Comparison of Different Methods of Molecular Detection of Erwinia amylovora in Plant Material
Abstract
1. Introduction
2. Materials and Methods
| Assay | Primer | Sequence | Source |
|---|---|---|---|
| LAMP | Ea_Shin2018_F3 | 5′-ATA ATA AGA GAA TGG CGC TAT G-3′ | EPPO 7/20 (3) [6] |
| Ea_Shin2018_B3 | 5′-TCT ACA TCT CCA CCT TTG G-3′ | ||
| Ea_Shin2018_FIP | 5′-TAA TGA AGT TGA ATC TCA GGC ATG AGA AAA AAT CCA TTG TAA AAC CTT CG-3′ | ||
| Ea_Shin2018_BIP | 5′-GAT GGA TTG CTT AGT GAG CTC AGC CAA TCT CTC CAC AAC CG-3′ | ||
| Ea_Shin2018_LoopF | 5′-AAA GTT GTT TTC ATC CCA CGG A-3′ | ||
| Real-time PCR | Ams116F (forward) | 5′-TCC CAC ATA CTG TGA ATC ATC CA-3 | |
| Ams189R (reverse) | 5′-GGG TAT TTG CGC TAA TTT TAT TCG-3′ | ||
| Ams141T (probe) | 5′-FAM-CCA GAA TCT GGC CCG CGT ATA CCG-TAMRA-3′ | ||
| 16S sequencing | Uni340F | 5′-CCT ACG GGR BGC ASC AG-3′ | [8] |
| Uni806R | 5′-GGA CTA CNN GGG TAT CTA AT-3′ |
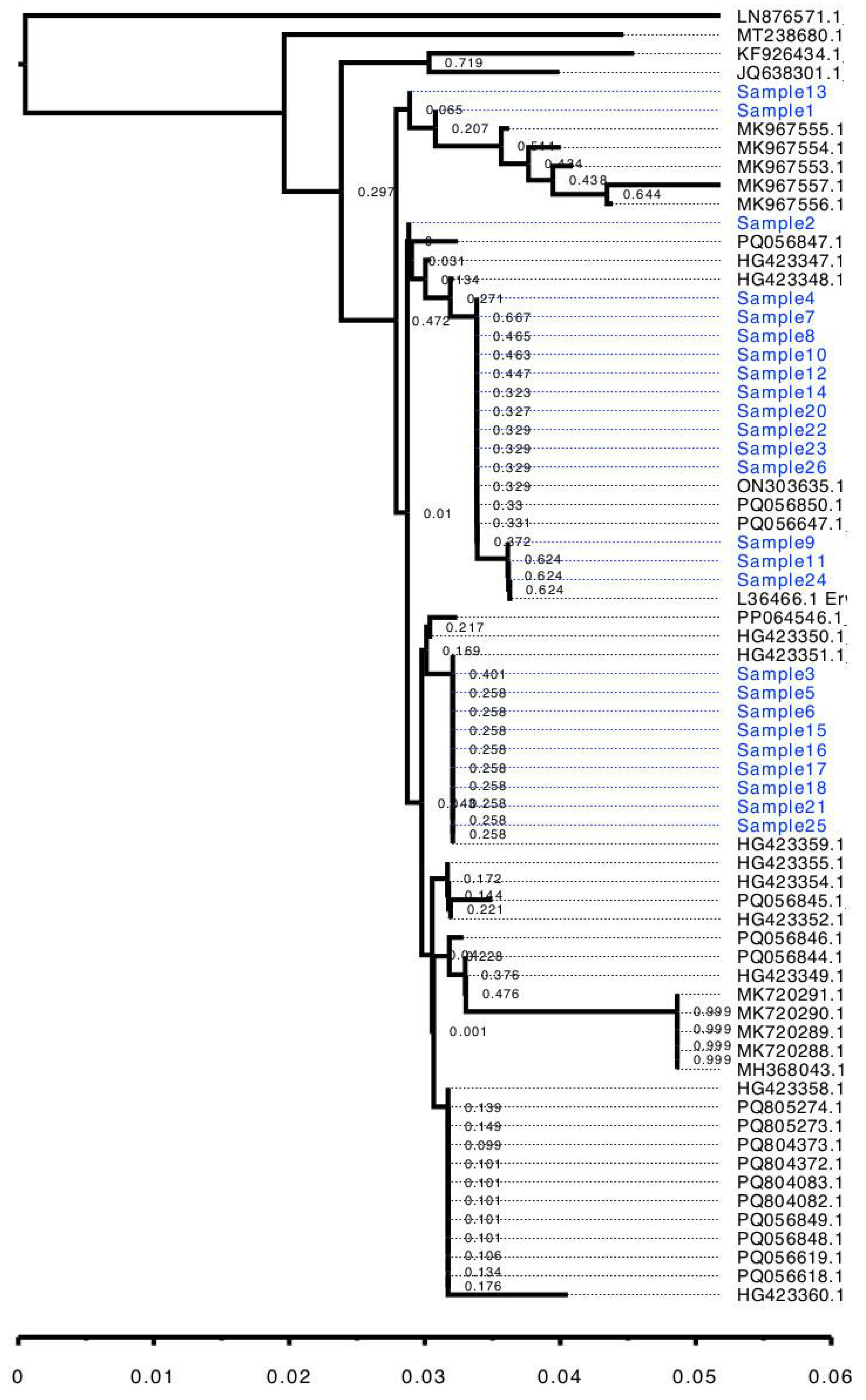
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Djaimurzina, A.; Umiralieva, Z.; Zharmukhamedova, G.; Born, Y.; Bühlmann, A.; Rezzonico, F. Detection of the Causative Agent of Fire Blight—Erwinia amylovora (Burrill) Winslow et al.—in the Southeast of Kazakhstan. Acta Hortic. 2014, 1056, 129–132. [Google Scholar] [CrossRef]
- Malakhova, N.P.; Skiba, Y.A.; Tezekbayeva, B.; Khasseyn, A.; Dmitriyeva, K.A.; Romashkin, V.N.; Zharmukhamedova, G.A.; Jumanov, Z.K.; Maltseva, E.R. Distribution of Erwinia amylovora on wild and culturally grown apple trees Malus spp. for the period of 2021. Eurasian J. Appl. Biotechnol. 2022, 2, 62–71. [Google Scholar] [CrossRef]
- Umiraliyeva, Z.Z.; Kopzhassarov, B.K.; Jaimurzina, A.A.; Niyazbekov, Z.B.; Issenova, G.Z.; Tursunova, A.K.; Berganayeva, G.E. Epidemiology of Fire Blight in Fruit Crops in Kazakhstan. AGRIVITA J. Agric. Sci. 2021, 43, 273–284. [Google Scholar] [CrossRef]
- Maltseva, E.R.; Berdygulova, Z.A.; Iskakova, G.; Zharmukhamedova, G.A.; Jumanova, Z.K.; Dosmagambet, Z.M.; Kuatbek, M.M.; Mendesh, A.M.; Ruslanuly, O.; Naizabayeva, D.A.; et al. Molecular Monitoring Reveals Two Distinct Erwinia amylovora Genotypes Threatening Cultivated Orchards and Wild Malus sieversii Reservoirs in Southern Kazakhstan. J. Plant Pathol. 2025, 1–8. [Google Scholar] [CrossRef]
- Forsline, P.L.; Luby, J.J.; Aldwinckle, H.S. Fire blight incidence on Malus sieversii grown in New York and Minnesota. Acta Hortic. 2008, 793, 345–350. [Google Scholar] [CrossRef]
- EPPO Standard on Diagnostics. PM 7/20 (3) Erwinia amylovora. EPPO Bull. 2022, 52, 198–224. [Google Scholar] [CrossRef]
- Sadanov, A.; Ismailova, E.; Alexyuk, M.; Shemshura, O.; Baimakhanova, G.; Baimakhanova, B.; Turlybaeva, Z.; Molzhigitova, A.; Yelubayeva, A.; Tleubekova, D.; et al. Whole Genome Sequence Data of Erwinia Amilovora Strain E22, from Kazakhstan. Data Brief. 2024, 57, 111090. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Horikoshi, K. Rapid Detection and Quantification of Members of the Archaeal Community by Quantitative PCR Using Fluorogenic Probes. Appl. Environ. Microbiol. 2000, 66, 5066–5072. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast Metagenomic Sequence Classification Using Exact Alignments. Genome Biol. 2014, 15, R46. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. Subgroup, 1000 Genome Project Data Processing the Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.J. Objections to Bootstrapping Phylogenies: A Critique. Syst. Biol. 1995, 44, 299–320. [Google Scholar] [CrossRef]
- Singh, J.; Cobb-Smith, D.; Higgins, E.; Khan, A. Comparative Evaluation of Lateral Flow Immunoassays, LAMP, and Quantitative PCR for Diagnosis of Fire Blight in Apple Orchards. J. Plant Pathol. 2021, 103, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Bühlmann, A.; Pothier, J.F.; Rezzonico, F.; Smits, T.H.M.; Andreou, M.; Boonham, N.; Duffy, B.; Frey, J.E. Erwinia amylovora Loop-Mediated Isothermal Amplification (LAMP) Assay for Rapid Pathogen Detection and on-Site Diagnosis of Fire Blight. J. Microbiol. Methods 2013, 92, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Serrano-Heras, G.; Castaño, M.J.; Solera, J. Real-Time PCR Detection Chemistry. Clin. Chim. Acta 2015, 439, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Liu, Y.; Zhou, X.; Yin, C.; Zhang, P.; Yang, Q.; Mao, L.; Shentu, X.; Yu, X. Nanopore Sequencing Technology and Its Application in Plant Virus Diagnostics. Front. Microbiol. 2022, 13, 939666. [Google Scholar] [CrossRef] [PubMed]
- Trontin, C.; Agstner, B.; Altenbach, D.; Anthoine, G.; Bagińska, H.; Brittain, I.; Chabirand, A.; Chappé, A.M.; Dahlin, P.; Dreo, T.; et al. VALITEST: Validation of Diagnostic Tests to Support Plant Health. EPPO Bull. 2021, 51, 198–206. [Google Scholar] [CrossRef]
- Veselovsky, V.; Romanov, M.; Zoruk, P.; Larin, A.; Babenko, V.; Morozov, M.; Strokach, A.; Zakharevich, N.; Khamidova, S.; Danilova, A.; et al. Comparative Evaluation of Sequencing Platforms: Pacific Biosciences, Oxford Nanopore Technologies, and Illumina for 16S rRNA-Based Soil Microbiome Profiling. Front. Microbiol. 2025, 16, 1633360. [Google Scholar] [CrossRef] [PubMed]

| Number | Region | Location (Coordinates, °) | Type | Number of Samples |
|---|---|---|---|---|
| 1 | Almaty | 43.226801; 76.916254 | Orchard | 3 |
| 2 | Almaty | N/A (Shelek village) | Orchard | 5 |
| 3 | Almaty | 43.510556402; 77.702780175 | Orchard | 4 |
| 4 | Almaty | 43.406389741; 77.423057951 | Orchard | 5 |
| 5 | Turkestan | 42.316667657; 70.616669068 | Orchard | 4 |
| 6 | Turkestan | 42.44023; 69.78490 | Orchard | 3 |
| 7 | Turkestan | 42.490000994; 70.290002409 | Orchard | 4 |
| 8 | Zhambyl | 43.035192625; 71.888579086 | Orchard | 2 |
| 9 | Zhambyl | 43.022715960; 71.817559086 | Orchard | 6 |
| 10 | Zhambyl | 43.026072641; 71.013099090 | Orchard | 3 |
| 11 | Zhambyl | 43.022852627; 71.823817419 | Orchard | 5 |
| 12 | Zhambyl | 42.863334271; 73.174169072 | Orchard | 3 |
| 13 | Zhetysu | 44.863056365; 78.764169105 | Orchard | 5 |
| 14 | Almaty | 43.293056816; 79.484169376 | Wild population | 6 |
| 15 | Almaty | 43.305278810; 79.751391374 | Wild population | 4 |
| 16 | Almaty | 43.364244848; 77.680407392 | Wild population | 3 |
| 17 | Zhetysu | 45.517460764; 80.722242446 | Wild population | 5 |
| 18 | Kyrgyz Republic | 41.210000953; 73.336669017 | Wild population | 4 |
| 19 | Kyrghyz Republic | 41.333334292; 72.933335690 | Wild population | 5 |
| 20 | Turkestan | 42.44863821; 69.78856815 | Abandoned orchard | 5 |
| 21 | Turkestan | 42.44779721; 69.79035526 | Abandoned orchard | 6 |
| 22 | Turkestan | 42.44968945; 69.78657079 | Abandoned orchard | 4 |
| 23 | Turkestan | 42.44874333; 69.76197175 | Abandoned orchard | 3 |
| 24 | Turkestan | 42.46041211; 69.74557239 | Abandoned orchard | 3 |
| 25 | Turkestan | 42.46230434; 69.75187984 | Abandoned orchard | 5 |
| 26 | Turkestan | 42.44522167; 69.84775302 | Abandoned orchard | 4 |
| 27 | Turkestan | 42.44401275; 69.85153749 | Abandoned orchard | 4 |
| 28 | Almaty | 43.55170546; 78.28706375 | Abandoned orchard | 3 |
| 29 | Almaty | 43.55202083; 78.28900855 | Abandoned orchard | 5 |
| 30 | Almaty | 43.54077255; 78.28506639 | Abandoned orchard | 3 |
| Total: | 124 |
| Sample Number | Sampling Population | Symptoms 1 | LAMP (Positive/Negative) | PCR (Ct) | 16S 2 | Sample Number | Sampling Population | Symptoms 1 | LAMP (Positive/Negative) | PCR (Ct) | 16S 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | + | 15.7951 | 101; 98.98% | 14 | 24 | 1 | + | 13.1110 | 100; 100% |
| 2 | 1 | 0 | + | 18.1443 | 90; 98.85% | 15 | 24 | 2 | + | 14.1351 | 86; 100% |
| 3 | 1 | 1 | + | 15.9387 | 97; 100% | 16 | 25 | 2 | + | 15.8117 | 86; 100% |
| 4 | 4 | 1 | + | 13.9387 | 100; 100% | 17 | 26 | 1 | + | 17.7767 | 97; 100% |
| 5 | 17 | 1 | + | 14.5635 | 97; 100% | 18 | 26 | 1 | − | 19.8467 | 97; 100% |
| 6 | 29 | 2 | + | 14.8362 | 97; 100% | 19 | 26 | 0 | + | - | - |
| 7 | 20 | 1 | + | 17.3393 | 100; 100% | 20 | 27 | 2 | + | 15.1253 | 100; 100% |
| 8 | 20 | 2 | + | 18.1095 | 100; 100% | 21 | 28 | 1 | + | 16.5928 | 97; 100% |
| 9 | 20 | 0 | + | 15.2245 | 100; 100% | 22 | 29 | 1 | + | 12.6914 | 104; 100% |
| 10 | 21 | 2 | + | 14.1580 | 100; 100% | 23 | 29 | 1 | + | 17.8952 | 100; 100% |
| 11 | 21 | 2 | + | 17.3028 | 101; 100% | 24 | 29 | 1 | + | 17.3102 | 100; 100% |
| 12 | 22 | 1 | + | 16.2087 | 100; 100% | 25 | 30 | 1 | + | 16.5955 | 86; 100% |
| 13 | 23 | 1 | − | 13.3125 | 99; 98.46% | 26 | 30 | 2 | + | 14.8070 | 102; 100% |
| Criterion | LAMP | Real-Time PCR | 16S/HTS (Target Sequencing) |
|---|---|---|---|
| Analytical sensitivity (LOD) | 104–105 cells/mL (for plant samples) [6,19] | 5 × 102–5 × 103 cells/mL (for plant samples) [6,19] | Depending on assay; not reported for E. amylovora |
| Diagnostic sensitivity | 65% [6,19] | 84% [6,19] | High with sufficient number of reads; risk of confusion with closely related genera at low depth [20] |
| Diagnostic specificity | 98% [6,19] | 97% [6,19] | |
| Analysis time | 20–60 min | 1.5–2.5 h | 8–12 h |
| Labor intensity | Low: minimal sample preparation, isothermal mode | Intermediate: DNA extraction, PCR, interpretation of analysis curves | High: library preparation and data analysis |
| Equipment requirements | Only a thermoblock or portable heater | RT-PCR thermal cycler | Sequencer + infrastructure for data analysis |
| Test cost | The cheapest method | The most expensive due to the cost of equipment | Average cost when using the ONT platform |
| Practical applicability | Rapid screening, field tests, nurseries, express diagnostics | Confirmatory method, quantitative determination, official phytosanitary inspections | Verification of results, disputed samples, analysis of complex matrices |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozharskiy, A.; Kostyukova, V.; Nizamdinova, G.; Gritsenko, D. Comparison of Different Methods of Molecular Detection of Erwinia amylovora in Plant Material. Curr. Issues Mol. Biol. 2025, 47, 1034. https://doi.org/10.3390/cimb47121034
Pozharskiy A, Kostyukova V, Nizamdinova G, Gritsenko D. Comparison of Different Methods of Molecular Detection of Erwinia amylovora in Plant Material. Current Issues in Molecular Biology. 2025; 47(12):1034. https://doi.org/10.3390/cimb47121034
Chicago/Turabian StylePozharskiy, Alexandr, Valeriya Kostyukova, Gulnaz Nizamdinova, and Dilyara Gritsenko. 2025. "Comparison of Different Methods of Molecular Detection of Erwinia amylovora in Plant Material" Current Issues in Molecular Biology 47, no. 12: 1034. https://doi.org/10.3390/cimb47121034
APA StylePozharskiy, A., Kostyukova, V., Nizamdinova, G., & Gritsenko, D. (2025). Comparison of Different Methods of Molecular Detection of Erwinia amylovora in Plant Material. Current Issues in Molecular Biology, 47(12), 1034. https://doi.org/10.3390/cimb47121034






