The Therapeutic Potential of Baicalin and Baicalein in Breast Cancer: A Systematic Review of Mechanisms and Efficacy
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Bibliometric Analysis of Publications on Baicalin and Baicalein
3.2. Characteristics of Included Studies
3.3. The Use of Baicalin in the Treatment of Breast Cancer
3.4. Studies Investigating Baicalein as an Anticancer Agent
3.5. Anticancer Effects of Plant Extracts Containing Baicalein and Baicalin in Breast Cancer Models
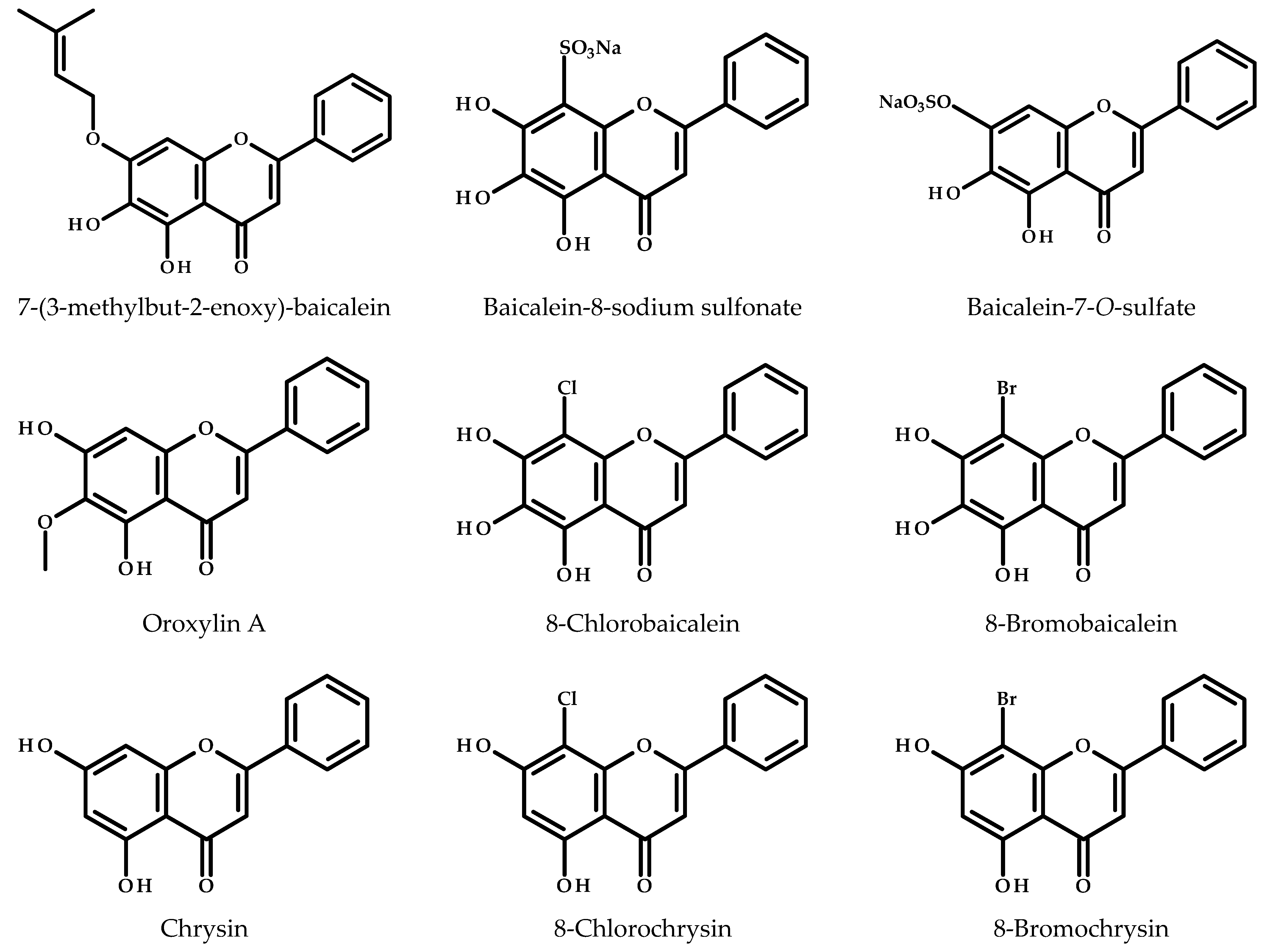
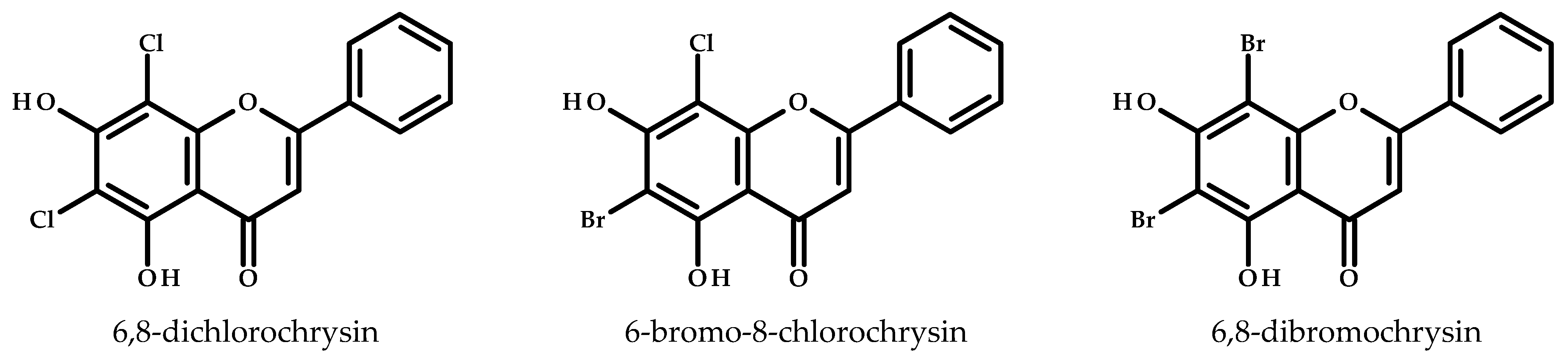
3.6. Derivatives of Baicalein in Anticancer Studies Against Breast Cancer
3.7. Synergistic Effects of Baicalin/Baicalein in Combination Therapies for Breast Cancer
3.8. Nanotechnology-Based Delivery Systems for Baicalin/Baicalein in Breast Cancer Research
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4T1 | Mouse mammary carcinoma cell line |
| AKT | Protein Kinase B |
| ALOX12/15 | Arachidonate 12/15-Lipoxygenase |
| AR | Androgen Receptor |
| AuNPs | Gold Nanoparticles |
| Bax | Bcl-2-Associated X Protein |
| BBB | Blood-Brain Barrier |
| Bcl-2 | B-Cell Lymphoma 2 |
| BCT | Breast-Conserving Therapy |
| CCID | Cell Cycle Inhibitory Dose |
| CDK4/6 | Cyclin-Dependent Kinase 4/6 |
| COX-2 | Cyclooxygenase-2 |
| CSC | Cancer Stem Cells |
| CYP1 | Cytochrome P450 Family 1 |
| CYP1B1 | Cytochrome P450 Family 1 Subfamily B Member 1 |
| DDIT4 | DNA Damage-Inducible Transcript 4 |
| Drp1 | Dynamin-Related Protein 1 |
| E2 | Estradiol |
| EGCG | Epigallocatechin Gallate |
| EMT | Epithelial-Mesenchymal Transition |
| ER | Estrogen Receptor |
| ERK | Extracellular Signal-Regulated Kinase |
| ERα | Estrogen Receptor Alpha |
| FAK | Focal Adhesion Kinase |
| GI Absorption | Gastrointestinal Absorption |
| GLOBOCAN | Global Cancer Observatory |
| GPR30 | G Protein-Coupled Estrogen Receptor 30 |
| H-Bond | Hydrogen Bond |
| HCT-116 | Human Colon Cancer Cell Line |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HIF-1α | Hypoxia-Inducible Factor 1-Alpha |
| HR+ | Hormone Receptor-Positive |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| IC50 | Half-Maximal Inhibitory Concentration |
| IFIT2 | Interferon-Induced Protein with Tetratricopeptide Repeats 2 |
| IGF-I | Insulin-Like Growth Factor 1 |
| IL-10 | Interleukin 10 |
| IL-6 | Interleukin 6 |
| IORT | Intraoperative Radiotherapy |
| JNK | c-Jun N-Terminal Kinase |
| LDH | Lactate Dehydrogenase |
| Log Kp | Logarithm of Skin Permeation Coefficient |
| LogPo/w | Logarithm of Octanol-Water Partition Coefficient (lipophilicity measure) |
| LogS | Logarithm of Aqueous Solubility |
| LOX-12 | Lipoxygenase-12 |
| MAPK | Mitogen-Activated Protein Kinase |
| MCF-10A | Non-Tumorigenic Breast Epithelial Cell Line |
| MDA-MB-231 | Human Triple-Negative Breast Cancer Cell Line |
| MDA-MB-468 | Human Breast Cancer Cell Line |
| miRNA | MicroRNA |
| MMP | Matrix Metalloproteinase |
| MMTV-PyMT | Mouse Mammary Tumor Virus-Polyoma Middle T Antigen |
| MOFs | Metal-Organic Frameworks |
| mTOR | Mammalian Target of Rapamycin |
| NF-κB | Nuclear Factor Kappa B |
| NLC | Nanostructured Lipid Carrier |
| NOD/SCID | Non-Obese Diabetic/Severe Combined Immunodeficiency |
| P-gp | P-glycoprotein (efflux transporter) |
| PARP | Poly (ADP-Ribose) Polymerase |
| PD-L1 | Programmed Death-Ligand 1 |
| PI3K | Phosphoinositide 3-Kinase |
| PIM-1 | Proto-oncogene serine/threonine-protein kinase |
| PLGA | Polylactic-co-Glycolic Acid |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROCR | Protein C Receptor |
| ROS | Reactive Oxygen Species |
| SATB1 | Special AT-Rich Sequence-Binding Protein 1 |
| SGNI | Shuganning Injection |
| SREBP1 | Sterol Regulatory Element-Binding Protein 1 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| TNBC | Triple-Negative Breast Cancer |
| TNF-α | Tumor Necrosis Factor Alpha |
| TPSA | Topological Polar Surface Area |
| u-PA | Urokinase-Type Plasminogen Activator |
| VEGF | Vascular Endothelial Growth Factor |
| ZEB1 | Zinc Finger E-Box Binding Homeobox 1 |
| ZIF-8 | Zeolitic Imidazolate Framework 8 |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Chen, H.-D.; Yu, Y.-W.; Li, N.; Chen, W.-Q. Changing Profiles of Cancer Burden Worldwide and in China: A Secondary Analysis of the Global Cancer Statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zuo, Z.; Wang, X.; Sun, Y.; Xu, D.; Liu, G.; Tong, Y.; Zhang, Z. Epidemiology, Risk Factors and Mechanism of Breast Cancer and Atrial Fibrillation. CardioOncology 2024, 10, 92. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288. [Google Scholar] [CrossRef]
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent Advances in the Treatment of Breast Cancer. Front. Oncol. 2018, 8, 227. [Google Scholar] [CrossRef]
- Murawa, P.; Murawa, D.; Adamczyk, B.; Połom, K. Breast Cancer: Actual Methods of Treatment and Future Trends. Rep. Pract. Oncol. Radiother. 2014, 19, 165–172. [Google Scholar] [CrossRef]
- da Silva Fernandes, T.; Gillard, B.M.; Dai, T.; Martin, J.C.; Chaudhry, K.A.; Dugas, S.M.; Fisher, A.A.; Sharma, P.; Wu, R.; Attwood, K.M.; et al. Inosine Monophosphate Dehydrogenase 2 (IMPDH2) Modulates Response to Therapy and Chemo-Resistance in Triple Negative Breast Cancer. Sci. Rep. 2025, 15, 1061. [Google Scholar] [CrossRef]
- Murphy, C.G.; Dickler, M.N. Endocrine Resistance in Hormone-Responsive Breast Cancer: Mechanisms and Therapeutic Strategies. Endocr. Relat. Cancer 2016, 23, R337–R352. [Google Scholar] [CrossRef]
- Anamika, F.; Chitkara, A.; Saharan, K.; Choudhary, T.; Soni, U.; Harmon, G.A.; Saeed, A. Trastuzumab-Induced Cardiotoxicity in Breast Cancer Patients: A Meta-Analysis and Review of the Literature (2012–2022). J. Clin. Oncol. 2023, 41, e24101. [Google Scholar] [CrossRef]
- Jabagi, M.J.; Goncalves, A.; Vey, N.; Le Tri, T.; Zureik, M.; Dray-Spira, R. Risk of Hematologic Malignant Neoplasms after Postoperative Treatment of Breast Cancer. Cancers 2019, 11, 1463. [Google Scholar] [CrossRef]
- Sharma, E.; Attri, D.C.; Sati, P.; Dhyani, P.; Szopa, A.; Sharifi-Rad, J.; Hano, C.; Calina, D.; Cho, W.C. Recent Updates on Anticancer Mechanisms of Polyphenols. Front. Cell Dev. Biol. 2022, 10, 1005910. [Google Scholar] [CrossRef] [PubMed]
- Asensi, M.; Ortega, A.; Mena, S.; Feddi, F.; Estrela, J.M. Natural Polyphenols in Cancer Therapy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 197–216. [Google Scholar] [CrossRef]
- Sauter, E.R. Cancer Prevention and Treatment Using Combination Therapy with Natural Compounds. Expert Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-W.; Hu, J.-J.; Fu, R.-Q.; Liu, X.; Zhang, Y.-H.; Li, J.; Liu, L.; Li, Y.-N.; Deng, Q.; Luo, Q.-S.; et al. Flavonoids Inhibit Cell Proliferation and Induce Apoptosis and Autophagy through Downregulation of PI3Kγ Mediated PI3K/AKT/mTOR/p70S6K/ULK Signaling Pathway in Human Breast Cancer Cells. Sci. Rep. 2018, 8, 11255. [Google Scholar] [CrossRef] [PubMed]
- Kursvietiene, L.; Kopustinskiene, D.M.; Staneviciene, I.; Mongirdiene, A.; Kubová, K.; Masteikova, R.; Bernatoniene, J. Anti-Cancer Properties of Resveratrol: A Focus on Its Impact on Mitochondrial Functions. Antioxidants 2023, 12, 2056. [Google Scholar] [CrossRef]
- Li-Weber, M. New Therapeutic Aspects of Flavones: The Anticancer Properties of Scutellaria and Its Main Active Constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, X.-Y.; Martin, C. Scutellaria baicalensis, the Golden Herb from the Garden of Chinese Medicinal Plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef]
- Parajuli, P.; Joshee, N.; Rimando, A.; Mittal, S.; Yadav, A. In Vitro Antitumor Mechanisms of Various Scutellaria Extracts and Constituent Flavonoids. Planta Med. 2009, 75, 41–48. [Google Scholar] [CrossRef]
- Guha, G.; Rajkumar, V.; Ashok Kumar, R.; Mathew, L. Aqueous Extract of Phyllanthus amarus Inhibits Chromium(VI)-Induced Toxicity in MDA-MB-435S Cells. Food Chem. Toxicol. 2010, 48, 396–401. [Google Scholar] [CrossRef]
- Beara, I.N.; Lesjak, M.M.; Cetojević-Simin, D.D.; Marjanović, Z.S.; Ristić, J.D.; Mrkonjić, Z.O.; Mimica-Dukić, N.M. Phenolic Profile, Antioxidant, Anti-Inflammatory and Cytotoxic Activities of Black (Tuber aestivum Vittad.) and White (Tuber magnatum Pico) Truffles. Food Chem. 2014, 165, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Hasibuan, P.A.Z.; Harahap, U.; Sitorus, P.; Satria, D. The Anticancer Activities of Vernonia amygdalina Delile. Leaves on 4T1 Breast Cancer Cells through Phosphoinositide 3-Kinase (PI3K) Pathway. Heliyon 2020, 6, e04449. [Google Scholar] [CrossRef] [PubMed]
- Al Kury, L.T.; Taha, Z.; Mahmod, A.I.; Talib, W.H. Xanthium spinosum L. Extracts Inhibit Breast Cancer in Mice by Apoptosis Induction and Immune System Modulation. Pharmaceuticals 2022, 15, 1504. [Google Scholar] [CrossRef]
- Yırtıcı, Ü.; Ergene, A.; Adem, Ş.; Atalar, M.N.; Eyüpoğlu, V.; Rawat, R.; Arat, E.; Hamzaoğlu, E. Centaurea mersinensis Phytochemical Composition and Multi-Dimensional Bioactivity Properties Supported by Molecular Modeling. J. Biomol. Struct. Dyn. 2024, 42, 2341–2357. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Y.; Zhao, C.; Zhao, B.; Wang, J. The Pharmacological Efficacy of Baicalin in Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 9317. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Yang, C.-X.; Zhang, L.; Yang, C.-Y.; Xu, X.-Q. Baicalein, as a Prooxidant, Triggers Mitochondrial Apoptosis in MCF-7 Human Breast Cancer Cells through Mobilization of Intracellular Copper and Reactive Oxygen Species Generation. Onco. Targets. Ther. 2019, 12, 10749–10761. [Google Scholar] [CrossRef]
- Hu, Z.; Guan, Y.; Hu, W.; Xu, Z.; Ishfaq, M. An Overview of Pharmacological Activities of Baicalin and Its Aglycone Baicalein: New Insights into Molecular Mechanisms and Signaling Pathways. Iran. J. Basic Med. Sci. 2022, 25, 14–26. [Google Scholar] [CrossRef]
- Morshed, A.K.M.H.; Paul, S.; Hossain, A.; Basak, T.; Hossain, M.S.; Hasan, M.M.; Hasibuzzaman, M.A.; Rahaman, T.I.; Mia, M.A.R.; Shing, P.; et al. Baicalein as Promising Anticancer Agent: A Comprehensive Analysis on Molecular Mechanisms and Therapeutic Perspectives. Cancers 2023, 15, 2128. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, M.; Dinda, S.; De, U.C. An Overview of Anti-SARS-CoV-2 and Anti-Inflammatory Potential of Baicalein and Its Metabolite Baicalin: Insights into Molecular Mechanisms. Eur. J. Med. Chem. 2023, 258, 115629. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of Therapeutic Targets for SARS-CoV-2 and Discovery of Potential Drugs by Computational Methods. Acta Pharm. Sin. B. 2020, 10, 766–788. [Google Scholar] [CrossRef] [PubMed]
- Formica, J.V.; Regelson, W. Review of the Biology of Quercetin and Related Bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Ikezoe, T.; Chen, S.S.; Heber, D.; Taguchi, H.; Koeffler, H.P. Baicalin Is a Major Component of PC-SPES Which Inhibits the Proliferation of Human Cancer Cells via Apoptosis and Cell Cycle Arrest. Prostate 2001, 49, 285–292. [Google Scholar] [CrossRef]
- Wang, N.; Tang, L.-J.; Zhu, G.-Q.; Peng, D.-Y.; Wang, L.; Sun, F.-N.; Li, Q.-L. Apoptosis Induced by Baicalin Involving Up-Regulation of P53 and Bax in MCF-7 Cells. J. Asian Nat. Prod. Res. 2008, 10, 1129–1135. [Google Scholar] [CrossRef]
- Chung, H.; Choi, H.S.; Seo, E.-K.; Kang, D.-H.; Oh, E.-S. Baicalin and Baicalein Inhibit Transforming Growth Factor-Β1-Mediated Epithelial-Mesenchymal Transition in Human Breast Epithelial Cells. Biochem. Biophys. Res. Commun. 2015, 458, 707–713. [Google Scholar] [CrossRef]
- Gao, Y.; Ni, Q.; Chen, H.; Wei, N.; Jia, Q. Up-Regulation of miR-200 b by Baicalin to Inhibit Migration of MCF-7 Breast Cancer Cells. Int. J. Clin. Exp. Med. 2017, 10, 2360–2366. [Google Scholar]
- Zhou, T.; Zhang, A.; Kuang, G.; Gong, X.; Jiang, R.; Lin, D.; Li, J.; Li, H.; Zhang, X.; Wan, J.; et al. Baicalin Inhibits the Metastasis of Highly Aggressive Breast Cancer Cells by Reversing Epithelial-to-Mesenchymal Transition by Targeting β-Catenin Signaling. Oncol. Rep. 2017, 38, 3599–3607. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, H.; Wang, H.; Hu, H.; He, H.; Gu, N.; Han, X.; Guo, Q.; Liu, D.; Cui, S.; et al. Baicalin Inhibits Breast Cancer Development via Inhibiting IĸB Kinase Activation in Vitro and in Vivo. Int. J. Oncol. 2018, 53, 2727–2736. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zeng, L.; Ge, A.; Chen, Z.; Bao, T.; Long, Z.; Ge, J.; Huang, L. Investigating the Regulation Mechanism of Baicalin on Triple Negative Breast Cancer’s Biological Network by a Systematic Biological Strategy. Biomed. Pharmacother. 2019, 118, 109253. [Google Scholar] [CrossRef]
- Duan, X.; Guo, G.; Pei, X.; Wang, X.; Li, L.; Xiong, Y.; Qiu, X. Baicalin Inhibits Cell Viability, Migration and Invasion in Breast Cancer by Regulating miR-338-3p and MORC4. Onco. Targets. Ther. 2019, 12, 11183–11193. [Google Scholar] [CrossRef]
- Liu, D.-K.; Dong, H.-F.; Liu, R.-F.; Xiao, X.-L. Baicalin Inhibits the TGF-Β1/p-Smad3 Pathway to Suppress Epithelial-Mesenchymal Transition-Induced Metastasis in Breast Cancer. Oncotarget 2020, 11, 2863–2872. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Huang, T.; Fang, Q.; Zhang, X.; Yuan, J.; Li, M.; Ge, H. Bone-Protective and Anti-Tumor Effect of Baicalin in Osteotropic Breast Cancer via Induction of Apoptosis. Breast Cancer Res. Treat. 2020, 184, 711–721. [Google Scholar] [CrossRef]
- Ge, A.; Liu, L.; Deng, X.; Luo, J.; Xu, Y. Exploring the Mechanism of Baicalin Intervention in Breast Cancer Based on MicroRNA Microarrays and Bioinformatics Strategies. Evid. Based. Complement. Alternat. Med. 2021, 2021, 7624415. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wei, W.; Yu, L.; Zhang, X.; Huang, F.; Zheng, Q.; Wang, L.; Cai, C. Baicalin Promotes Mammary Gland Development via Steroid-like Activities. Front. Cell Dev. Biol. 2021, 9, 682469. [Google Scholar] [CrossRef]
- Wang, A.; Guo, D.; Cheng, H.; Jiang, H.; Liu, X.; Yun, Z. Transcriptome Sequencing Explores the Mechanism of Baicalin on Bone Cancer Pain. J. Inflamm. Res. 2021, 14, 5999–6010. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Lin, Q.; Chen, H.; Liu, L.; Liao, H.; Cheng, Y.; Zhang, X.; Wang, Z.; Shen, A.; et al. Baicalin Suppresses the Migration and Invasion of Breast Cancer Cells via the TGF-β/lncRNA-MALAT1/miR-200c Signaling Pathway. Medicine 2022, 101, e29328. [Google Scholar] [CrossRef]
- Taiyab, A.; Choudhury, A.; Haidar, S.; Yousuf, M.; Rathi, A.; Koul, P.; Chakrabarty, A.; Islam, A.; Shamsi, A.; Hassan, M.I. Exploring MTH1 Inhibitory Potential of Thymoquinone and Baicalin for Therapeutic Targeting of Breast Cancer. Biomed. Pharmacother. 2024, 173, 116332. [Google Scholar] [CrossRef]
- Jia, Q.; Zhou, Y.; Song, L.; Shi, X.; Jiang, X.; Tao, R.; Wang, A.; Wu, Y.; Wei, Z.; Zhang, Y.; et al. Baicalin Reduces Chronic Stress-Induced Breast Cancer Metastasis via Directly Targeting Β2-Adrenergic Receptor. J. Pharm. Anal. 2024, 14, 100934. [Google Scholar] [CrossRef] [PubMed]
- So, F.V.; Guthrie, N.; Chambers, A.F.; Moussa, M.; Carroll, K.K. Inhibition of Human Breast Cancer Cell Proliferation and Delay of Mammary Tumorigenesis by Flavonoids and Citrus Juices. Nutr. Cancer 1996, 26, 167–181. [Google Scholar] [CrossRef] [PubMed]
- So, F.V.; Guthrie, N.; Chambers, A.F.; Carroll, K.K. Inhibition of Proliferation of Estrogen Receptor-Positive MCF-7 Human Breast Cancer Cells by Flavonoids in the Presence and Absence of Excess Estrogen. Cancer Lett. 1997, 112, 127–133. [Google Scholar] [CrossRef]
- Kuntz, S.; Wenzel, U.; Daniel, H. Comparative Analysis of the Effects of Flavonoids on Proliferation, Cytotoxicity, and Apoptosis in Human Colon Cancer Cell Lines. Eur. J. Nutr. 1999, 38, 133–142. [Google Scholar] [CrossRef]
- Tong, W.-G.; Ding, X.-Z.; Adrian, T.E. The Mechanisms of Lipoxygenase Inhibitor-Induced Apoptosis in Human Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2002, 296, 942–948. [Google Scholar] [CrossRef]
- Po, L.S.; Chen, Z.-Y.; Tsang, D.S.C.; Leung, L.K. Baicalein and Genistein Display Differential Actions on Estrogen Receptor (ER) Transactivation and Apoptosis in MCF-7 Cells. Cancer Lett. 2002, 187, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Markaverich, B.M.; Crowley, J.; Rodriquez, M.; Shoulars, K.; Thompson, T. Tetrahydrofurandiol Stimulation of Phospholipase A2, Lipoxygenase, and Cyclooxygenase Gene Expression and MCF-7 Human Breast Cancer Cell Proliferation. Environ. Health Perspect. 2007, 115, 1727–1731. [Google Scholar] [CrossRef][Green Version]
- Lin, C.-W.; Yang, L.-Y.; Shen, S.-C.; Chen, Y.-C. IGF-I plus E2 Induces Proliferation via Activation of ROS-Dependent ERKs and JNKs in Human Breast Carcinoma Cells. J. Cell. Physiol. 2007, 212, 666–674. [Google Scholar] [CrossRef]
- Günther, S.; Ruhe, C.; Derikito, M.G.; Böse, G.; Sauer, H.; Wartenberg, M. Polyphenols Prevent Cell Shedding from Mouse Mammary Cancer Spheroids and Inhibit Cancer Cell Invasion in Confrontation Cultures Derived from Embryonic Stem Cells. Cancer Lett. 2007, 250, 25–35. [Google Scholar] [CrossRef]
- Lee, J.-H.; Li, Y.-C.; Ip, S.-W.; Hsu, S.-C.; Chang, N.-W.; Tang, N.-Y.; Yu, C.-S.; Chou, S.-T.; Lin, S.-S.; Lino, C.-C.; et al. The Role of Ca2+ in Baicalein-Induced Apoptosis in Human Breast MDA-MB-231 Cancer Cells through Mitochondria- and Caspase-3-Dependent Pathway. Anticancer Res. 2008, 28, 1701–1711. [Google Scholar]
- Androutsopoulos, V.P.; Ruparelia, K.; Arroo, R.R.J.; Tsatsakis, A.M.; Spandidos, D.A. CYP1-Mediated Antiproliferative Activity of Dietary Flavonoids in MDA-MB-468 Breast Cancer Cells. Toxicology 2009, 264, 162–170. [Google Scholar] [CrossRef]
- Ragazzon, P.A.; Bradshaw, T.; Matthews, C.; Iley, J.; Missailidis, S. The Characterisation of Flavone-DNA Isoform Interactions as a Basis for Anticancer Drug Development. Anticancer Res. 2009, 29, 2273–2283. [Google Scholar] [PubMed]
- Zhou, Q.-M.; Wang, S.; Zhang, H.; Lu, Y.-Y.; Wang, X.-F.; Motoo, Y.; Su, S.-B. The Combination of Baicalin and Baicalein Enhances Apoptosis via the ERK/P38 MAPK Pathway in Human Breast Cancer Cells. Acta Pharmacol. Sin. 2009, 30, 1648–1658. [Google Scholar] [CrossRef]
- Wang, L.; Ling, Y.; Chen, Y.; Li, C.-L.; Feng, F.; You, Q.-D.; Lu, N.; Guo, Q.-L. Flavonoid Baicalein Suppresses Adhesion, Migration and Invasion of MDA-MB-231 Human Breast Cancer Cells. Cancer Lett. 2010, 297, 42–48. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Xing, Q.; Yan, J.; Senthil, M.; Akmal, Y.; Kowolik, C.M.; Kang, J.; Lu, D.M.; Zhao, M.; et al. Identification of a Natural Compound by Cell-Based Screening That Enhances Interferon Regulatory Factor-1 Activity and Causes Tumor Suppression. Mol. Cancer Ther. 2011, 10, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.T.; Moon, J.; Song, Y.; Viet, P.Q.; Van Phuc, P.; Lee, J.M.; Yi, T.-H.; Cho, M.; Cho, S.K. Ginsenoside F2 Induces Apoptosis Accompanied by Protective Autophagy in Breast Cancer Stem Cells. Cancer Lett. 2012, 321, 144–153. [Google Scholar] [CrossRef]
- Viola, K.; Kopf, S.; Huttary, N.; Vonach, C.; Kretschy, N.; Teichmann, M.; Giessrigl, B.; Raab, I.; Stary, S.; Krieger, S.; et al. Bay11-7082 Inhibits the Disintegration of the Lymphendothelial Barrier Triggered by MCF-7 Breast Cancer Spheroids; the Role of ICAM-1 and Adhesion. Br. J. Cancer 2013, 108, 564–569. [Google Scholar] [CrossRef]
- Aryal, P.; Kim, K.; Park, P.-H.; Ham, S.; Cho, J.; Song, K. Baicalein Induces Autophagic Cell Death through AMPK/ULK1 Activation and Downregulation of mTORC1 Complex Components in Human Cancer Cells. FEBS J. 2014, 281, 4644–4658. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, E.; Xing, Q.; Yan, J.; Arrington, A.; Wang, C.; Tully, D.; Kowolik, C.M.; Lu, D.M.; Frankel, P.H.; et al. Baicalein Upregulates DDIT4 Expression Which Mediates mTOR Inhibition and Growth Inhibition in Cancer Cells. Cancer Lett. 2015, 358, 170–179. [Google Scholar] [CrossRef]
- Chang, H.-T.; Chou, C.-T.; Kuo, D.-H.; Shieh, P.; Jan, C.-R.; Liang, W.-Z. The Mechanism of Ca2+ Movement in the Involvement of Baicalein-Induced Cytotoxicity in ZR-75-1 Human Breast Cancer Cells. J. Nat. Prod. 2015, 78, 1624–1634. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Xue, X.-H.; Ma, Y.-N.; Zhang, S.-Q. Effect of Baicalein on the Expression of SATB1 in Human Breast Cancer Cells. Exp. Ther. Med. 2015, 9, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Li, Z.; Zhu, Z.; Chen, H.; Zhao, L.; Wang, X.; Chen, Y. Baicalein Suppresses 17-β-Estradiol-Induced Migration, Adhesion and Invasion of Breast Cancer Cells via the G Protein-Coupled Receptor 30 Signaling Pathway. Oncol. Rep. 2015, 33, 2077–2085. [Google Scholar] [CrossRef]
- Ma, X.; Yan, W.; Dai, Z.; Gao, X.; Ma, Y.; Xu, Q.; Jiang, J.; Zhang, S. Baicalein Suppresses Metastasis of Breast Cancer Cells by Inhibiting EMT via Downregulation of SATB1 and Wnt/β-Catenin Pathway. Drug Des. Devel. Ther. 2016, 10, 1419–1441. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hong, D.-Y.; Wang, J.; Ling-Hu, J.; Zhang, Y.-Y.; Pan, D.; Xu, Y.-N.; Tao, L.; Luo, H.; Shen, X.-C. Baicalein, Unlike 4-Hydroxytamoxifen but Similar to G15, Suppresses 17β-Estradiol-Induced Cell Invasion, and Matrix Metalloproteinase-9 Expression and Activation in MCF-7 Human Breast Cancer Cells. Oncol. Lett. 2017, 14, 1823–1830. [Google Scholar] [CrossRef]
- Grigalius, I.; Petrikaite, V. Relationship between Antioxidant and Anticancer Activity of Trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Hong, D.-Y.; Chen, L.; Zhang, Y.-Y.; Xu, Y.-N.; Pan, D.; Fu, L.-Y.; Tao, L.; Luo, H.; et al. Baicalein Has Protective Effects on the 17β-Estradiol-Induced Transformation of Breast Epithelial Cells. Oncotarget 2017, 8, 10470–10484. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cao, Y.; Tang, L.; Yang, Y.; Chen, F.; Xia, J. Baicalein Inhibits Breast Cancer Growth via Activating a Novel Isoform of the Long Noncoding RNA PAX8-AS1-N. J. Cell. Biochem. 2018, 119, 6842–6856. [Google Scholar] [CrossRef]
- Yan, W.; Ma, X.; Zhao, X.; Zhang, S. Baicalein Induces Apoptosis and Autophagy of Breast Cancer Cells via Inhibiting PI3K/AKT Pathway in Vivo and Vitro. Drug Des. Devel. Ther. 2018, 12, 3961–3972. [Google Scholar] [CrossRef]
- Terabayashi, T.; Hanada, K.; Motani, K.; Kosako, H.; Yamaoka, M.; Kimura, T.; Ishizaki, T. Baicalein Disturbs the Morphological Plasticity and Motility of Breast Adenocarcinoma Cells Depending on the Tumor Microenvironment. Genes Cells 2018, 23, 466–479. [Google Scholar] [CrossRef]
- Marcial-Medina, C.; Ordoñez-Moreno, A.; Gonzalez-Reyes, C.; Cortes-Reynosa, P.; Perez Salazar, E. Oleic Acid Induces Migration through a FFAR1/4, EGFR and AKT-Dependent Pathway in Breast Cancer Cells. Endocr. Connect. 2019, 8, 252–265. [Google Scholar] [CrossRef]
- Koh, S.Y.; Moon, J.Y.; Unno, T.; Cho, S.K. Baicalein Suppresses Stem Cell-like Characteristics in Radio- and Chemoresistant MDA-MB-231 Human Breast Cancer Cells through up-Regulation of IFIT2. Nutrients 2019, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, L.; Hong, D.; Chen, Z.; Zhang, J.; Fu, L.; Pan, D.; Zhang, Y.; Xu, Y.; Gan, S.; et al. Baicalein Inhibits Fibronectin-Induced Epithelial-Mesenchymal Transition by Decreasing Activation and Upregulation of Calpain-2. Cell Death Dis. 2019, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Eichsteininger, J.; Kirisits, K.; Smöch, C.; Stadlbauer, C.; Nguyen, C.H.; Jäger, W.; Özmen, A.; Ecker, G.; Krupitza, G.; Krenn, L. Structural Insight into the in Vitro Anti-Intravasative Properties of Flavonoids. Sci. Pharm. 2019, 87, 23. [Google Scholar] [CrossRef]
- Susmitha, G.D.; Miyazato, K.; Ogura, K.; Yokoyama, S.; Hayakawa, Y. Anti-Metastatic Effects of Baicalein by Targeting STAT3 Activity in Breast Cancer Cells. Biol. Pharm. Bull. 2020, 43, 1899–1905. [Google Scholar] [CrossRef]
- An, L.; Dou, X.; Wang, M.; Luo, W.; Ma, Q.; Liu, X. Involvement of TNF-Alpha and IL-10 in Breast Cancer and Patient Survival. Trop. J. Pharm. Res. 2020, 19, 2033–2039. [Google Scholar] [CrossRef]
- He, S.; Wang, S.; Liu, S.; Li, Z.; Liu, X.; Wu, J. Baicalein Potentiated M1 Macrophage Polarization in Cancer through Targeting PI3Kγ/NF-κB Signaling. Front. Pharmacol. 2021, 12, 743837. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-T.; Liu, C.-H.; Wong, S.H.; Pan, Y.-C.; Lin, L.-T. Small Molecules Baicalein and Cinnamaldehyde Are Potentiators of Measles Virus-Induced Breast Cancer Oncolysis. Phytomedicine 2021, 89, 153611. [Google Scholar] [CrossRef]
- Dong, Y.; He, G.; Chen, K.; He, X.; Pan, M.; Huang, X.; Yu, X.; Xia, J. Baicalein Promotes KDM4E to Induce BICD1 and Inhibit Triple-Negative Breast Cancer Progression by Blocking PAR1 Signaling. Mol. Carcinog. 2024, 63, 1288–1302. [Google Scholar] [CrossRef]
- Liu, M.; Li, C.; Qu, J.; Sun, S.; Zhao, Z.; Wang, W.; Lv, W.; Zhang, Y.; Cai, Y.; Zhao, F.; et al. Baicalein Enhances Immune Response in TNBC by Inhibiting Leptin Expression of Adipocytes. Cancer Sci. 2023, 114, 3834–3847. [Google Scholar] [CrossRef]
- He, G.; Huang, X.; Dong, Y.; Chen, K.; He, X.; Pan, M.; Zeng, W.; Yu, X.; Xia, J. Preliminary Investigation on the Mechanism of Baicalein Regulating the Effects of Nischarin on Invasion and Apoptosis of Human Breast Cancer Cells MCF-7 through Wnt3α/β-Catenin Pathway. Int. Immunopharmacol. 2024, 143, 113262. [Google Scholar] [CrossRef]
- Syeda Abida Ejaz, M.; Sarfraz, M.; Tanveer, A.; Wani, T.; Ruby, S.; Zargar, M.; Arafat, C. Evaluation of Cytotoxic Activity and Apoptosis-Inducing Potential of 5,6,7-Trihydroxyflavone against Breast Cancer and Cervical Cancer Cell Lines. J. Biol. Regul. Homeost. Agents 2024, 38, 303–317. [Google Scholar]
- He, T.-M.; Liu, J.-X.; Duan, C.-C.; Li, X.-F.; Zhang, J.-Y. Effective Material Basis and Mechanism Analysis of Compound Banmao Capsule against Tumors Using Integrative Network Pharmacology and Molecular Docking. Evid. Based. Complement. Alternat. Med. 2021, 2021, 6653460. [Google Scholar] [CrossRef]
- Qi, X.; Xu, H.; Zhang, P.; Chen, G.; Chen, Z.; Fang, C.; Lin, L. Investigating the Mechanism of Scutellariae barbata Herba in the Treatment of Colorectal Cancer by Network Pharmacology and Molecular Docking. Evid. Based. Complement. Alternat. Med. 2021, 2021, 3905367. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xin, G.; Fang, B. Molecular Docking and Molecular Dynamics Simulation Studies on the Effect of Baicalein on Breast and Ovarian Cancers. Trop. J. Pharm. Res. 2023, 22, 1395–1402. [Google Scholar] [CrossRef]
- Rathi, A.; Khan, A.; Haider, S.; Roy, S.; Taiyab, A.; Mahendru, S.; Hussain, A.; Chakrabarty, A.; Islam, A.; Imtaiyaz Hassan, M.; et al. Exploring the Potential of Baicalin and Resveratrol as PIM-1 Kinase Inhibitors: Therapeutic Targeting of Prostate and Breast Cancers. J. Mol. Liq. 2024, 396, 124026. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Li, X.-L.; Wang, Q.-F.; Mehendale, S.R.; Yuan, C.-S. Selective Fraction of Scutellaria baicalensis and Its Chemopreventive Effects on MCF-7 Human Breast Cancer Cells. Phytomedicine 2010, 17, 63–68. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, Z.; Zhang, H.; Zhen, Z.; Calway, T.; Wang, Y.; Yuan, C.-S.; Wang, C.-Z. Pretreatment of Baicalin and Wogonoside with Glycoside Hydrolase: A Promising Approach to Enhance Anticancer Potential. Oncol. Rep. 2013, 30, 2411–2418. [Google Scholar] [CrossRef]
- Park, J.R.; Lee, M.C.; Moon, S.-C.; Kim, J.; Ha, K.-T.; Park, E.J.; Hong, C.; Seo, B.-D.; Kim, B.J. Scutellaria baicalensis Georgi Induces Caspase-Dependent Apoptosis via Mitogen Activated Protein Kinase Activation and the Generation of Reactive Oxygen Species Signaling Pathways in MCF-7 Breast Cancer Cells. Mol. Med. Rep. 2017, 16, 2302–2308. [Google Scholar] [CrossRef]
- Du, J.; Wang, L.; Huang, X.; Zhang, N.; Long, Z.; Yang, Y.; Zhong, F.; Zheng, B.; Lan, W.; Lin, W.; et al. Shuganning Injection, a Traditional Chinese Patent Medicine, Induces Ferroptosis and Suppresses Tumor Growth in Triple-Negative Breast Cancer Cells. Phytomedicine 2021, 85, 153551. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-C.; Lee, D.-Y.; Yeh, P.-Y. A Novel Chinese Herbal and Corresponding Chemical Formula for Cancer Treatment by Targeting Tumor Maintenance, Progression, and Metastasis. Front. Pharmacol. 2022, 13, 907826. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wu, X.-J.; Shi, J.-B.; Shi, X.-W.; Zhao, N.; Xiong, Y.; Wang, L.-P. Sanhuang Xiexin Decoction Ameliorates TNBC by Modulating JAK2-STAT3 and Lipid Metabolism. Chin. J. Integr. Med. 2024, 30, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhu, Y.; Long, F.; Ma, Y.; Tang, Q.; Wang, T. Exploring the Potential of Huangqin Tang in Breast Cancer Treatment Using Network Pharmacological Analysis and Experimental Verification. BMC Complement. Med. Ther. 2024, 24, 221. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Wu, J.; Chen, Y.; Chen, B.; Hu, J.; Zhou, J. Exploring Therapeutic Potentials of Baicalin and Its Aglycone Baicalein for Hematological Malignancies. Cancer Lett. 2014, 354, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.P.; Camões, A.; de São José Nascimento, M.; Cidade, H.; Sousa, M.E.; Pinto, M.M.M. Effects of a Prenyl-Baicalein Derivative on ER (+) MCF-7 and ER (−) MDA-MB-231 Breast Tumor Cell Lines. Med. Chem. Res. 2012, 21, 3154–3160. [Google Scholar] [CrossRef]
- Wang, N.; Ren, D.; Deng, S.; Yang, X. Differential Effects of Baicalein and Its Sulfated Derivatives in Inhibiting Proliferation of Human Breast Cancer MCF-7 Cells. Chem. Biol. Interact. 2014, 221, 99–108. [Google Scholar] [CrossRef]
- An, D.; Song, Z.; Yi, Y.; Zhang, Q.; Liu, J.; Zhang, Y.; Zhou, J.; Zhao, G.; Cong, D.; Li, N.; et al. Oroxylin A, a Methylated Metabolite of Baicalein, Exhibits a Stronger Inhibitory Effect than Baicalein on the CYP1B1-Mediated Carcinogenic Estradiol Metabolite Formation: Oroxylin A Has a Stronger Inhibition than Baicalein for CYP1B1. Phytother. Res. 2019, 33, 1033–1043. [Google Scholar] [CrossRef]
- Marzec, E.; Świtalska, M.; Winiewska-Szajewska, M.; Wójcik, J.; Wietrzyk, J.; Maciejewska, A.M.; Poznański, J.; Mieczkowski, A. The Halogenation of Natural Flavonoids, Baicalein and Chrysin, Enhances Their Affinity to Human Protein Kinase CK2. IUBMB Life 2020, 72, 1250–1261. [Google Scholar] [CrossRef]
- Yasuda, N.; Ali, S.; Aman, A.; Krusong, K.; Herfindo, N.; Chavasiri, W.; Choowongkomon, K.; Wolschann, P.; Mahalapbutr, P.; Rungrotmongkol, T.; et al. In Vitro and in Silico Studies of the Inclusion Complexation of 8-Bromobaicalein with β-Cyclodextrins. J. Mol. Graph. Model. 2024, 132, 108840. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Franek, K.J.; Zhou, Z.; Zhang, W.-D.; Chen, W.Y. In Vitro Studies of Baicalin Alone or in Combination with Salvia miltiorrhiza Extract as a Potential Anti-Cancer Agent. Int. J. Oncol. 2005, 26, 217–224. [Google Scholar] [CrossRef]
- Chang, W.-T.; Li, J.; Haung, H.-H.; Liu, H.; Han, M.; Ramachandran, S.; Li, C.-Q.; Sharp, W.W.; Hamann, K.J.; Yuan, C.-S.; et al. Baicalein Protects against Doxorubicin-Induced Cardiotoxicity by Attenuation of Mitochondrial Oxidant Injury and JNK Activation. J. Cell. Biochem. 2011, 112, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Chen, Y.; Liu, R.; Zhang, H.; Zhang, Y. Potentiation of Paclitaxel Activity by Curcumin in Human Breast Cancer Cell by Modulating Apoptosis and Inhibiting EGFR Signaling. Arch. Pharm. Res. 2014, 37, 1086–1095. [Google Scholar] [CrossRef]
- An, H.; Yu, X.; Xiang, C.; Zhang, Y.; Xia, J.; Wang, Y. Baicalein and U0126 Suppress Human Breast Cancer Cell Line MCF-7 through Regulating MAPK Signaling Pathway. Int. J. Clin. Exp. Pathol. 2016, 9, 10266–10273. [Google Scholar]
- Xi, Q.; Qi, H.; Li, Y.; Xi, Y.; Zhang, L. Low Frequency Ultrasound Combined with Baicalein Can Reduced the Invasive Capacity of Breast Cancer Cells by down Regulating the Expression of MMP-2, MMP-9, and u-PA. Transl. Cancer Res. 2016, 5, 838–844. [Google Scholar] [CrossRef]
- Talik Sisin, N.N.; Abdul Razak, K.; Zainal Abidin, S.; Che Mat, N.F.; Abdullah, R.; Ab Rashid, R.; Khairil Anuar, M.A.; Rahman, W.N. Synergetic Influence of Bismuth Oxide Nanoparticles, Cisplatin and Baicalein-Rich Fraction on Reactive Oxygen Species Generation and Radiosensitization Effects for Clinical Radiotherapy Beams. Int. J. Nanomed. 2020, 15, 7805–7823. [Google Scholar] [CrossRef]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Luo, X.; Zhang, Q.; Song, L. Baicalin, a Potent Inhibitor of NF-κB Signaling Pathway, Enhances Chemosensitivity of Breast Cancer Cells to Docetaxel and Inhibits Tumor Growth and Metastasis Both in Vitro and in Vivo. Front. Pharmacol. 2020, 11, 879. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Cheng, W.-T.; Cheng, H.-C.; Chou, W.-C.; Chen, H.-I.; Ou, H.-C.; Tsai, K.-L. Baicalin Enhances Chemosensitivity to Doxorubicin in Breast Cancer Cells via Upregulation of Oxidative Stress-Mediated Mitochondria-Dependent Apoptosis. Antioxidants 2021, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Shehatta, N.H.; Okda, T.M.; Omran, G.A.; Abd-Alhaseeb, M.M. Baicalin; a Promising Chemopreventive Agent, Enhances the Antitumor Effect of 5-FU against Breast Cancer and Inhibits Tumor Growth and Angiogenesis in Ehrlich Solid Tumor. Biomed. Pharmacother. 2022, 146, 112599. [Google Scholar] [CrossRef]
- Sisin, N.N.T.; Mat, N.F.C.; Rashid, R.A.; Dollah, N.; Razak, K.A.; Geso, M.; Algethami, M.; Rahman, W.N. Natural Baicalein-Rich Fraction as Radiosensitizer in Combination with Bismuth Oxide Nanoparticles and Cisplatin for Clinical Radiotherapy. Int. J. Nanomed. 2022, 17, 3853–3874. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Abd El-Alim, A.E.-A.F.; El-Hafeez, M.A.; Metwally, M.M.M.; Khamis, T.; Galal, A.A.A. Baicalein Prevents Capecitabine-Induced Heart Damage in Female Wistar Rats and Enhances Its Anticancer Potential in MCF-7 Breast Cancer Cells. Life Sci. 2023, 319, 121523. [Google Scholar] [CrossRef]
- Hua, F.; Xiao, Y.-Y.; Qu, X.-H.; Li, S.-S.; Zhang, K.; Zhou, C.; He, J.-L.; Zhu, Y.; Wan, Y.-Y.; Jiang, L.-P.; et al. Baicalein Sensitizes Triple Negative Breast Cancer MDA-MB-231 Cells to Doxorubicin via Autophagy-Mediated down-Regulation of CDK1. Mol. Cell. Biochem. 2023, 478, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Bernasinska-Slomczewska, J.; Hikisz, P.; Pieniazek, A.; Koceva-Chyla, A. Baicalin and Baicalein Enhance Cytotoxicity, Proapoptotic Activity, and Genotoxicity of Doxorubicin and Docetaxel in MCF-7 Breast Cancer Cells. Molecules 2024, 29, 2503. [Google Scholar] [CrossRef]
- Awajan, D.; Abu-Humaidan, A.H.A.; Talib, W.H. Study of the Antitumor Activity of the Combination Baicalin and Epigallocatechin Gallate in a Murine Model of Vincristine-Resistant Breast Cancer. Farmatsiia 2024, 71, 1–20. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhao, Y.-G.; Zhang, Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules 2024, 29, 4854. [Google Scholar] [CrossRef]
- Kavithaa, K.; Paulpandi, M.; Padma, P.R.; Sumathi, S. Induction of Intrinsic Apoptotic Pathway and Cell Cycle Arrest via Baicalein Loaded Iron Oxide Nanoparticles as a Competent Nano-Mediated System for Triple Negative Breast Cancer Therapy. RSC Adv. 2016, 6, 64531–64543. [Google Scholar] [CrossRef]
- Lee, D.; Ko, W.-K.; Hwang, D.-S.; Heo, D.N.; Lee, S.J.; Heo, M.; Lee, K.-S.; Ahn, J.-Y.; Jo, J.; Kwon, I.K. Use of Baicalin-Conjugated Gold Nanoparticles for Apoptotic Induction of Breast Cancer Cells. Nanoscale Res. Lett. 2016, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Xia, X.; Yang, Y.; Ye, J.; Dong, W.; Ma, P.; Jin, Y.; Liu, Y. Co-Encapsulation of Paclitaxel and Baicalein in Nanoemulsions to Overcome Multidrug Resistance via Oxidative Stress Augmentation and P-Glycoprotein Inhibition. Int. J. Pharm. 2016, 513, 8–16. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Pu, G.; Zhang, F.; Liu, H.; Zhang, Y. Co-Delivery of Baicalein and Doxorubicin by Hyaluronic Acid Decorated Nanostructured Lipid Carriers for Breast Cancer Therapy. Drug Deliv. 2016, 23, 1364–1368. [Google Scholar] [CrossRef]
- Kavithaa, K.; Sumathi, S.; Padma, P.R. Intracellular Uptake of PEG-Funtionalized Baicalein Loaded Iron Oxide Nanoparticles Regulates Apoptotic Genes in Triple Negative Breast Cancer Cells: Mitochondrial Pathway Targeted Therapy for Breast Cancer. J. Cluster Sci. 2017, 28, 2057–2073. [Google Scholar] [CrossRef]
- El-Gogary, R.; Gaber, S.A.A.; Nasr, M. Polymeric Nanocapsular Baicalin: Chemometric Optimization, Physicochemical Characterization and Mechanistic Anticancer Approaches on Breast Cancer Cell Lines. Sci. Rep. 2019, 9, 11064. [Google Scholar] [CrossRef]
- Mi, X.; Hu, M.; Dong, M.; Yang, Z.; Zhan, X.; Chang, X.; Lu, J.; Chen, X. Folic Acid Decorated Zeolitic Imidazolate Framework (ZIF-8) Loaded with Baicalin as a Nano-Drug Delivery System for Breast Cancer Therapy. Int. J. Nanomed. 2021, 16, 8337–8352. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Liu, F.; Lan, M.; Zou, T.; Li, L.; Cai, T.; Cai, Y. Preparation and Evaluation of Folate-Modified Albumin Baicalin-Loaded Nanoparticles for the Targeted Treatment of Breast Cancer. J. Drug Deliv. Sci. Technol. 2021, 65, 102603. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, S.; Saraf, S.A.; Chourasia, M.K.; Mathew, J.; Pandey, A.C. Encapsulation of Baicalein in Cinnamon Essential Oil Nanoemulsion for Enhanced Anticancer Efficacy against MDA-MB-231 Cells. Bionanoscience 2021, 11, 1049–1060. [Google Scholar] [CrossRef]
- Wang, T.; Yang, J.; Kang, H.; Zhang, L.; Chen, H. Facile Preparation of a Novel Hyaluronic Acid-Modified Metal-Polyphenol Photothermal Nanoformulation for Tumor Therapy. Int. J. Biol. Macromol. 2022, 222, 3066–3076. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lan, M.; Ren, B.; Li, L.; Zou, T.; Kong, Z.; Fan, D.; Cai, T.; Cai, Y. Baicalin-Loaded Folic Acid-Modified Albumin Nanoparticles (FA-BSANPs/BA) Induce Autophagy in MCF-7 Cells via ROS-Mediated P38 MAPK and Akt/mTOR Pathway. Cancer Nanotechnol. 2022, 13, 2. [Google Scholar] [CrossRef]
- Gharari, Z.; Hanachi, P.; Sadeghinia, H.; Walker, T.R. Eco-Friendly Green Synthesis and Characterization of Silver Nanoparticles by Scutellaria multicaulis Leaf Extract and Its Biological Activities. Pharmaceuticals 2023, 16, 992. [Google Scholar] [CrossRef]

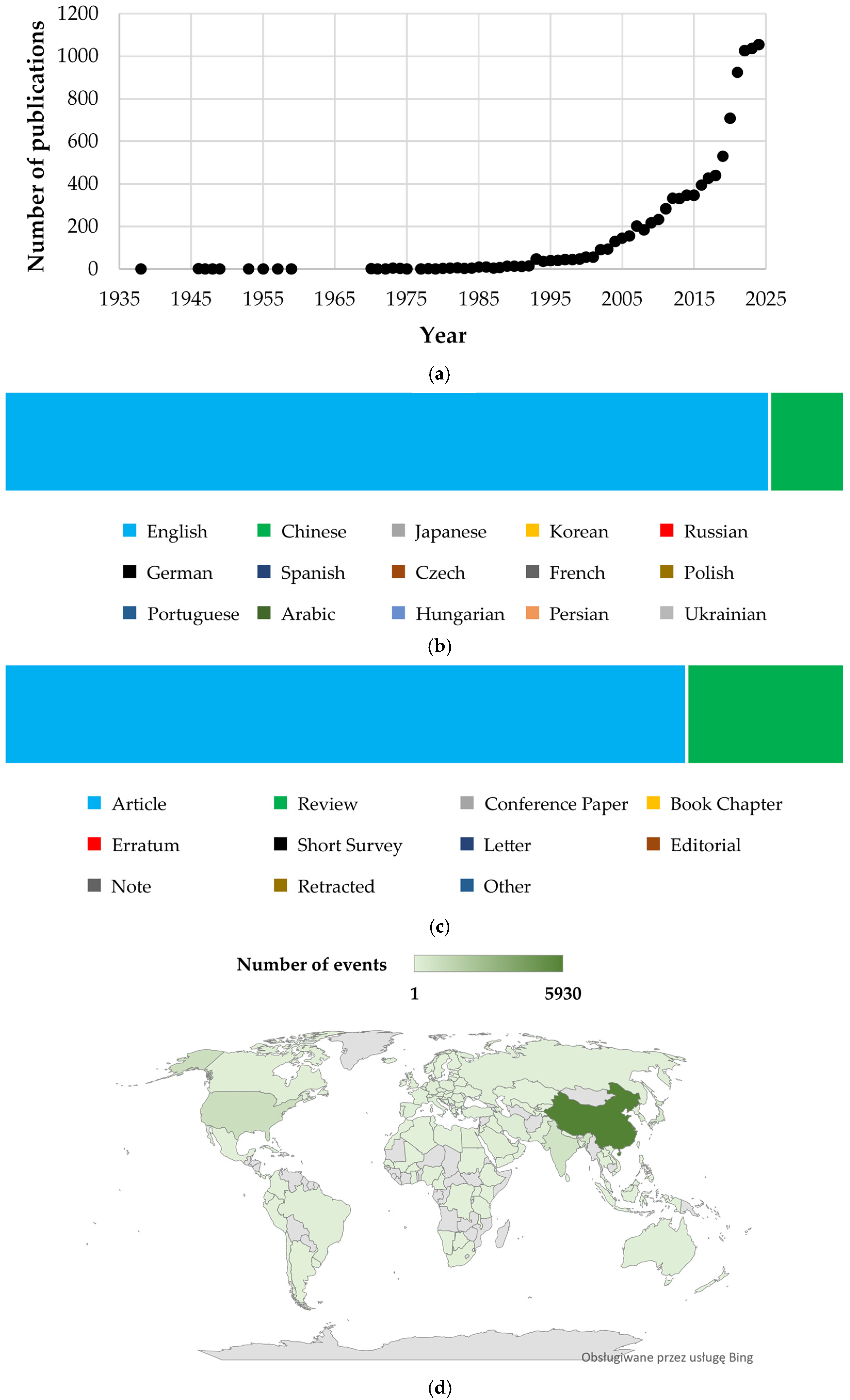
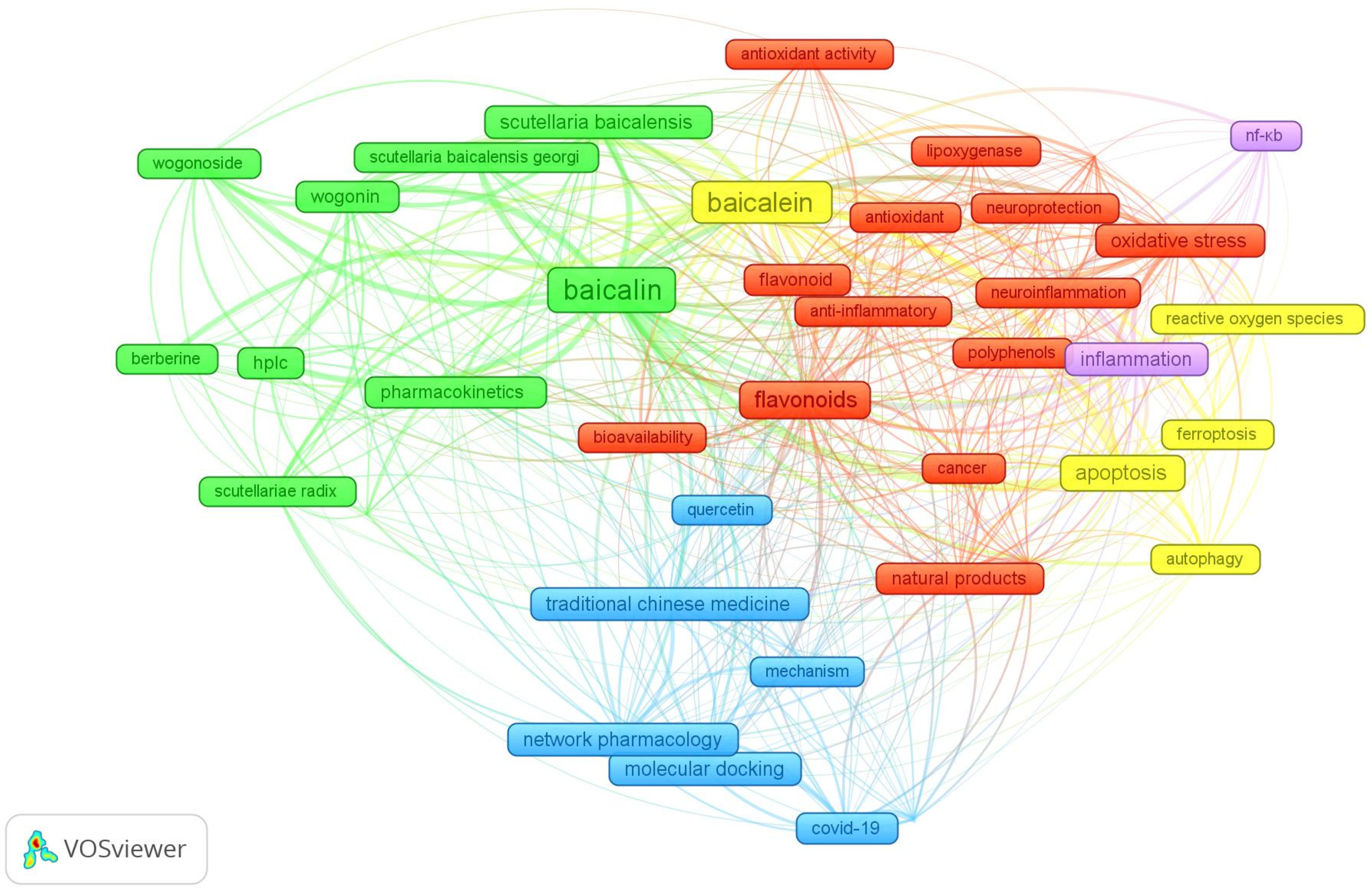


| Rank | Authors | Title | Year | Journal | Total Citation | Reference |
|---|---|---|---|---|---|---|
| 1. | Cushnie, T.P.T.; Lamb, A.J. | Antimicrobial Activity of Flavonoids | 2005 | International Journal of Antimicrobial Agents | 3287 | [31] |
| 2. | Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. | Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases | 2013 | Antioxidants and Redox Signaling | 2067 | [32] |
| 3. | Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. | Analysis of Therapeutic Targets for SARS-CoV-2 and Discovery of Potential Drugs by Computational Methods. | 2020 | Acta Pharmaceutica Sinica B | 1712 | [33] |
| 4. | Formica, J.V.; Regelson, W. | Review of the Biology of Quercetin and Related Bioflavonoids. | 1995 | Food and Chemical Toxicology | 1617 | [34] |
| 5. | Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. | Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review | 2018 | Chinese Medicine | 1331 | [35] |
| Cell Lines | Animal Models | Tested Concentrations | The Outcome of the Study | Reference |
|---|---|---|---|---|
| PC-3, DU145, LNCaP, MCF-7, HL-60, and NB4 | - | 0.1–20 µM | The MCF-7 cell line was insensitive to growth inhibition by baicalin | [36] |
| MCF-7 | - | 50–400 µM | Inhibition of MCF-7 cell proliferation in a dose- and time-dependent manner (IC50 = 206 µM after 24 h) At 200 µM baicalin induced potent cytotoxicity and morphological changes indicative of cell death Cells arrested in G0/G1 phase and chromatin condensation confirmed apoptosis Upregulation of protein p53 and the bax gene | [37] |
| MCF-10A and MDA-MB-231 | - | 2–5 µM | Inhibition of NF-κB activation and TGF-β1-induced EMT in both cell lines Suppression of TGF-β1-induced migration and colony formation in MDA-MB-231 breast cancer cells | [38] |
| MCF-7 | - | 10–160 µM | Baicalin at 160 µM significantly reduced the survival of MCF-7 cells, and concentrations of 10–80 µM had no significant effect Inhibition of MCF-7 cell migration by upregulating miR-200b and E-cadherin | [39] |
| MDA-MB-231 and 4T1 | - | 10–100 µM | Baicalin (up to 100 µM) did not affect the viability of MDA-MB-231 and 4T1 cells Baicalin suppressed breast cancer metastasis by inhibiting migration, invasion, and EMT through the β-catenin pathway | [40] |
| MCF-7, MDA-MB-231, and MCF-10 | Female BALB/c nude mice (5 weeks old) | 20–30 µM | Inhibition of breast cancer cell proliferation, invasion, and migration by inducing G1/S arrest and suppressing the NF-κB pathway Reduction of tumor growth in vivo and modulated inflammatory responses | [41] |
| MDA-MB-231 | - | 10–40 µg/mL | Inhibition of MDA-MB-231 cell growth, proliferation, migration, and invasion, with effects increasing at higher concentrations (40 μg/mL was the most effective) Reducing Vimentin, β-catenin, c-Myc, and MMP-7 mRNA expression while increasing E-cadherin expression | [42] |
| MCF-10A, MCF-7, and MDA-MB-231 | - | 25–200 µM | Inhibition of viability, migration, and invasion of breast cancer cells, but no cytotoxicity to normal MCF-10A cells Inhibition of breast cancer progression by upregulating miR-338-3p | [43] |
| SK-BR-2 and MCF-7 | Female BALB/c-null and normal mice (6 weeks old) | 100 mg/kg | Baicalin bonded directly to TGF-β1 and downregulated its expression, along with p-Smad3 and vimentin, while upregulating E-cadherin Baicalin suppressed tumor growth and metastasis, with upregulated E-cadherin and downregulated TGF-β1, vimentin, and p-Smad3 in tumors | [44] |
| MDA-MB-231 and MCF-7 | - | 0.25–100 nM | Induction of apoptosis in breast cancer cells (MDA-MB-231, MCF-7) by increasing cytochrome c release, DNA fragmentation, and caspase-3, -8, and -9 activity Inhibition of the mTOR pathway, reducing phosphorylation of mTOR and p70 S6 kinase—Baicalin had minimal effects on osteoblasts and bone cells | [45] |
| MCF-7 | - | 50–200 µM | Inhibition of MCF-7 cell proliferation in a time- and concentration-dependent manner (0–200 µM, 24–72 h) At 150 µM baicalin, miRNA microarray analysis identified 92 upregulated and 35 downregulated miRNAs | [46] |
| Eph4, MDA-MB-231, ZR-75-1, and 293T | C57BL/6 and Actin-DsRed mice (3 weeks old) | 50–200 mg/kg | Baicalin suppressed luminal breast cancer (ZR-75-1) growth by downregulating ESR1 but promoted triple-negative breast cancer (MDA-MB-231) growth by upregulating PROCR Baicalin exhibited steroid hormone-like activity, regulating genes involved in Hippo signaling and cell cycle while suppressing breast cancer-related genes | [47] |
| MADB-106 | Sprague-Dawley (SD) rats | 30 mg/kg | Reversing bone loss in bone cancer pain rats Inhibition of osteoclast activation and reduction of inflammation Downregulating cancer-related genes and modulating pathways like NF-κB and TNF signaling. | [48] |
| MDA-MB-231 | - | 12.5–50 µM | Reduction of cell viability of MDA-MB-231 cells after 48–72 h of treatment Inhibition of migration and invasion of breast cancer cells Downregulation of the TGF-β/ZEB1 pathway, reducing TGF-β1, ZEB1, and N-cadherin (mesenchymal markers) while upregulating E-cadherin (epithelial marker) at both mRNA and protein levels Downregulation of lncRNA-MALAT1 and upregulated miR-200c | [49] |
| MCF-7 | - | 0–200 µM | Anti-proliferative and cytotoxic activity against breast cancer cells (IC50 = 34.8 μM) Baicalin triggered apoptosis and heightened ROS production in MCF7 cells | [50] |
| MCF-7, MDA-MB-231, MCF-7 ADRB2^OE | Female BALB/c and C57BL/6J mice (6 weeks old) | 25–100 µM | Baicalin suppressed epinephrine-induced migration and invasion of breast cancer cells in wound healing, transwell, and 3D spheroid assays Protection against chronic stress-promoted breast cancer metastasis by binding to β2-AR and blocking its activation on tumors | [51] |
| Cell Lines | Animal Models | Tested Concentrations | The Outcome of the Study | Reference |
|---|---|---|---|---|
| MDA-MB-435 | Female Sprague-Dawley rats | 0–250 µg/mL | Inhibition of MDA-MB-435 breast cancer cell proliferation in vitro, with an IC50 of 5.9 µg/mL | [52] |
| MCF-7 | - | 0–2 mg/mL | Baicalein inhibits the proliferation of MCF-7 breast cancer cells through a non-estrogenic mechanism (IC₅₀ = 5.3 µg/mL) | [53] |
| Caco-2, HT-29, LLC-PK1, and MCF-7 | - | 1–1000 µM | MCF-7 breast cancer cells were less sensitive to baicalein, requiring 6.6-fold higher concentrations to achieve 50% growth inhibition compared to intestinal cells | [54] |
| MCF-7 and MDA-MB-231 | - | 1.25–20 µM | Inhibition of the proliferation of both MDA-MB-231 and MCF-7 breast cancer cells Induction of apoptosis through mitochondrial pathways, caspase activation, and modulation of Bcl-2 family proteins | [55] |
| MCF-7 | - | 10–100 µM | Induction of apoptosis in MCF-7 cells at 10 µM Baicalein antagonized estradiol-induced ER transactivation, showing consistent antiestrogenic effects | [56] |
| MCF-7 | - | 5 µM | Inhibition of the proliferative effects of THF-diols and E2 on MCF-7 breast cancer cells by targeting LOX-12 | [57] |
| MCF-7 | - | 25–50 µM | Baicalein, but not its glycoside inhibited E2/IGF-I-induced proliferation, c-Jun expression, and ROS production | [58] |
| 4T1 | - | 1–100 µM | Demonstration of anti-proliferative, anti-metastatic, and antioxidant properties in 4T1 tumor spheroids | [59] |
| MDA-MB-231 | - | 25–100 µM | Induction of apoptosis in MDA-MB-231 breast cancer cells through ROS production, mitochondrial dysfunction, Ca2+ signaling, and regulation of apoptotic proteins | [60] |
| MDA-MB-468 and MCF-10A | - | 10 µM | Antiproliferative effects in breast cancer cells (MDA-MB-468), Baicalein was not metabolized by CYP1 enzymes | [61] |
| MCF-7 and CCRFCEM leukemia cells | - | 1–1000 µM | Baicalein (and baicalin) induced G1-phase arrest in MCF-7 and CCRFCEM cells—Moderate anticancer activity (IC50 = 69.6 μM for baicalein and 78.8 μM for baicalin against MCF-7 cells) Baicalein-induced DNA damage in MCF-7 cells (Baicalin had no effect) | [62] |
| MCF-7 and MDA-MB-231 | - | 25–400 µM | The combination of baicalin and baicalein synergistically inhibited breast cancer cell growth, induced apoptosis, and caused cell cycle arrest by activating the ERK/p38 MAPK pathway | [63] |
| MDA-MB-231 | - | 2–50 µM | Baicalein demonstrates potent anti-metastatic effects by inhibiting adhesion, motility, and invasion of MDA-MB-231 breast cancer cells Suppression of the activity and expression of MMP-2 and MMP-9, likely through the downregulation of the MAPK signaling pathway | [64] |
| AGS, MDA468, BT549, SKBR3, C3L5, and MDA468-C23 | Female SCID-Bg mice (Charles River, 6 weeks old) | 0.5–10 µM | Baicalein enhanced IRF-1 activity, upregulated tumor-suppressive pathways, and inhibited cancer cell growth both in vitro and in vivo | [65] |
| MCF-7 and breast cancer stem cells (CSCs) | - | 0–120 µM | Baicalein exhibits limited effectiveness against breast CSCs compared to ginsenoside F2 | [66] |
| MCF-7 | - | 100 µM | Inhibition of ALOX12/15 and reduction of 12(S)-HETE production | [67] |
| PC-3, MDA-MB-231 and DU145 | - | 2.5–20 µg/mL | Induction of autophagy, dysfunction of mitochondria, and cell cycle arrest | [68] |
| MDA-MB-468, SKBR3, Hs578T, and BT549 | SCID-Beige mice | 5–80 µM | Baicalein-induced DDIT4 (DNA Damage-Inducible Transcript 4) expression in multiple breast cancer cell lines Baicalein treatment significantly suppressed tumor growth without toxicity in an orthotopic mouse model of triple-negative breast cancer | [69] |
| ZR-75-1 | - | 10–40 µM | Baicalein increased intracellular Ca2+ concentration through Ca2+ release from the ER and Ca2+ entry via SOCCs, leading to ROS production, caspase activation, and apoptosis in ZR-75-1 cells | [70] |
| MDA-MB-231 | - | 5–80 µM | Inhibition of proliferation and migration of MDA-MB-231 cells Downregulation of SATB1 protein expression | [71] |
| MCF-7 and SK-BR-3 | - | 5–50 µM | Inhibition of E2-induced migration, adhesion, invasion, and GPR30 signaling in breast cancer cells | [72] |
| MCF-10A, MCF7, SKBR3, and MDA-MB-231 | Female Balb/c nude mice (4–8 weeks old) | 10–40 µM | Suppression of proliferation, migration, and invasion of cancer cells Downregulation of SATB1 and the Wnt/β-catenin pathway Reversing EMT and reducing metastasis in vivo | [73] |
| MCF-7 | - | 10 µM | Inhibition of E2-induced invasion and MMP-9 activity in MCF-7 breast cancer cells by targeting GPR30 signaling | [74] |
| A549, MCF-7, and U87 | - | 3.125–100 µM | Moderate anti-proliferative activity against the MCF-7 breast cancer cell line (EC50 = 26.1 µM) | [75] |
| MCF-10A and MCF-12A | Female non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice (6–9 weeks old) | 2–8 µM | Baicalein effectively prevents estradiol (E2)-induced neoplastic transformation in mammary epithelial cells by inhibiting cell growth, migration, invasion, and tumorigenesis, and by blocking ERα and GPR30 signaling pathways | [76] |
| MDA-MB-231 and MCF-7 | Seventy-six pairs of breast cancer and adjacent normal tissues from patients after surgery | 50–400 µM | Upregulation of PAX8-AS1-N, resulting in the inhibition of tumor growth and progression by modulating miR-17-5p and its downstream targets | [77] |
| MCF-7 and MDA-MB-231 | Female BALB/c nude mice (3–6 weeks old) | 10–40 µM | Inhibition of proliferation and induction of apoptosis and autophagy Suppression of the PI3K/AKT/mTOR signaling pathway | [78] |
| MDA-MB-231, MCF-10A, and HeLa | - | 2–50 µM | Inhibition of breast cancer cell motility by disrupting paracrine interactions mediated by laminin-332 | [79] |
| MDA-MB-231 and MCF-7 | - | 20 µM | Baicalein inhibited oleic acid-induced migration and invasion in breast cancer cells Blocking of AKT2 and FAK activation Reduction of 12(S)-HETE secretion through 12-LOX inhibition Altering focal contact formation | [80] |
| MDA-MB-231 and MDA-MB-231/IR | - | 20–100 µM | Reversion of IFIT2 expression, suppression of stem cell-like properties, and induction of apoptosis | [81] |
| MCF-10A | Female MMTV-PyMT transgenic mice (about 3 weeks old) | 2.5–10 µM | Inhibition of fibronectin-induced migration, invasion, and epithelial-mesenchymal transition in breast epithelial cells by suppressing calpain-2 activity and modulating Ca2+ levels and ERK signaling In vivo, baicalein delays tumor progression, reduces metastasis, and prolongs survival in a breast cancer mouse model | [82] |
| MCF-7 and MCF-10A | - | 12.5–200 µM | Inhibition of MCF-7 breast cancer cell proliferation and induction of apoptosis through a copper-dependent mechanism involving ROS generation and mitochondrial dysfunction | [26] |
| MCF-7 | - | 10–75 µM | Exhibition of weak activity in the CCID assay (IC50 = 130.2 µM) | [83] |
| 4T1 and MDA-MB-231 | - | 10–100 µM | Baicalein suppressed STAT3 activity by inhibiting its phosphorylation and reduces IL-6 production, leading to anti-proliferative, cytotoxic, and anti-metastatic effects | [84] |
| MCF-7 | - | 20 µM | Antiproliferative effect on MCF-7 cells Inhibition of TNF-α and IL-10 | [85] |
| B16-F10, 4T1, THP-1 | - | 20–80 µM | Baicalein exerted its anti-tumor effects by promoting M1 macrophage polarization through the NF-κB/TNF-α signaling pathway | [86] |
| MCF-7 | - | 20–84 µM | Exhibition of the cytotoxicity in MCF-7 cells (CC₅₀ = 56.46 µM)—Baicalein inactivated free virus particles and modestly reduced MV infection when added concurrently or immediately post-infection | [87] |
| MDA-MB-231, BT549, MDA-MB-468, MCF-7, ZR-75-1, T47D, and MCF-10A | BALB/c nude mice (6–8 weeks old) | 10–100 µM | Anti-tumor effects by targeting the KDM4E/BICD1/PAR1 signaling pathway | [88] |
| MDA-MB- 231, BT549, 4T1, and 3T3-L1 | Female BALB/C mice (4 weeks old) | 10–40 µM | Baicalein significantly slowed tumor growth and downregulated PD-L1, LEP, SREBP1, and p-STAT3 in tumors | [89] |
| MCF-7 | - | 25–400 µM | Inhibition of MCF-7 cells viability, induction of apoptosis, and suppression of the migration and invasion by modulating the Wnt3α/β-catenin signaling pathway Upregulation of Nischarin | [90] |
| MDA-MB-231, MCF-7, and HeLa | - | 3.23–22.2 µM | Induction of LDH release and increased caspase-3-activity, indicating cell membrane damage and apoptosis | [91] |
| Cell Lines | Animal Models | Plant/Materials | Baicalein Content | Baicalin Content | The Outcome of the Study | Reference |
|---|---|---|---|---|---|---|
| U87-MG, U251, MDA-MB-231, HMEC, and PC3 | - | thirteen Scutellaria species | 0.21–2.34 μg/mg extract | 0.92–25.34 μg/mg extract | Scutellaria species inhibited the proliferation of MDA-MB-231 cells and induced apoptosis | [19] |
| MDA-MB-435S | - | Phyllanthus amarus (whole plant) | present | not specified | The application of the aqueous extract mitigated Cr(VI)-induced oxidative toxicity in MDA-MB-435S in a dosage-dependent manner | [20] |
| MCF-7 | - | the roots of S. baicalensis | 40.54% in the baicalin-deprived fraction, 0.00–14.24% in other fractions | 0.51–19.58% | The presence of baicalin in the isolated fractions reduced their antiproliferative effects Baicalin-deprived fraction primarily contained baicalein, and wogonin demonstrated the strongest anticancer activity, primarily through cell cycle arrest and apoptosis induction | [96] |
| HCT-116 and MCF-7 | - | S. baicalensis root powder | 8.5–68.6% | 1.0–43.3% | Cellulase treatment significantly enhanced the antiproliferative potential of S. baicalensis extracts against cancer cells and promoted apoptosis through S-phase cell cycle arrest | [97] |
| MCF-7, HeLa, HT-29, and MRC-5 | - | black (Tuber aestivum Vittad.) and white (T. magnatum Pico) truffles | 2.01–15.73 µg/g dw in T. aestivum | - | LC-MS/MS analysis identified 14 phenolic compounds, with T. aestivum rich in p-hydroxybenzoic acid, baicalein, and kaempferol, and T. magnatum in epicatechin and catechin T. aestivum and T. magnatum extracts showed significant growth inhibition in MCF-7 and HeLa cells | [21] |
| MCF-7 | - | S. baicalensis Georgi extract | not specified | present | The extract exhibited potent anti-cancer effects on MCF-7 cells through mechanisms involving apoptosis induction via mitochondrial pathways, caspase activation, and ROS generation | [98] |
| 4T1 | - | leaves of Vernonia amygdalina Delile | present | not present | Ethyl acetate fraction of V. amygdalina Delile demonstrated anticancer potential by inducing cell cycle arrest, apoptosis/necrosis, and inhibiting PI3K/mTOR pathways | [22] |
| SK-BR-3, MDA-MB-231, MDA-MB-468, BT-549, MCF10A, 786-O, A2780, HCT116, HepG2, A549, Lo2, and WPMY-1, U-87 MG, and HT1080 | Female nude mice (4–6 weeks old) | Shuganning injection | not specified | not specified | It inhibited TNBC cell proliferation more effectively than non-TNBC cells It induces non-apoptotic, lipid peroxidation-dependent cell death (ferroptosis) In vivo experiments with nude mice showed that SGNI significantly inhibited tumor growth without adverse effects | [99] |
| MCF-7, Huh7, T47D, HCC 1954, AGS, LoVo, Mia-Paca2, U2OS, and MDA-MB-231 | Female C57BL/6 mice (6 weeks of age) | “Heirloom recipe” (Formula X) | not present | 5.07 mg/g | Formula X suppressed the growth of four breast cancer cell lines (T47D, MCF7, MDA-MB231, HCC1954) in a dose-dependent manner Reduction of the expression of the oncogenic proteins Berberine, baicalin, and saponin mixture reduced tumor growth in mouse models | [100] |
| MCF-7, T47D, Vero, and EMT6/P | BALB/c female mice (4–6 weeks old) | Xanthium spinosum | present in methanol fraction | present in hexane fraction | In vivo studies showed significant tumor size reduction in mice treated with X. spinosum extracts | [23] |
| 4T1 | Female BALB/c mice (4–6 weeks old) | Sanhuang Xiexin Decoction (SXD) | not present | 46.04 mg/g | SXD demonstrated significant antitumor effects against 4T1 breast cancer in mice through modulation of lipid profiles, cytokine levels, angiogenesis inhibition, and low toxicity | [101] |
| MCF-7 and MDA-MB-231 | - | Huangqin Tang (HQT) | present | not specified | HQT inhibited proliferation, induced apoptosis, induced G2/M phase arrest, and reduced HIF-1α protein levels in breast cancer cells | [102] |
| MCF-7, MDA-MB-231, SKBR-3, and L929 | - | aerial parts of Centaurea mersinensis | 11.12 µg/g | 648.28 µg/g | The methanol extract showed moderate cytotoxicity against breast cancer cell lines (IC50 = 22.17–46.20 µg/mL) | [24] |
| Derivative(s) | Cell Lines | Animal Models | The Outcome of the Study | Reference |
|---|---|---|---|---|
| Prenylated baicalein | MCF-7 and MDA-MB-231 | - | The prenylated baicalein derivative demonstrated antiproliferative activity in MCF-7 cells, likely mediated through estrogen receptor interactions, and showed potential for synergistic effects with 4-hydroxytamoxifen and fulvestrant | [104] |
| Baicalein-8-sodium sulfonate and Baicalein-7-O-sulfate | MCF-7 and H184B5F5/M10 | - | Baicalein-8-sodium sulfonate exhibited a higher anticancer effect than its precursor, induced apoptosis, cell cycle arrest, and ROS-mediated oxidative stress in MCF-7 cells | [105] |
| Oroxylin A | MCF-7 | - | Oroxylin A inhibited CYP1B1-mediated 4-hydroxylation of 17β-estradiol Oroxylin A was more potent than baicalein | [106] |
| 8-Chlorobaicalein, 8-bromobaicalein, chrysin, 8-chlorochrysin, 8-bromochrysin, 6,8-dichlorochrysin, 6-bromo-8-chlorochrysin, and 6,8-dibromochrysin | A549, LoVo, MV4-11, MCF-7, and MCF-10A | - | Baicalein and its halogenated derivatives showed increased cytotoxicity towards breast cancer cells (MCF-7) but were also toxic to non-tumorigenic breast cells (MCF-10A) | [107] |
| 8-Bromobaicalein | MCF-7 | - | 8-bromobaicalein and 2,6-di-O-methyl-β-cyclodextrin complexes demonstrated enhanced cytotoxic activity against MCF-7 breast cancer cells, with IC50 values significantly lower than free 8-bromobaicalein | [108] |
| Property/Activity | Baicalin | Baicalein |
|---|---|---|
| Molecular Formula | C21H18O11 | C15H10O5 |
| Molecular Weight (g/mol) | 446.36 | 270.24 |
| H-Bond Acceptors | 11 | 5 |
| H-Bond Donors | 6 | 3 |
| Molar Refractivity | 106.72 | 73.99 |
| TPSA (Å2) | 187.12 | 90.90 |
| Consensus LogPo/w | 0.25 | 2.24 |
| Water Solubility LogS | −3.41 | −4.03 |
| GI Absorption | Low | High |
| BBB Permeant | No | No |
| P-gp Substrate | Yes | No |
| CYP Inhibition | None | CYP1A2, CYP2D6, CYP3A4 |
| Skin Permeation (Log Kp) (cm/s) | −8.23 | −5.70 |
| Breast Cancer Cell Lines Tested | 4T1, MADB-106, MCF-7, MCF-7 ADRB2^OE, MDA-MB-231, SK-BR-2, ZR-75-1 | 4T1, BT549, Hs578T, MCF-7, MDA-MB-231, MDA-MB-231/IR, MDA-MB-435, MDA-MB-468, MDA-MB-468-C23, SK-BR-3, T47D, ZR-75-1 |
| Bioavailability Issues | Poor water solubility Poor intestinal absorption | Better absorption than baicalin Rapid glucuronidation in liver/enterocytes |
| Derivatives | No derivatives were examined | Prenylated, sulfonated, and halogenated derivatives improved efficacy |
| Anti-Proliferative | Suppressed cell growth via ESR1 downregulation | Inhibition of cells via mitochondrial pathways and DDIT4 induction |
| Apoptotic | p53/Bax upregulation, caspase-3/-8/-9 activation, and ROS production | Activated mitochondrial apoptosis, caspase cascades, and Bcl-2 modulation, and increased Ca2+, ROS, and DNA damage |
| Anti-Metastatic | Inhibited EMT via β-catenin/TGF-β | Blocked MMP-2/9, SATB1/Wnt pathways |
| Pathway Targets | NF-κB, mTOR, miRNAs | STAT3, PI3K/AKT/mTOR, Wnt/β-catenin |
| Combination of: | Cell Lines | Animal Models | The Outcome of the Study | Reference |
|---|---|---|---|---|
| Baicalin and Salvia miltiorrhiza extract | MCF-7, T-47D, FaDu, and CAL-27 | - | Combining baicalin with S. miltiorrhiza extract resulted in synergistic effects, significantly enhancing the inhibition of MCF-7, T-47D, and CAL-27 cells | [110] |
| Baicalin and Doxorubicin | MCF-7 and chick cardiomyocytes | - | Baicalein protected against doxorubicin-induced cardiotoxicity by reducing ROS generation, preserving mitochondrial function, and inhibiting JNK-mediated apoptosis Baicalein did not interfere with doxorubicin anticancer activity in MCF-7 cells | [111] |
| Baicalein and Paclitaxel | MCF-7 | Female Kunming mice (4–6 weeks old) | When combined with paclitaxel, baicalein showed antagonistic effects, i.e., reduced the growth-inhibitory effect of paclitaxel | [112] |
| Baicalein and U0126 | MCF-7 | - | The combination of baicalein with U0126 enhanced inhibition of cell proliferation, induction of apoptosis and cell cycle arrest, and suppression of migration, likely through modulation of the MAPK/ERK signaling pathway | [113] |
| Baicalein and ultrasounds | MDA-MB-231 | - | Synergistic effect of the combination of baicalein and ultrasound Both baicalein and low-frequency ultrasound effectively reduce the invasive activity of MDA-MB-231 cells by downregulating the expression of MMP-2, MMP-9, and u-PA at both the mRNA and protein levels | [114] |
| Bismuth Oxide Nanoparticles, Cisplatin, and Baicalein-Rich Fraction from O. indicum | MCF-7, MDA-MB-231, and NIH/3T3 | - | The combination of bismuth oxide nanoparticles and cisplatin was the most effective in enhancing ROS generation and radiosensitization in breast cancer cells The addition of a baicalein-rich fraction had significant radiosensitization effects in normal cells | [115] |
| Baicalin and Docetaxel | MDA-MB-231, MDA-MB-453, and 4T1 | Female BALB/c mice (6–8 weeks old) | Baicalin inhibited cell proliferation, induced mitochondria-mediated apoptosis, suppressed migration and invasion via the NF-κB pathway, and enhanced chemosensitivity to docetaxel Baicalin inhibited tumor growth and pulmonary metastasis | [116] |
| Baicalin and Doxorubicin | MDA-MB-231 and MCF-7 | - | Baicalin enhanced doxorubicin anticancer effects by increasing oxidative stress, calcium levels, and mitochondrial dysfunction in breast cancer cells | [117] |
| Baicalin and 5-fluorouracil | - | Swiss albino mice | The combination of baicalin and 5-fluorouracil demonstrated the most effective antitumor activity, significantly inhibiting tumor growth, promoting apoptosis, and reducing tumor-related biomarkers | [118] |
| Bismuth Oxide Nanoparticles, Cisplatin, and Baicalein-Rich Fraction from O. indicum | MCF-7, MDA-MB-231, and NIH/3T3 | - | The combination demonstrated the most potent radiosensitization effects across all radiation types in cancer cells | [119] |
| Baicalein and Capecitabine | MCF-7 | Thirty-two adult female albino rats | In MCF-7 cells, the combination of compounds enhanced cytotoxicity, cell cycle arrest, and apoptosis compared to individual treatments In rats, baicalein partially mitigated capecitabine-induced cardiotoxicity, oxidative stress, and inflammation | [120] |
| Baicalin and Doxorubicin | MDA-MB-231 | - | Baicalein enhanced the sensitivity of MDA-MB-231 cells to doxorubicin by activating autophagy and mitophagy, down-regulating CDK1, and inhibiting Drp1-mediated mitochondrial fission | [121] |
| Baicalin/baicalein and Doxorubicin or Docetaxel | MCF-7 and HUVEC-ST | - | Baicalin or baicalein enhanced the cytotoxicity of doxorubicin and docetaxel in MCF-7 cells Both flavonoids increased doxorubicin uptake in MCF-7 cells, induced apoptosis, and caused DNA damage | [122] |
| Baicalin, Epigallocatechin Gallate (EGCG), and Vincristine | EMT-6/P and EMT-6/V | Female BALB/c mice (4–6 weeks old) | Baicalin and EGCG demonstrate strong anti-proliferative effects, especially in vincristine-resistant cancer cells The combination with vincristine had a synergistic effect in resistant cells Combined therapy significantly reduced tumor size and achieved high cure rates in both models with no liver or kidney toxicity | [123] |
| Formula | Cell Lines | Animal Models | The Outcome of the Study | Reference |
|---|---|---|---|---|
| Baicalein-loaded iron oxide nanoparticles | MDA-MB-231 and HBL-100 | - | Baicalein-loaded nanoparticles showed significant cytotoxicity against MDA-MB-231 cells (IC50 = 22 µg/mL) Baicalein-loaded nanoparticles disrupted the cell cycle and induced apoptosis by downregulation of the anti-apoptotic protein Bcl-2 and upregulation of pro-apoptotic proteins (Bax, cytochrome-c, caspase-3, PARP, and p53) | [125] |
| Baicalin-loaded gold nanoparticles (AuNPs) conjugated with thiolated beta-cyclodextrin | MCF-7 | - | The obtained delivery system enhanced the apoptotic effects of baicalin | [126] |
| Baicalein and paclitaxel nanoemulsion | MCF-7 and MCF-7/Tax | Female BALB/c nude mice (6–8 weeks old) | The obtained nanoemulsions enhanced anticancer efficacy through synergistic effects, increased cellular uptake, oxidative stress induction, and apoptosis activation In an MCF-7/Tax xenograft model, nanoemulsion showed the highest tumor inhibition rate (77.0%) and minimal weight loss | [127] |
| Hyaluronic acid-decorated nanostructured lipid carriers (NLCs) as nanocarriers for co-delivery of baicalein and doxorubicin | MCF-7/ADR | Kunming mice (4–6 weeks old) | The co-delivery system exhibited enhanced targeting, sustained drug release, and synergistic anticancer effects (IC50 = 0.056 mg/mL) The obtained formula demonstrated the highest tumor inhibition rate (88%) in mice bearing human breast cancer | [128] |
| Baicalein-loaded iron oxide nanoparticles | MDA-MB-231 | - | Baicalein-loaded iron oxide nanoparticles effectively internalized into MDA-MB-231 cells, localized in critical cellular regions, and induced apoptosis through mitochondrial dysfunction, DNA damage, and cell cycle arrest. | [129] |
| Baicalin-loaded polylactide-glycolide (PLGA) nanocapsules | MCF-7 and MDA-MB-231 | - | Nanocapsules demonstrated superior anticancer activity and induced apoptosis, sustained drug release, and enhanced cellular uptake | [130] |
| Methoxy poly(ethylene glycol)-folic acid-decorated zeolitic imidazolate framework (ZIF-8) loaded with baicalin | MCF-7 and L929 | Female BALB/c mice | The nano-delivery system demonstrated excellent tumor-targeting capability, pH-responsive drug release, and potent anticancer activity both in vitro and in vivo. | [131] |
| Folate-modified albumin baicalin-loaded nanoparticles | MCF-7 | Female BALB/c nude mice (6–8 weeks old) | Nanoparticles demonstrated excellent tumor-targeting capability, sustained drug release, and potent anticancer activity both in vitro and in vivo The obtained system enhanced cellular uptake, induced apoptosis, and significantly inhibited tumor growth | [132] |
| Baicalein and cinnamon essential oil nanoemulsion | MDA-MB-231 | - | Nanoemulsion demonstrated enhanced anticancer activity 19-fold and 23-fold higher cytotoxicity compared to free baicalein after 12 h and 24 h, respectively, against breast cancer cells The anticancer activity is attributed to the combined effect of baicalein and cinnamon oil, along with improved drug permeability and cellular uptake via endocytosis | [133] |
| Hyaluronic acid-modified ferrous baicalein nanoparticle | HUVECs and 4T1 | Female BALB/c mice (8 weeks old) | In 4T1 tumor-bearing mice, nanoparticles and near-infrared significantly inhibited tumor growth (a combination of photothermal therapy and chemotherapy), showed tumor cell necrosis/apoptosis with no damage to major organs | [134] |
| Folate-modified albumin baicalin-loaded nanoparticles | MCF-7 | Female BALB/c nude mice | Nanoparticles demonstrated sustained drug release, enhanced cellular uptake, and potent anti-cancer effects through induction of cell cycle arrest, apoptosis, and autophagy | [135] |
| Silver nanoparticles obtained by S. multicaulis leaf extract | MDA-MB-231 and HFF2 | - | The green-synthesized silver nanoparticles demonstrated significant antioxidant, anticancer, and apoptotic activities | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieniuk, B.; Uğur, Ş. The Therapeutic Potential of Baicalin and Baicalein in Breast Cancer: A Systematic Review of Mechanisms and Efficacy. Curr. Issues Mol. Biol. 2025, 47, 181. https://doi.org/10.3390/cimb47030181
Zieniuk B, Uğur Ş. The Therapeutic Potential of Baicalin and Baicalein in Breast Cancer: A Systematic Review of Mechanisms and Efficacy. Current Issues in Molecular Biology. 2025; 47(3):181. https://doi.org/10.3390/cimb47030181
Chicago/Turabian StyleZieniuk, Bartłomiej, and Şuheda Uğur. 2025. "The Therapeutic Potential of Baicalin and Baicalein in Breast Cancer: A Systematic Review of Mechanisms and Efficacy" Current Issues in Molecular Biology 47, no. 3: 181. https://doi.org/10.3390/cimb47030181
APA StyleZieniuk, B., & Uğur, Ş. (2025). The Therapeutic Potential of Baicalin and Baicalein in Breast Cancer: A Systematic Review of Mechanisms and Efficacy. Current Issues in Molecular Biology, 47(3), 181. https://doi.org/10.3390/cimb47030181







