Antineoplastic Activity of Methyl rosmarinate in Glioblastoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Methyl rosmarinate
2.2. Cell Lines and Conditions for Treatment
2.3. Cell Viability Assay
2.4. Flow Cytometric Analysis of DNA Cell Cycle
2.5. Combination Treatment with RM and TMZ
2.6. Scratch Wound Healing Assay
2.7. Statistical Analysis
3. Results
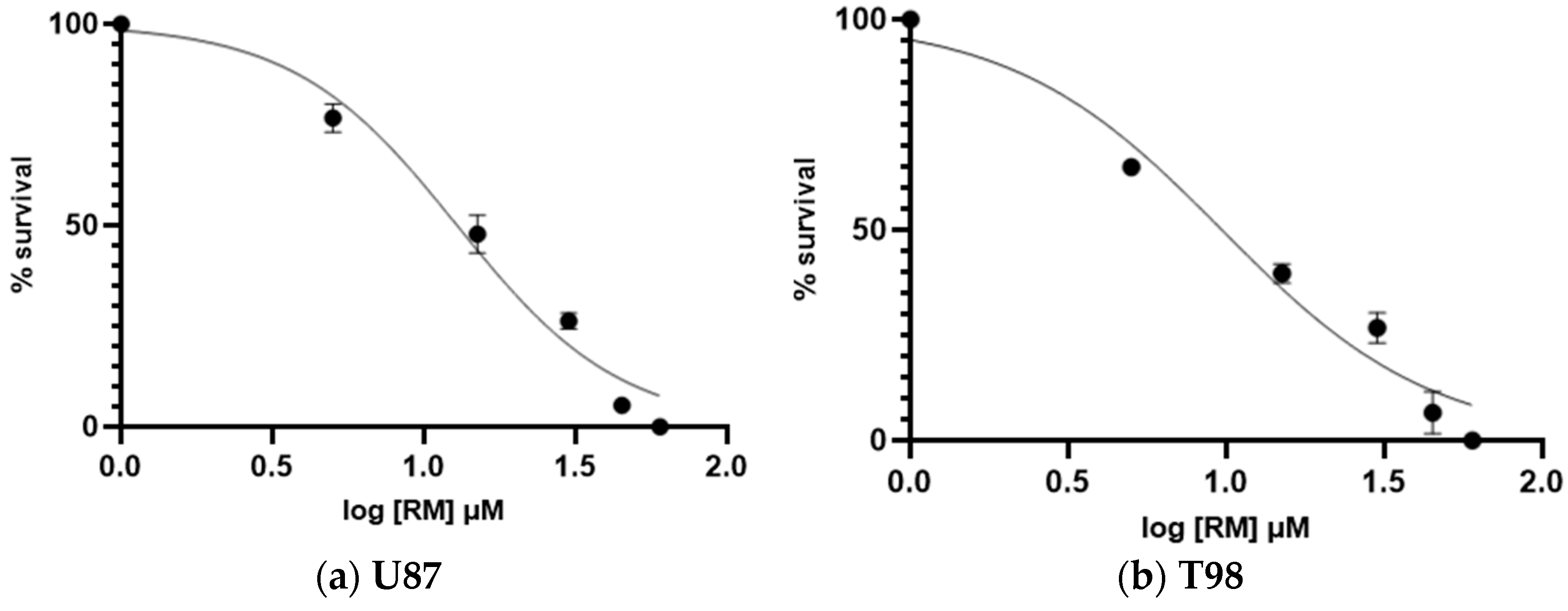
3.1. Calculation of Half Maximal Inhibitory Concentration of Methyl rosmarinate and GMB Viability
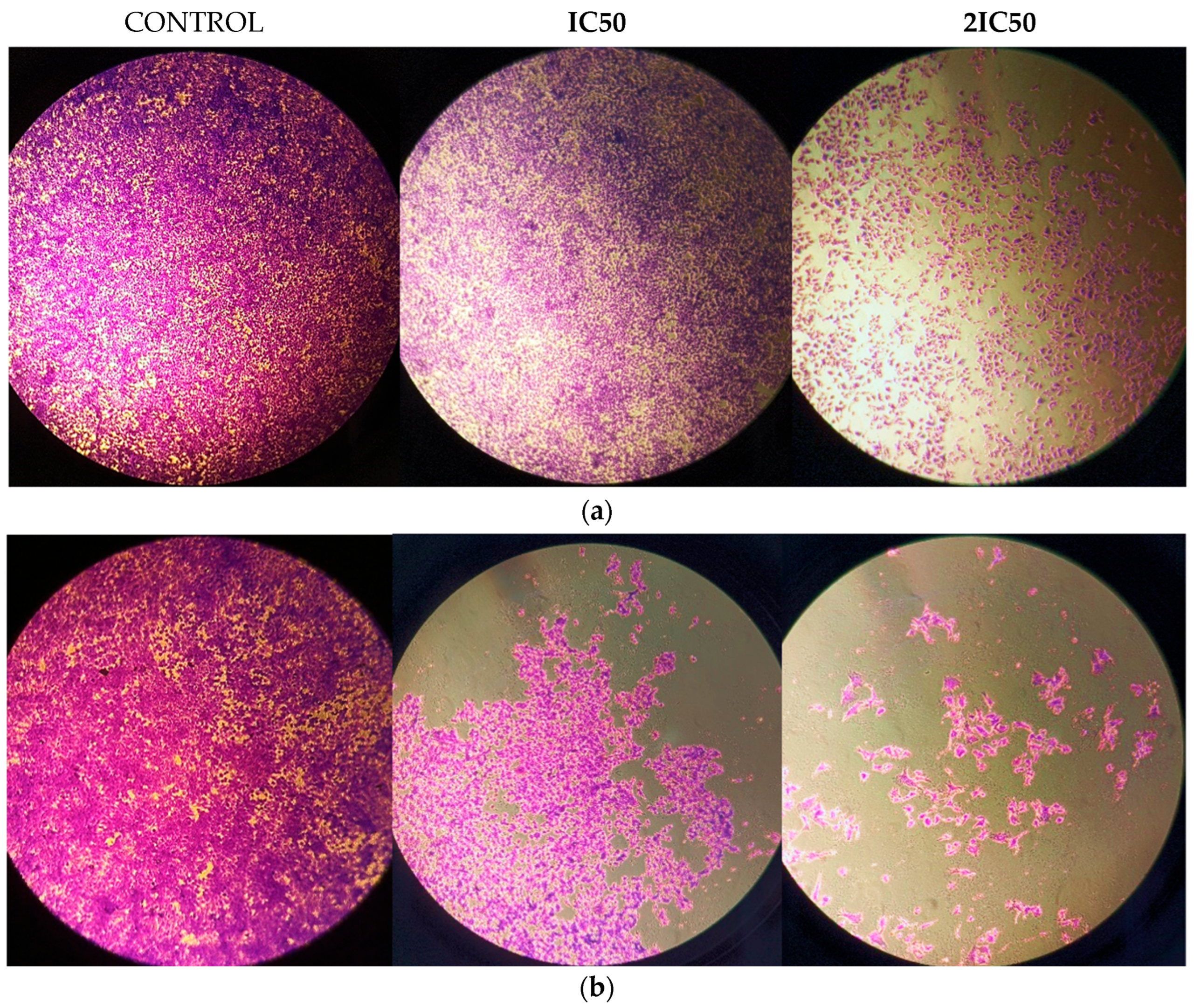
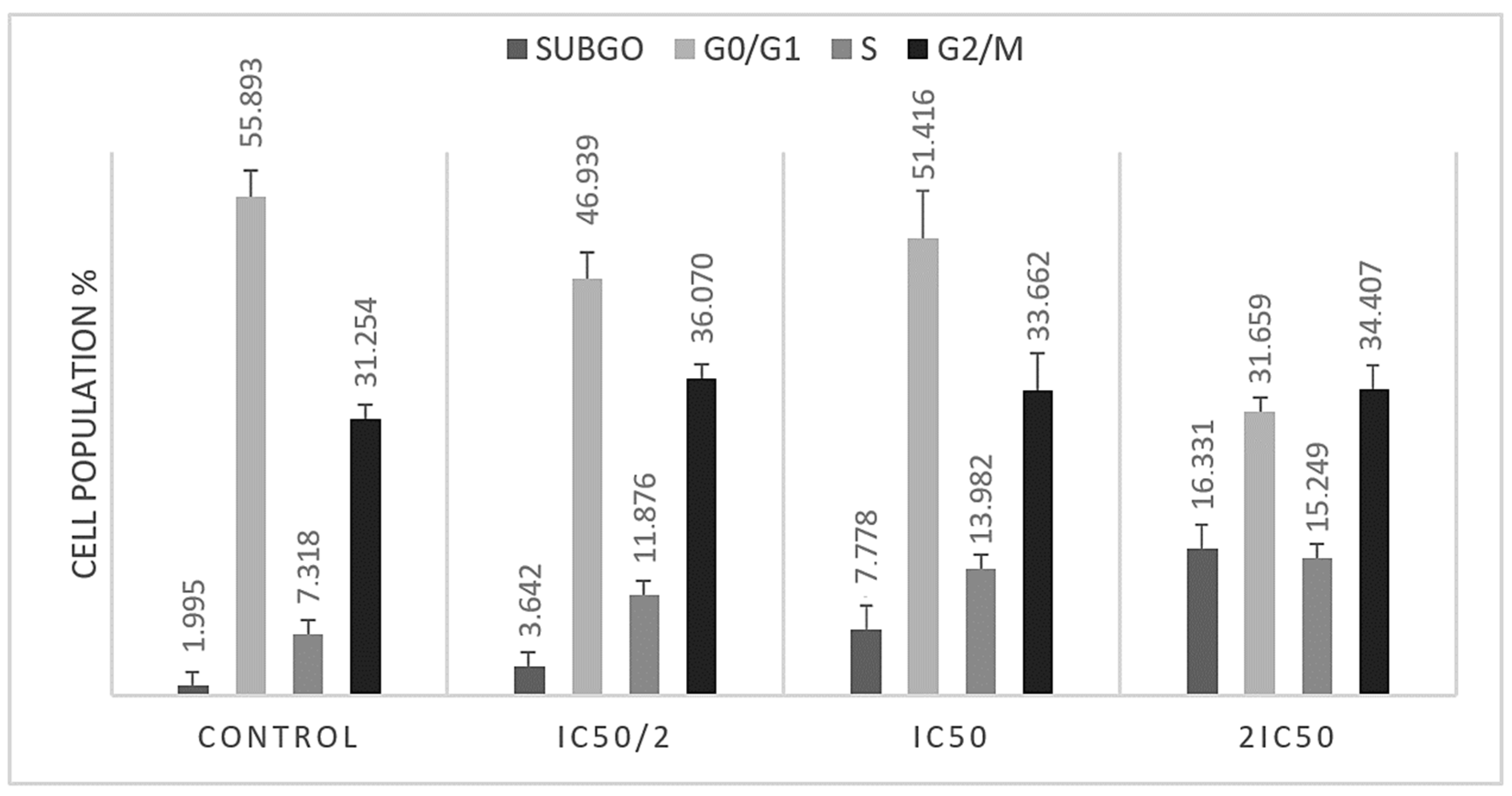
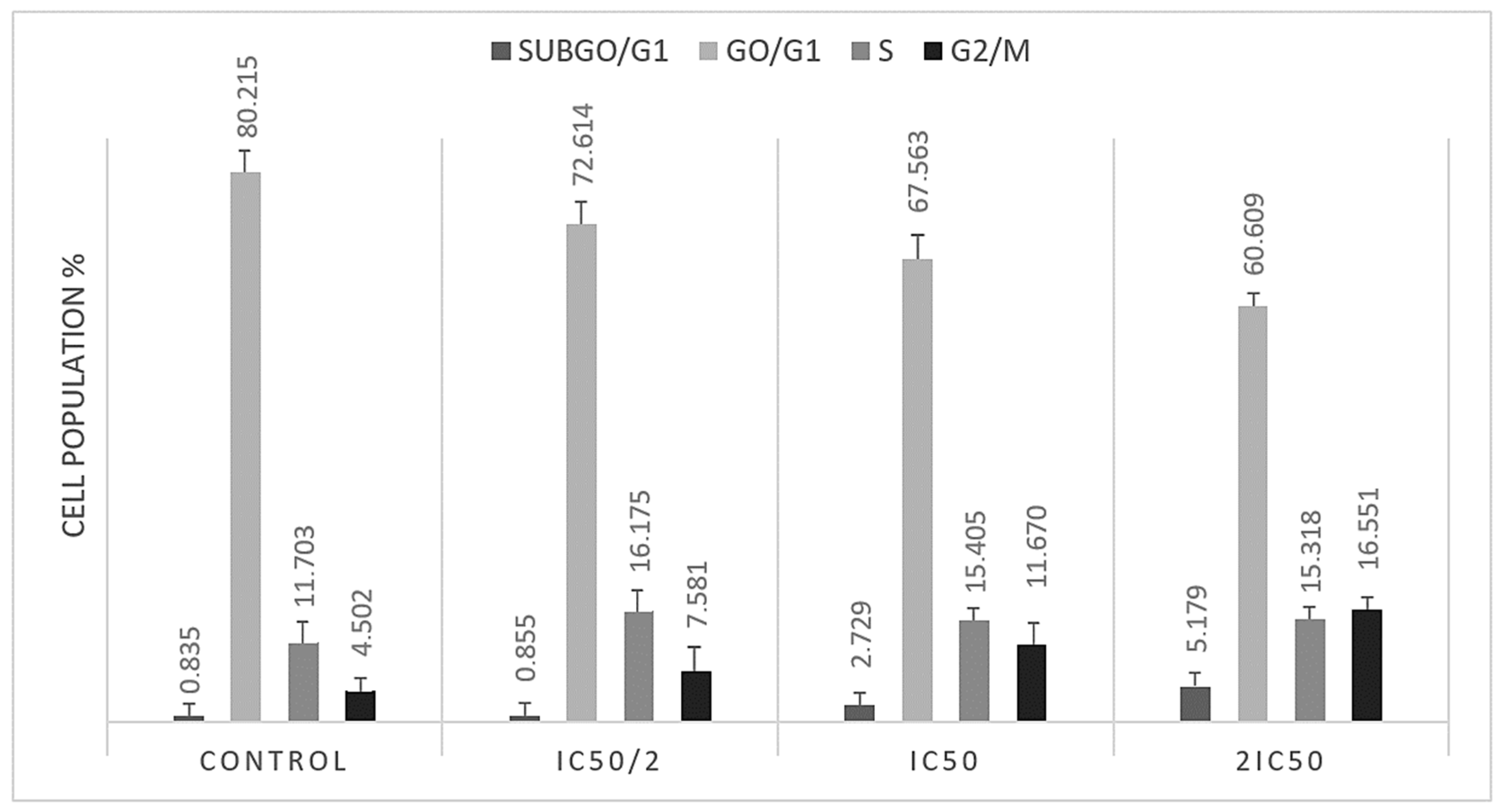
3.2. S and G2/M Cell Cycle Arrest Caused by Methyl rosmarinate
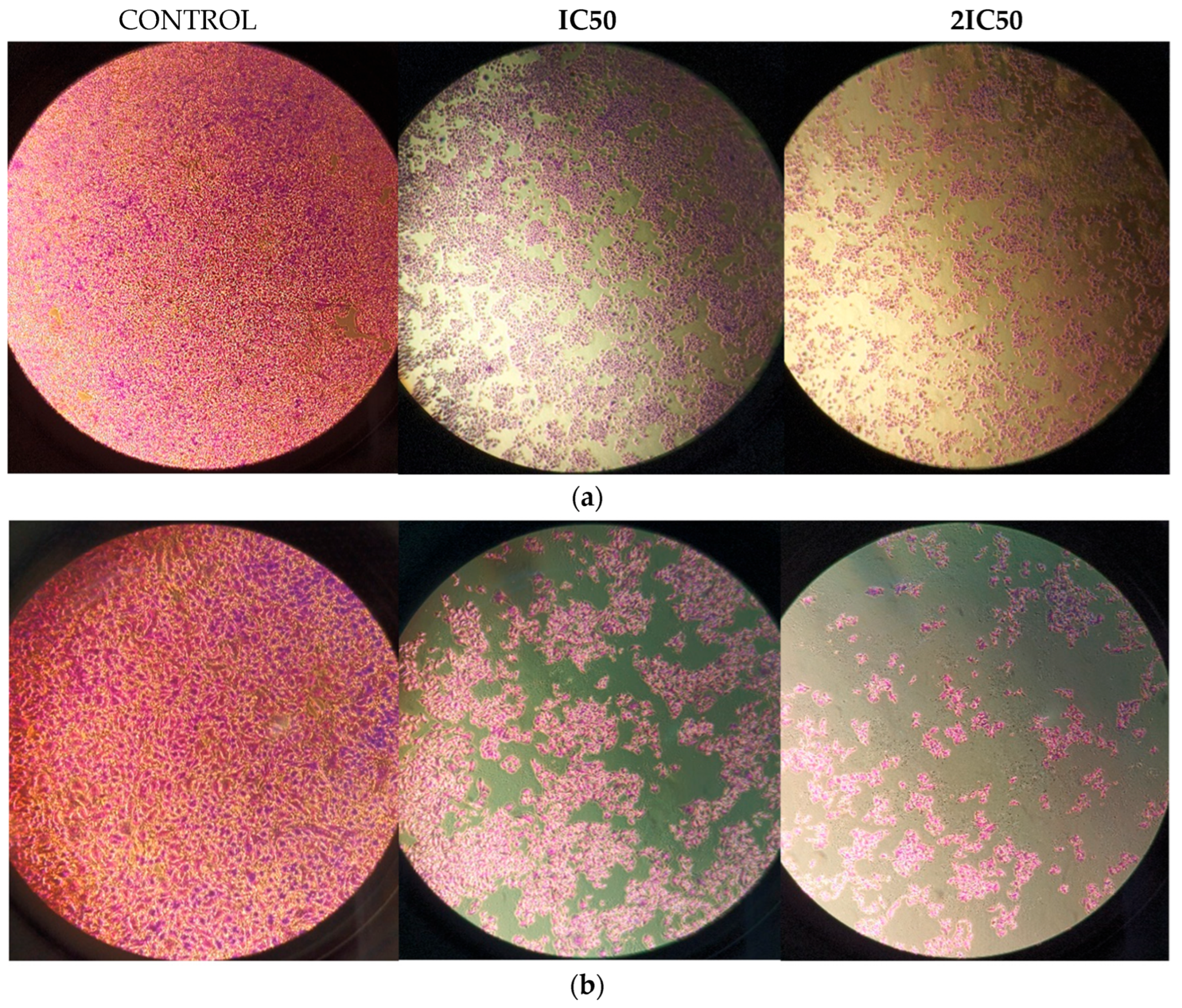
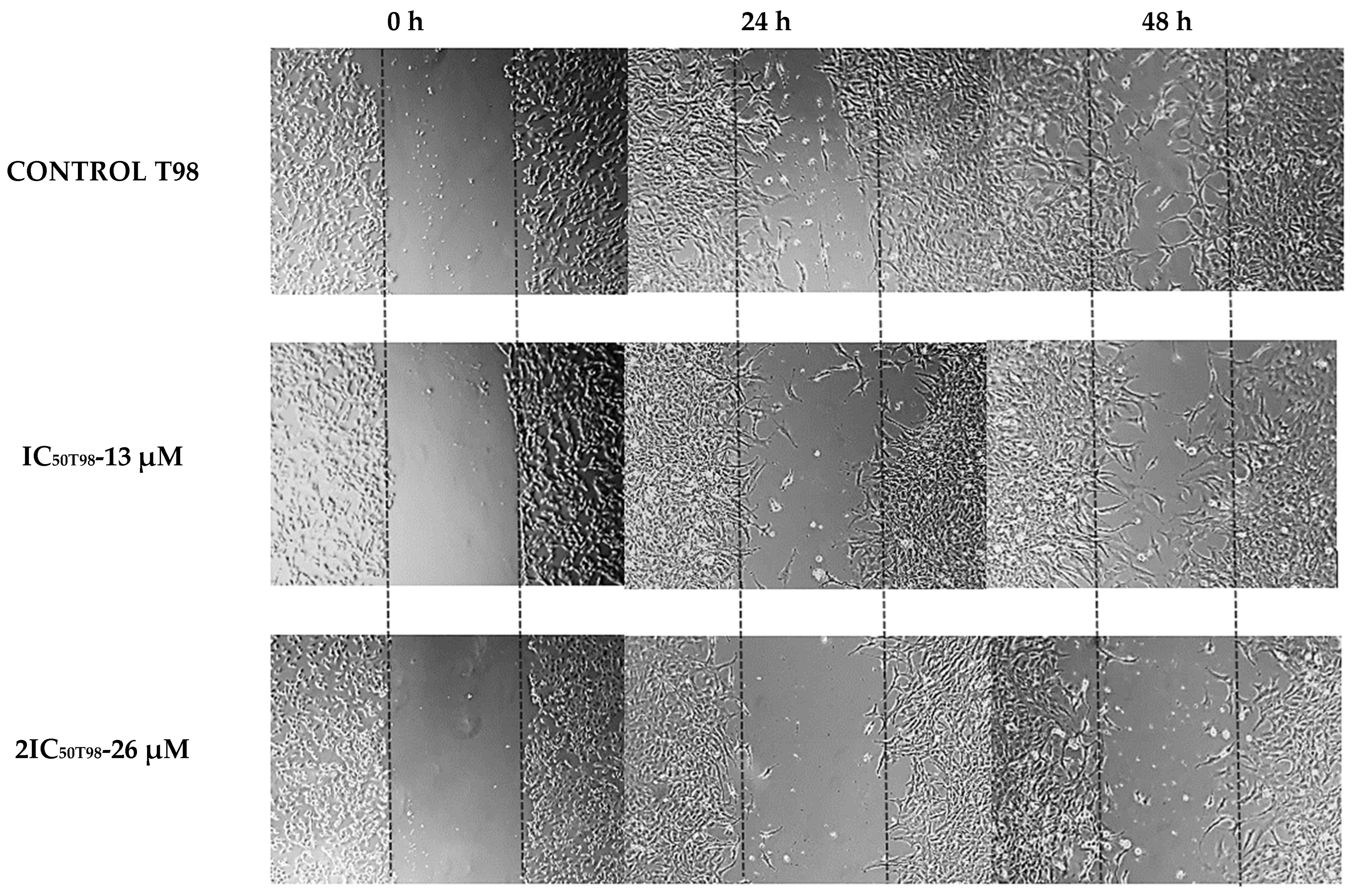
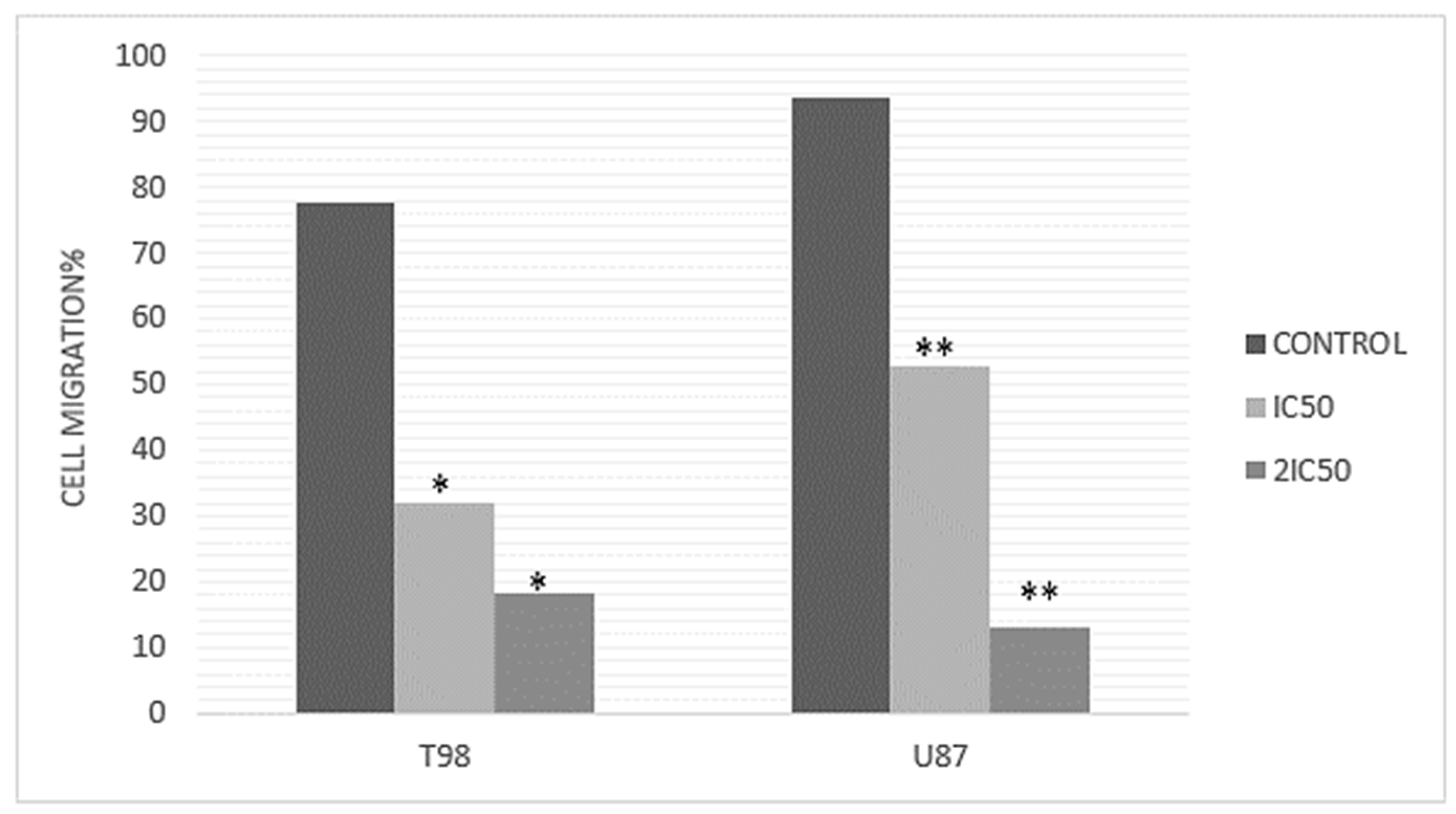
3.3. Methyl rosmarinate Inhibited U87 and T98 Cell Migration
3.4. Methyl rosmarinate and TMZ Have a Synergistic Effect on the U87 Cell Line
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD3OD | Deuterated Methanol |
| CI | Combination Index |
| BBB | Blood–brain barrier |
| DMSO | DimethylSulfoxide |
| FBS | Fetal Bovine Serum |
| GBM | Glioblastoma |
| H2SO4 | Sulfuric acid |
| MEK | Mitogen-activated protein kinase |
| NMR | Nuclear Magnetic Resonance |
| PBS | Phosphate-Buffered Saline |
| PI | Propidium Iodide |
| PI3K | Phosphoinositide 3—kinase |
| RM | Methyl rosmarinate |
| TMZ | Temozolomide |
| VEGFR2 | Vascular Endothelial Growth Factor Receptor 2 |
References
- Loras, A.; Gonzalez-Bonet, L.G.; Gutierrez-Arroyo, J.L.; Martinez-Cadenas, C.; Marques-Torrejon, M.A. Neural Stem Cells as Potential Glioblastoma Cells of Origin. Life 2023, 13, 905. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Reid, T.R.; Oronsky, A.; Sandhu, N.; Knox, S.J. A Review of Newly Diagnosed Glioblastoma. Front. Oncol. 2021, 10, 574012. [Google Scholar] [CrossRef] [PubMed]
- Groves, M.D.; Maor, M.H.; Meyers, C.; Kyritsis, A.P.; Jaeckle, K.A.; Yung, W.K.; Sawaya, R.E.; Hess, K.; Bruner, J.M.; Peterson, P.; et al. A phase II trial of high-dose bromodeoxyuridine with accelerated fractionation radiotherapy followed by procarbazine, lomustine, and vincristine for glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Li, X.; Zhang, X. Glioblastoma: An Update in Pathology, Molecular Mechanisms and Biomarkers. Int. J. Mol. Sci. 2024, 25, 3040. [Google Scholar] [CrossRef]
- DeCordova, S.; Shastri, A.; Tsolaki, A.G.; Yasmin, H.; Klein, L.; Singh, S.K.; Kishore, U. Molecular Heterogeneity and Immunosuppressive Microenvironment in Glioblastoma. Front. Immunol. 2020, 11, 1402. [Google Scholar] [CrossRef]
- Kyritsis, A.P.; Levin, V.A. An algorithm for chemotherapy treatment of recurrent glioma patients after temozolomide failure in the general oncology setting. Cancer Chemother. Pharmacol. 2011, 67, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Kyritsis, A.P.; Bondy, M.L.; Levin, V.A. Modulation of glioma risk and progression by dietary nutrients and antiinflammatory agents. Nutr. Cancer 2011, 63, 174–184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M.A. Status and Challenges of Plant-Anticancer Compounds in Cancer Treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Toma, M.M.; Gitea, D.; et al. Current Perspective on the Natural Compounds and Drug Delivery Techniques in Glioblastoma Multiforme. Cancers 2021, 13, 2765. [Google Scholar] [CrossRef]
- Blogspot.com. 2014. Available online: https://florahellenica.blogspot.com/2014/04/thymus-thracicus.html (accessed on 5 November 2024).
- Velen|Flora of Greece—An Annotated Checklist. Cybertaxonomy.org. 2024. Available online: https://portal.cybertaxonomy.org/flora-greece/cdm_dataportal/taxon/e8995434-a703-4c81-b149-aa6c87b0c70d (accessed on 5 November 2024).
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, A.M. Biomedical features and therapeutic potential of rosmarinic acid. Arch. Pharm. Res. 2022, 45, 205–228. [Google Scholar] [CrossRef]
- Kernou, O.-N.; Azzouz, Z.; Madani, K.; Rijo, P. Application of Rosmarinic Acid with Its Derivatives in the Treatment of Microbial Pathogens. Molecules 2023, 28, 4243. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Roumy, V.; Mahieux, S.; Biabiany, M.; Standaert-Vitse, A.; Rivière, C.; Sahpaz, S.; Bailleul, F.; Neut, C.; Hennebelle, T. Rosmarinic Acid and Its Methyl Ester as Antimicrobial Components of the Hydromethanolic Extract of Hyptis atrorubens Poit. (Lamiaceae). Evid.-Based Complement. Altern. Med. 2013, 2013, 604536. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, X.; Zhang, M.; Bai, B.; Yang, Y.; Fan, S. The antibacterial mechanism of perilla rosmarinic acid. Biotechnol. Appl. Biochem. 2021, 69, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudah, M.A.; Tashtoush, H.I.; Khlaifat, E.F.; Ibrahim, S.O.; Saleh, A.M.; Al-Jaber, H.I.; Abu Zarga, M.H.; Abu Orabi, S.T. Chemical constituents of the aerial parts of Salvia judaica Boiss. from Jordan. Nat. Prod. Res. 2019, 34, 2981–2985. [Google Scholar] [CrossRef]
- Lin, L.; Dong, Y.; Zhao, H.; Wen, L.; Yang, B.; Zhao, M. Comparative evaluation of rosmarinic acid, Methyl rosmarinate and pedalitin isolated from Rabdosia serra (MAXIM.) HARA as inhibitors of tyrosinase and α-glucosidase. Food Chem. 2011, 129, 884–889. [Google Scholar] [CrossRef]
- Içen, M.S.; Gürbüz, İ.; Bedir, E.; Günbatan, T.; Demirci, F. Isolation of rosmarinic acid and Methyl rosmarinate as lipoxygenase inhibitors from Salvia palaestina Benth. by activity-guided fractionation. S. Afr. J. Bot. 2021, 141, 177–182. [Google Scholar] [CrossRef]
- Li, H.; Sun, M.; Lei, F.; Liu, J.; Chen, X.; Li, Y.; Wang, Y.; Lu, J.; Yu, D.; Gao, Y.; et al. Methyl rosmarinate is an allosteric inhibitor of SARS-CoV-2 3 C L protease as a potential candidate against SARS-CoV-2 infection. Antivir. Res. 2024, 224, 105841. [Google Scholar] [CrossRef]
- Senol Deniz, F.S.; Eren, G.; Orhan, I.E.; Sener, B.; Ozgen, U.; Aldaba, R.; Calis, I. Outlining In Vitro and In Silico Cholinesterase Inhibitory Activity of Twenty-Four Natural Products of Various Chemical Classes: Smilagenin, Kokusaginine, and Methyl rosmarinate as Emboldening Inhibitors. Molecules 2021, 26, 2024. [Google Scholar] [CrossRef]
- Xian, Y.; Jiang, W.; Liu, R.; Tu, K.; Gao, S.; Wang, J.; Zhang, L. Methyl rosmarinate induces cell apoptosis in human hepatocellular carcinoma cells via inhibiting the Akt/mTOR signaling pathways. J. Xi’an Jiaotong Univ. (Med. Sci.) 2023, 57, 802–808. [Google Scholar]
- Tzitiridou, P.; Zoi, V.; Papagrigoriou, T.; Lazari, D.; Sioka, C.; Alexiou, G.A.; Kyritsis, A.P. Antineoplastic Activity of 9″-Lithospermic Acid Methyl Ester in Glioblastoma Cells. Int. J. Mol. Sci. 2024, 25, 2094. [Google Scholar] [CrossRef]
- Hou, Y.-Z.; Chen, K.-K.; Deng, X.-L.; Fu, Z.-L.; Chen, D.-F.; Wang, Q. Anti-complementary constituents of Anchusa italica. Nat. Prod. Res. 2017, 31, 2572–2574. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulou, M.; Dimitriadis, K.; Koromili, M.; Zoi, V.; Vartholomatos, E.; Galani, V.; Kyritsis, A.P.; Alexiou, G.A.; Lazari, D. Siderol Inhibits Proliferation of Glioblastoma Cells and Acts Synergistically with Temozolomide. Biomedicines 2022, 10, 3216. [Google Scholar] [CrossRef] [PubMed]
- Zoi, V.; Papagrigoriou, T.; Tsiftsoglou, O.S.; Alexiou, G.A.; Giannakopoulou, M.; Tzima, E.; Tsekeris, P.; Zikou, A.; Kyritsis, A.P.; Lazari, D.; et al. Therapeutic Potential of Linearol in Combination with Radiotherapy for the Treatment of Glioblastoma In Vitro. Int. J. Mol. Sci. 2023, 24, 3760. [Google Scholar] [CrossRef] [PubMed]
- Burster, T.; Traut, R.; Yermekkyzy, Z.; Mayer, K.; Westhoff, M.-A.; Bischof, J.; Knippschild, U. Critical View of Novel Treatment Strategies for Glioblastoma: Failure and Success of Resistance Mechanisms by Glioblastoma Cells. Front. Cell Dev. Biol. 2021, 9, 695325. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Mamun, A.A.; Sarwar, M.S.; Nasrin, F.; Emran, T.B.; Alanazi, I.S.; Rauf, A.; Albadrani, G.M.; Sayed, A.A.; et al. Natural Small Molecules Targeting NF-κB Signaling in Glioblastoma. Front. Pharmacol. 2021, 12, 703761. [Google Scholar] [CrossRef]
- Prakash, V.; Gabrani, R. An Insight into Emerging Phytocompounds for Glioblastoma Multiforme Therapy. Cardiovasc. Hematol. Agents Med. Chem. 2023, 22, 336–347. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Valdés, A.; Gallego, R.; Suárez-Montenegro, Z.J.; Alarcón, M.; Ibañez, E.; Alvarez-Rivera, G.; Cifuentes, A. Blood–Brain Barrier Permeability Study of Potential Neuroprotective Compounds Recovered From Plants and Agri-Food by-Products. Front. Nutr. 2022, 9, 924596. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, W.; Wang, L.; Shan, L.; Li, H.; Huang, J.; Sun, Q.; Zhang, W. Synthesis of derivatives of Methyl rosmarinate and their inhibitory activities against matrix metalloproteinase-1 (MMP-1). Eur. J. Med. Chem. 2013, 62, 148–157. [Google Scholar] [CrossRef]
- Ismael, D. Systems Pharmacology and Molecular Docking Reveals the Potential Beneficial effect of Rabdosia serra in Treating liver Cancer. J. Pharm. Pharmacol. Res. 2023, 7, 145–156. [Google Scholar]
- Eclarin, P.R.; Nas, J.S. Potential cytotoxic and antiangiogenic properties of Ehretia microphylla leaf ethanolic extract through the modulation of MEK and VEGFR 2. Explor. Anim. Med. Res. 2023, 13, 158–168. [Google Scholar] [CrossRef]
- Verdugo, E.; Puerto, I.; Medina, M.Á. An update on the molecular biology of glioblastoma, with clinical implications and progress in its treatment. Cancer Commun. 2022, 42, 1083–1111. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, C.; Chen, N.; Gu, H.; Yen, A.; Cao, L.; Wang, E.; Wang, L. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget 2016, 7, 33440–33450. [Google Scholar] [CrossRef] [PubMed]
- Dewdney, B.; Jenkins, M.R.; Best, S.A.; Freytag, S.; Prasad, K.; Holst, J.; Endersby, R.; Johns, T.G. From signalling pathways to targeted therapies: Unravelling glioblastoma’s secrets and harnessing two decades of progress. Signal Transduct. Target. Ther. 2023, 8, 400. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.-Y.; Chien, Y.; Yang, Y.-P.; Chen, M.-T.; Lin, L.-T. Overview of the molecular mechanisms of migration and invasion in glioblastoma multiforme. J. Chin. Med. Assoc. 2021, 84, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Lieftink, C.; Beijersbergen, R.L. It takes two to tango, and the right music: Synergistic drug combinations with cell-cycle phase-dependent sensitivities. eBioMedicine 2021, 69, 103448. [Google Scholar] [CrossRef]
- Proto, M.C.; Fiore, D.; Piscopo, C.; Laezza, C.; Bifulco, M.; Gazzerro, P. Modified Adenosines Sensitize Glioblastoma Cells to Temozolomide by Affecting DNA Methyltransferases. Front. Pharmacol. 2022, 13, 815646. [Google Scholar] [CrossRef]
- Patyka, M.; Sharifi, Z.; Petrecca, K.; Mansure, J.; Jean-Claude, B.; Sabri, S. Sensitivity to PRIMA-1MET is associated with decreased MGMT in human glioblastoma cells and glioblastoma stem cells irrespective of p53 status. Oncotarget 2016, 7, 60245–60269. [Google Scholar] [CrossRef]
- Butler, M.; Pongor, L.; Su, Y.-T.; Xi, L.; Raffeld, M.; Quezado, M.; Trepel, J.; Aldape, K.; Pommier, Y.; Wu, J. MGMT Status as a Clinical Biomarker in Glioblastoma. Trends Cancer 2020, 6, 380–391. [Google Scholar] [CrossRef]
- Dymova, M.A.; Kuligina, E.V.; Richter, V.A. Molecular Mechanisms of Drug Resistance in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 6385. [Google Scholar] [CrossRef]
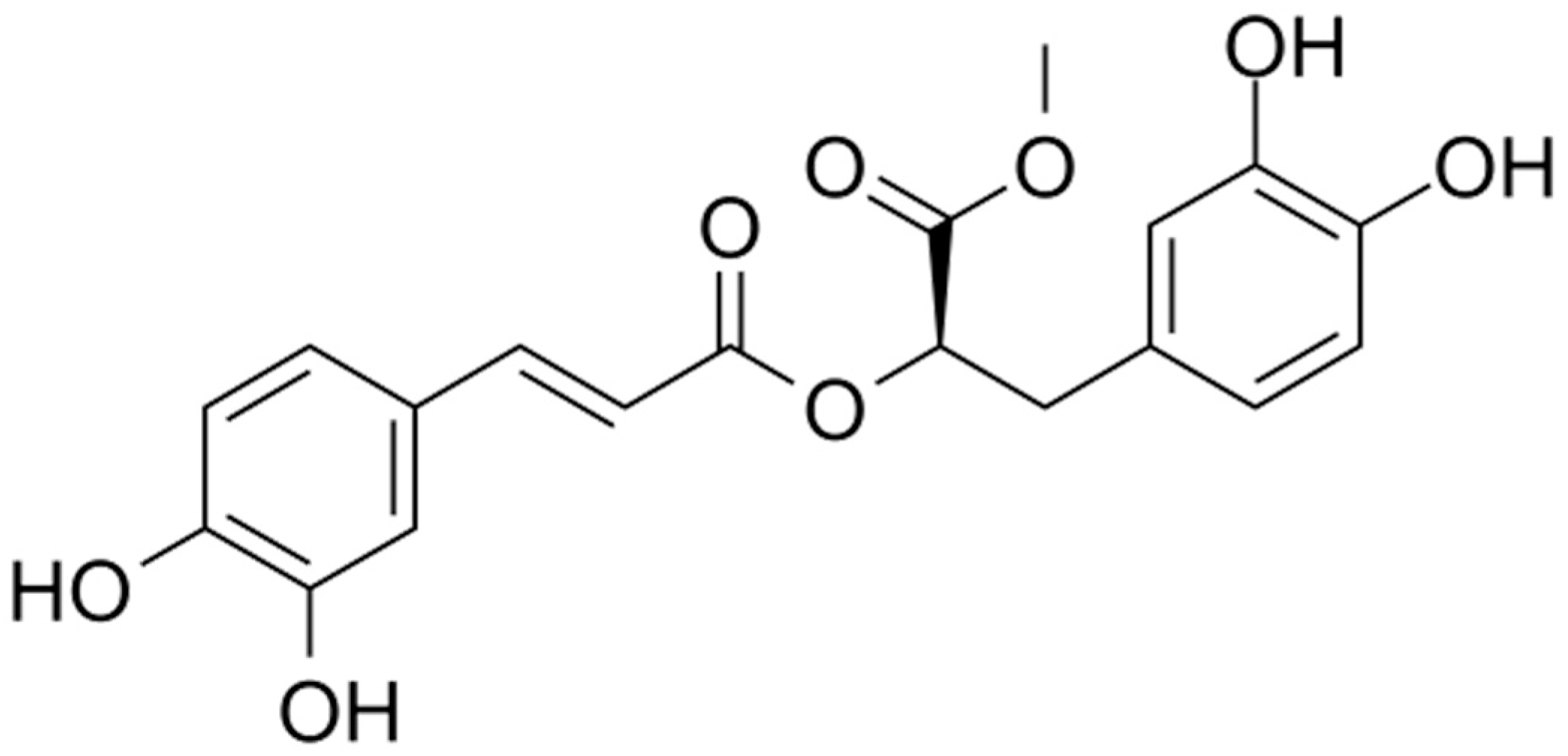
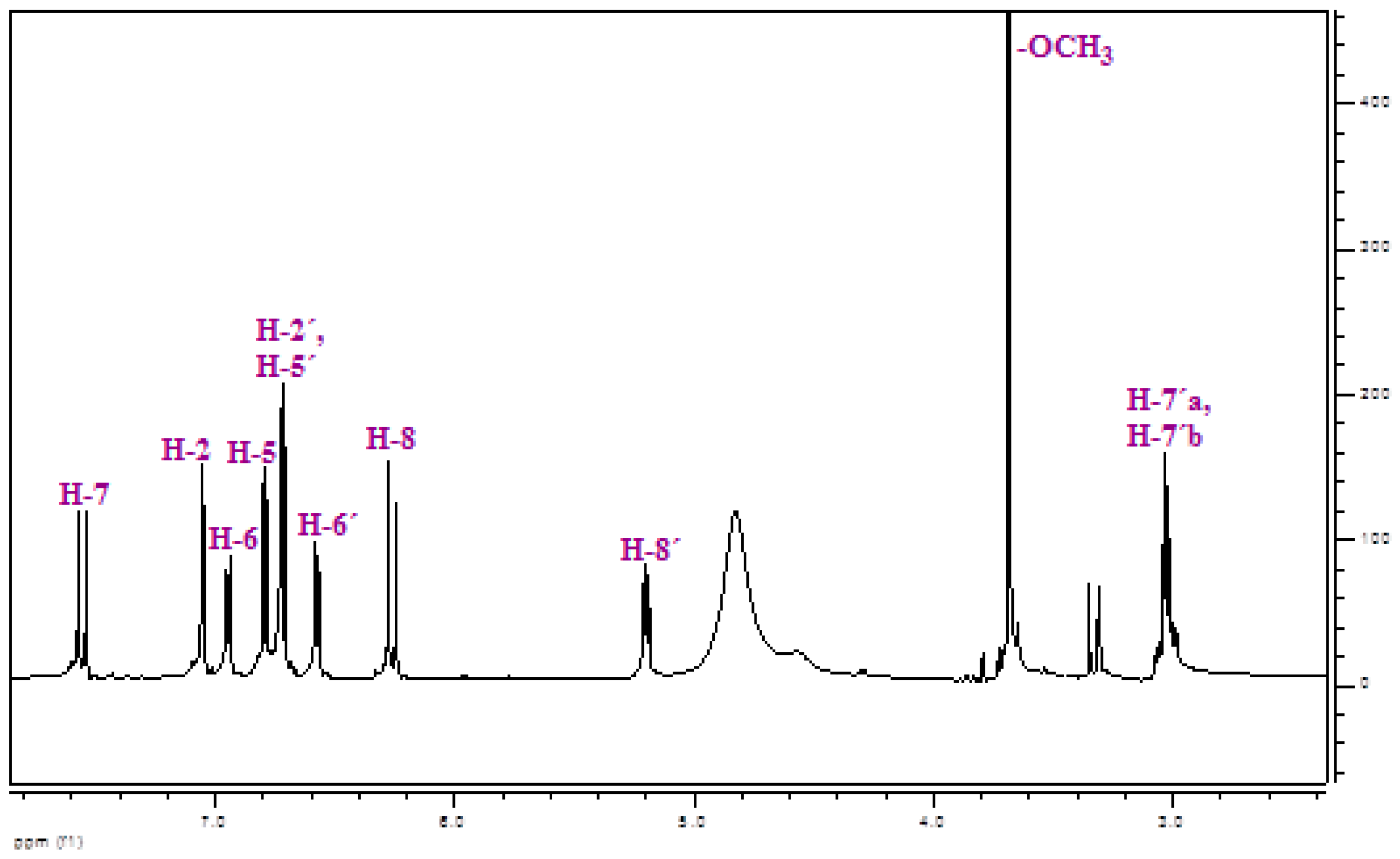


| δ (ppm) | Multiplicity | J (Hz) | no H | Identification |
|---|---|---|---|---|
| 7.55 | d | 16.1 | 1 | H-7 |
| 7.05 | d | 2.0 | 1 | H-2 |
| 6.94 | dd | 8.3, 2.0 | 1 | H-6 |
| 6.79 | d | 8.3 | 1 | H-5 |
| 6.72 | d | 2.0 | 1 | H-2′ |
| 6.71 | d | 8.3 | 1 | H-5′ |
| 6.57 | dd | 8.3, 2.0 | 1 | H-6′ |
| 6.26 | d | 16.1 | 1 | H-8 |
| 5.20 | dd | 7.4, 4.8 | 1 | H-8′ |
| 3.69 | s | - | 3 | -OCH3 |
| 3.05 | dd | 14.1, 4.8 | 1 | H-7′a |
| 3.01 | dd | 14.1, 7.4 | 1 | H-7′b |
| % | subG0 | G0/G1 | S | G2/M |
|---|---|---|---|---|
| Control | 1.9945 ± 0.448 | 55.893 ± 1.975 | 7.318 ± 0.316 | 31.254 ± 1.079 |
| IC50/2 | 3.642 ± 0.523 * | 46.939 ± 2.420 * | 11.876 ± 0.732 * | 36.070 ± 0.653 * |
| IC50 | 7.778 ± 1.676 * | 51.083 ± 6.369 * | 13.982 ± 1.019 * | 35.181 ± 3.801 * |
| 2IC50 | 16.331 ± 1.287 * | 31.659 ± 1.084 * | 15.249 ± 0.544 * | 34.407 ± 0.742 * |
| % | subG0 | G0/G1 | S | G2/M |
|---|---|---|---|---|
| Control | 0.835 ± 0.212 | 80.215 ± 1.549 | 11.703 ± 1.188 | 4.502 ± 0.256 |
| IC50/2 | 0.855 ± 0.076 * | 72.614 ± 2.291 * | 16.175 ± 1.016 * | 7.581 ± 1.735 * |
| IC50 | 2.729 ± 0.906 * | 67.563 ± 2.263 * | 15.405 ± 0.686 * | 11.670 ± 1.264 * |
| 2IC50 | 5.179 ± 0.441 * | 60.609 ± 0.149 * | 15.318 ± 0.304 * | 16.551 ± 0.315 * |
| Methyl rosmarinate RM (μΜ) | Temozolomide ΤΜΖ (μΜ) | Effect | CI | Conclusion |
|---|---|---|---|---|
| U87 cells | ||||
| 4.9 | 25 | 0.76 | 0.34767 | SYNERGY |
| 9.8 | 50 | 0.87 | 0.39376 | SYNERGY |
| 14.7 | 75 | 0.94 | 0.3095 | SYNERGY |
| 19.6 | 100 | 0.99 | 0.10174 | SYNERGY |
| T98 cells | ||||
| 6.5 | 60 | 0.65 | 1.28268 | ANTAGONISM |
| 13 | 120 | 0.88 | 1.5547 | ANTAGONISM |
| 19.5 | 180 | 0.92 | 1.98022 | ANTAGONISM |
| 26 | 240 | 0.99 | 1.21169 | ANTAGONISM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benekou, M.V.; Tzitiridou, P.; Papagrigoriou, T.; Galani, V.; Sioka, C.; Kyritsis, A.P.; Lazari, D.; Alexiou, G.A. Antineoplastic Activity of Methyl rosmarinate in Glioblastoma Cells. Curr. Issues Mol. Biol. 2025, 47, 180. https://doi.org/10.3390/cimb47030180
Benekou MV, Tzitiridou P, Papagrigoriou T, Galani V, Sioka C, Kyritsis AP, Lazari D, Alexiou GA. Antineoplastic Activity of Methyl rosmarinate in Glioblastoma Cells. Current Issues in Molecular Biology. 2025; 47(3):180. https://doi.org/10.3390/cimb47030180
Chicago/Turabian StyleBenekou, Maria Vasiliki, Panagiota Tzitiridou, Theodora Papagrigoriou, Vasiliki Galani, Chrissa Sioka, Athanassios P. Kyritsis, Diamanto Lazari, and George A. Alexiou. 2025. "Antineoplastic Activity of Methyl rosmarinate in Glioblastoma Cells" Current Issues in Molecular Biology 47, no. 3: 180. https://doi.org/10.3390/cimb47030180
APA StyleBenekou, M. V., Tzitiridou, P., Papagrigoriou, T., Galani, V., Sioka, C., Kyritsis, A. P., Lazari, D., & Alexiou, G. A. (2025). Antineoplastic Activity of Methyl rosmarinate in Glioblastoma Cells. Current Issues in Molecular Biology, 47(3), 180. https://doi.org/10.3390/cimb47030180









