, and Implications in China. Current Issues in Molecular Biology. 2025; 47(4):242. https://doi.org/10.3390/cimb47040242
Chicago/Turabian StyleZou, Weikun, Shi-Peng Chen, Zhijian Yang, and Xuanyang Chen. 2025. "Key Sweet Potato Viruses in Fujian Province and Their Distribution, Harmfulness, and Implications in China" Current Issues in Molecular Biology 47, no. 4: 242. https://doi.org/10.3390/cimb47040242
APA StyleZou, W., Chen, S.-P., Yang, Z., & Chen, X. (2025). Key Sweet Potato Viruses in Fujian Province and Their Distribution, Harmfulness, and Implications in China. Current Issues in Molecular Biology, 47(4), 242. https://doi.org/10.3390/cimb47040242







