Correlation of PD-L1 and HIF-1 Alpha Expression with KRAS Mutation and Clinicopathological Parameters in Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sections and Immunohistochemical Examination
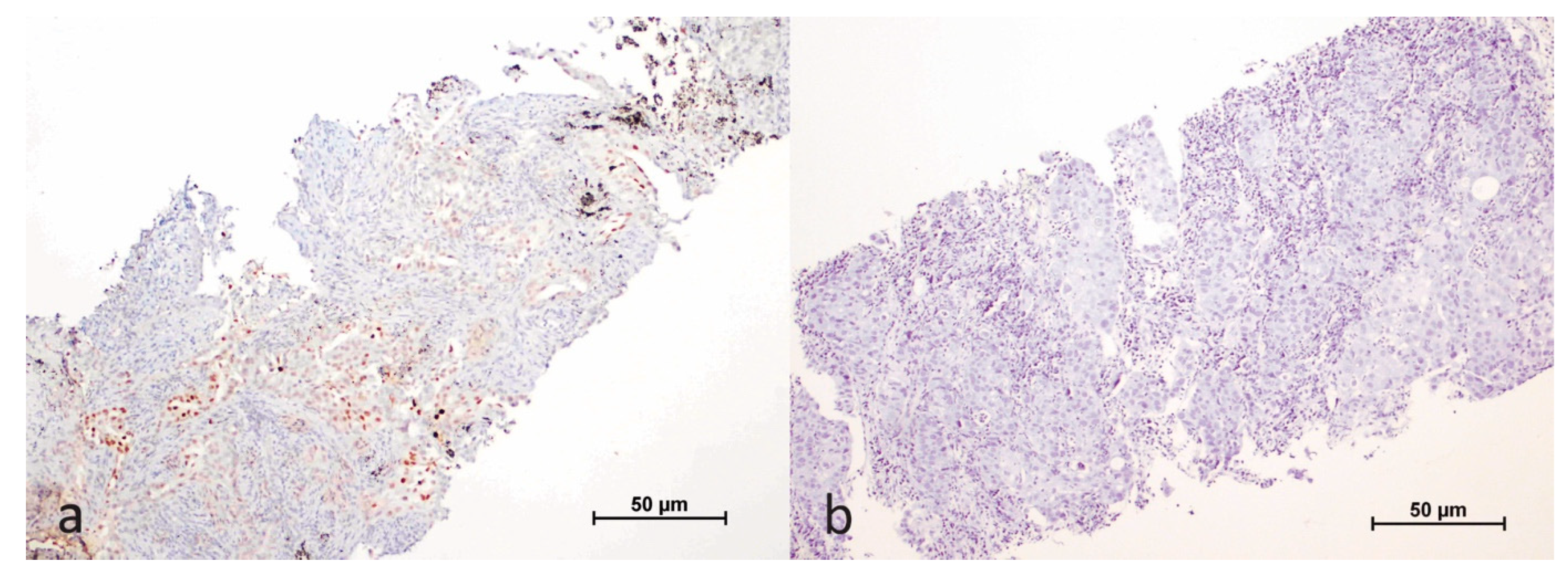
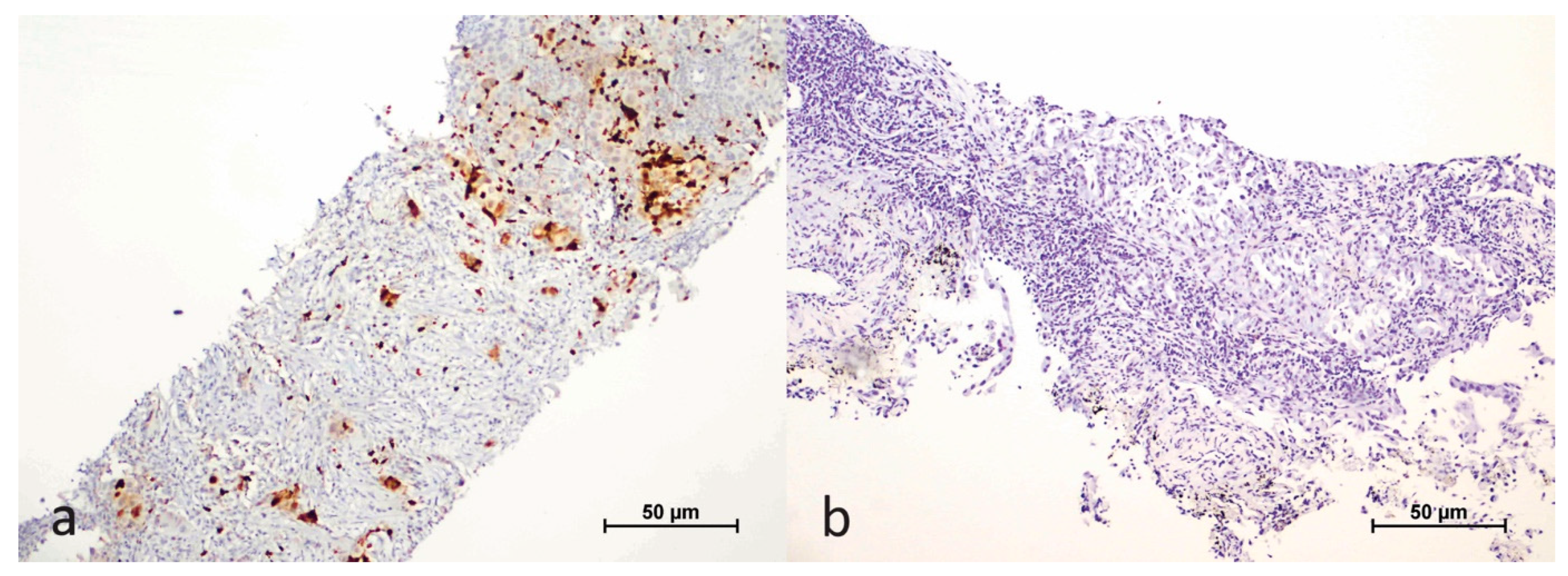
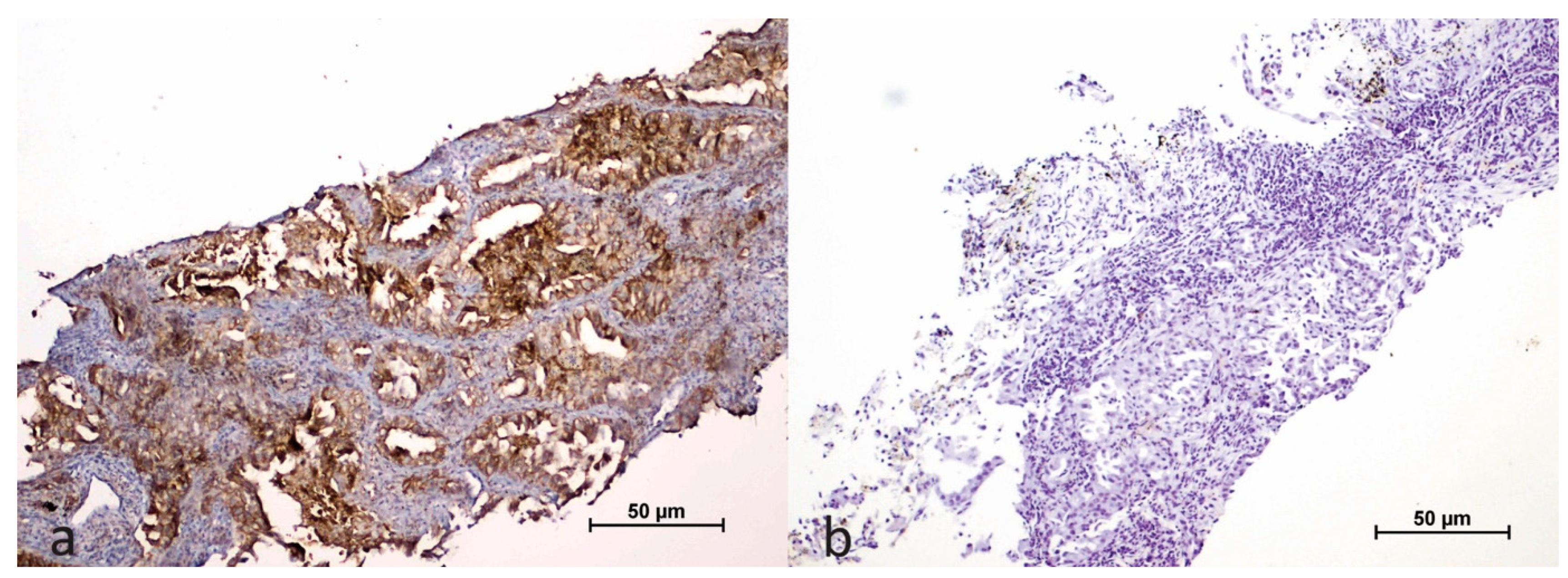
2.2. Evaluation of Immunohistochemical Study Results
2.3. Molecular Analysis: Real-Time PCR Tests for KRAS
2.4. Statistical Analysis
3. Results
3.1. Demographic Data of the Cases
3.2. Evaluation of KRAS Mutation Status with HIF-1α and PD-L1 Expression
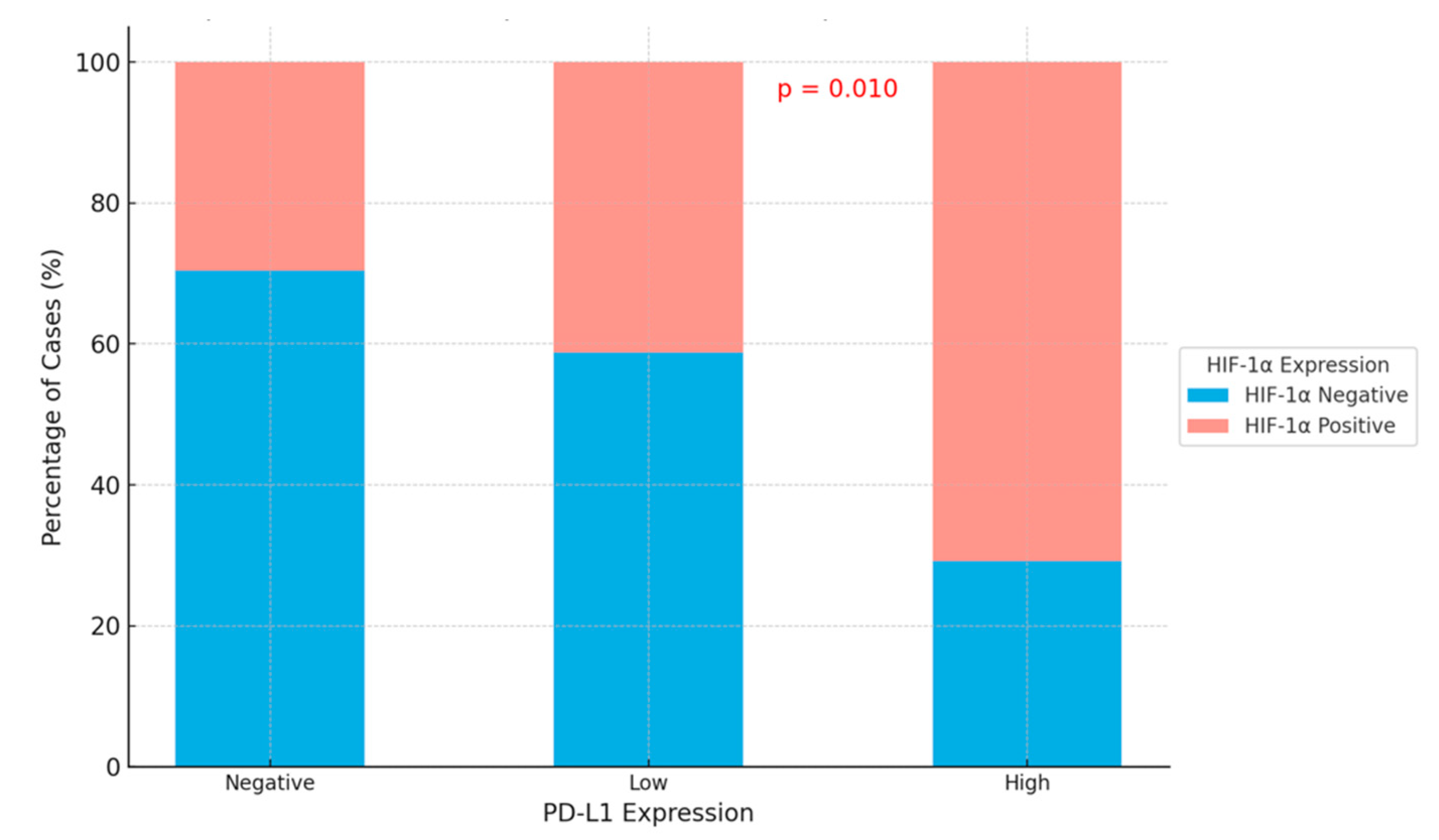
3.3. Relationship Between HIF-1α in Tumor Microenvironment and PD-L1
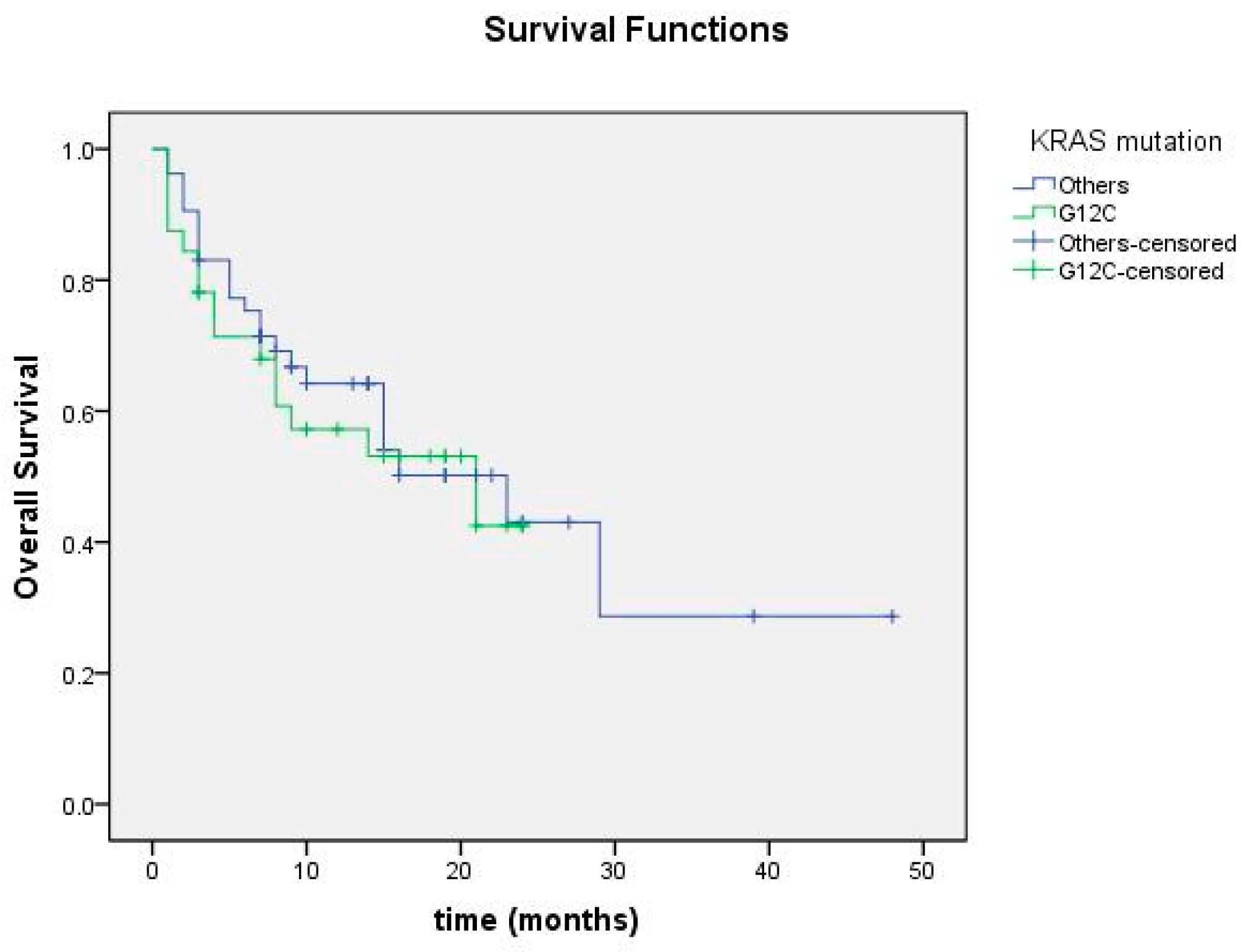
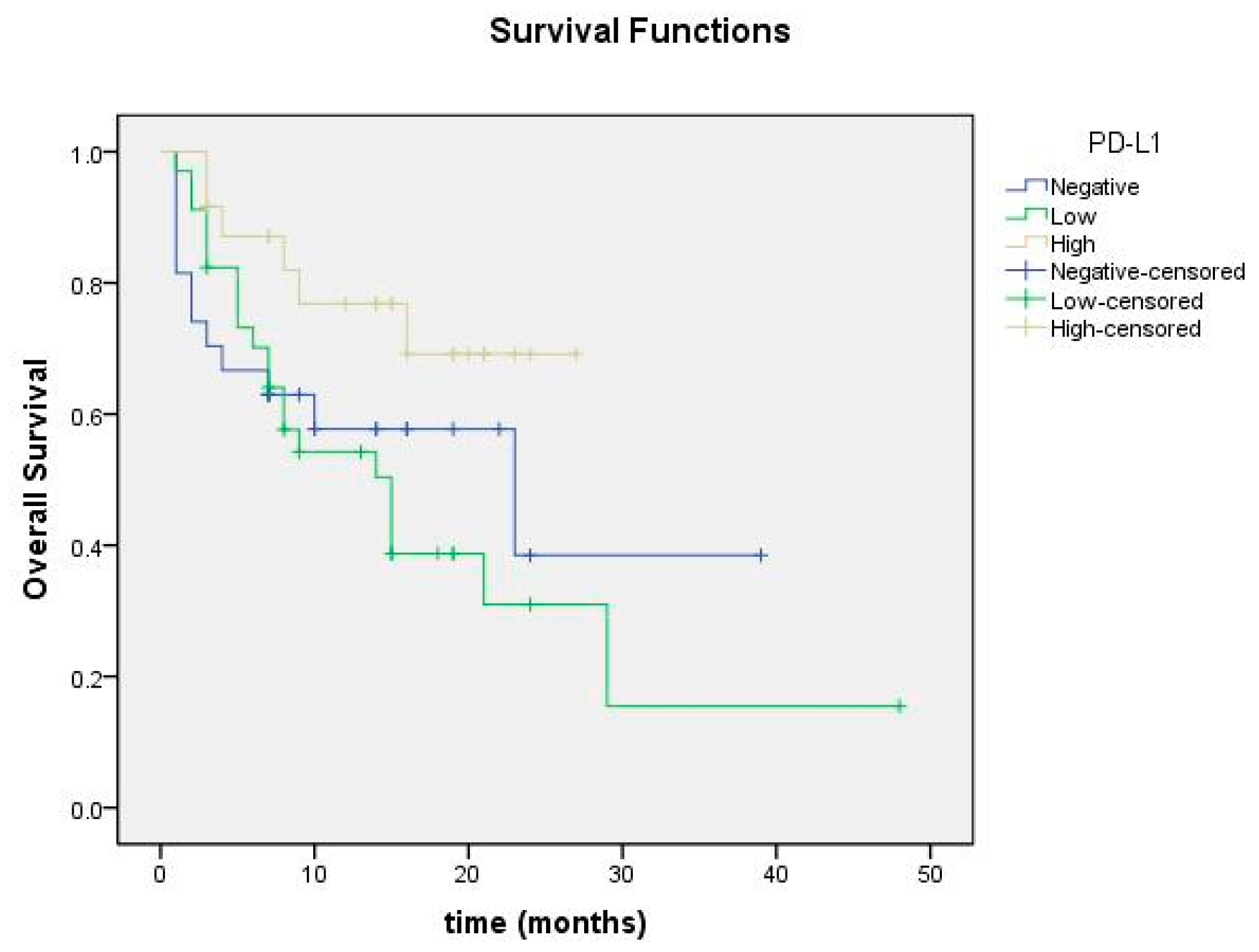
3.4. Correlation with Prognostic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberg, A.J.; Ford, J.G.; Samet, J.M. Epidemiology of Lung Cancer: ACCP Evidence-Based Clinical Practice Guidelines (2nd Edition). Chest 2007, 132, 29S–55S. [Google Scholar] [CrossRef]
- MacConaill, L.E. Advancing Personalized Cancer Medicine in Lung Cancer. Arch. Pathol. Lab. Med. 2012, 136, 1210–1216. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.; Yatabe, Y.; Ishikawa, Y.; Wistuba, I.; Flieder, D.B.; Franklin, W.; et al. Diagnosis of Lung Cancer in Small Biopsies and Cytology: Implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification. Arch. Pathol. Lab. Med. 2013, 137, 668–684. [Google Scholar] [CrossRef] [PubMed]
- Pentel, P.; Malin, D.; van Beurden, W.J.C.; Dekhuijzen, P.N.R.; Harff, G.A.; Smeenk, F.W.J.M.; Zureik, M.; Orehek, J.; Fietze, I.; Glos, M.; et al. Pattern of Lung Cancer in Turkey, 1994–1998. Respiration 2002, 69, 207–210. [Google Scholar] [CrossRef]
- Riely, G.J.; Marks, J.; Pao, W. KRAS Mutations in Non-Small Cell Lung Cancer. Proc. Am. Thorac. Soc. 2009, 6, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Johnson, R.S. Hypoxia: A Key Regulator of Angiogenesis in Cancer. Cancer Metastasis Rev. 2007, 26, 281–290. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for Cancer Therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ning, Y.; Zhan, Y.; Liu, S.; Yang, Y.; Wen, Q.; Fan, S. Co-Expression of PD-L1 and HIF-1alpha Predicts Poor Prognosis in Patients with Non-Small Cell Lung Cancer after Surgery. J. Cancer 2021, 12, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.D.; Fesik, S.W.; Kimmelman, A.C.; Luo, J.; Der, C.J. Drugging the Undruggable RAS: Mission Possible? Nat. Rev. Drug Discov. 2014, 13, 828–851. [Google Scholar] [CrossRef] [PubMed]
- Falk, A.T.; Yazbeck, N.; Thon, L.; Guibert, N.; Hofman, V.; Zahaf, K.; Lespinet, V.; Bordone, O.; Tanga, V.; Washetine, K.; et al. Impact of Kras Mutant Subtypes on PD-L1 Expression in Lung Adenocarcinoma. Ann. Oncol. 2016, 27, vi28. [Google Scholar] [CrossRef][Green Version]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L.; et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef]
- Aggarwal, C.; Maity, A.P.; Bauml, J.M.; Long, Q.; Aleman, T.; Ciunci, C.; D’Avella, C.; Volpe, M.; Anderson, E.; Jones, L.M.; et al. A Phase II Open-Label Trial of Binimetinib and Hydroxychloroquine in Patients With Advanced KRAS-Mutant Non-Small Cell Lung Cancer. Oncologist 2023, 28, 644–e564. [Google Scholar] [CrossRef] [PubMed]
- Janne, P.A.; Shaw, A.T.; Pereira, J.R.; Jeannin, G.; Vansteenkiste, J.; Barrios, C.; Franke, F.A.; Grinsted, L.; Zazulina, V.; Smith, P.; et al. Selumetinib plus Docetaxel for KRAS-Mutant Advanced Non-Small-Cell Lung Cancer: A Randomised, Multicentre, Placebo-Controlled, Phase 2 Study. Lancet Oncol. 2013, 14, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Janne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRAS(G12C) Mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar] [CrossRef]
- Wankhede, D.; Bontoux, C.; Grover, S.; Hofman, P. Prognostic Role of KRAS G12C Mutation in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 3043. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, P.; Cakar, B.; Gunenc, D.; Nart, D.; Cinkooglu, A.; Katgi, N. PDL-1 Expression and Survival in Metastatic Non-Small Cell Lung Cancer Patients Who Received Chemotherapy as First-Line Treatment. Turk. Thorac. J. 2022, 23, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.K.; Ye, F.; Wu, X.; An, H.X.; Wu, J.X. Clinicopathological and Prognostic Significance of Programmed Cell Death Ligand1 (PD-L1) Expression in Patients with Non-Small Cell Lung Cancer: A Meta-Analysis. J. Thorac. Dis. 2015, 7, 462–470. [Google Scholar] [CrossRef]
- Schmidt, L.H.; Kummel, A.; Gorlich, D.; Mohr, M.; Brockling, S.; Mikesch, J.H.; Grunewald, I.; Marra, A.; Schultheis, A.M.; Wardelmann, E.; et al. PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. PLoS ONE 2015, 10, e0136023. [Google Scholar] [CrossRef]
- Velcheti, V.; Schalper, K.A.; Carvajal, D.E.; Anagnostou, V.K.; Syrigos, K.N.; Sznol, M.; Herbst, R.S.; Gettinger, S.N.; Chen, L.; Rimm, D.L. Programmed Death Ligand-1 Expression in Non-Small Cell Lung Cancer. Lab. Investig. 2014, 94, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Taron, M.; Rosell, R.; Mendez, P.; Queralt, C.; Ronco, M.S.; Sanchez, J.J.; Sanchez, J.M.; Maestre, J.; Majo, J. Clinical Significance of Hypoxia-Inducible Factor-1a Messenger RNA Expression in Locally Advanced Non-Small-Cell Lung Cancer after Platinum Agent and Gemcitabine Chemotherapy Followed by Surgery. Clin. Lung Cancer 2005, 6, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Pardoll, D.M. Immune Checkpoint Inhibitors: Making Immunotherapy a Reality for the Treatment of Lung Cancer. Cancer Immunol. Res. 2013, 1, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer Immunotherapies Targeting the PD-1 Signaling Pathway. J. Biomed. Sci. 2017, 24, 26. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, E.; Papadimitrakopoulou, V.; Welsh, J.; Tang, C.; Tsao, A.S. Immunotherapy in Lung Cancer. Transl. Lung Cancer Res. 2014, 3, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Raez, L.E.; Cassileth, P.A.; Schlesselman, J.J.; Sridhar, K.; Padmanabhan, S.; Fisher, E.Z.; Baldie, P.A.; Podack, E.R. Allogeneic Vaccination with a B7.1 HLA-A Gene-Modified Adenocarcinoma Cell Line in Patients with Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2004, 22, 2800–2807. [Google Scholar] [CrossRef]
- Zhou, G.W.; Xiong, Y.; Chen, S.; Xia, F.; Li, Q.; Hu, J. Anti-PD-1/PD-L1 Antibody Therapy for Pretreated Advanced Nonsmall-Cell Lung Cancer: A Meta-Analysis of Randomized Clinical Trials. Medicine 2016, 95, e4611. [Google Scholar] [CrossRef]
- Tanvetyanon, T.; Creelan, B.C.; Antonia, S.J. The Safety and Efficacy of Nivolumab in Advanced (Metastatic) Non-Small Cell Lung Cancer. Expert Rev. Anticancer Ther. 2016, 16, 903–910. [Google Scholar] [CrossRef]
- Ferrara, R.; Imbimbo, M.; Malouf, R.; Paget-Bailly, S.; Calais, F.; Marchal, C.; Westeel, V. Single or Combined Immune Checkpoint Inhibitors Compared to First-Line Platinum-Based Chemotherapy with or without Bevacizumab for People with Advanced Non-Small Cell Lung Cancer. Cochrane Database Syst. Rev. 2021, 4, CD013257. [Google Scholar] [CrossRef]
- Cefali, M.; Epistolio, S.; Ramelli, G.; Mangan, D.; Molinari, F.; Martin, V.; Freguia, S.; Mazzucchelli, L.; Froesch, P.; Frattini, M.; et al. Correlation of KRAS G12C Mutation and High PD-L1 Expression with Clinical Outcome in NSCLC Patients Treated with Anti-PD1 Immunotherapy. J. Clin. Med. 2022, 11, 1627. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.Y.; Johnson, C.; Washburn, J.G.; Cruz-Correa, M.R.; Dang, D.T.; Dang, L.H. Oncogenic KRAS Modulates Mitochondrial Metabolism in Human Colon Cancer Cells by Inducing HIF-1alpha and HIF-2alpha Target Genes. Mol. Cancer 2010, 9, 293. [Google Scholar] [CrossRef] [PubMed]



| Age of Diagnosis | 66.40 ± 8.62 |
| Gender | |
| Women | 19 (22.4%) |
| Men | 66 (77.6%) |
| Tumor type | |
| Adenocarcinoma | 70 (82.4%) |
| NSCLC, NOS | 5 (5.9%) |
| Squamous Cell Carcinoma | 10 (11.8%) |
| KRAS mutation type | |
| G12C | 32 (37.6%) |
| G12X | 27 (31.8%) |
| G13D | 5 (5.9%) |
| A146X | 9 (10.6%) |
| Q61X | 12 (14.1%) |
| Stage | |
| II | 7 (8.2%) |
| III | 16 (18.8%) |
| IV | 62 (72.9%) |
| Current status | |
| Alive | 46 (54.1%) |
| Exitus | 39 (45.9%) |
| KRAS Mutation | p-Value | ||
|---|---|---|---|
| mKRAS G12C n (%) | Other KRAS Mutations n (%) | ||
| PD-L1 % | 19 (0–90) 33.94 ± 35.10 | 4 (0–90) 18.42 ± 25.14 | 0.789 |
| PD-L1 expression | |||
| Negative | 9 (28.1) | 18 (34.0) | 0.041 |
| Low | 9 (28.1) | 25 (47.2) | |
| High | 14 (43.8) | 10 (18.9) | |
| HIF-1α Tumor % | 0 (0–70) 6.66 ± 15.120 | (0–50) 3.96 ± 9.12 | 0.099 |
| HIF-1α Tumor | |||
| Negative | 18 (56.3) | 28 (52.8) | 0.759 |
| Positive | 14 (43.8) | 25 (47.2) | |
| HIF-1α in Tumor Cells | p-Value | ||
|---|---|---|---|
| Negative | Positive | ||
| PD-L1 expression | |||
| Negative | 19 (41.3) | 8 (20.5) | 0.010 |
| Low | 20 (43.5) | 14 (35.9) | |
| High | 7 (15.2) | 17 (43.6) | |
| KRAS Mutation | |||
|---|---|---|---|
| HIF-1α Microenvironment | mKRAS G12C n (%) | Other KRAS Mutations n (%) | p-Value |
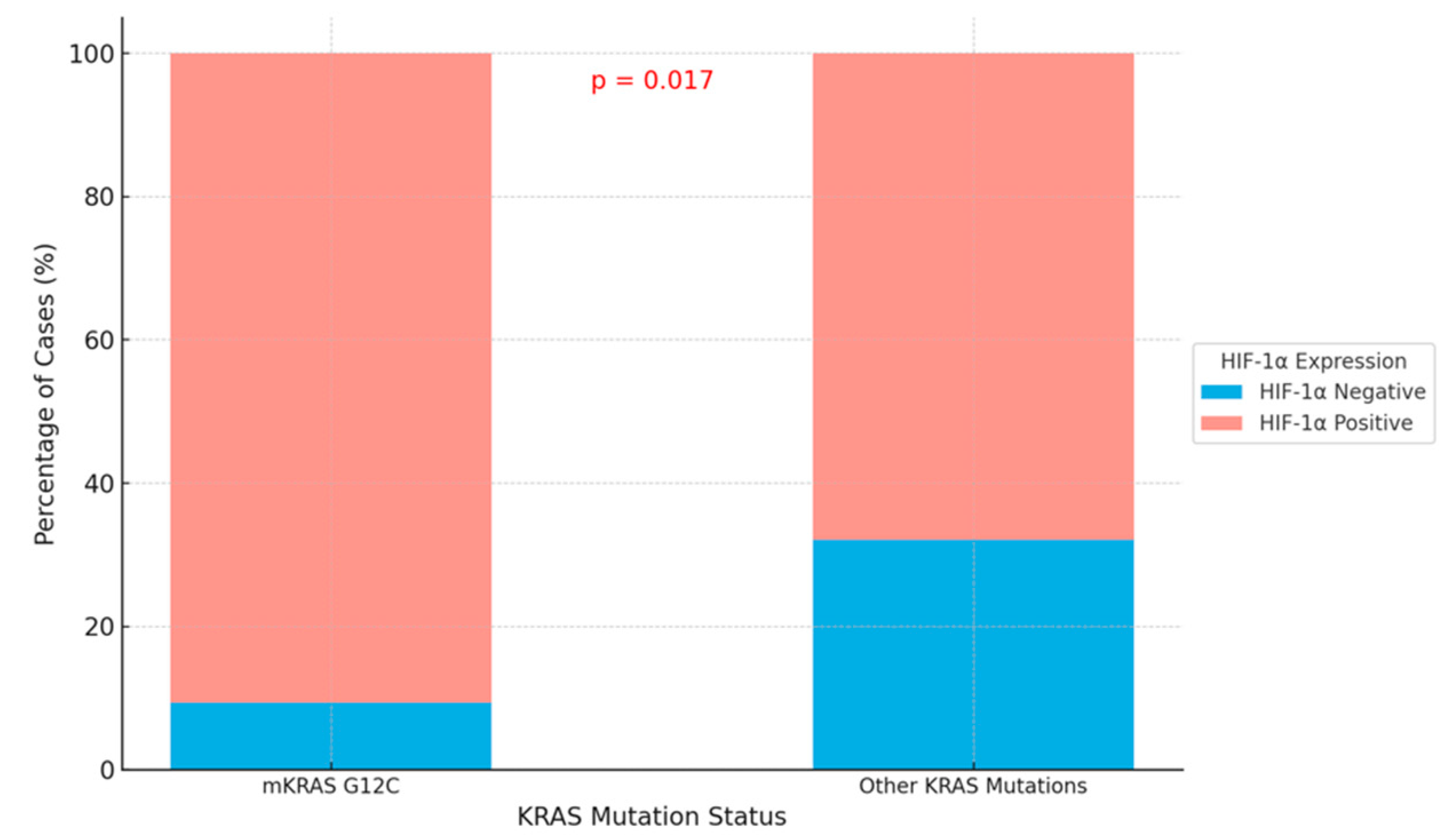
| Negative | 3 (9.4) | 17 (37.1) | 0.017 |
| Positive | 29 (90.6) | 36 (67.9) | |
| HIF-1α Tumor Microenvironment | p-Value | ||
|---|---|---|---|
| Negative | Positive | ||
| PD-L1 expression | |||
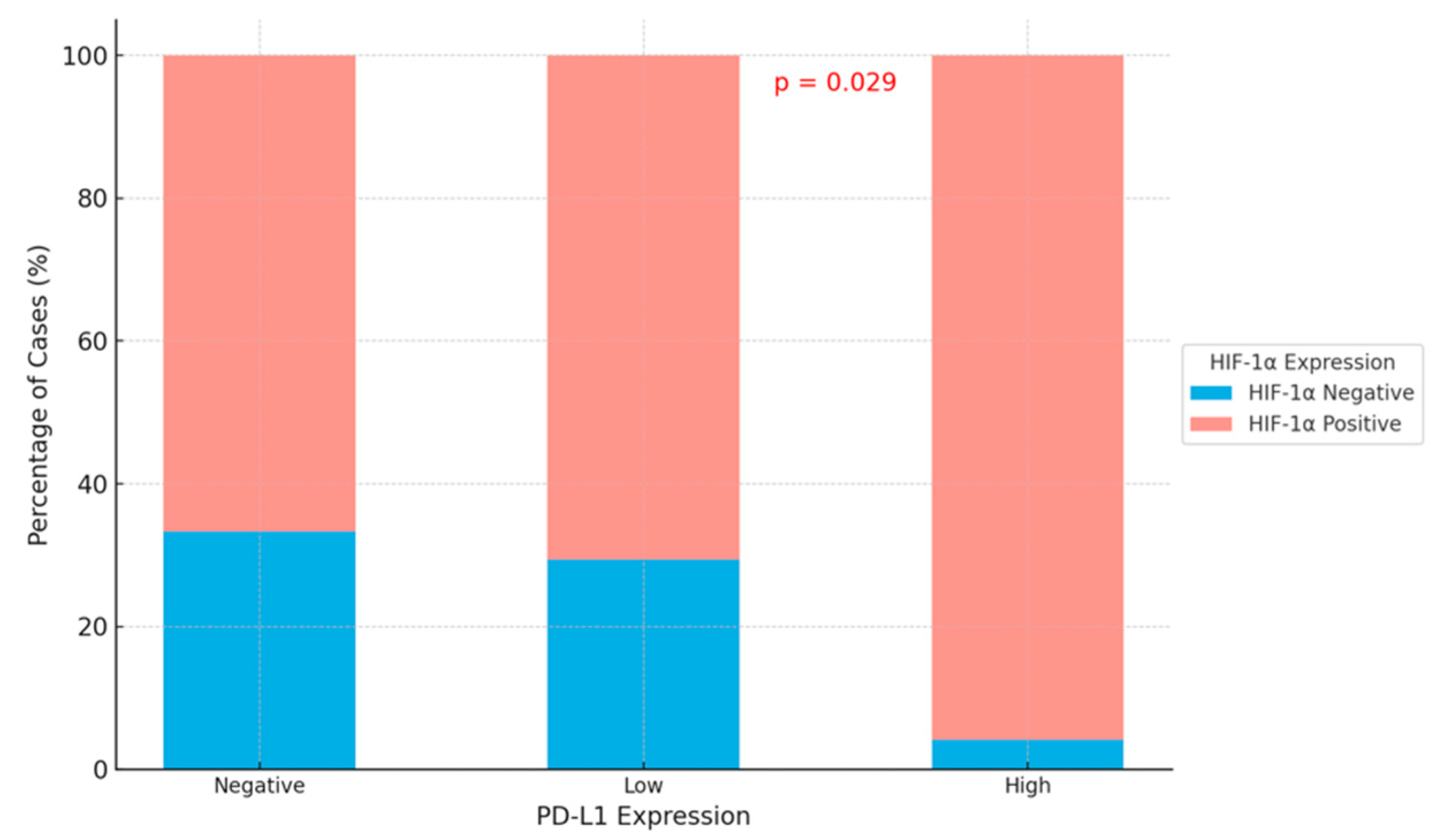
| Negative | 9 (33.3) | 18 (66.7) | 0.029 |
| Low | 10 (29.4) | 24 (70.6) | |
| High | 1 (4.2) | 23 (95.8) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Er Özilhan, S.; Efil, S.C.; Çanakçı, D.; Ağaçkıran, Y.; Şener Dede, D.; Onak Kandemir, N.; Doğan, M.; Ünal, T.D.K.; Kıran, M.M.; Kayaçetin, S.; et al. Correlation of PD-L1 and HIF-1 Alpha Expression with KRAS Mutation and Clinicopathological Parameters in Non-Small Cell Lung Cancer. Curr. Issues Mol. Biol. 2025, 47, 121. https://doi.org/10.3390/cimb47020121
Er Özilhan S, Efil SC, Çanakçı D, Ağaçkıran Y, Şener Dede D, Onak Kandemir N, Doğan M, Ünal TDK, Kıran MM, Kayaçetin S, et al. Correlation of PD-L1 and HIF-1 Alpha Expression with KRAS Mutation and Clinicopathological Parameters in Non-Small Cell Lung Cancer. Current Issues in Molecular Biology. 2025; 47(2):121. https://doi.org/10.3390/cimb47020121
Chicago/Turabian StyleEr Özilhan, Seda, Safa Can Efil, Doğukan Çanakçı, Yetkin Ağaçkıran, Didem Şener Dede, Nilüfer Onak Kandemir, Mehmet Doğan, Tuba Dilay Kökenek Ünal, Merve Meryem Kıran, Serra Kayaçetin, and et al. 2025. "Correlation of PD-L1 and HIF-1 Alpha Expression with KRAS Mutation and Clinicopathological Parameters in Non-Small Cell Lung Cancer" Current Issues in Molecular Biology 47, no. 2: 121. https://doi.org/10.3390/cimb47020121
APA StyleEr Özilhan, S., Efil, S. C., Çanakçı, D., Ağaçkıran, Y., Şener Dede, D., Onak Kandemir, N., Doğan, M., Ünal, T. D. K., Kıran, M. M., Kayaçetin, S., Balta, H., & Tatlı Doğan, H. (2025). Correlation of PD-L1 and HIF-1 Alpha Expression with KRAS Mutation and Clinicopathological Parameters in Non-Small Cell Lung Cancer. Current Issues in Molecular Biology, 47(2), 121. https://doi.org/10.3390/cimb47020121






