Exploring Migraine Pathogenesis: Transcriptomic Insights and Pathway Analysis in Nitroglycerin-Induced Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Migraine Model Animals
2.3. Preparation of Tissues
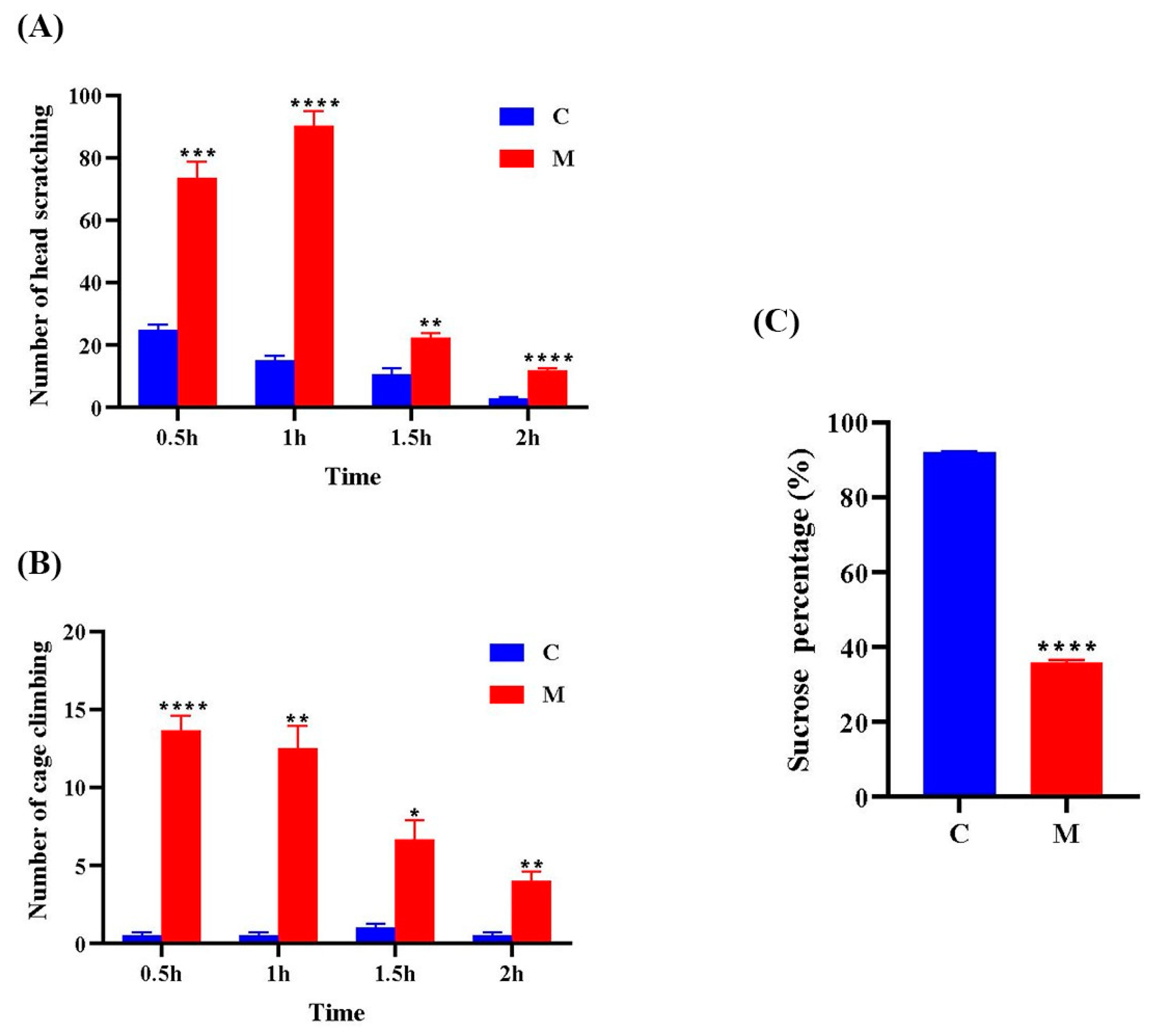
2.4. Behavioral Experiments
2.5. Sucrose Preference Test
2.6. Transcriptome Sequencing Analysis
2.6.1. RNA Library Construction and Sequencing
2.6.2. Data Quality Control
2.6.3. Differentially Expressed Genes and Enrichment Analysis
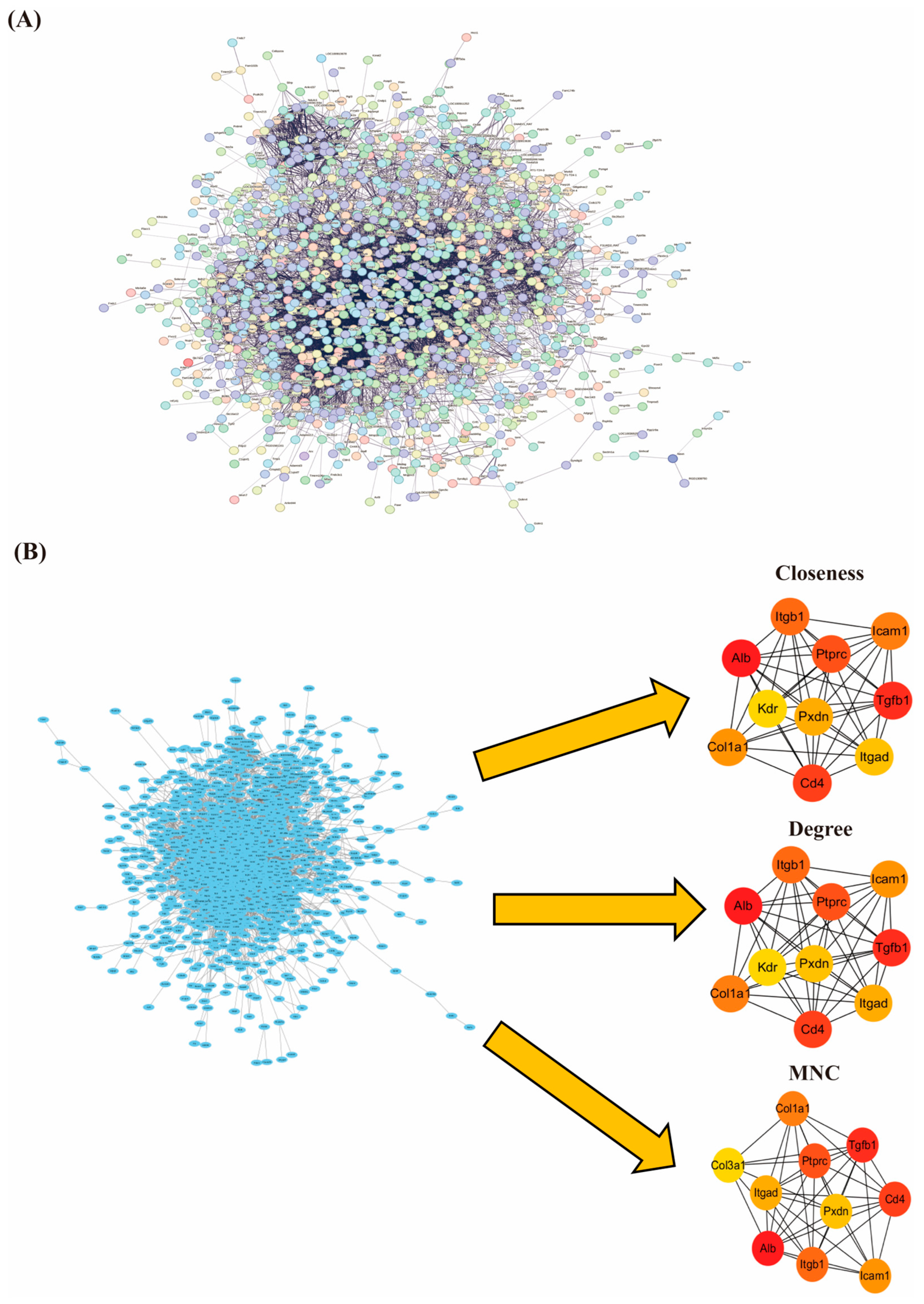
2.6.4. Construction of Protein Interaction Networks and Screening Hub Genes
2.7. Statistical Methods
3. Results
3.1. Behavioral Observations
3.2. Raw Data Quality Assessment
3.3. Analysis of Transcriptional Patterns in the Migraine Model Group
3.4. NTG Treatment Activates the PI3K–Akt Signaling Pathway in Migraine Rats
3.5. NTG Treatment Activates Cytokine–Cytokine Receptor Interaction Signaling Pathway in Migraine Rats
3.6. PPI Network Construction and Screening of Hub Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia Int. J. Headache 2013, 33, 629–808. [Google Scholar]
- Fan, X.-X.; Ye, L.; Yang, Y.-H.; Huang, W.-J.; Ko, C.-Y. Migraine Duration as a Potential Amplifier of Obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2024, 17, 1025–1037. [Google Scholar]
- Krause, D.N.; Warfvinge, K.; Haanes, K.A.; Edvinsson, L. Hormonal influences in migraine-interactions of oestrogen, oxytocin and CGRP. Nat. Rev. Neurol. 2021, 17, 621–633. [Google Scholar]
- Steiner, T.J.; Stovner, L.J.; Jensen, R.; Uluduz, D.; Katsarava, Z. Lifting The Burden: The Global Campaign Against Headache. Migraine remains second among the world’s causes of disability, and first among young women: Findings from GBD2019. J. Headache Pain 2020, 21, 137. [Google Scholar] [PubMed]
- Yu, S.-Y.; Cao, X.-T.; Zhao, G.; Yang, X.-S.; Qiao, X.-Y.; Fang, Y.-N.; Feng, J.-C.; Liu, R.-Z.; Steiner, T.J. The burden of headache in China: Validation of diagnostic questionnaire for a population-based survey. J. Headache Pain 2011, 12, 141–146. [Google Scholar]
- Karsan, N.; Goadsby, P.J. Biological Insights from the Premonitory Symptoms of Migraine. Nat. Rev. Neurol. 2018, 14, 699–710. [Google Scholar]
- Denuelle, M.; Fabre, N.; Payoux, P.; Chollet, F.; Geraud, G. Hypothalamic Activation in Spontaneous Migraine Attacks. Headache 2007, 47, 1418–1426. [Google Scholar] [CrossRef]
- Meylakh, N.; Marciszewski, K.K.; Di Pietro, F.; Macefield, V.G.; Macey, P.M.; Henderson, L.A. Altered Regional Cerebral Blood Flow and Hypothalamic Connectivity Immediately Prior to a Migraine Headache. Cephalalgia 2020, 40, 448–460. [Google Scholar]
- Stankewitz, A.; Keidel, L.; Rehm, M.; Irving, S.; Kaczmarz, S.; Preibisch, C.; Witkovsky, V.; Zimmer, C.; Schulz, E.; Toelle, T.R. Migraine Attacks as a Result of Hypothalamic Loss of Control. NeuroImage Clin. 2021, 32, 102784. [Google Scholar] [CrossRef]
- Pavlovic, J.M.; Buse, D.C.; Sollars, C.M.; Haut, S.; Lipton, R.B. Trigger Factors and Premonitory Features of Migraine Attacks: Summary of Studies. Headache 2014, 54, 1670–1679. [Google Scholar]
- Zhang, L.; Yu, W.; Xu, M.; Cui, F.; Song, W.; Yan, M.; Cao, Z.; Zhang, Z. The hypothalamus may mediate migraine and ictal photophobia: Evidence from Granger causality analysis. Neurol. Sci. 2022, 43, 6021–6030. [Google Scholar]
- Zhang, X.-F.; Zhang, W.-J.; Dong, C.-L.; Hu, W.-L.; Sun, Y.-Y.; Bao, Y.; Zhang, C.-F.; Guo, C.-R.; Wang, C.-Z.; Yuan, C.-S. Analgesia Effect of Baicalein against NTG-Induced Migraine in Rats. Biomed. Pharmacother. 2017, 90, 116–121. [Google Scholar] [PubMed]
- Demartini, C.; Greco, R.; Zanaboni, A.M.; Sances, G.; De Icco, R.; Borsook, D.; Tassorelli, C. Nitroglycerin as a Comparative Experimental Model of Migraine Pain: From Animal to Human and Back. Prog. Neurobiol. 2019, 177, 15–32. [Google Scholar]
- Akerman, S.; Karsan, N.; Bose, P.; Hoffmann, J.R.; Holland, P.R.; Romero-Reyes, M.; Goadsby, P.J. Nitroglycerine Triggers Triptan-Responsive Cranial Allodynia and Trigeminal Neuronal Hypersensitivity. Brain 2019, 142, 103–119. [Google Scholar] [PubMed]
- Pedersen, S.H.; Ramachandran, R.; Amrutkar, D.V.; Petersen, S.; Olesen, J.; Jansen-Olesen, I. Mechanisms of Glyceryl Trinitrate Provoked Mast Cell Degranulation. Cephalalgia 2015, 35, 1287–1297. [Google Scholar] [PubMed]
- Conti, P.; D’Ovidio, C.; Conti, C.; Gallenga, C.E.; Lauritano, D.; Caraffa, A.; Kritas, S.K.; Ronconi, G. Progression in migraine: Role of mast cells and pro-inflammatory and anti-inflammatory cytokines. Eur. J. Pharmacol. 2019, 844, 87–94. [Google Scholar]
- Thangameeran, S.I.M.; Tsai, S.-T.; Liew, H.-K.; Pang, C.-Y. Examining Transcriptomic Alterations in Rat Models of Intracerebral Hemorrhage and Severe Intracerebral Hemorrhage. Biomolecules 2024, 14, 678. [Google Scholar]
- Ye, J.; Huang, F.; Zeng, H.; Xu, X.; Wu, G.; Tian, S.; Zhao, J.; Zhang, W. Multi-Omics and Network Pharmacology Study Reveals the Effects of Dengzhan Shengmai Capsule against Neuroinflammatory Injury and Thrombosis Induced by Ischemic Stroke. J. Ethnopharmacol. 2023, 305, 116092. [Google Scholar]
- Chen, H.; Tang, X.; Li, J.; Hu, B.; Yang, W.; Zhan, M.; Ma, T.; Xu, S. IL-17 Crosses the Blood-Brain Barrier to Trigger Neuroinflammation: A Novel Mechanism in Nitroglycerin-Induced Chronic Migraine. J. Headache Pain 2022, 23, 1. [Google Scholar] [CrossRef]
- Feng, S.; Shu, X.; Zhang, H. Effects of Jieyu Zhitong decoction on behavior and brain-derived neurotrophic factor expression in cerebral tissue of rats with migraine and depression. J. Mod. Integr. Chin. West. Med. 2022, 31, 1786–1791+1796. [Google Scholar]
- Yao, G.; Huang, Q.; Wang, M.; Yang, C.-L.; Liu, C.-F.; Yu, T.-M. Behavioral Study of a Rat Model of Migraine Induced by CGRP. Neurosci. Lett. 2017, 651, 134–139. [Google Scholar] [PubMed]
- Motaghinejad, M.; Motevalian, M.; Shabab, B. Neuroprotective effects of various doses of topiramate against methylphenidate induced oxidative stress and inflammation in rat isolated hippocampus. Clin. Exp. Pharmacol. Physiol. 2016, 43, 360–371. [Google Scholar]
- Liu, W.; Lv, Y.; Ren, F. PI3K/Akt Pathway Is Required for Spinal Central Sensitization in Neuropathic Pain. Cell Mol. Neurobiol. 2018, 38, 747–755. [Google Scholar]
- Han, X.; Cheng, X.; Xu, J.; Liu, Y.; Zhou, J.; Jiang, L.; Gu, X.; Xia, T. Activation of TREM2 Attenuates Neuroinflammation via PI3K/Akt Signaling Pathway to Improve Postoperative Cognitive Dysfunction in Mice. Neuropharmacology 2022, 219, 109231. [Google Scholar] [PubMed]
- Cianciulli, A.; Porro, C.; Calvello, R.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Microglia Mediated Neuroinflammation: Focus on PI3K Modulation. Biomolecules 2020, 10, 137. [Google Scholar] [CrossRef]
- Hou, J.; Wang, L.; Ni, L.; Hou, B.; Wang, K.; Zhu, N.; Yang, H. Chuanxiong Qingnao Granules (CQG) Alleviates Nitroglycerin-Induced Migraine-like Pain in Rats by Glycerophospholipid Metabolism and PI3K/Akt Signaling Pathway. Phytomedicine 2025, 136, 156336. [Google Scholar] [PubMed]
- Zhu, X.; Liu, S.; Tian, L.; Li, X.; Yao, R.; Zhao, Y.; Gao, Z.; Liu, X.-R.; Liu, X.-Q.; Huo, F.-Q.; et al. Spinal Interleukin-16 Mediates Inflammatory Pain via Promoting Glial Activation. Int. Immunopharmacol. 2024, 127, 111411. [Google Scholar]
- Dai, C.; Basilico, P.; Cremona, T.P.; Collins, P.; Moser, B.; Benarafa, C.; Wolf, M. CXCL14 Displays Antimicrobial Activity against Respiratory Tract Bacteria and Contributes to Clearance of Streptococcus Pneumoniae Pulmonary Infection. J. Immunol. 2015, 194, 5980–5989. [Google Scholar]
- Saleem, M.; Martin, H.; Coates, P. Prolactin Biology and Laboratory Measurement: An Update on Physiology and Current Analytical Issues. Clin. Biochem. Rev. 2018, 39, 3–16. [Google Scholar]
- Chen, Y.; Navratilova, E.; Dodick, D.W.; Porreca, F. An Emerging Role for Prolactin in Female-Selective Pain. Trends Neurosci. 2020, 43, 635–648. [Google Scholar]
- Noori-Zadeh, A.; Karamkhani, M.; Seidkhani-Nahal, A.; Khosravi, A.; Darabi, S. Evidence for Hyperprolactinemia in Migraineurs: A Systematic Review and Meta-Analysis. Neurol. Sci. 2020, 41, 91–99. [Google Scholar] [PubMed]
- Masoud, S.A.; Fakharian, E. Serum Prolactin and Migraine. Ann. Saudi Med. 2005, 25, 489–491. [Google Scholar]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The Antioxidant Properties of Serum Albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [PubMed]
- Yang, Z.; Xu, P.; Geng, C.; Zhang, H. Evaluation of Simple Antioxidant Blood Parameters in Patients with Migraine. Front. Neurol. 2022, 13, 939363. [Google Scholar]
- Moisset, X.; Giraud, P.; Dallel, R. Migraine in Multiple Sclerosis and Other Chronic Inflammatory Diseases. Rev. Neurol. 2021, 177, 816–820. [Google Scholar]
- Badry, R.; Gamal, R.M. Different Types of Headache in Patients with Systemic Lupus Erythematosus. Int. J. Neurosci. 2015, 125, 357–360. [Google Scholar]
- Sutherland, H.G.; Albury, C.L.; Griffiths, L.R. Advances in Genetics of Migraine. J. Headache Pain 2019, 20, 72. [Google Scholar] [PubMed]
- Sanjabi, S.; Zenewicz, L.A.; Kamanaka, M.; Flavell, R.A. Anti-Inflammatory and pro-Inflammatory Roles of TGF-β, IL-10, and IL-22 in Immunity and Autoimmunity. Curr. Opin. Pharmacol. 2009, 9, 447–453. [Google Scholar]
- Bø, S.; Davidsen, E.; Gulbrandsen, P.; Dietrichs, E.; Bovim, G.; Stovner, L.; White, L. Cerebrospinal Fluid Cytokine Levels in Migraine, Tension-Type Headache and Cervicogenic Headache. Cephalalgia 2009, 29, 365–372. [Google Scholar]
- Güzel, I.; Taşdemir, N.; Çelik, Y. Evaluation of Serum Transforming Growth Factor Β1 and C-Reactive Protein Levels in Migraine Patients. Neurol. I Neurochir. Pol. 2013, 47, 357–362. [Google Scholar]
- Ishizaki, K.; Takeshima, T.; Fukuhara, Y.; Araki, H.; Nakaso, K.; Kusumi, M.; Nakashima, K. Increased Plasma Transforming Growth Factor-Beta1 in Migraine. Headache 2005, 45, 1224–1228. [Google Scholar] [CrossRef]
- Long, E.O. ICAM-1: Getting a Grip on Leukocyte Adhesion. J. Immunol. 2011, 186, 5021–5023. [Google Scholar] [CrossRef]
- Wang, F.; He, Q.; Ren, Z.; Li, F.; Chen, W.; Lin, X.; Zhang, H.; Tai, G. Association of Serum Levels of Intercellular Adhesion Molecule-1 and Interleukin-6 with Migraine. Neurol. Sci. 2015, 36, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Alberti, A.; Baldi, A.; Coppola, F.; Rossi, C.; Pierguidi, L.; Floridi, A.; Calabresi, P. Proinflammatory Cytokines, Adhesion Molecules, and Lymphocyte Integrin Expression in the Internal Jugular Blood of Migraine Patients Without Aura Assessed Ictally. Headache 2006, 46, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.; Lu, Y.-C.W. Deciphering CD4+ T Cell-Mediated Responses against Cancer. Mol. Carcinog. 2024, 63, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Fu, J.; Dong, X.; Chen, B.; Hong, H.; Cui, Z. Identification of Hub Genes in the Subacute Spinal Cord Injury in Rats. BMC Neurosci. 2022, 23, 51. [Google Scholar] [CrossRef]
- Wang, L.; Song, D.; Wei, C.; Chen, C.; Yang, Y.; Deng, X.; Gu, J. Telocytes Inhibited Inflammatory Factor Expression and Enhanced Cell Migration in LPS-Induced Skin Wound Healing Models in Vitro and in Vivo. J. Transl. Med. 2020, 18, 60. [Google Scholar] [CrossRef]
- Buechler, M.B.; Fu, W.; Turley, S.J. Fibroblast-Macrophage Reciprocal Interactions in Health, Fibrosis, and Cancer. Immunity 2021, 54, 903–915. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Kan, C.; Chen, B.; Qu, N.; Hou, N.; Liu, Y.; Han, F. COL1A1: A Novel Oncogenic Gene and Therapeutic Target in Malignancies. Pathol. Res. Pract. 2022, 236, 154013. [Google Scholar] [CrossRef]
- Papageorgiou, A.-P.; Heymans, S. Peroxidasin-like Protein: Expanding the Horizons of Matrix Biology. Cardiovasc. Res. 2014, 101, 342–343. [Google Scholar] [CrossRef]
- Liu, S.; Xu, X.; Omari-Siaw, E.; Yu, J.; Deng, W. Progress of Reprogramming Astrocytes into Neuron. Mol. Cell. Neurosci. 2024, 130, 103947. [Google Scholar] [PubMed]




| Sample | Clean Reads | Total Reads | Clean Bases | GC Content | ≥Q30 | Mapped Reads |
|---|---|---|---|---|---|---|
| C1 | 20,220,350 | 40,440,700 | 6,054,753,132 | 46.13% | 97.87% | 97.41% |
| C2 | 19,291,371 | 38,582,742 | 5,777,712,820 | 46.96% | 98.35% | 97.93% |
| C3 | 20,526,329 | 41,052,658 | 6,146,641,140 | 46.59% | 97.97% | 97.58% |
| C4 | 28,648,350 | 57,296,700 | 8,579,723,824 | 46.75% | 98.37% | 97.82% |
| C5 | 18,317,506 | 36,635,012 | 5,486,122,250 | 46.80% | 98.36% | 97.90% |
| C6 | 27,359,047 | 54,718,094 | 8,194,954,072 | 46.90% | 98.16% | 98.10% |
| M1 | 21,243,334 | 42,486,668 | 6,360,036,630 | 47.39% | 97.86% | 97.37% |
| M2 | 20,496,560 | 40,993,120 | 6,138,110,930 | 46.36% | 98.69% | 97.83% |
| M3 | 26,050,892 | 52,101,784 | 7,802,078,532 | 47.00% | 98.41% | 97.76% |
| M4 | 18,567,057 | 37,134,114 | 5,560,463,154 | 46.99% | 98.27% | 97.90% |
| M5 | 20,497,889 | 40,995,778 | 6,136,901,752 | 46.64% | 97.87% | 97.48% |
| M6 | 20,334,317 | 40,668,634 | 6,086,114,090 | 47.56% | 99.12% | 97.29% |
| Gene | Encoded Protein | Function | Clinical/Study Findings | log2FoldChange |
|---|---|---|---|---|
| Alb | Serum albumin | Effective removal of reactive oxygen species and active nitrogen. | Migraine patients, especially females, exhibit significantly reduced serum albumin levels compared to the general population [33]. | −0.6639 |
| Tgfb1 | Transforming growth factor-β1 protein | Multifunctional cytokines are closely related to immune regulation and inflammatory responses. | TGF-β1 levels are significantly elevated in the serum and cerebrospinal fluid of individuals with migraine [39,40], and remain elevated in platelets even during headache-free intervals [41]. | 0.744684 |
| Icam-1 | Intercellular adhesion molecule-1 (ICAM1/CD54) | Mediates the migration and infiltration of white blood cells at the site of inflammation. | Soluble ICAM1 levels in internal jugular vein blood are transiently elevated within two hours following a migraine attack [44]. | 1.282695 |
| CD4 | CD4 glycoprotein | Involved in immune response and inflammation regulation. | No evidence currently links these genes to migraine. | 1.613812 |
| Ptprc | CD45 glycoprotein | Involved in T cell antigen receptor signal transduction. | No evidence currently links these genes to migraine. | 1.176863 |
| Itgb1 | Integrin β1 | Involved in extracellular matrix-cell receptor interactions. | No evidence currently links these genes to migraine. | 0.613643 |
| Itgad | Integrin subunit CD11d | Involved in immune responses such as leukocyte adhesion and migration. | No evidence currently links these genes to migraine. | 1.604032 |
| Col1a1 | α1 chain of type I collagen | Involved in cell proliferation and metastasis. | No evidence currently links these genes to migraine. | 2.617393 |
| Pxdn | peroxidase | Antioxidant enzyme that catalyzes the oxidation of a wide range of substrates. | No evidence currently links these genes to migraine. | 0.638802 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.-W.; Meng, R.-T.; Ko, C.-Y. Exploring Migraine Pathogenesis: Transcriptomic Insights and Pathway Analysis in Nitroglycerin-Induced Rat Model. Curr. Issues Mol. Biol. 2025, 47, 241. https://doi.org/10.3390/cimb47040241
Chen Q-W, Meng R-T, Ko C-Y. Exploring Migraine Pathogenesis: Transcriptomic Insights and Pathway Analysis in Nitroglycerin-Induced Rat Model. Current Issues in Molecular Biology. 2025; 47(4):241. https://doi.org/10.3390/cimb47040241
Chicago/Turabian StyleChen, Qiao-Wen, Run-Tian Meng, and Chih-Yuan Ko. 2025. "Exploring Migraine Pathogenesis: Transcriptomic Insights and Pathway Analysis in Nitroglycerin-Induced Rat Model" Current Issues in Molecular Biology 47, no. 4: 241. https://doi.org/10.3390/cimb47040241
APA StyleChen, Q.-W., Meng, R.-T., & Ko, C.-Y. (2025). Exploring Migraine Pathogenesis: Transcriptomic Insights and Pathway Analysis in Nitroglycerin-Induced Rat Model. Current Issues in Molecular Biology, 47(4), 241. https://doi.org/10.3390/cimb47040241







