Modulating Skin Aging Molecular Targets and Longevity Drivers Through a Novel Natural Product: Rose-Derived Polydeoxyribonucleotide (Rose PDRN)
Abstract
1. Introduction
2. Materials and Methods
2.1. Rose-Derived PDRN, Material, DNA Extraction, and Characterization
2.2. Primary Cultured Keratinocytes
2.3. Organotypic 3D Skin Model (Human Skin Explants)
2.4. Western Blot and ELISA Assays
2.5. Mitochondrial Function Analysis via Mitotracker®
2.6. ATP/ADP Detection and Quantification
2.7. Detection, Visualization, and Quantification of Biomarkers on Cells
2.8. Detection, Visualization, and Quantification of Biomarkers on Skin Explant Sections
2.9. Data Integration and Statistics
- (a)
- a baseline variation (% of induction) representing the stimulatory effect of PDRN, when applied without stress exposure, compared to the experimental group “control” was obtained by using the following equation:Baseline variation (% of induction group X vs. group Control) =
((Biomarker_Levels_ group_X/Biomarker_Levels_ group_Control) − 1) * 100 - (b)
- a protective value (% of efficacy) was used to describe the relative effect of PDRN under stress conditions and to indicate the positioning of each condition with respect to the non-stressed control (baseline control). This % of efficacy was calculated by setting the control group as the reference for maximum efficiency (100%) and the stress group as the reference for minimum efficiency (0%). Each experimental condition was then positioned within this range to provide a clearer interpretation of how PDRN counteracts stress-induced alterations relative to baseline. The following equation was employed for calculation:Efficacy (% of efficacy group X) = ((Biomarker_Levels_Group_Stress − Biomarker_Levels_Group_ X)/(Biomarker_Levels_Group_Stress − Biomarker_Levels_ group_Control) * 100
3. Results and Discussion
3.1. In Vitro Assessments (On Human Keratinocytes)
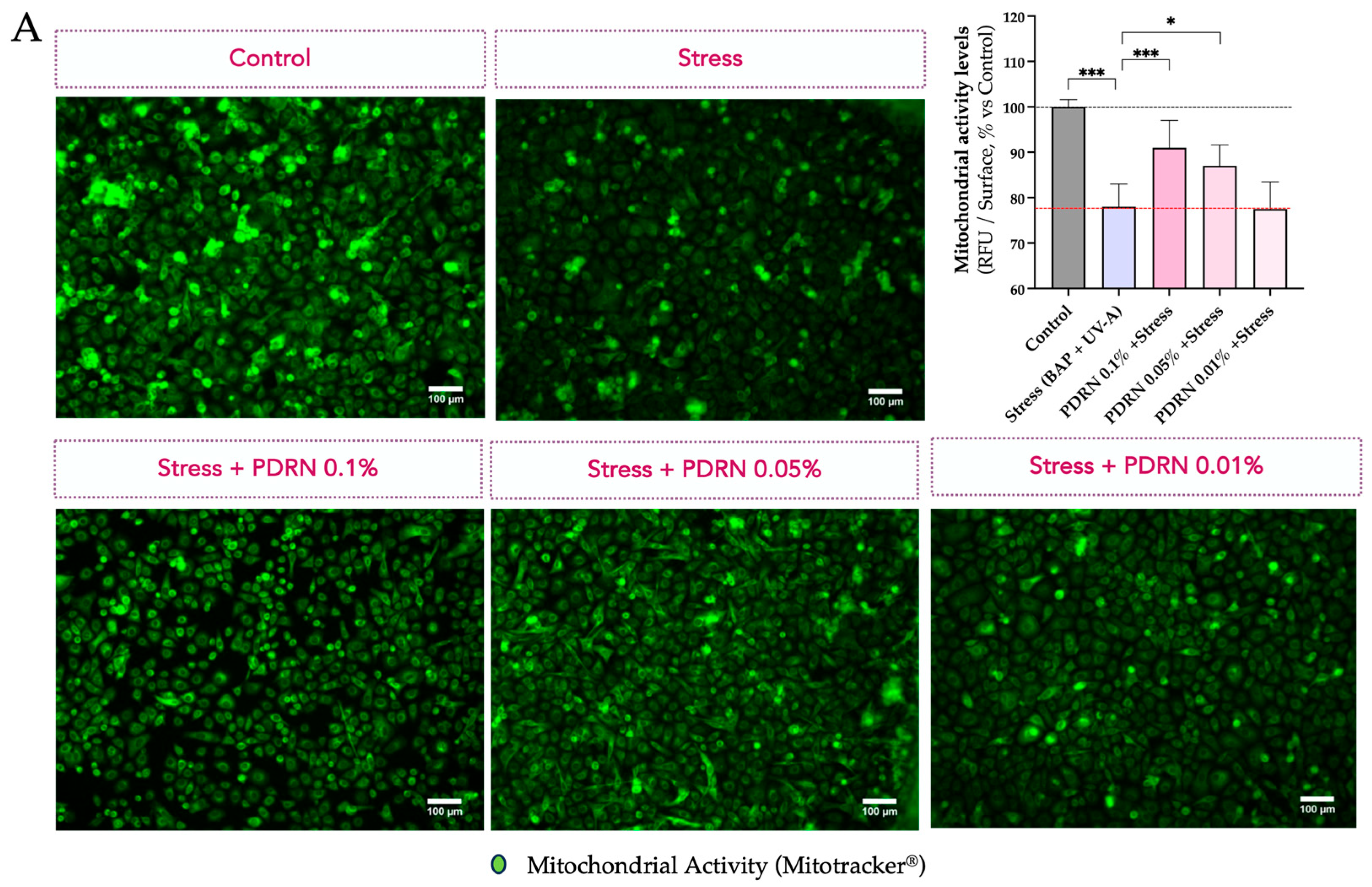
3.1.1. Mitochondrial Membrane Polarization
3.1.2. Autophagy and Mitophagy
3.1.3. Mitochondrial Function and Content
3.2. Ex Vivo (On Skin Explants) Assessments
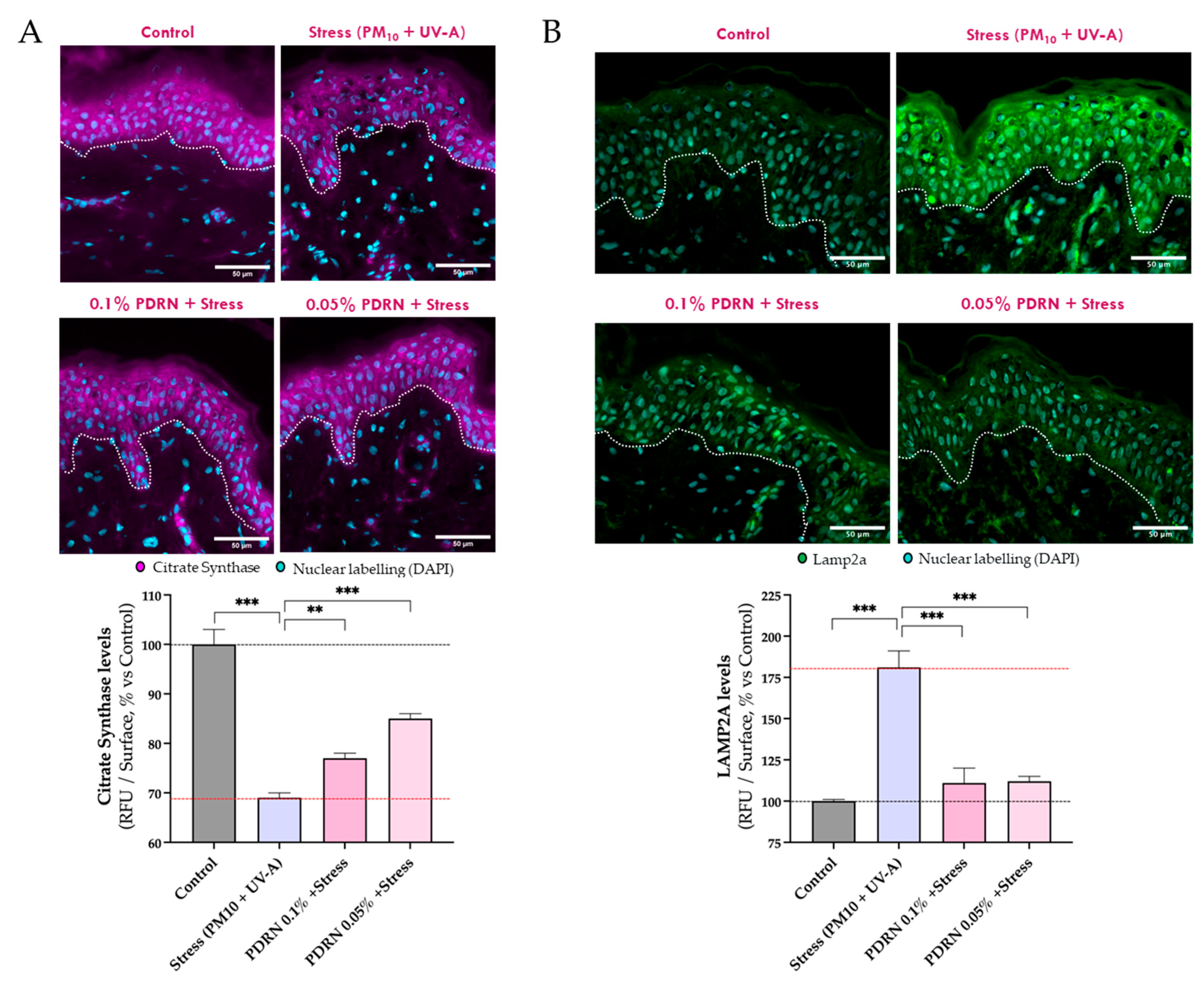
3.2.1. Mitochondrial Function
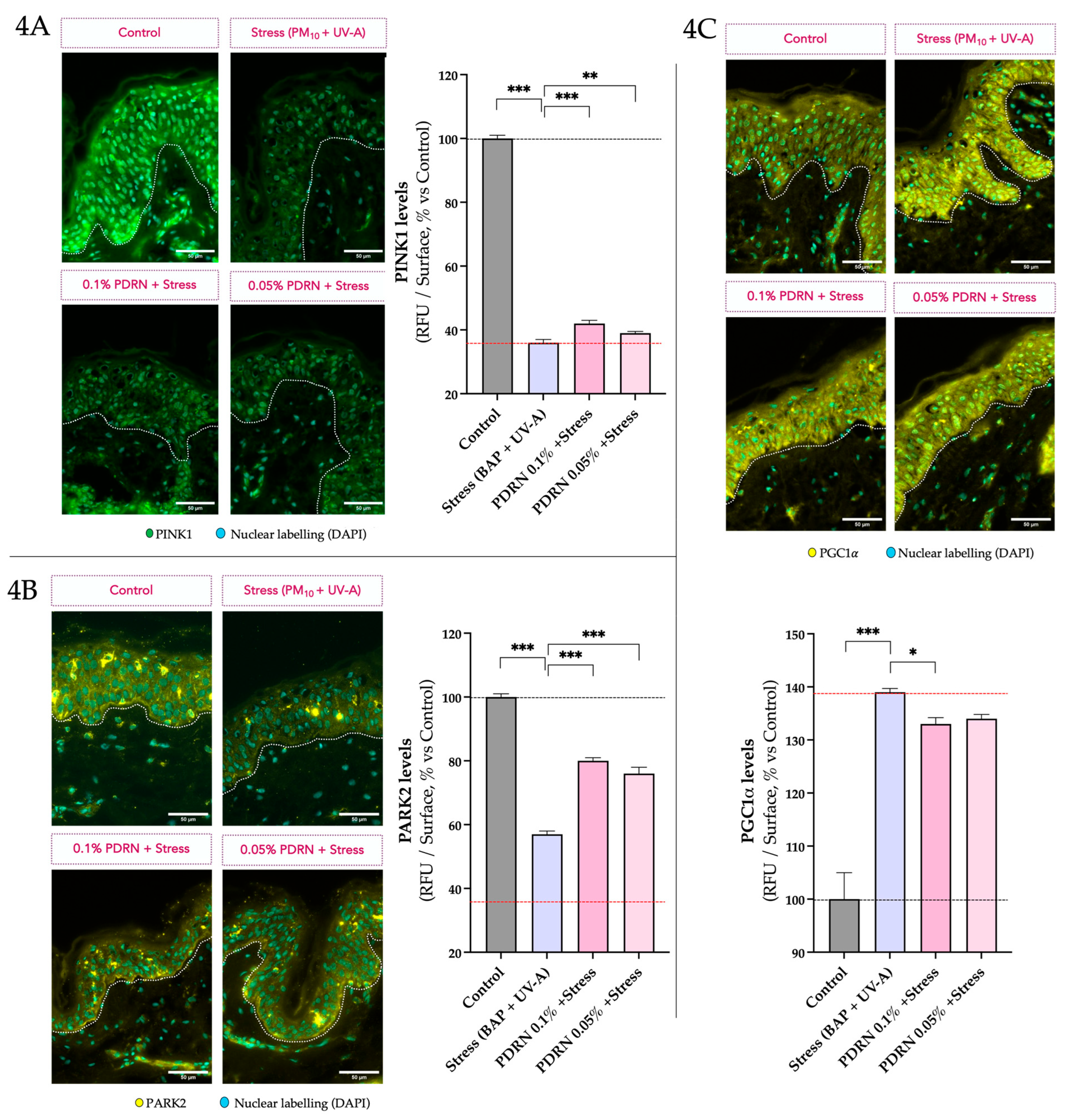
3.2.2. Autophagy and Mitochondrial Homeostasis
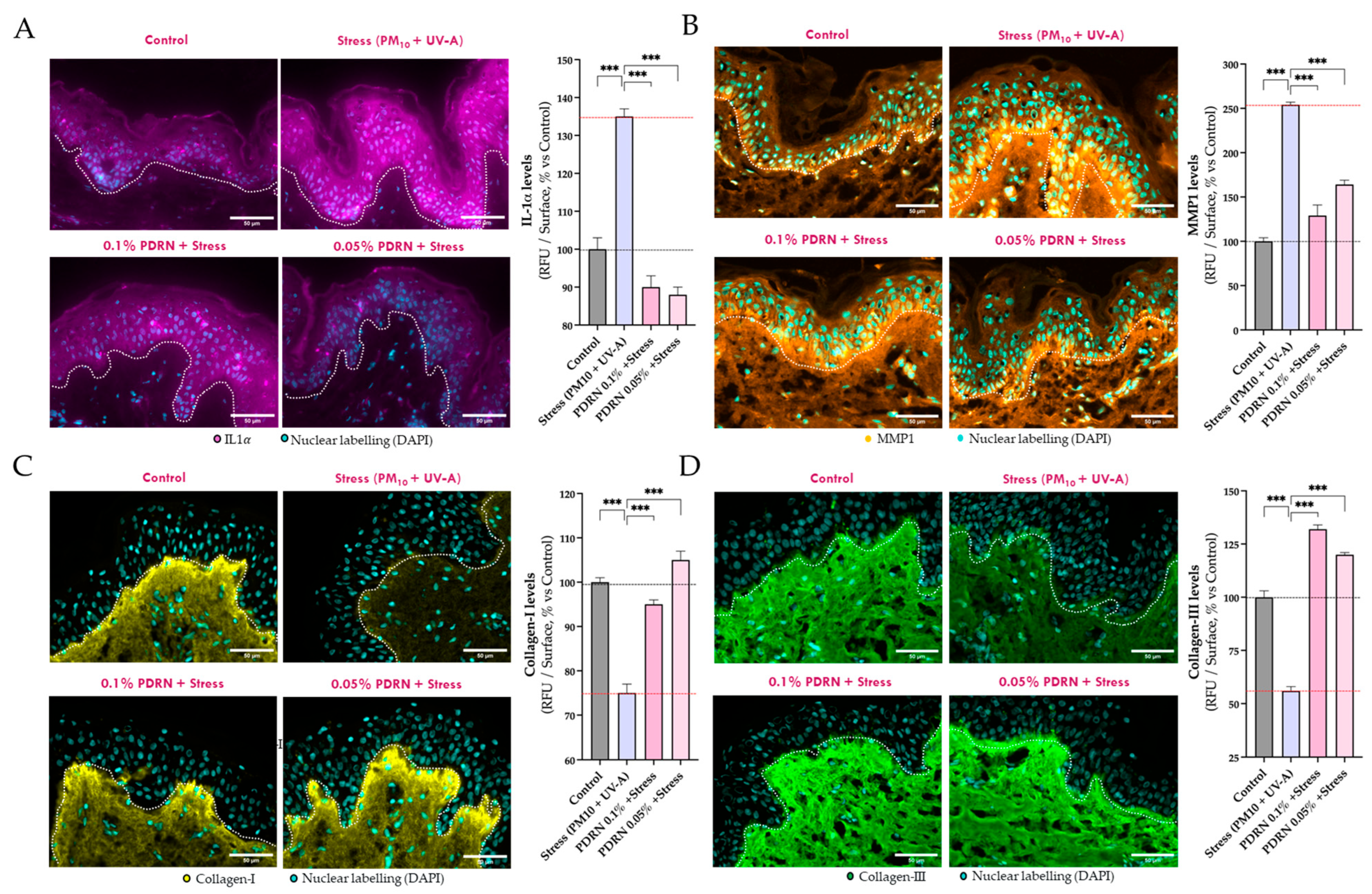
3.2.3. Evaluation of SASP Markers and Dermal Matrix Integrity
3.2.4. Proteostasis and Oxidative Stress
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The Skin Aging Exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Hartung, F.; Krutmann, J.; Haarmann-Stemmann, T. Evidence that the aryl hydrocarbon receptor orchestrates oxinflammatory responses and contributes to airborne particulate matter-induced skin aging. Free. Radic. Biol. Med. 2025, 233, 264–278. [Google Scholar] [CrossRef]
- Hüls, A.; Vierkötter, A.; Gao, W.; Krämer, U.; Yang, Y.; Ding, A.; Stolz, S.; Matsui, M.; Kan, H.; Wang, S.; et al. Traffic-related air pollution contributes to development of facial lentigines: Further epidemiological evidence from Caucasians and Asians. J. Investig. Dermatol. 2016, 136, 1053–1061. [Google Scholar] [CrossRef]
- Brem, R.; Macpherson, P.; Guven, M.; Karran, P. Oxidative stress induced by UVA photoactivation of the tryptophan UVB photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) inhibits nucleotide excision repair in human cells. Sci. Rep. 2017, 7, 4310. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L. Pollution and Sun Exposure: A Deleterious Synergy. Mechanisms and Opportunities for Skin Protection. Curr. Med. Chem. 2018, 25, 5469–5486. [Google Scholar] [CrossRef]
- Hui, J.; Lin, Z.; Pang, T.; Wu, J.; Zhao, C.; Zhang, Y.; Lei, Y.; Li, Q.; Yao, Y.; Zhao, M.; et al. Effects and mechanisms of polycyclic aromatic hydrocarbons in inflammatory skin diseases. Sci. Total Environ. 2024, 925, 171492. [Google Scholar]
- Larnac, E.; Méthot, S.; Pelchat, F.; Millette, M.-A.; Montoni, A.; Salesse, C.; Haydont, V.; Marrot, L.; Rochette, P.J. Synergistic Toxicity of Pollutant and Ultraviolet Exposure from a Mitochondrial Perspective. Int. J. Mol. Sci. 2024, 25, 9146. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Lee, Y.I.; Jang, S.; Song, S.Y.; Lee, W.J.; Lee, J.H. Particulate matter-induced atmospheric skin aging is aggravated by UVA and inhibited by a topical L-ascorbic acid compound. J. Dermatol. Sci. 2021, 103, 123–131. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Maier, A.B.; Cuervo, A.M.; Gladyshev, V.N.; Ferrucci, L.; Gorbunova, V.; Kennedy, B.K.; Rando, T.A.; Seluanov, A.; Sierra, F.; et al. From geroscience to precision geromedicine: Understanding and managing aging. Cell 2025, 188, 2043–2062. [Google Scholar] [CrossRef]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological activity and clinical use of PDRN. Front. Pharmacol. 2017, 8, 224, Erratum in Front. Pharmacol. 2022, 13, 1073510. [Google Scholar] [CrossRef]
- Belletti, S.; Uggeri, J.; Gatti, R.; Govoni, P.; Guizzardi, S. Polydeoxyribonucleotide promotes cyclobutane pyrimidine dimer repair in UVB-exposed dermal fibroblasts. Photodermatol. Photoimmunol. Photomed. 2007, 23, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Wang, G.; Zhou, F.; Gong, L.; Zhang, J.; Qi, L.; Cui, H. Polydeoxyribonucleotide: A promising skin anti-aging agent. Chin. J. Plast. Reconstr. Surg. 2022, 4, 187–193. [Google Scholar] [CrossRef]
- Naik, V.; Chodankar, S.; Pereira, M.; Loliyekar, A. The impact of polydeoxyribonucleotide on wound healing: A systematic review. Int. Surg. J. 2025, 12, 568–574. [Google Scholar] [CrossRef]
- Marques, C.; Porcello, A.; Cerrano, M.; Hadjab, F.; Chemali, M.; Lourenço, K.; Hadjab, B.; Raffoul, W.; Applegate, L.A.; Laurent, A.E. From Polydeoxyribonucleotides (PDRNs) to Polynucleotides (PNs): Bridging the Gap Between Scientific Definitions, Molecular Insights, and Clinical Applications of Multifunctional Biomolecules. Biomolecules 2025, 15, 148. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, S.; Wang, H.; Lee, G.; Kim, S.; Ryu, Y.H.; Chang, N.; Kang, Y.W. Analysis of skin regeneration and barrier-improvement efficacy of polydeoxyribonucleotide isolated from Panax ginseng C.A. Meyer adventitious root. Molecules 2023, 28, 7240. [Google Scholar] [CrossRef]
- Choi, J.H.; Qiu, F.; Shi, J.; Huang, W.; Zhao, C.; Han, Q. PDRN prevents SIRT1 degradation by attenuating autophagy during UVB-induced skin aging. PLoS ONE 2023, 18, e0321005. [Google Scholar]
- Bitto, A.; Polito, F.; Altavilla, D.; Minutoli, L.; Migliorato, A.; Squadrito, F. Polydeoxyribonucleotide (PDRN) restores blood flow in an experimental model of peripheral artery occlusive disease. J. Vasc. Surg. 2008, 48, 1292–1300. [Google Scholar] [CrossRef]
- Green, D.R.; Galluzzi, L. Mitochondria and the autophagy–inflammation–cell death axis in organismal aging. Science 2011, 333, 1109–1112. [Google Scholar] [CrossRef]
- Passos, J.F.; Saretzki, G.; Ahmed, S.; Nelson, G.; Richter, T.; Peters, H.; Wappler, I.; Birket, M.J.; Harold, G.; Schaeuble, K.; et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007, 5, e110. [Google Scholar] [CrossRef]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schrøder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Dice, J.F. Age-related decline in chaperone-mediated autophagy due to reduced lysosomal receptor levels. J. Biol. Chem. 2000, 275, 31505–31513. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Wong, E. Chaperone-mediated autophagy: Roles in disease and aging. Cell Res. 2014, 24, 92–104. [Google Scholar] [CrossRef]
- Tasset, I.; Cuervo, A.M. Role of chaperone-mediated autophagy in metabolism. FEBS J. 2016, 283, 2403–2418. [Google Scholar] [CrossRef] [PubMed]
- Kiffin, R.; Christian, C.; Knecht, E.; Cuervo, A.M. Activation of chaperone-mediated autophagy during oxidative stress. Mol. Biol. Cell 2007, 15, 4829–4840. [Google Scholar] [CrossRef] [PubMed]
- Valdor, R.; Martinez-Vicente, M. The role of chaperone-mediated autophagy in tissue homeostasis and disease pathogenesis. Biomedicines 2024, 12, 257–278. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhang, X.; Zhang, S.; Sun, Y.; Wang, H.; Chen, Y.; Zhang, H.; Zou, P.; Feng, Y.; Lu, X.; et al. Chaperone-mediated autophagy manipulates PGC1α stability and governs energy metabolism under thermal stress. Nat. Commun. 2025, 16, 4455. [Google Scholar] [CrossRef]
- Banarase, T.A.; Sammeta, S.S.; Wankhede, N.L.; Mangrulkar, S.V.; Rahangdale, S.R.; Aglawe, M.M.; Taksande, B.G.; Upaganlawar, A.B.; Umekar, M.J.; Kale, M.B. Mitophagy regulation in aging and neurodegenerative disease. Biophys. Rev. 2023, 15, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, X.; Li, M.; Yu, X.; Huang, F.; Wang, Y.; Yan, Y.; Zhang, H.; Shi, Y.; He, X. The role of mitochondrial quality surveillance in skin aging: Focus on mitochondrial dynamics, biogenesis and mitophagy. Ageing Res. Rev. 2023, 87, 101917. [Google Scholar] [CrossRef]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.-F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, M.; Sliter, D.A.; Kane, L.A.; Sarraf, S.A.; Wang, C.; Burman, J.L.; Sideris, D.P.; Fogel, A.I.; Youle, R.J. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 2015, 524, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ikeda, A.; Murata, D.; Wang, H.; Zhang, C.; Khare, P.; Adachi, Y.; Ito, F.; Quirós, P.M.; Blackshaw, S.; et al. Dual regulation of mitochondrial fusion by Parkin–PINK1 and OMA1. Nature 2025, 639, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhu, Y.; Deng, C.; Liang, Z.; Chen, J.; Chen, Y.; Wang, X.; Liu, Y.; Tian, Y.; Yang, Y. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases. Signal Transduct. Target. Ther. 2024, 9, 50. [Google Scholar] [CrossRef]
- Luo, Y.; Bollag, W.B. The role of PGC-1α in aging skin barrier function. Cells 2024, 13, 1135–1153. [Google Scholar] [CrossRef]
- Tang, Q.-Q.; Otto, T.C.; Lane, M.D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 2004, 101, 9607–9611. [Google Scholar] [CrossRef]
- Tu, Y.; Quan, T. Oxidative Stress and Human Skin Connective Tissue Aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Hamon, M.P.; Ahmed, E.K.; Baraibar, M.A.; Friguet, B. Proteome Oxidative Modifications and Impairment of Specific Metabolic Pathways During Cellular Senescence and Aging. Proteomics 2020, 20, e1800421. [Google Scholar] [CrossRef]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Martic, I.; Jansen-Dürr, P.; Cavinato, M. Effects of Air Pollution on Cellular Senescence and Skin Aging. Cells 2022, 11, 2220. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.; Gil, J. Senescence and the SASP: Many Therapeutic Avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- Cuollo, L.; Antonangeli, F.; Santoni, A.; Soriani, A. The Senescence-Associated Secretory Phenotype (SASP) in the Challenging Future of Cancer Therapy and Age-Related Diseases. Biology 2020, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Jaffri, J. Reactive Oxygen Species and Antioxidant System in Selected Skin Disorders. Malays. J. Med. Sci. 2023, 30, 7–20. [Google Scholar] [CrossRef]
- Baraibar, M.A.; Friguet, B. Oxidative Proteome Modifications Target Specific Cellular Pathways during Oxidative Stress, Cellular Senescence and Aging. Exp. Gerontol. 2013, 48, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Le Boulch, M.; Ahmed, E.K.; Rogowska-Wrzesinska, A.; Baraibar, M.A.; Friguet, B. Proteome Oxidative Carbonylation during Oxidative Stress-Induced Premature Senescence of WI-38 Human Fibroblasts. Mech. Ageing Dev. 2018, 170, 59–71. [Google Scholar] [CrossRef]
- Gonos, E.S.; Kapetanou, M.; Sereikaite, J.; Bartosz, G.; Naparło, K.; Grzesik, M.; Sadowska-Bartosz, I. Origin and Pathophysiology of Protein Carbonylation, Nitration and Chlorination in Age-Related Brain Diseases and Aging. Aging 2018, 10, 868–901. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The Proteostasis Network and Its Decline in Ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Qomaladewi, N.P.; Lee, J.; Park, S.H.; Cho, J.Y. The Role of Autophagy in Skin Fibroblasts, Keratinocytes, Melanocytes, and Epidermal Stem Cells. J. Investig. Dermatol. 2020, 140, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Nyström, T. Role of Oxidative Carbonylation in Protein Quality Control and Senescence. EMBO J. 2005, 24, 1311–1317. [Google Scholar] [CrossRef]
- Hyundai Bioland Co., Ltd., L’Oréal. Cosmetic Compositions Containing Rose-Derived DNA as an Active Ingredient for Skin Barrier Improvement and Regeneration Effect. KR 10-2024-0085418, PCT/KR2024/012702.
- Baraibar, M.A.; Ladouce, R.; Friguet, B. Proteomic quantification and identification of carbonylated proteins upon oxidative stress and during cellular aging. J. Proteom. 2013, 92, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cavagnino, A.; Bobier, A.; Baraibar, M. The skin oxi-proteome as a molecular signature of exposome stress. Househ. Pers. Care Today 2019, 15, 28–32. [Google Scholar]
- Gouin, O.; Cavagnino, A.; Azadiguian, G.; Jäger, S.; Comte, G.; Bendahmane, M.; Breton, L.; Baraibar, M.A.; Black, A.F. Exploring Skin Longevity Pathways: Rosa hybrid Extract-Mediated AMP-Activated Protein Kinase Activation, Antioxidant, and Autophagic Mechanisms in Human Keratinocytes. Cosmetics 2025, 12, 57. [Google Scholar] [CrossRef]



| Description | Target and Reference |
|---|---|
| Primary antibodies | Collagen I (Abcam/ab138492) Collagen III (Abcam, ab6310) |
| IL-1alpha (Proteintech, 16765-1-AP) | |
| MMP1 (Abcam, ab137332) PINK1 (Abcam, ab216144) PARKIN/PARK2 (Abcam, ab77924) PGC1alpha (Abcam, ab191838) LAMP2A (Invitrogen, 51-2200) Citrate Synthase (Abcam, ab96600) | |
| Secondary antibodies | Anti-mouse Alexafluor 647 (Invitrogen A21235) |
| Anti-Rabbit Alexafluor 647 (Invitrogen A21244) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavagnino, A.; Azadiguian, G.; Breton, L.; Baraibar, M.; Black, A.F. Modulating Skin Aging Molecular Targets and Longevity Drivers Through a Novel Natural Product: Rose-Derived Polydeoxyribonucleotide (Rose PDRN). Curr. Issues Mol. Biol. 2025, 47, 971. https://doi.org/10.3390/cimb47120971
Cavagnino A, Azadiguian G, Breton L, Baraibar M, Black AF. Modulating Skin Aging Molecular Targets and Longevity Drivers Through a Novel Natural Product: Rose-Derived Polydeoxyribonucleotide (Rose PDRN). Current Issues in Molecular Biology. 2025; 47(12):971. https://doi.org/10.3390/cimb47120971
Chicago/Turabian StyleCavagnino, Andrea, Gayané Azadiguian, Lionel Breton, Martin Baraibar, and Annie F. Black. 2025. "Modulating Skin Aging Molecular Targets and Longevity Drivers Through a Novel Natural Product: Rose-Derived Polydeoxyribonucleotide (Rose PDRN)" Current Issues in Molecular Biology 47, no. 12: 971. https://doi.org/10.3390/cimb47120971
APA StyleCavagnino, A., Azadiguian, G., Breton, L., Baraibar, M., & Black, A. F. (2025). Modulating Skin Aging Molecular Targets and Longevity Drivers Through a Novel Natural Product: Rose-Derived Polydeoxyribonucleotide (Rose PDRN). Current Issues in Molecular Biology, 47(12), 971. https://doi.org/10.3390/cimb47120971






