Abstract
Artemisia argyi H. Lév. & Vaniot (A. argyi) is a perennial herb belonging to the Asteraceae family and is a medicinal plant widely used in traditional medicine. In the field of plant physiology, JAZ proteins play a central role in the jasmonic acid (JA) signaling pathway, significantly affecting plant growth and development as well as responses to biotic and abiotic stresses. This study aims to identify and analyze the JAZ gene family of A. argyi. Through a genome-wide analysis of A. argyi. 18 JAZ genes were identified and classified into three subfamilies, based on phylogenetic relationships. Additionally, for this study, we comprehensively analyzed the physical and chemical properties, gene structure, chromosomal locations, conserved domains, cis-acting elements, and evolutionary relationships of these genes. The tissue-specific expression patterns of JAZ genes were obtained from transcriptome data, revealing distinct expression profiles across different tissues in A. argyi. Finally, this research identified a candidate JAZ gene, AarJAZ18, which is involved in the development of glandular trichomes in the leaves of A. argyi. Subsequently, the relative expression levels of AarJAZ18 in different tissues were validated using quantitative real-time PCR (qRT-PCR). In summary, this study provides a foundation for further investigation into the functions of A. argyi JAZ genes and offers valuable gene resources for breeding superior varieties and enhancing germplasm innovation.
1. Introduction
Jasmonic acid is an important plant hormone, and jasmonates (JAs), including jasmonic acid and its bioactive derivatives, play a significant role in the plant’s response to biotic and abiotic stresses, as well as in plant growth and development [1,2,3]. In plants, JA is primarily produced through the oxygenated phospholipid biosynthesis pathway, which originates from the α-linolenic acid released from membrane lipids. The JAZ protein family comes from a large protein family known as TIFY, which was previously named ZIM [4]. The TIFY family also includes three other subfamilies: TIFY, PPD, and ZML [4,5]. All sub-families contain a conserved TIFY domain, as well as one or two different additional domains. The JAZ protein family consists of two highly conserved functional domains, namely, the TIFY (also known as ZIM) and Jas (also known as CCT_2) domains [6]. The TIFY domain (PF06200) typically consists of 28 amino acids and has a conserved sequence “TIF[F/Y]XG” [4], located at the N-terminal of the JAZ protein sequence. There are also some TIFY domain variants, like TMLYGG, SFFYGG, TIFYNG, and TIFING [7]. The Jas domain (PF09425) is characterized by a “SLX2FX2KRX2RX5PY” motif, a variant of the CCT domain [8,9], located at the C-terminal of the JAZ protein sequence. Both the TIFY and Jas domains, which are highly conserved in JAZ proteins, are crucial in the JA signaling pathway. The TIFY domain mediates the interaction between JAZ proteins and the NINJA-TPL complex, thereby cooperatively suppressing the signal transduction of the JA pathway [10]. Additionally, the Jas domain mediates the interaction between JAZ and other genes (such as COI1 and MYC), thereby inhibiting their transcriptional activity [11,12,13]. Therefore, JAZ proteins are vital in the JA-triggered signaling cascade.
In 2007, JAZ proteins were first discovered in Arabidopsis thaliana [6]. Since then, with the rapid development of gene sequencing technology, an increasing number of plant JAZ genes have been identified. For example, 12, 15, 9, 26, and 23 JAZ genes have been found in Populus trichocarpa [14], Oryza sativa [15], Artemisia annua [16], Solanum lycopersicum [17], and Zea mays [18], respectively. As more JAZ genes are identified, their functions in plant resistance to biotic and abiotic stresses and in regulating plant development are gradually being uncovered. For instance, Arabidopsis AtJAZ4 is involved in regulating the development of roots, hypocotyls, and petioles [19]; OsJAZ9 in O. sativa may participate in tolerance to water deficit stress by regulating the width of O. sativa leaves and stomatal density [20]; VqJAZ7 in grapevines can enhance resistance to powdery mildew by regulating cell death and the accumulation of superoxide anions [21]; and the overexpression of GsJAZ2 in soybean significantly enhances the transgenic lines’ resistance to salt stress [22]. These pieces of evidence all indicate that JAZ genes play an important role in plant growth and development, as well as in stress resistance.
A. argyi is a perennial herbaceous plant in the Asteraceae family, with over three hundred species that are widely distributed in Asian countries such as China, Korea, and Japan [23]. As a perennial herbaceous plant, its lifespan can reach 3–5 years in suitable environments. Compared to the harsh growing conditions of some other medicinal plants, A. argyi can grow in a variety of habitats, including grasslands, hillsides, and along riverbanks, thriving in temperate regions with well-drained soils. For thousands of years, A. argyi has been extensively used in traditional Chinese medicine, both internally and externally, for treating conditions such as dysmenorrhea, abdominal pain, and inflammation [24]. These medicinal values are inseparable from the rich bioactive components in A. argyi leaves, mainly including polysaccharides, flavonoids, alkaloids, and volatile oils [25]. A. argyi glandular trichomes have been found to be the site of the synthesis and storage of volatile oils, as well as the key tissue in the production of flavonoids and terpenoids [26,27]. In recent years, due to factors such as long-term monocropping, pest and disease infestations, genetic diversity issues, and improper management, some cultivation areas have experienced a decline in the yield and quality of A. argyi, significantly impacting both its quality and production. Therefore, it is imperative to initiate genetic research aimed at identifying those genes that can contribute to the enhancement of high-quality core A. argyi cultivars. However, most of the current research on A. argyi is limited to its chemical composition, pharmacological effects, and bioactivity [28,29,30]. Through previous studies [14,15,16,17,18,19,20,21,22], we believe that the JAZ gene also plays an important role in the growth and development of A. argyi. Therefore, based on the reported A. argyi genomic data, this study identified those members of the JAZ gene family in A. argyi and analyzed their functions through bioinformatics, identifying a total of 18 A. argyi JAZ genes and uncovering a JAZ gene that may affect the development of A. argyi glandular trichomes. This provides a theoretical basis for further research on molecular biology in A. argyi and some potential genetic resources for the creation of superior A. argyi varieties in the future.
2. Materials and Methods
2.1. Identification and Physicochemical Properties Analysis of JAZ Genes in A. argyi
In this study, we obtained the whole-genome data and annotation files for A. argyi and A. thaliana from the A. argyi Gene Database [31] (https://ngdc.cncb.ac.cn/search/?dbId=gwh&q=PRJCA010808&page=, accessed on 14 September 2024) and the TAIR Arabidopsis Genome Database (https://www.arabidopsis.org, accessed on 14 September 2024), respectively. To comprehensively identify the members of the A. argyi JAZ gene family, we employed two strategies. First, bidirectional BLAST analysis was performed using the Arabidopsis JAZ protein sequences as queries to identify corresponding A. argyi JAZ protein sequences with similar structural characteristics, with a minimum hit e-value = 1e–5. Second, we downloaded HMM-format files containing the conserved domains of TIFY and Jas (accession numbers PF06200 and PF09425, respectively) from the Pfam database. The Simple HMM search function in TBtoolsv2.056 was then used to identify A. argyi protein sequences containing both conserved domains, where the minimum hit e-value = 0.01.
2.2. Physicochemical Properties Analysis of JAZ Genes in A. argyi
The physicochemical properties of each candidate gene, including protein length, isoelectric point, hydrophobicity, and instability index, were analyzed using the ExPASy ProtParam tool (https://web.expasy.org/cgi-bin/protparam/protparam, accessed on 19 September 2024) [32]. Additionally, the subcellular localization of the proteins was predicted using Plant-mPLoc (http://www.csbio.sjtu.edu.cn, accessed on 19 September 2024) by providing the protein sequences.
2.3. Phylogenetic Analysis of JAZ Genes in A. argyi
To perform the phylogenetic analysis, JAZ protein sequences from dicotyledonous plants, including the model species A. thaliana and Artemisia annua, were retrieved from the NCBI database. In addition, JAZ protein sequences from monocotyledonous plants such as O. sativa and Z. mays were also obtained. Detailed sequence information is provided in Table S4. These sequences, together with the identified JAZ protein sequences from A. argyi, were introduced into MEGA11 software for multiple sequence alignment using the MUSCLE algorithm [33]. For phylogenetic analysis, the neighbor-joining method was applied, using the most appropriate substitution model and 1000 bootstrap replicates. The phylogenetic tree was visualized and enhanced using EvolView (http://www.evolgenius.info/evolview, accessed on 19 September 2024) [34].
2.4. Gene Structure and Conservative Domain Analysis of JAZ Genes in A. argyi
Gene structure analysis was performed using the A. argyi genome annotation data in GFF format. TBtools was employed to analyze the intron-exon structures of the 18 identified JAZ genes. For further functional analysis of AarJAZs, the protein sequences were submitted to the MEME suite (https://meme-suite.org/meme/doc, accessed on 20 September 2024) to identify conserved motifs. The search parameters included a maximum of 10 motifs, with motif repetitions set to 0 or 1. The motif data from MEME, along with the gene structure and conserved domains, was integrated into a composite diagram using TBtools. This diagram includes the phylogenetic tree, conserved domains, gene structure, and motifs, facilitating a deeper understanding of the structural features and functional implications of the JAZ genes in A. argyi.
2.5. Gene Duplication and Collinearity Analysis of JAZ Family Genes in A. argyi
Gene density analysis of the A. argyi genome was conducted using TBtools, and the results were visualized on chromosomes. The positions of the AarJAZ genes were determined using the genome annotation file, and a distribution map of all AarJAZ genes was generated. Gene duplication and collinearity analysis of the JAZ family members were performed using the One-Step MCScanX function in TBtools. The chromosomal distribution and segmental duplication events of the 18 JAZ genes in A. argyi were visualized using the Advanced Circos function. Additionally, genome data and annotation files for A. thaliana, O. sativa, Z. mays, and A. annua were retrieved from the NCBI database (https://www.ncbi.nlm.nih.gov/genome, accessed on 25 September 2024). To examine the evolutionary relationships of segmental duplication events, the Ka, Ks, and Ka/Ks values of the segmental duplication gene pairs were calculated using the Simple Ka/Ks Calculator function in TBtools.
2.6. GO Enrichment Analysis of JAZ Genes in A. argyi
The 18 JAZ protein sequences of A. argyi were submitted to eggNOG-mapper (http://eggnog-mapper.embl.de, accessed on 29 September 2024) for GO enrichment annotation [35]. The analysis was performed with the following parameters: minimum hit e-value = 0.001, minimum hit bit-score = 60, percentage identity = 40. The annotation data were then processed and visualized using TBtools software.
2.7. Expression Analysis of JAZ Genes in A. argyi
The RNA-seq expression profiles of AarJAZs were mined from the transcriptome data by Cui et al. [36]. Expression heat maps were generated using TBtools software and row scaling was performed. The A. argyi plants used in this study were sourced from Qichun County, Hubei Province, in 2023. The collected roots, stems, and leaves of A. argyi were grown in a light-controlled incubator under conditions of 25 °C and a 16/8-h light/dark cycle. During the active growth phase, samples from the roots, stems, and leaves were immediately frozen in liquid nitrogen and stored at −80 °C for later use. Following sample collection, tissues were rapidly frozen in liquid nitrogen, and primers were designed based on sequence specificity. Total RNA was extracted using Trizol (Sangon), and cDNA synthesis was performed using a cDNA reverse transcription kit with 1 μg of RNA. For qRT-PCR analysis, each result was derived from independent RNA samples, with three biological replicates and three technical replicates per experiment, and the relative expression levels of transcripts were calculated via the 2−∆∆Ct method. The A. argyi actin gene was used as an internal control for each PCR experiment. The primers used for the qRT-PCR analysis are listed in Table S5.
3. Results
3.1. Identification and Characterization of JAZ Genes in A. argyi
This study employed two retrieval strategies to identify sequences containing the TIFY and Jas domains within the genome database of A. argyi, ultimately identifying 33 candidate A. argyi JAZ gene sequences. After removing redundant entries and verifying the presence of conserved domains, 18 distinct JAZ gene sequences were confirmed in A. argyi, with the full-length protein sequences provided in Table S1. The sequences were designated as AarJAZ1 to AarJAZ18, based on their chromosomal distribution (Table 1). The lengths of the amino acid sequences ranged from 162 to 318 amino acids (aa), with an average length of 221 aa. The molecular weights varied between 17.56 and 33.54 KDa, with an average of 28.23 kDa, and the isoelectric points (pI) ranged from 5.16 to 9.52. Among the 18 A. argyi JAZ proteins, only AarJAZ2, AarJAZ11, and AarJAZ15 were classified as acidic proteins (pI < 7), while the remaining 15 were basic proteins (pI > 7). The instability coefficients varied from 37.8 to 67.19, with AarJAZ8 being the only protein with an instability coefficient below 40. The other 17 AarJAZ proteins exhibited instability coefficients greater than 40, indicating potential instability [37]. Regarding hydrophobicity, the grand average of hydropathy (GRAVY) values for all 18 AarJAZ protein sequences were negative, suggesting that these proteins are hydrophilic. Predictions of subcellular localization indicated that all 18 AarJAZ proteins are localized in the nucleus, implying that they may function as transcription factors.

Table 1.
Physiochemical parameters and subcellular location of the 18 JAZ genes in A. argyi.
3.2. Phylogenetic Analyses and Classification of the A.argyi JAZ Gene Family
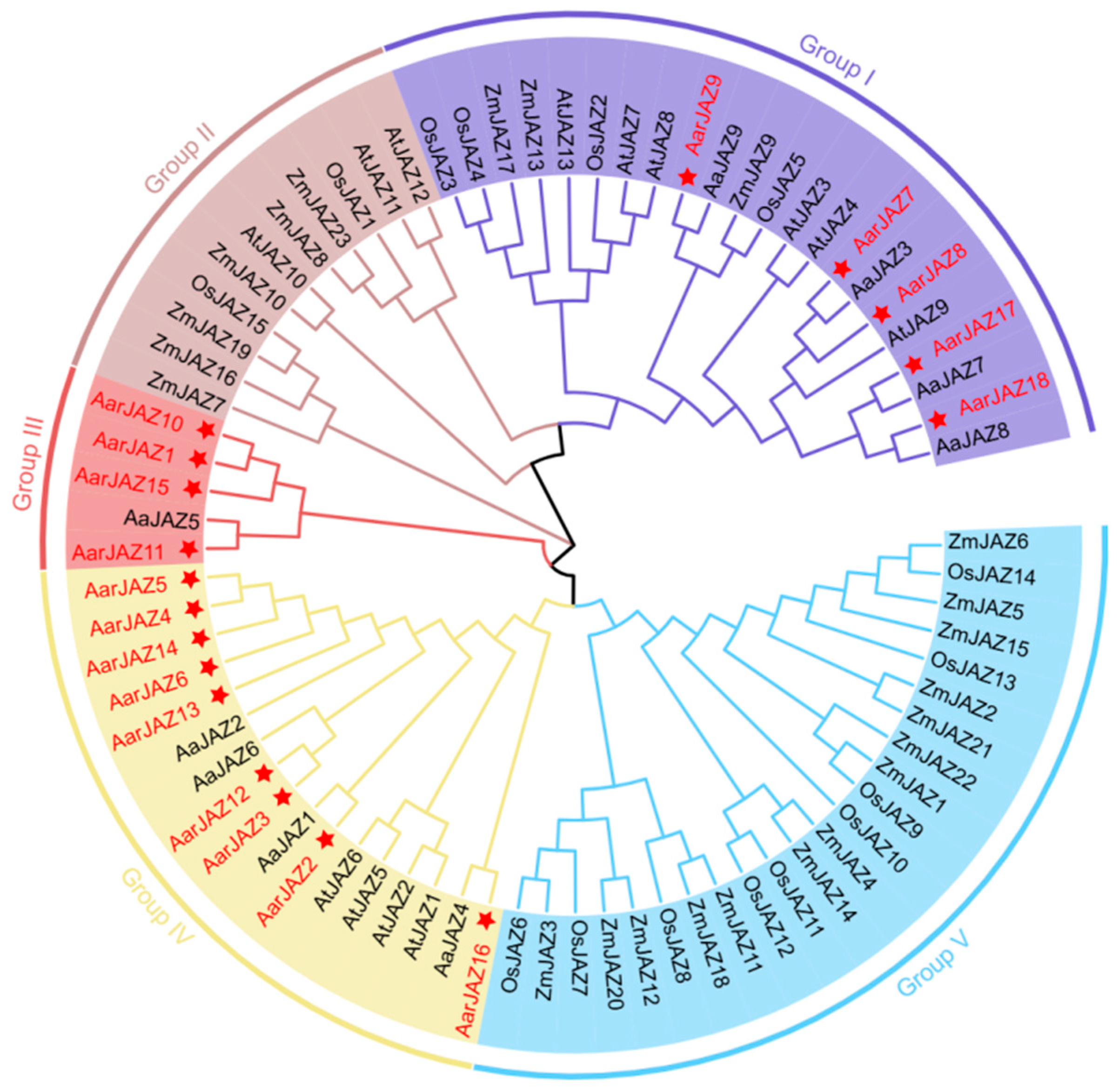
To investigate the systematic evolutionary relationships of the JAZ gene family in A. argyi, we aligned 60 JAZ protein sequences using MEGA11 software, including 13 from A. thaliana, 9 from A. annua, 15 from O. sativa, and 23 from Z. mays. A phylogenetic tree was then constructed using the neighbor-joining method, which does not rely on complex evolutionary models and can infer phylogenetic relationships based on sequence distance. For our gene family data, the neighbor-joining method effectively reflects gene similarities and differences, and the resulting tree is easy to interpret. Based on this phylogenetic tree (Figure 1), the JAZ protein sequences were classified into five subfamilies. Notably, members of the A. argyi JAZ gene family are distributed across Groups I, III, and IV, with no members found in Groups II and V, which are predominantly populated by JAZ gene family members from monocot plants such as Z. mays and O. sativa. Group IV contains the largest number of members, with nine A. argyi JAZ genes, while Groups I and III contain five and four members, respectively. Additionally, the distribution pattern indicates that the A. argyi JAZ family members are closely related to those of A. annua, suggesting a high degree of homology between the JAZ gene families of these two species.
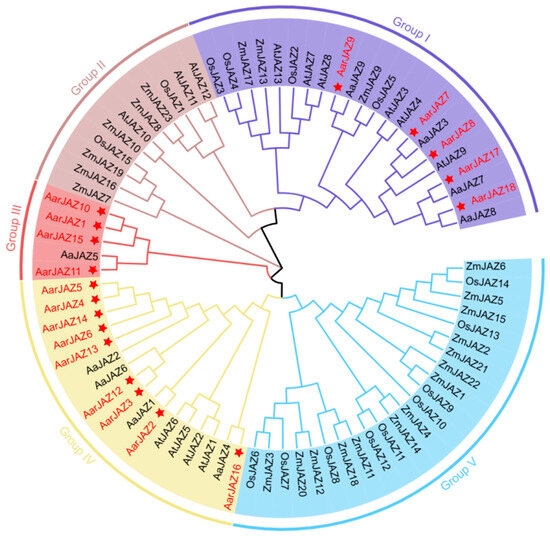
Figure 1.
Phylogenetic tree of JAZ proteins from A. thaliana, A. argyi, A.annua, O. sativa, and Z. mays. The tree was constructed using MEGA11 by the neighbor-joining method, with 1000 bootstrap replicates.
3.3. Conserved Motifs, Conserved Structural Domains, and Gene Structure Analysis of the JAZ Gene Family in A. argyi
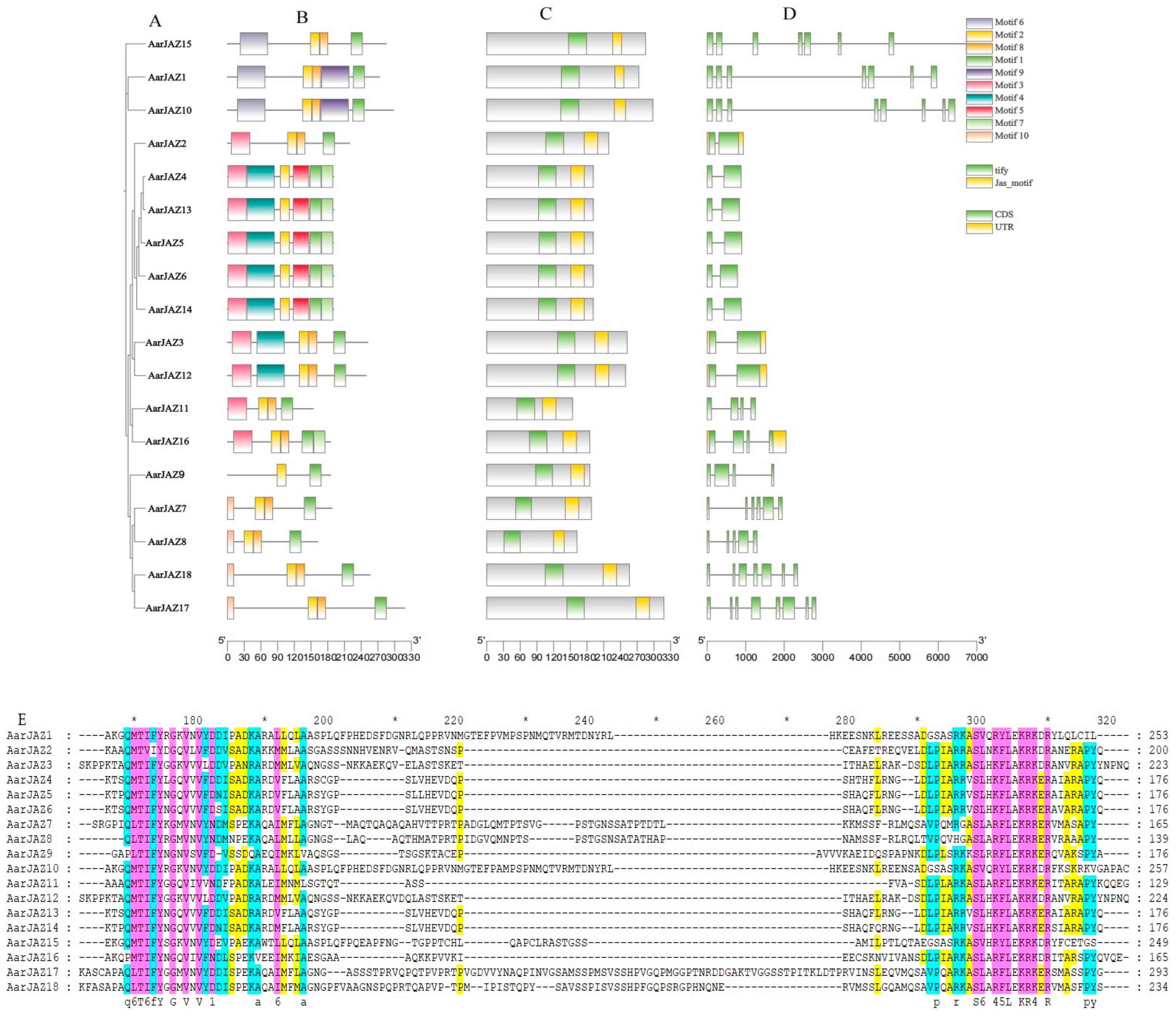
To further investigate the structural features and conserved domains of JAZ proteins, we analyzed 18 JAZ protein sequences using the MEME Suite, identifying 10 conserved motifs and predicting additional motifs in the JAZ proteins, which were then visualized (Figure 2B). Detailed motif information is provided in Table S2. A search of the NCBI-CDD database revealed that all JAZ protein sequences from A. argyi contain the conserved TIFY and CCT_2 domains. Further analysis of the conservation patterns of the TIFY and Jas domains across the 18 JAZ proteins was conducted using GeneDoc2.7 software, and the results indicated that these domains are not entirely conserved (Figure 2E).
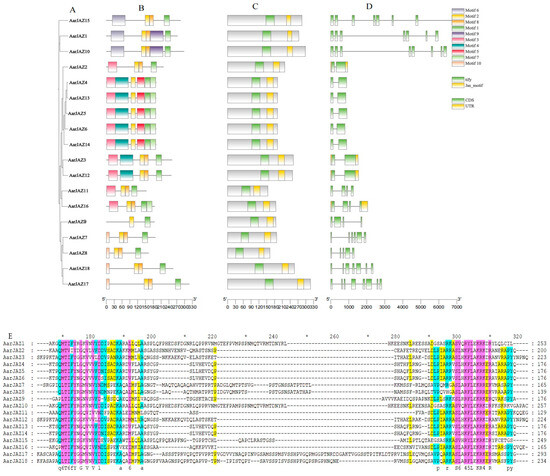
Figure 2.
Phylogenetic relationship, conserved motif, and gene structure analyses of AarJAZs. (A) Phylogenetic tree. (B) Distribution of conserved domains in AarJAZs. Boxes of ten different colors represent different conserved domains. (C) Prediction and analysis of conserved domains in AarJAZs through NCBI-CDD. (D) Exon/intron distribution of AarJAZs. (E) Sequence alignment of TIFY and Jas domains in AarJAZs.
Intron-exon structure analysis was performed to assess the diversity of the A. argyi JAZ gene architecture. As shown in Figure 2A, the number of exons in these JAZ genes varies from two to eight, with the majority (six genes) containing two introns, including AarJAZ2, AarJAZ4, AarJAZ5, AarJAZ6, AarJAZ13, and AarJAZ14. Notably, only two JAZ genes, AarJAZ10 and AarJAZ17, contain eight exons. Additionally, it was observed that only AarJAZ2, AarJAZ3, AarJAZ12, and AarJAZ16 possess untranslated regions (UTRs) at both the 5′ and 3′ ends (Figure 2D). Among the 18 AarJAZ proteins, 10 conserved motifs were identified, with each protein containing 2 to 6 motifs. Most members of the same subfamily exhibited a similar number and type of motifs, with similar distribution patterns, suggesting that proteins within the same subfamily may share functional similarities (Figure 2B). Motif1 and Motif2 were present in all 18 protein sequences, indicating that these motifs are widely distributed and highly conserved across the AarJAZ proteins. Additionally, members of the same subfamily showed similar distributions of the TIFY and Jas domains (Figure 2C). Finally, sequence alignments conducted using GeneDoc software revealed that the TIFY and Jas domains of the 18 JAZ proteins from A. argyi are poorly conserved (Figure 2E). For example, the TIFY domain in AarJAZ2 is represented as TVIY, and the Jas domain in AarJAZ1, AarJAZ5, and AarJAZ10 exhibited partial amino acid deletions (notably lacking the PY motif).
3.4. Chromosome Distribution of the JAZ Gene Family in A. argyi
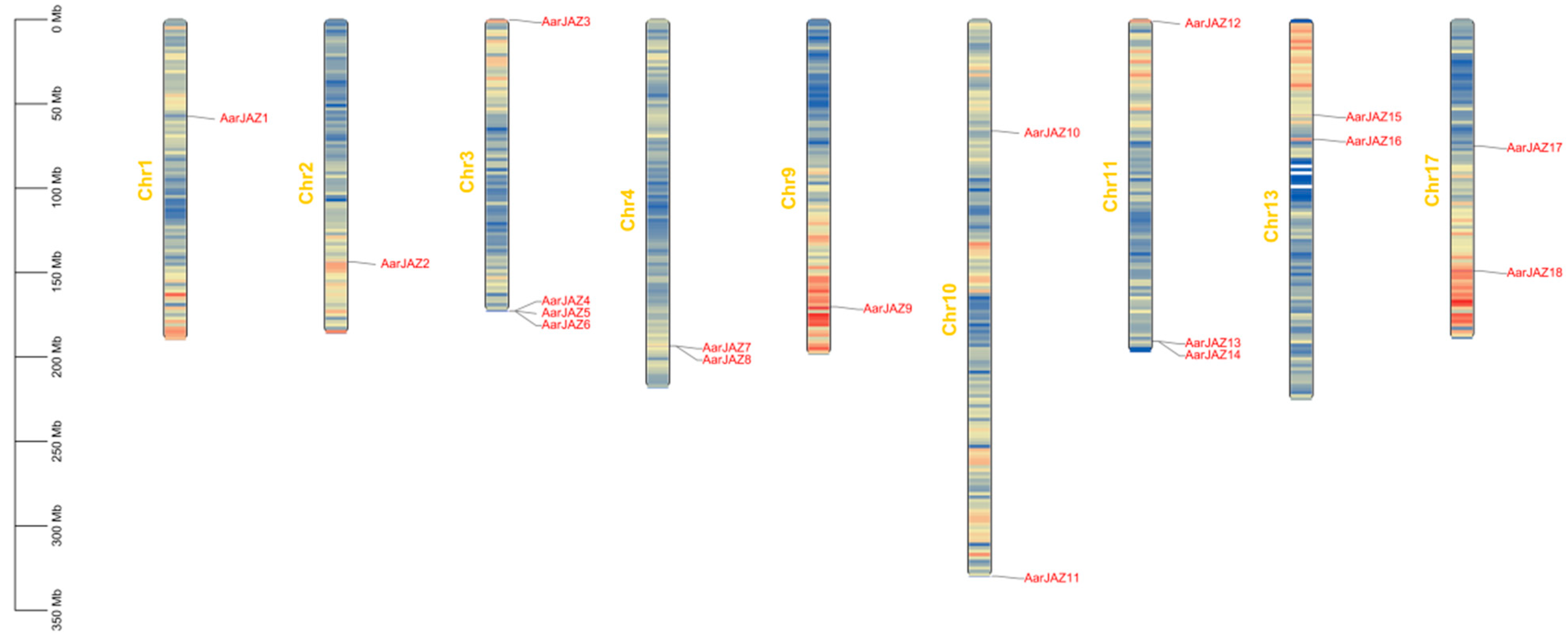
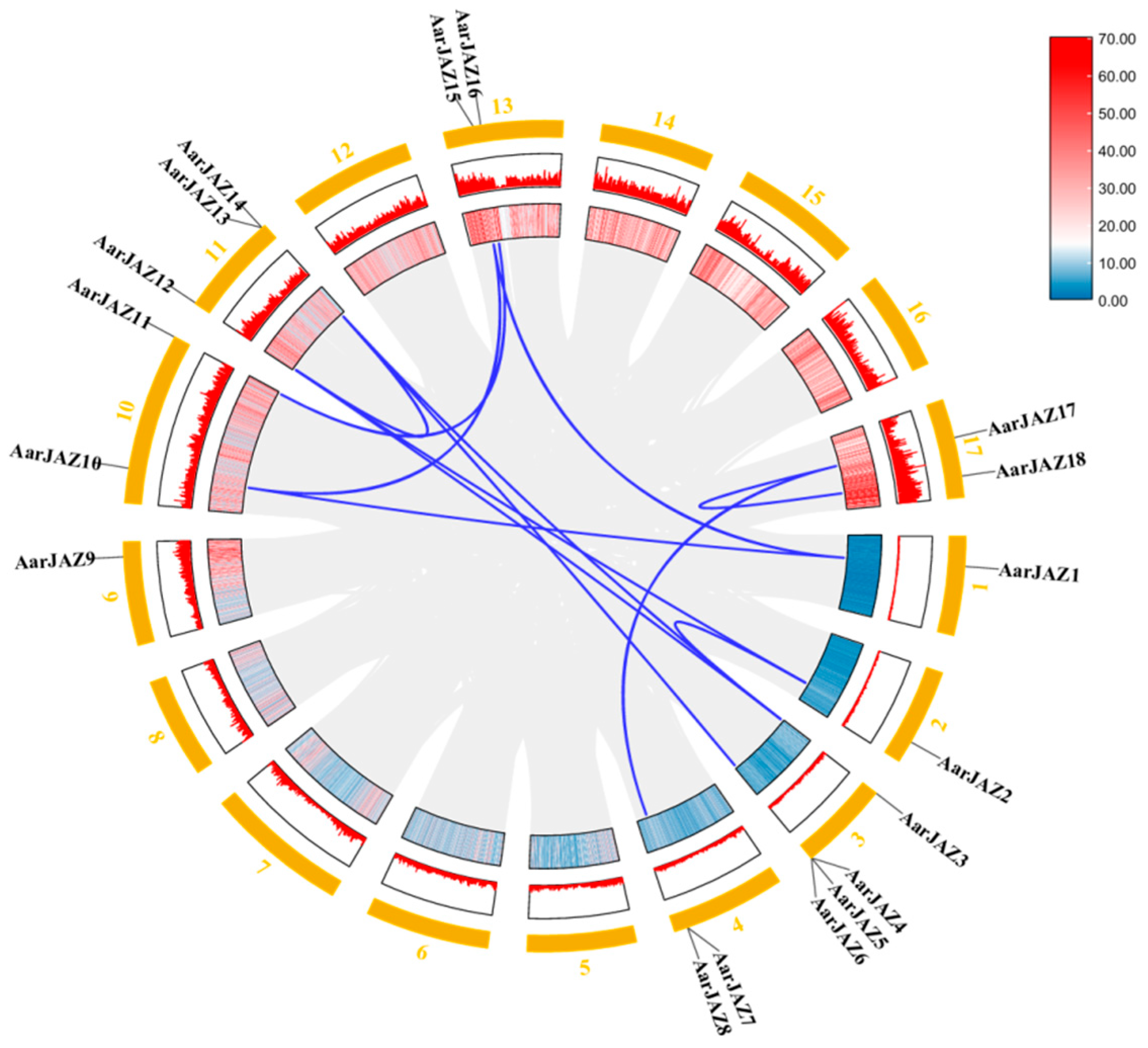
Chromosomal localization analysis of members of the JAZ gene family indicates that 18 AarJAZ genes are distributed among 9 of the 17 chromosomes. The distribution of AarJAZ genes across chromosomes is not uniform, with Chr3 containing the most JAZ genes (four genes), Chr11 containing three JAZ genes, Chr4, Chr10, Chr13, and Chr17 each containing two JAZ genes, and the remaining Chr1, Chr2, and Chr9 each containing one JAZ gene. This distribution pattern suggests that there is no significant correlation between chromosome length and the distribution of AarJAZ genes (Figure 3).
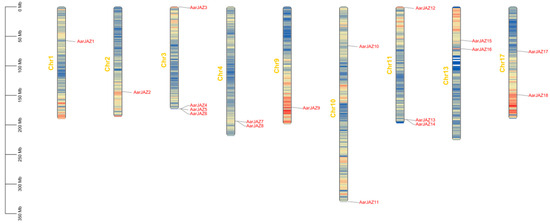
Figure 3.
Chromosomal localization distribution of AarJAZs.
3.5. Analysis of Cis-Acting Elements in AarJAZ Promoters
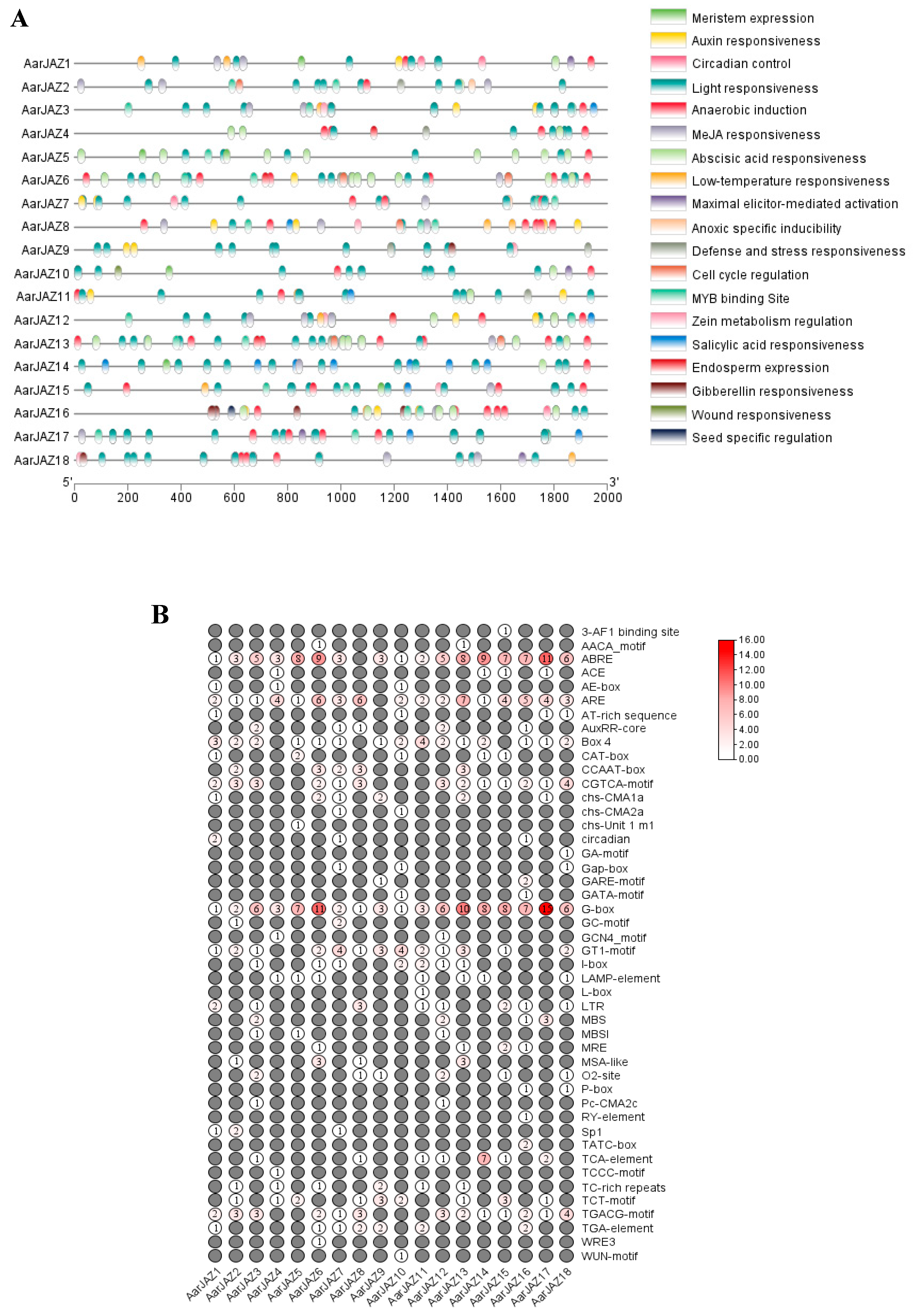
Cis-acting elements serve as molecular switches that are closely associated with the regulation of gene expression under both biotic and abiotic stresses [38]. The analysis of cis-elements warrants further functional investigation of the A. argyi JAZ gene family. To this end, the 2000-bp upstream region of the JAZ genes was extracted for cis-element identification. The results revealed that the largest numbers of photoresponsive elements were located within the promoter regions of the JAZ genes, with the G-box element being widely present in various JAZ genes (Figure 4A). This suggests that JAZ genes may be regulated by light. Furthermore, many JAZ genes were found to harbor numerous cis-acting elements associated with hormone responses, including ABA response elements (ABRE), JA response elements (the TGACG and CGTCA motifs), IAA response elements (the TGA-element), GA response elements (P-box, GARE-motif, and TATC-box), and SA response elements (the TCA-element). Additionally, the JAZ genes exhibited a significant number of cis-elements that are involved in stress responses (Figure 4B), such as low-temperature-responsive elements (LTR), drought-responsive elements (MBS), and defense/stress-responsive elements (TC-rich repeats), among others. These findings suggest that the A. argyi JAZ genes play a crucial role in plant growth and development, in regulating hormonal signaling networks, and in mediating responses to both biotic and abiotic stresses.
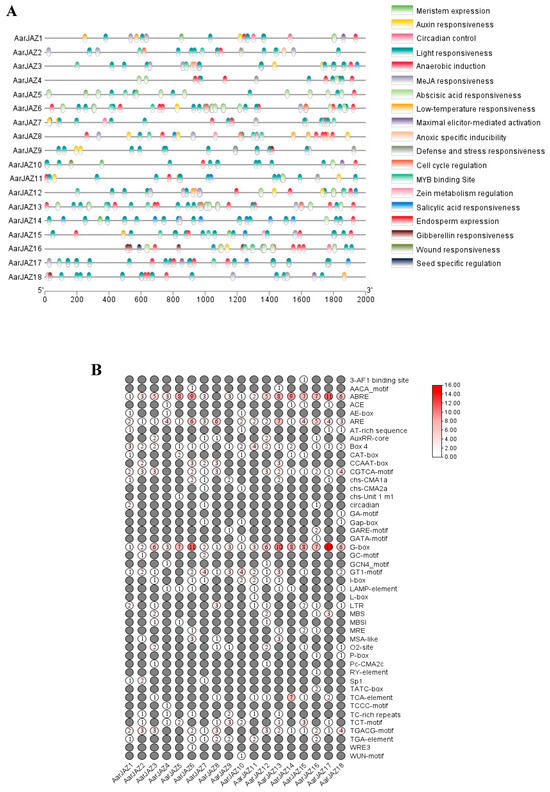
Figure 4.
Predicted cis-elements in AarJAZ gene promoters. (A) The distribution and functional classification of the cis-acting elements 2000 bp upstream from the JAZs. (B) The numbers of each cis-acting element in the JAZ gene promoter.
3.6. Intraspecific Collinearity Analyses of the JAZ Gene Family in A. argyi
Gene duplication events contribute to the expansion of gene families and play a critical role in evolutionary adaptation by facilitating the acquisition of new gene functions. Given the significance of gene duplication in the evolution of plant gene families, we investigated the duplication patterns of the 18 JAZ family genes in the A. argyi genome and identified 12 pairs of homologous duplicates (Figure 5). To assess the evolutionary rate and selective pressures acting on the JAZ gene family in A. argyi, we performed Ka and Ks analyses (Table 2). The results revealed that the Ka/Ks ratios for all twelve pairs of duplicates were less than 1, indicating that these 12 pairs of JAZ genes in A. argyi have undergone a strong purifying selection process.
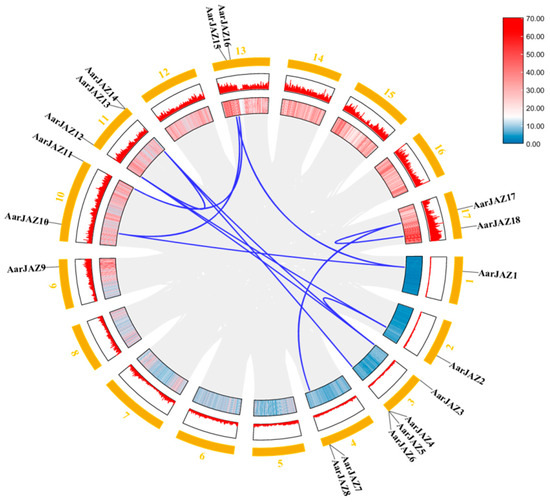
Figure 5.
Synteny analysis of AarJAZs. The colors represent gene density, and the outermost numbers represent the individual chromosomes. Red means high gene density, blue means low gene density. The blue lines indicate segmentally duplicated gene pairs.

Table 2.
Estimated Ka/Ks ratios of the duplicated JAZ genes in A. argyi.
3.7. Expression Analysis and Gene Ontology Enrichment of the JAZ Gene Family in A. argyi
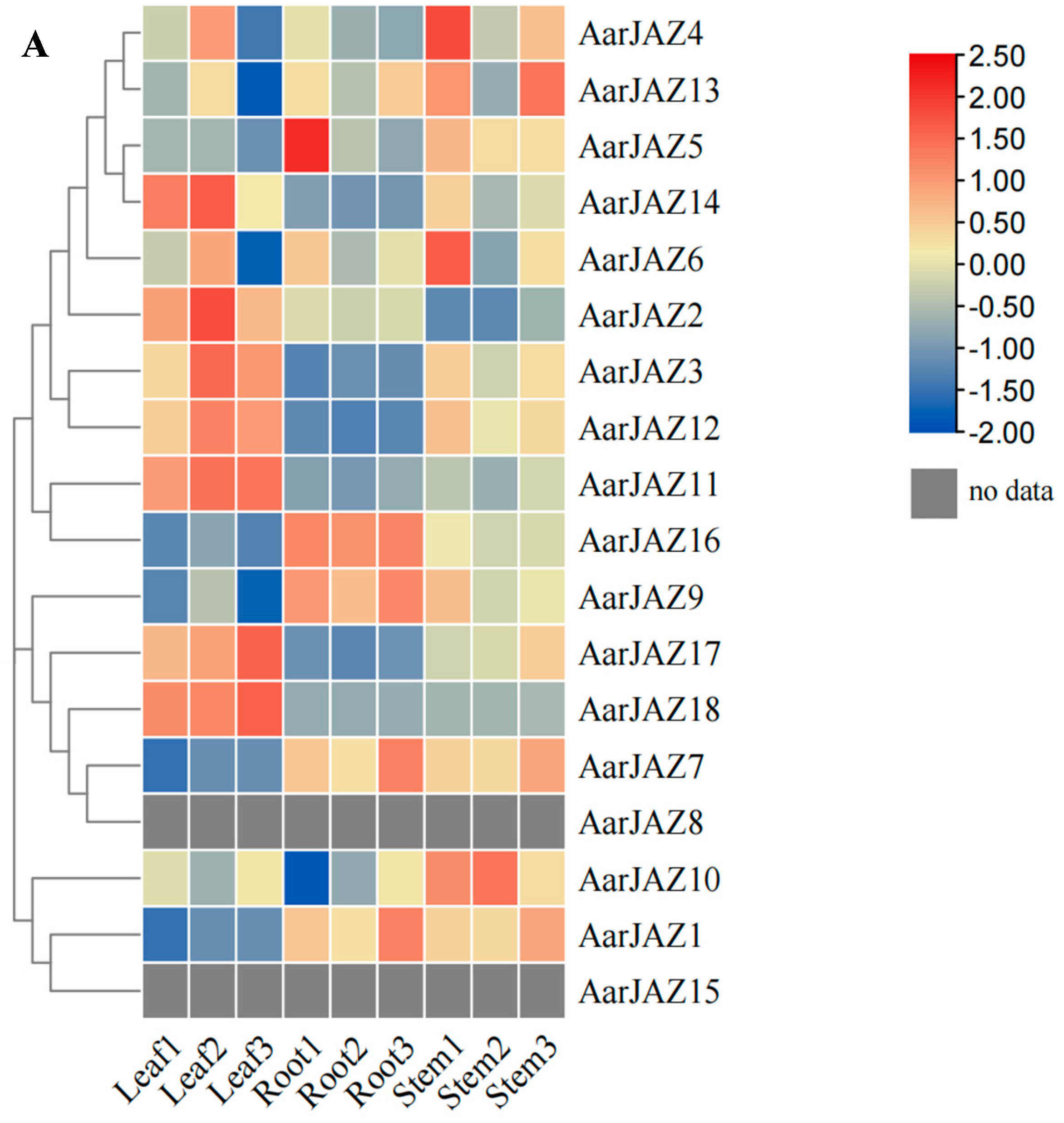
To investigate the expression patterns of AarJAZ genes in different tissues, we performed an expression analysis of the JAZ gene family in A. argyi using publicly available transcriptome data from various tissues (root, stem, and leaf) [36]. Among the transcriptome data, we identified 18 homologous JAZ gene sequences. After performing BLAST filtering, 15 genes with a sequence alignment identity greater than 95% were selected. The expression levels of these genes were visualized using a clustering heatmap, with different colors representing varying expression intensities. The results (Figure 6A) showed that the expression of AarJAZ14, AarJAZ2, AarJAZ3, AarJAZ12, AarJAZ11, AarJAZ17, and AarJAZ18 in the leaf tissues was significantly higher than in the stem and root tissues.
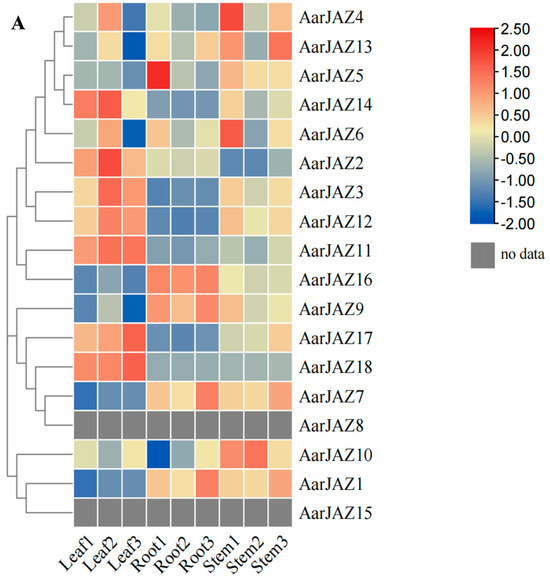
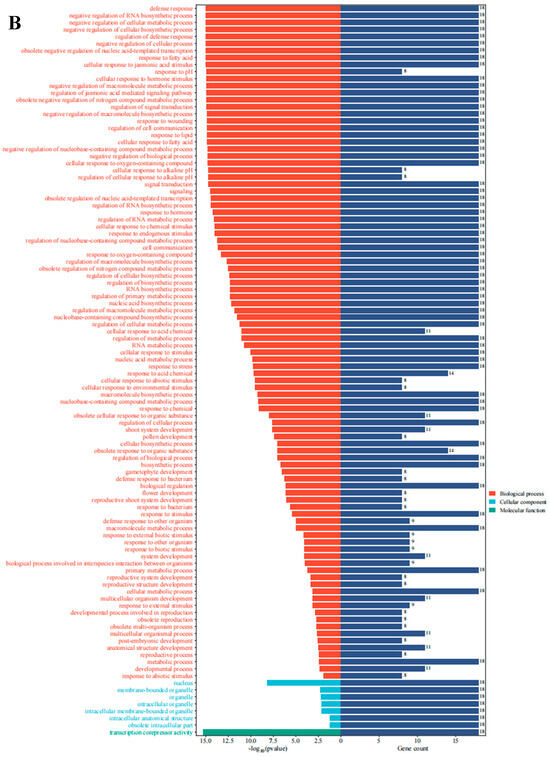
Figure 6.
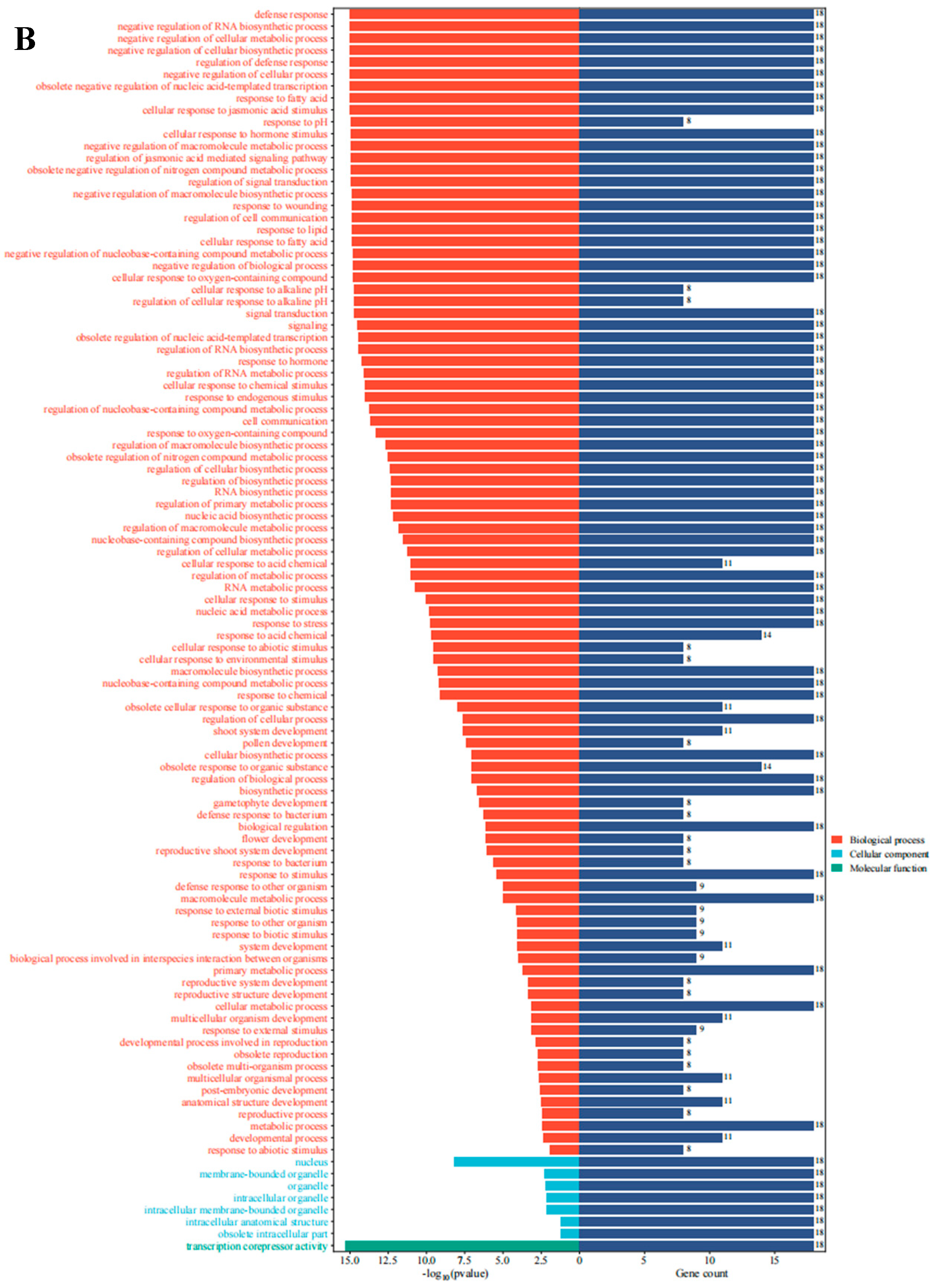
Expression analysis and gene ontology enrichment of AarJAZs. (A) Expression analysis of AarJAZs in different tissues. The expression level was calculated according to the FRKM. Red rectangles indicate high expression, while the blue rectangles represent low expression. (B) Gene ontology enrichment of AarJAZs.
To further investigate the functional roles of AarJAZs in biological processes, we conducted a GO enrichment analysis of 18 JAZ genes. The results revealed that these 18 genes were significantly enriched in 104 GO terms (Table S3). Of these, the majority (96 terms) were categorized as biological processes, while 7 terms were associated with cellular components and 1 with molecular function (Figure 6B). In the biological process category, key terms included stress response, hormone signaling, and negative regulation. Regarding cellular components, the prominent categories included the nucleus, membrane-bounded organelles, organelles, and intracellular organelles. In terms of molecular function, the only enriched term was transcription corepressor activity.
3.8. Screening of Candidate AarJAZ Genes for Glandular Trichome Development and the Relative Expression Levels in Different Tissues
In recent years, JAZ genes have been found to regulate the development of glandular trichomes in plants. In A. thaliana, JAZ proteins regulate the jasmonic acid (JA)-mediated initiation of non-glandular trichomes by inhibiting the formation of the WD-repeat/bHLH/MYB complex [39]. In Nicotiana tabacum, the overexpression of NbJAZ3 has been shown to increase trichome density [40]. In A. annua, AaJAZ8 has been demonstrated to inhibit the transcriptional activity of AaHD1 and AaGL3 through protein interactions, thereby influencing glandular trichome density [16,41]. In Gossypium hirsutum, GmJAZ2 negatively regulates fiber initiation by interacting with the R2R3-MYB transcription factor GhMYB25-like [42]. These findings highlight the critical role of JAZ proteins in the molecular mechanisms underlying glandular trichome development.
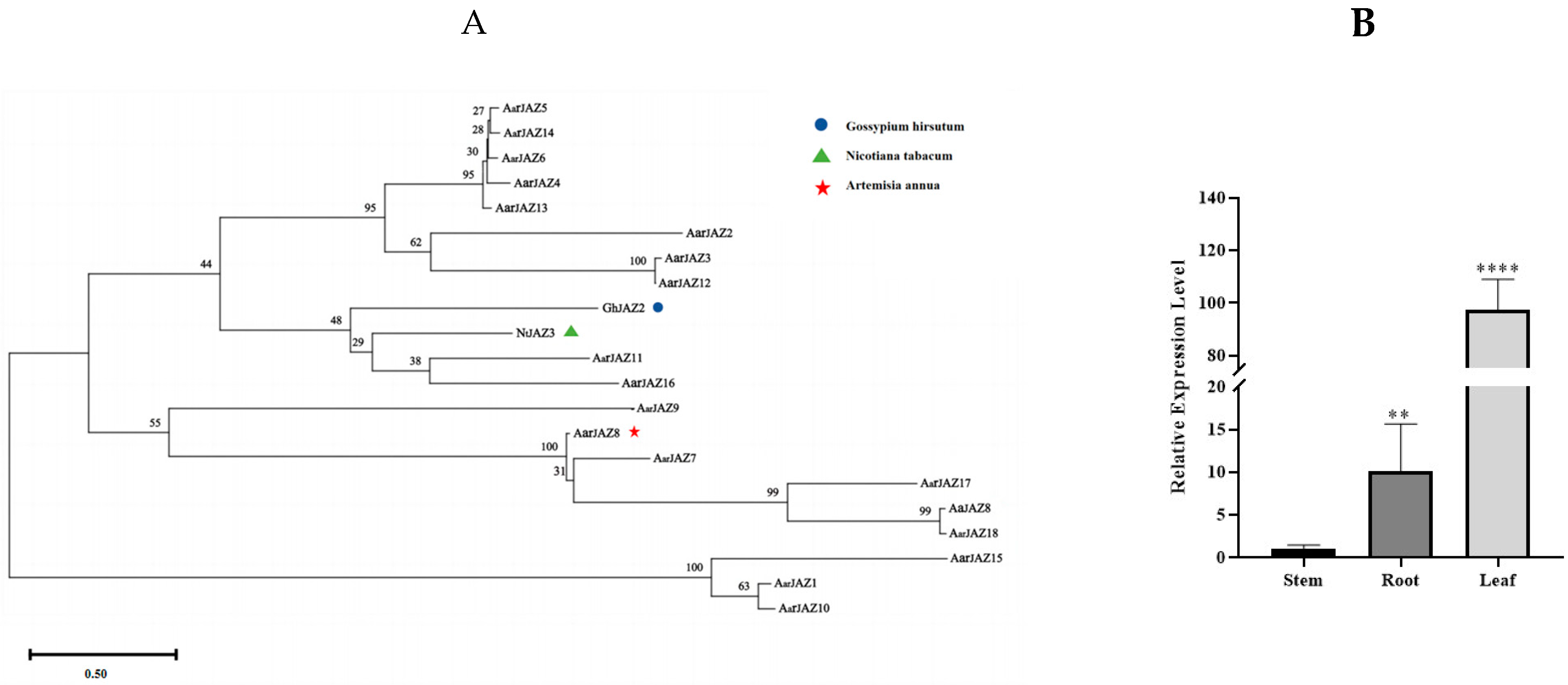
To further investigate the functional similarity between JAZ proteins, we compared the reported protein sequences of AaJAZ8, GhJAZ2, and NtJAZ3 with the A. argyi JAZ protein sequences using MEGA11 (Figure 7A). Our results reveal that AarJAZ18 shares a close evolutionary relationship with AaJAZ8. Sequence alignment via NCBI BLAST showed that the amino acid sequence identity between AarJAZ18 and AaJAZ8 is as high as 98%. This suggests that AarJAZ18 is likely to have a similar function to AaJAZ8; therefore, AarJAZ18 may play a comparable role in regulating glandular trichome development. We further validated the expression levels of AarJAZ18 in various organs using qRT-PCR. The results (Figure 7B) indicated that AarJAZ18 was predominantly expressed in the leaves, which is consistent with the transcriptome data, further supporting the potential role of this gene in the glandular trichomes of A. argyi leaves.
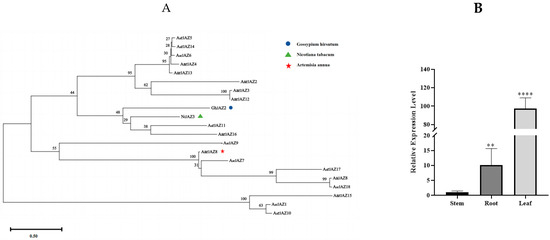
Figure 7.
Phylogenetic tree of AaJAZS in A. argyi and JAZs in other species, and the qRT-PCR analysis of AarJAZ18 in different tissues. (A) Phylogenetic tree of the AaJAZS, NbJAZ3, AaJAZ8, and GmJAZ2 proteins. (B) qRT-PCR analysis of AarJAZ18 in different tissues. Statistically significant differences were assessed using Student’s t-test (** p < 0.01, **** p < 0.0001).
4. Discussion
JAZ proteins are plant-specific and play a crucial role in various physiological processes, including plant growth and stress responses, through their involvement in the JA signaling pathway. However, research on JAZ proteins has primarily focused on model plants and studies on JAZ proteins within the genus Artemisia are limited, with reports mainly confined to A. annua [15]. In A. argyi, the lack of comprehensive genomic and transcriptomic data has resulted in only a few gene families being characterized. It was not until 2022 and 2023 that two genomic datasets for A. argyi were published [31,43]. Therefore, this study utilized bioinformatics tools to identify 18 AarJAZ genes in the A. argyi genome; it was also found that the conserved motifs in the protein sequences of these genes are not completely conserved. These amino acid variations may contribute to functional differences, suggesting that distinct JAZ proteins in A. argyi may have divergent roles. The analysis of these genes and proteins offers a theoretical foundation for future research on the functions of JAZ genes in A. argyi.
Gene duplication events have significantly contributed to the formation of new gene family members throughout the evolutionary history of plants. These events facilitate the generation of new genes during plant genome evolution, thereby enhancing the adaptability of plants to their environments. Gene duplication is also considered a key driver of plant evolution and innovation, providing an essential genetic source for the evolution of novel functions [44]. Consequently, the study of gene duplication events deepens our understanding of the processes underlying gene and species evolution. Whole-genome duplication, segmental duplication, and tandem duplication are the primary mechanisms of gene duplication [45]. Segmental duplication plays a dominant role in the generation and maintenance of gene families, serving as the main source of gene structural variation and innovation [46]. This study identified 18 AarJAZ genes within the A. argyi genome. Through collinearity analysis, we determined that 13 of these genes originated from segmental duplication, while the remaining 5 were generated by tandem or whole-genome duplication. These findings suggest that the abundant JAZ genes in A. argyi are primarily the result of segmental duplication, a mechanism also observed in species such as Petunia hybrida (morning glory) and Raphanus sativus (radish) [47,48]. Furthermore, the diversity of conserved protein domains among the 18 AarJAZ proteins not only indicates a high degree of evolutionary refinement in the JA signaling pathway of A. argyi but also suggests the significant adaptability of A. argyi to its environmental conditions.
Transcription factors regulate gene expression by interacting with the promoter regions of target genes, which are crucial for these regulatory processes [49]. Thus, analyzing the cis-acting elements in the promoter regions of the JAZ genes in A. argyi provides valuable insights into their potential functions. Our analysis of the cis-acting elements in the A. argyi JAZ genes revealed a high abundance of light-responsive elements, suggesting that JAZ genes may play a significant role in light-regulated plant development. Additionally, we identified numerous hormone-responsive elements, including those for abscisic acid (ABA), indole-3-acetic acid (IAA), salicylic acid (SA), gibberellic acid (GA), and jasmonic acid (JA). These findings highlight the involvement of A. argyi JAZ genes in hormone-mediated growth and development. The presence of multiple stress-responsive elements further supports their essential role in stress responses. GO enrichment analysis further emphasizes that JAZ genes are closely associated with stress responses, hormone signaling, and negative regulation. Moreover, these genes are predominantly localized in the nucleus, where they exert negative regulatory effects on the expression of target genes through interactions with various transcription factors.
Among the species in the genus Artemisia, A. argyi is one of the most well-known. The 2015 Nobel Prize in Physiology or Medicine was awarded for the discovery of artemisinin, an antimalarial compound isolated from A. annua, which has spurred global interest in the study of other Artemisia species [50,51]. In A. annua, much of the research has focused on increasing artemisinin production by enhancing the density of glandular trichomes [52,53,54]. This research provides valuable insights for trait improvement and cultivar breeding in A. argyi, where glandular trichomes are key sites for the synthesis and storage of essential oils, as well as metabolites such as flavonoids and terpenoids. Thus, increasing glandular trichome density in A. argyi could enhance the production of secondary metabolites, thereby increasing the medicinal value of the plant. In this study, we constructed a phylogenetic tree of several JAZ proteins that are known to regulate trichome development (Figure 6). We identified AarJAZ18, which is highly homologous to AaJAZ8, with an amino acid sequence similarity of 98%. This suggests that AarJAZ18 may function similarly to AaJAZ8 in regulating protein function. Based on these findings, we propose that AarJAZ18 is a key candidate gene involved in the regulation of glandular trichome development in A. argyi leaves. Further tissue-specific expression analysis and subcellular localization studies revealed that AarJAZ18 is primarily localized in the nucleus and is most highly expressed in the leaves, suggesting that AarJAZ18 may function as a transcription factor regulating trichome development in medicinally important leaf tissues. Although no effective transgenic transformation system for A. argyi has been reported so far, we believe that developing such a system is crucial for further investigating the role of AarJAZ18 in A. argyi. Future research will focus on constructing a reliable transformation system to validate our hypothesis. Additionally, potential experiments could include the investigation of AarJAZ18’s interactions with other transcription factors and its response to environmental stressors. Further exploration into its regulatory mechanisms under different developmental stages and stress conditions would provide more comprehensive insights. Expanding on tissue-specific expression patterns and functional validation through transgenic models would be key to elucidating the precise role of AarJAZ18 in glandular trichome development. These studies could significantly contribute to advancing breeding strategies aimed at enhancing the medicinal properties of A. argyi.
5. Conclusions
JAZ genes are known to play crucial roles in various aspects of plant growth and development. However, the identification and functional analysis of JAZ proteins in A. argyi have not yet been explored. In this study, we have identified 18 putative JAZ genes in A. argyi, expanding our understanding of the JAZ gene family in this species. Phylogenetic analysis revealed that AarJAZs are divided into three subfamilies (subfamilies I, III, and IV), shedding light on their evolutionary relationships. Our promoter cis-element analysis demonstrated that AarJAZs are regulated by light, multiple plant hormones, and stress factors, indicating that these genes may play an important role in A. argyi’s stress tolerance mechanisms. Further functional insights, derived from GO enrichment analysis, suggest that AarJAZs are integral to stress responses, hormone signaling, and negative regulation, and are primarily localized in the nucleus. These findings imply that AarJAZs may act as negative regulators by interacting with the transcription factors involved in these processes. Notably, our study highlights AarJAZ18 as a potential key regulator in glandular trichome development, which could have significant implications for the breeding and genetic improvement of A. argyi. Moving forward, further functional characterization of these genes, particularly in relation to glandular trichome development and stress responses, would be crucial to validate their roles and explore their potential for crop improvement. Additionally, investigating the interactions between AarJAZs and other transcription factors under different environmental conditions will provide further insights into their regulatory networks.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cimb47020100/s1, Table S1: Protein sequences of JAZ genes in A. argyi; Table S2: Motif logos and sequences of Motifs 1–10, obtained using the MEME online website; Table S3: Gene ontology enrichment of the JAZ gene family; Table S4: Protein sequences of the JAZ genes in Arabidopsis, Artemisia annua, Oryza sativa, and Zea mays; Table S5: List of primers used in this study.
Author Contributions
Z.G.: Methodology, visualization, analysis of the data, and writing of the manuscript. X.W.: Data curation and validation. Y.L.: Investigation and data curation. T.Z.: Software and validation. Z.Y.: funding acquisition. Y.W.: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Smart Agricultural Facility Technology Innovation and Integration Demonstration: 2024ZY-CGZY-01; Introduction of Famous Varieties of Facility Vegetables, Melons, and Fruits and the Construction of Standardized Demonstration Bases: QYXTZX-AL2023-07; Shaanxi Province 100 Billion Facility Agriculture Special Project in 2021: K3030821094.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A. Metabolic end run to jasmonate. Nat. Chem. Biol. 2018, 14, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Gimenez-Ibanez, S.; Goossens, A.; Solano, R. Redundancy and specificity in jasmonate signalling. Curr. Opin. Plant Biol. 2016, 33, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, B.; Grunewald, W.; Bateman, A.; Kohchi, T.; Gheysen, G. The tify family previously known as ZIM. Trends Plant Sci. 2007, 12, 239–244. [Google Scholar] [CrossRef]
- Bai, Y.H.; Meng, Y.J.; Huang, D.L.; Qi, Y.H.; Chen, M. Origin and evolutionary analysis of the plant-specific TIFY transcription factor family. Genomics 2011, 98, 128–136. [Google Scholar] [CrossRef]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, Q.; Li, M.; Zhai, J.; Wu, S.; Ahmad, S.; Lan, S.; Peng, D.; Liu, Z.J. Genome-Wide Identification and Expression Pattern Analysis of TIFY Family Genes Reveal Their Potential Roles in Phalaenopsis aphrodite Flower Opening. Int. J. Mol. Sci. 2024, 25, 5422. [Google Scholar] [CrossRef]
- Staswick, P.E. JAZing up jasmonate signaling. Trends Plant Sci. 2008, 13, 66–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, M.; Singer, S.D.; Fei, Z.; Wang, H.; Wang, X. Genome-wide identification and analysis of the TIFY gene family in grape. PLoS ONE 2012, 7, e44465. [Google Scholar] [CrossRef]
- Geerinck, J.; Pauwels, L.; De Jaeger, G.; Goossens, A. Dissection of the one-MegaDalton JAZ1 protein complex. Plant Signal. Behav. 2010, 5, 1039–1041. [Google Scholar] [CrossRef][Green Version]
- Melotto, M.; Mecey, C.; Niu, Y.; Chung, H.S.; Katsir, L.; Yao, J.; Zeng, W.Q.; Thines, B.; Staswick, P.; Browse, J.; et al. A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1F-box protein. Plant J. 2008, 55, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Figueroa, P.; Browse, J. Characterization of JAZ-interacting bHLH transcription factors that regulate jasmonate responses in Arabidopsis. J. Exp. Bot. 2011, 62, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calvo, P.; Chini, A.; Fernández-Barbero, G.; Chico, J.M.; Gimenez-Ibanez, S.; Geerinck, J.; Eeckhout, D.; Schweizer, F.; Godoy, M.; Franco-Zorrilla, J.M.; et al. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 2011, 23, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, F.; Chen, D.; Chu, W.; Liu, H.; Xiang, Y. Genome-wide identification and analysis of the Populus trichocarpa TIFY gene family. Plant Physiol. Biochem. 2017, 115, 360–371. [Google Scholar] [CrossRef]
- Ye, H.Y.; Du, H.; Tang, N.; Li, X.H.; Xiong, L.Z. Identification and expression profiling analysis of TIFY family genes involved in stress and phytohormone responses in rice. Plant Mol. Biol. 2009, 71, 291–305. [Google Scholar] [CrossRef]
- Yan, T.; Chen, M.; Shen, Q.; Li, L.; Fu, X.; Pan, Q.; Tang, Y.; Shi, P.; Lv, Z.; Jiang, W.; et al. HOMEODOMAIN PROTEIN 1 is required for jasmonate-mediated glandular trichome initiation in Artemisia annua. New Phytol. 2017, 213, 1145–1155. [Google Scholar] [CrossRef]
- Sun, Y.G.; Liu, C.X.; Liu, Z.B.; Zhao, T.T.; Jiang, J.B.; Li, J.F.; Xu, X.Y.; Yang, H.H. Genome-Wide Identification, Characterization and Expression Analysis of the JAZ Gene Family in Resistance to Gray Leaf Spots in Tomato. Int. J. Mol. Sci. 2021, 22, 9974. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Li, X.L.; Yu, R.; Han, M.; Wu, Z.Y. Isolation, structural analysis, and expression characteristics of the maize TIFY gene family. Mol. Genet. Genom. 2015, 290, 1849–1858. [Google Scholar] [CrossRef]
- Oblessuc, P.R.; Obulareddy, N.; DeMott, L.; Matiolli, C.C.; Thompson, B.K.; Melotto, M. JAZ4 is involved in plant defense, growth, and development in Arabidopsis. Plant J. 2020, 101, 371–383. [Google Scholar] [CrossRef]
- Singh, A.P.; Mani, B.; Giri, J. OsJAZ9 is involved in water-deficit stress tolerance by regulating leaf width and stomatal density in rice. Plant Physiol. Biochem. 2021, 162, 161–170. [Google Scholar] [CrossRef]
- Hanif, M.; Rahman, M.U.; Gao, M.; Yang, J.; Ahmad, B.; Yan, X.; Wang, X. Heterologous Expression of the Grapevine JAZ7 Gene in Arabidopsis Confers Enhanced Resistance to Powdery Mildew but Not to Botrytis cinerea. Int. J. Mol. Sci. 2018, 19, 3889. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Pan, X.; Yu, Y.; Zhu, Y.; Kong, F.; Sun, X.; Wang, F. Overexpression of a TIFY family gene, GsJAZ2, exhibits enhanced tolerance to alkaline stress in soybean. Mol. Breed. 2020, 40, 33. [Google Scholar] [CrossRef]
- Mei, Q.X.; Chen, X.L.; Xiang, L.; Liu, Y.; Su, Y.Y.; Gao, Y.Q.; Dai, W.B.; Dong, P.P.; Chen, S.L. DNA Barcode for Identifying Folium Artemisiae Argyi from Counterfeits. Biol. Pharm. Bull. 2016, 39, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission 2020 Pharmacopoeia of the People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2020.
- Hu, W.J.; Yu, A.Q.; Bi, H.Z.; Gong, Y.; Wang, H.; Kuang, H.X.; Wang, M. Recent advances in Artemisia argyi Levl. et Vant. polysaccharides: Extractions, purifications, structural characteristics, pharmacological activities, and existing and potential applications. Int. J. Biol. Macromol. 2024, 279, 135250. [Google Scholar] [CrossRef]
- Cui, Z.; Li, M.; Han, X.; Liu, H.; Li, C.; Peng, H.; Liu, D.; Huang, X.; Zhang, Z. Morphogenesis, ultrastructure, and chemical profiling of trichomes in Artemisia argyi H. Lév. & Vaniot (Asteraceae). Planta 2022, 255, 102. [Google Scholar]
- Turner, G.W.; Gershenzon, J.; Croteau, R.B. Distribution of peltate glandular trichomes on developing leaves of peppermint. Plant Physiol. 2000, 124, 655–664. [Google Scholar] [CrossRef]
- Shi, X.S.; Li, H.L.; Li, X.M.; Wang, D.J.; Li, X.; Meng, L.H.; Zhou, X.W.; Wang, B.G. Highly oxygenated polyketides produced by Trichoderma koningiopsis QA-3, an endophytic fungus obtained from the fresh roots of the medicinal plant Artemisia argyi. Bioorg. Chem. 2020, 94, 103448. [Google Scholar] [CrossRef]
- Tseng, C.P.; Huang, Y.L.; Chang, Y.W.; Liao, H.R.; Chen, Y.L.; Hsieh, P.W. Polysaccharide-containing fraction from Artemisia argyi inhibits tumor cell-induced platelet aggregation by blocking interaction of podoplanin with C-type lectin-like receptor 2. J. Food Drug Anal. 2020, 28, 115–123. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Shao, Z.T.; Bi, G.M.; Sun, Y.W.; Wang, Y.M.; Meng, D.L. Chemical constituents and biological activities of Artemisia argyi H.Lév. & vaniot. Nat. Prod. Res. 2023, 37, 1401–1405. [Google Scholar]
- Miao, Y.; Luo, D.; Zhao, T.; Du, H.; Liu, Z.; Xu, Z.; Guo, L.; Chen, C.; Peng, S.; Li, J.X.; et al. Genome sequencing reveals chromosome fusion and extensive expansion of genes related to secondary metabolism in Artemisia argyi. Plant Biotechnol. J. 2022, 20, 1902–1915. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Gao, S.; Lercher, M.J.; Chen, W.H.; Hu, S. Evolview v2: An online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016, 44, W236–W241. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.J.; Julien, P.; Kuhn, M.; von Mering, C.; Muller, J.; Doerks, T.; Bork, P. eggNOG: Automated construction and annotation of orthologous groups of genes. Nucleic Acids Res. 2008, 36, D250–D254. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Gao, X.; Wang, J.; Shang, Z.; Zhang, Z.; Zhou, Z.; Zhang, K. Full-Length Transcriptome Analysis Reveals Candidate Genes Involved in Terpenoid Biosynthesis in Artemisia argyi. Front. Genet. 2021, 12, 659962. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar]
- Nakashima, K.; Ito, Y.; Yamaguchi-Shinozaki, K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol. 2009, 149, 88–95. [Google Scholar] [CrossRef]
- Qi, T.; Song, S.; Ren, Q.; Wu, D.; Huang, H.; Chen, Y.; Fan, M.; Peng, W.; Ren, C.; Xie, D. The Jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate Jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 2011, 23, 1795–1814. [Google Scholar] [CrossRef]
- Yan, X.; Cui, L.; Liu, X.; Cui, Y.; Wang, Z.; Zhang, H.; Chen, L.; Cui, H. NbJAZ3 is required for jasmonate-meditated glandular trichome development in Nicotiana benthamiana. Physiol. Plant. 2022, 174, e13666. [Google Scholar] [CrossRef]
- Ahmad Khan, R.; Mohammad; Kumar, A.; Abbas, N. AaGL3-like is jasmonate-induced bHLH transcription factor that positively regulates trichome density in Artemisia annua. Gene 2024, 904, 148213. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; He, X.; Tu, L.; Zhu, L.; Zhu, S.; Ge, Z.; Zhang, X. GhJAZ2 negatively regulates cotton fiber initiation by interacting with the R2R3-MYB transcription factor GhMYB25-like. Plant J. 2016, 88, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, M.; Dong, S.; Wu, X.; Zhang, G.; He, L.; Jiao, Y.; Chen, S.; Li, L.; Luo, H. A chromosome-scale genome assembly of Artemisia argyi reveals unbiased subgenome evolution and key contributions of gene duplication to volatile terpenoid diversity. Plant Commun. 2023, 4, 100516. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.C.; Purugganan, M.D. The evolutionary dynamics of plant duplicate genes. Curr. Opin. Plant Biol. 2005, 8, 122–128. [Google Scholar] [CrossRef]
- Howe, G.A.; Yoshida, Y. Evolutionary Origin of JAZ Proteins and Jasmonate Signaling. Mol. Plant 2019, 12, 153–155. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Tian, S.; Liu, S.; Wang, Y.; Wang, K.; Yin, C.; Yue, Y.; Hu, H. Genome-Wide Identification and Characterization of JAZ Protein Family in Two Petunia Progenitors. Plants 2019, 8, 203. [Google Scholar] [CrossRef]
- Jia, K.; Yan, C.; Zhang, J.; Cheng, Y.; Li, W.; Yan, H.; Gao, J. Genome-wide identification and expression analysis of the JAZ gene family in turnip. Sci. Rep. 2021, 11, 21330. [Google Scholar] [CrossRef]
- Ahmadizadeh, M.; Heidari, P. Bioinformatics study of transcription factors involved in cold stress. Biharean Biol. 2014, 8, 83–86. [Google Scholar]
- Efferth, T.; Zacchino, S.; Georgiev, M.I.; Liu, L.; Wagner, H.; Panossian, A. Nobel Prize for artemisinin brings phytotherapy into the spotlight. Phytomedicine 2015, 22, A1–A3. [Google Scholar] [CrossRef]
- Su, X.Z.; Miller, L.H. The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. Sci. China Life Sci. 2015, 58, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Li, J.; Qiu, S.; Qi, F.; Su, H.; Bu, Q.; Jiang, R.; Tang, K.; Zhang, L.; Chen, W. The transcription factors TLR1 and TLR2 negatively regulate trichome density and artemisinin levels in Artemisia annua. J. Integr. Plant Biol. 2022, 64, 1212–1228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tan, H.; Li, Q.; Li, Q.; Wang, Y.; Bu, Q.; Li, Y.; Wu, Y.; Chen, W.; Zhang, L. TRICHOME AND ARTEMISININ REGULATOR 2 positively regulates trichome development and artemisinin biosynthesis in Artemisia annua. New Phytol. 2020, 228, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Judd, R.; Bagley, M.C.; Li, M.; Zhu, Y.; Lei, C.; Yuzuak, S.; Ekelöf, M.; Pu, G.; Zhao, X.; Muddiman, D.C.; et al. Artemisinin Biosynthesis in Non-glandular Trichome Cells of Artemisia annua. Mol. Plant 2019, 12, 704–714. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).