Integrating Network Pharmacology and Metabolomics to Elucidate the Mechanism of Cryptotanshinone Against Platelet Aggregation
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Animal Experimentation
2.2.1. Experimental Animals and Grouping
2.2.2. Drug Administration and Model Establishment
2.2.3. Sample Collection and Processing
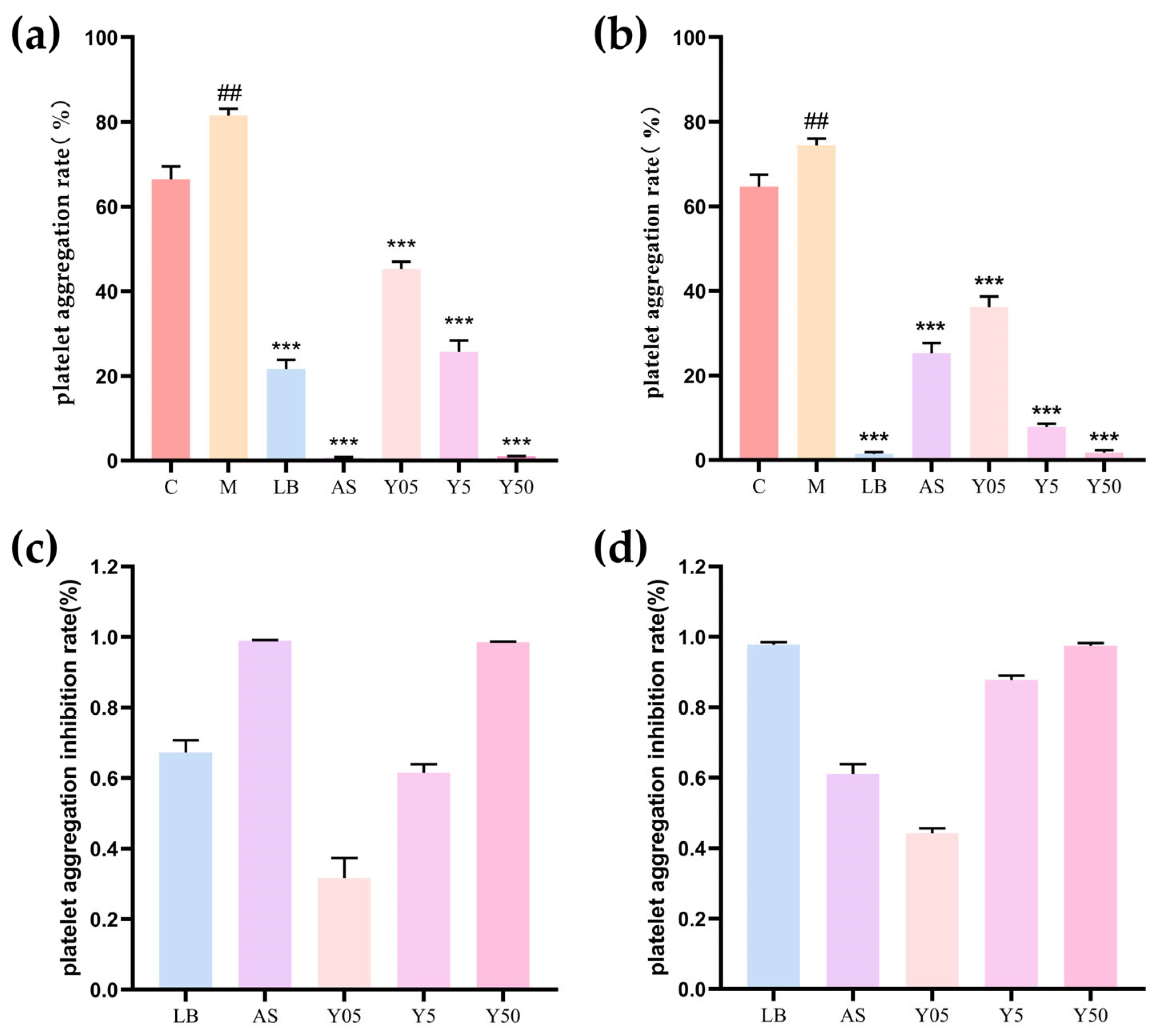
2.3. Platelet Aggregation Assay
2.4. Network Pharmacology
2.4.1. Collection of Potential Compound Targets
2.4.2. Disease Target Acquisition
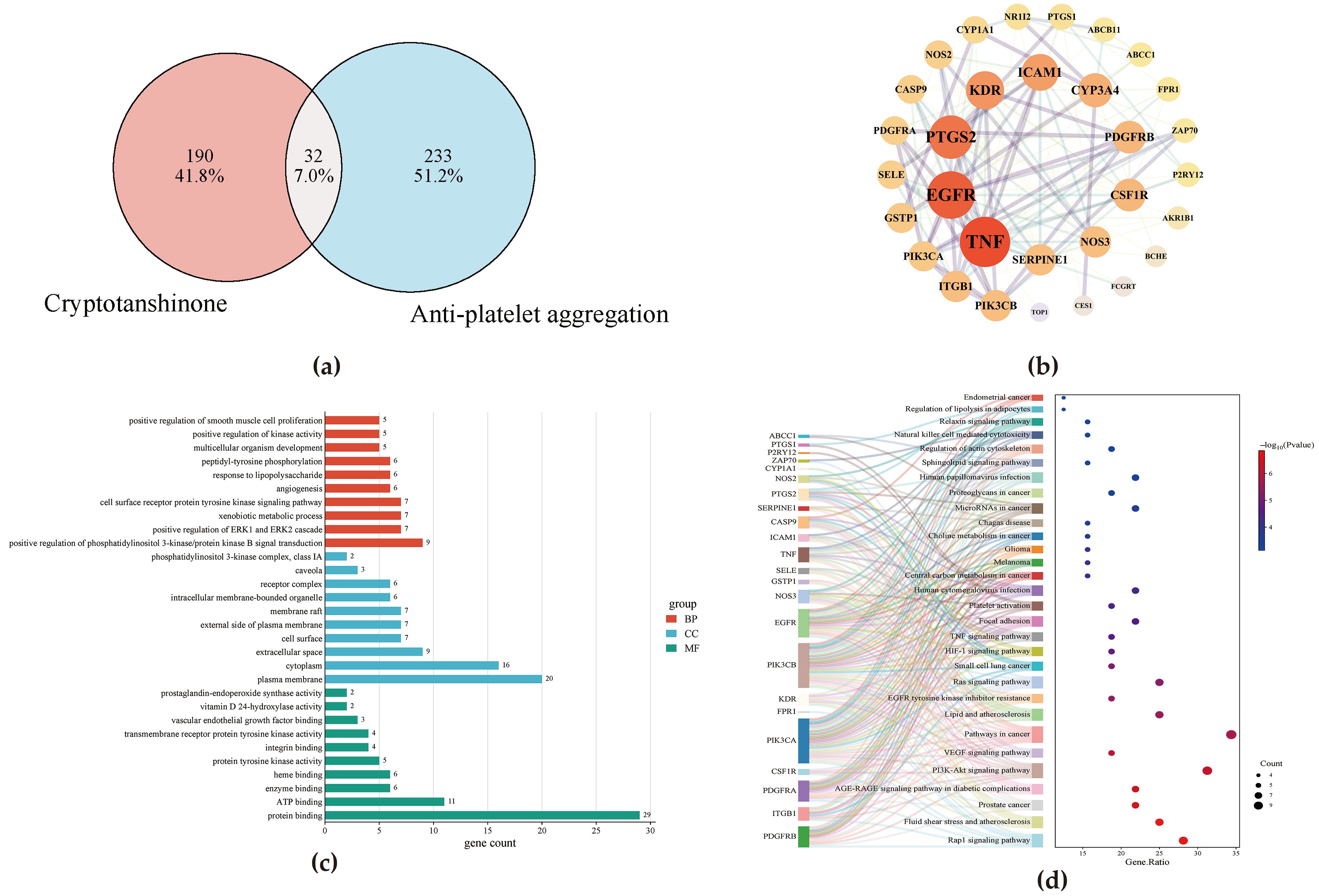
2.4.3. Protein–Protein Interaction Network Construction and Core Target Selection
2.4.4. GO and KEGG Enrichment Analysis
2.5. Untargeted Metabolomics Analysis
2.5.1. Extraction of Serum Metabolites
2.5.2. LC-MS Analysis Conditions
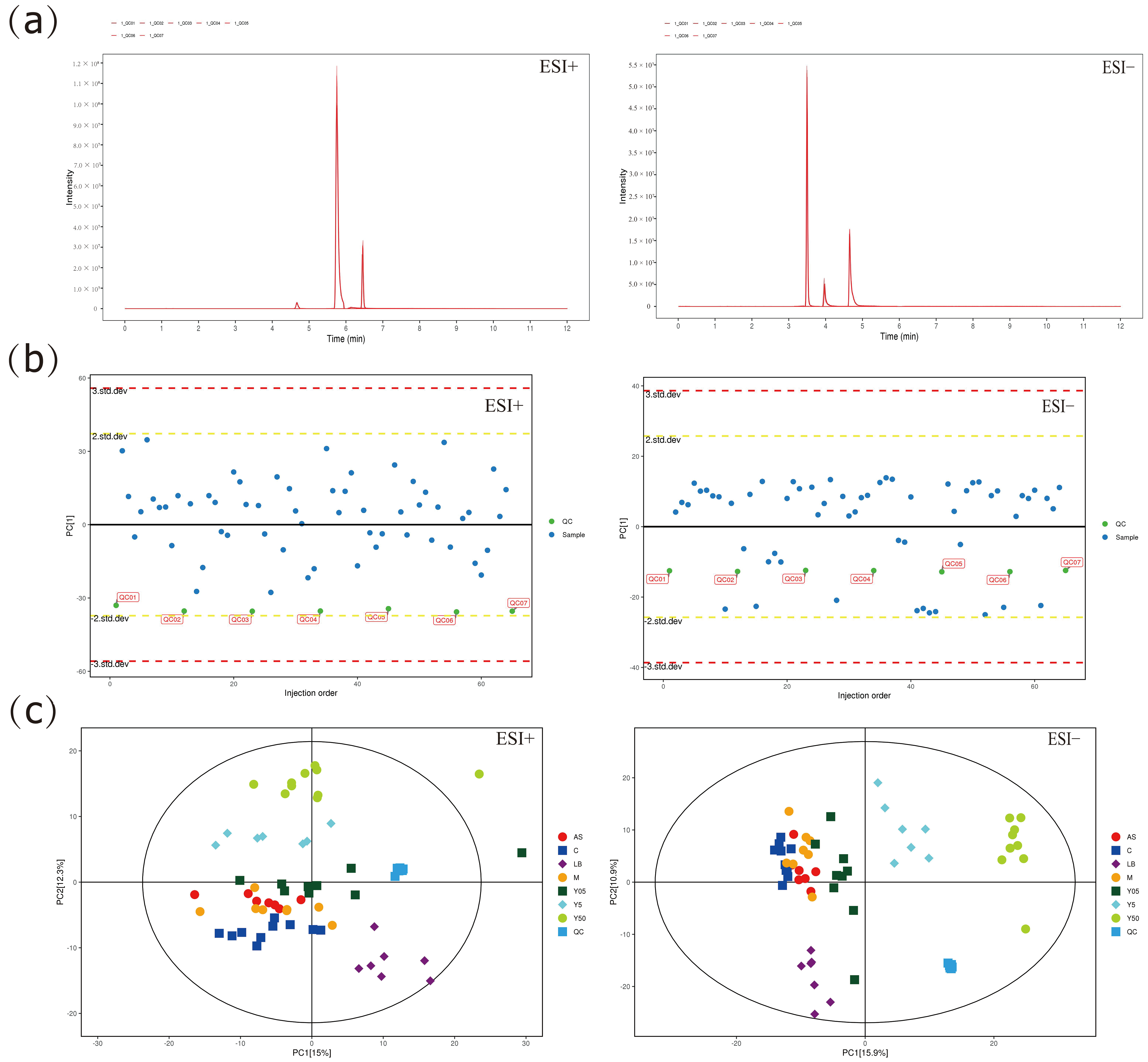
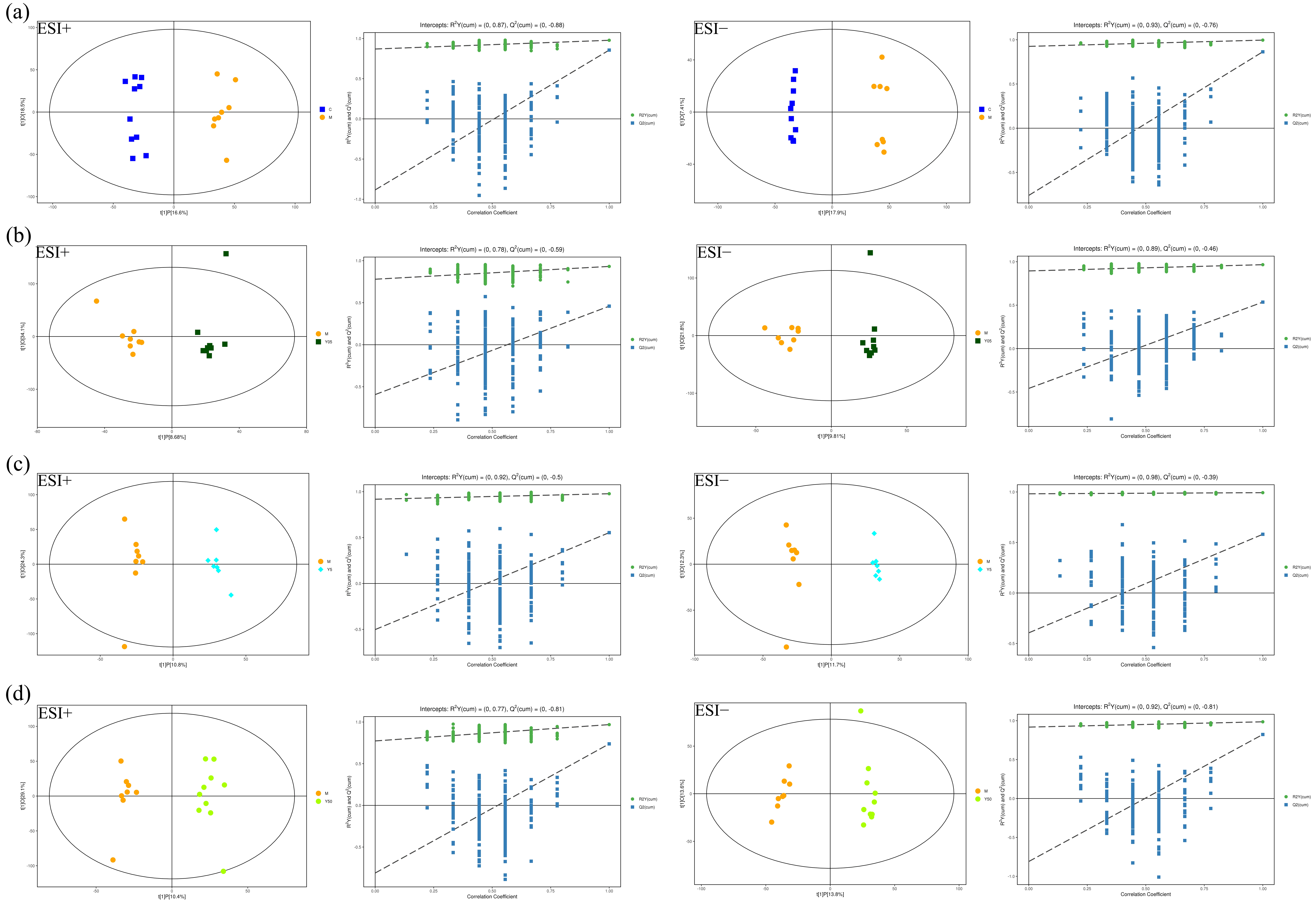
2.5.3. Data Processing and Analysis
2.6. Integrated Network-Pharmacology and Metabolomics Analysis
2.7. Molecular Docking
2.8. Statistical Analysis
3. Results
3.1. Effect on Platelet Aggregation Rate in Rat Acute Blood Stasis Model
3.2. Network Pharmacology Analysis
3.3. Untargeted Metabolomics
3.3.1. Multivariate Statistical Analysis
3.3.2. Screening and Identification of Differential Metabolites
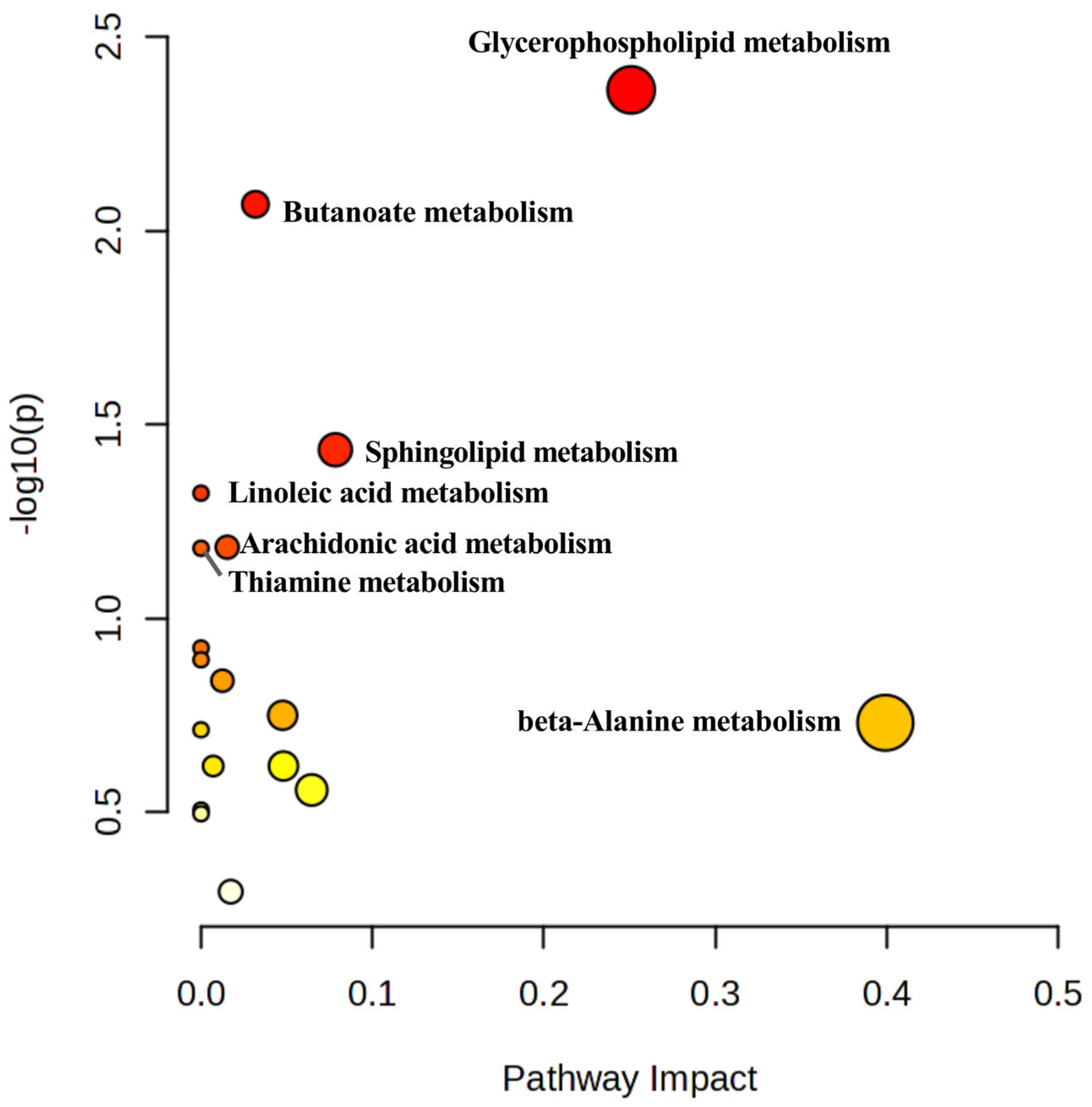
3.3.3. KEGG Pathway Analysis of Differential Metabolites
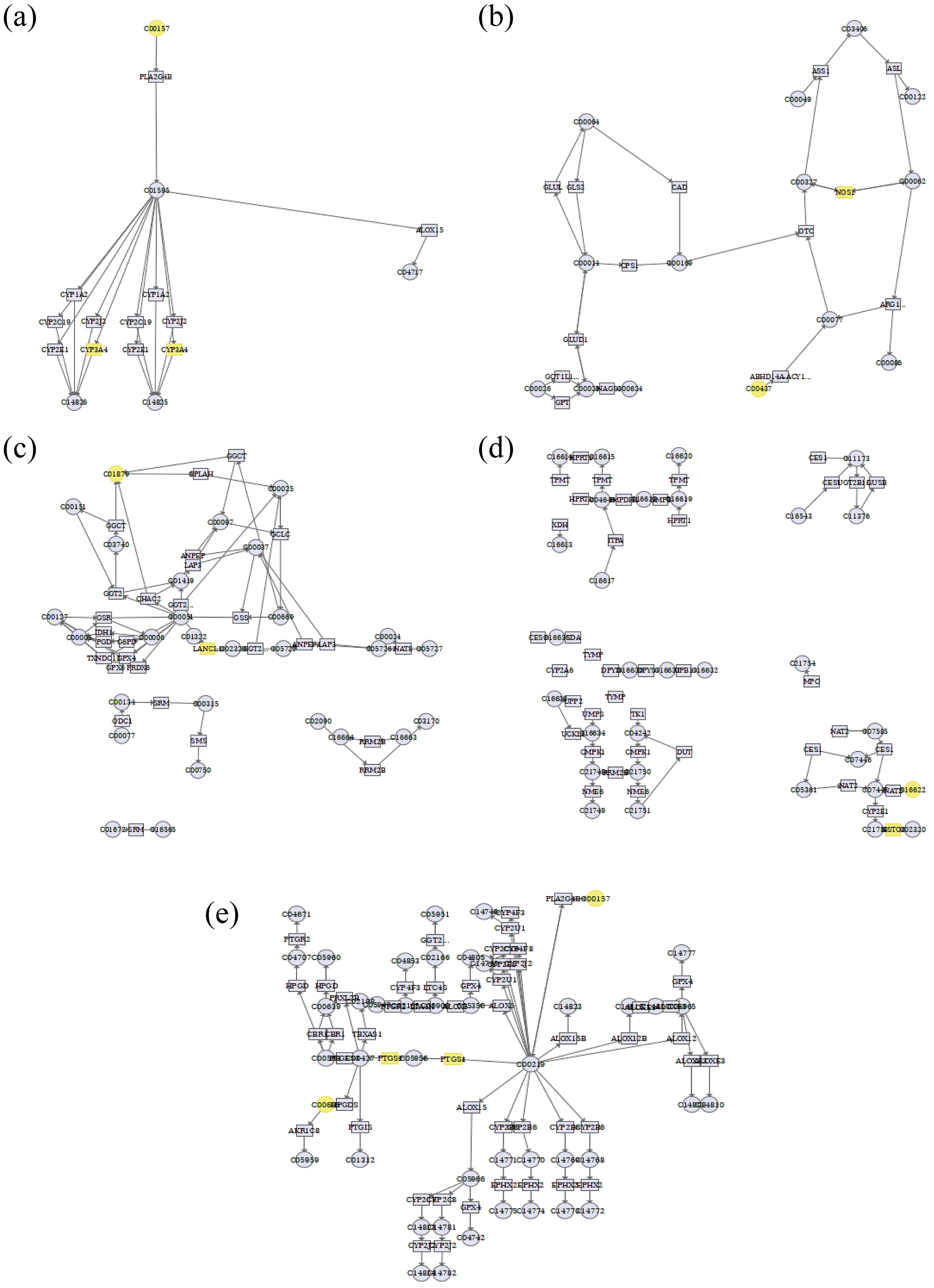
3.4. Integrated Analysis of Metabolomics and Network Pharmacology
3.5. Analysis of Molecular Docking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTS | Cryptotanshinone |
| AA | Arachidonic acid |
| ADP | Adenosine diphosphate |
| LB | Clopidogrel (positive control group) |
| PPI | Protein-protein interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| BP | Biological process |
| CC | Cellular component |
| MF | Molecular function |
| QC | Quality control |
| PCA | Principal component analysis |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| HMDB | Human Metabolome Database |
| EpOMEs | Epoxy-octadecenoic acids |
| NOX | NADPH oxidase |
References
- Jackson, S.P. The Growing Complexity of Platelet Aggregation. Blood 2007, 109, 5087–5095. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk. JACC 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Coenen, D.M.; Heinzmann, A.C.A.; Karel, M.F.A.; Cosemans, J.M.E.M.; Koenen, R.R. The Multifaceted Contribution of Platelets in the Emergence and Aftermath of Acute Cardiovascular Events. Atherosclerosis 2021, 319, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C.; Morais, J.; Baigent, C.; Collet, J.-P.; Fitzgerald, D.; Halvorsen, S.; Rocca, B.; Siegbahn, A.; Storey, R.F.; Vilahur, G. Antiplatelet Agents for the Treatment and Prevention of Coronary Atherothrombosis. J. Am. Coll. Cardiol. 2017, 70, 1760–1776. [Google Scholar] [CrossRef]
- Pereira, N.L.; Cresci, S.; Angiolillo, D.J.; Batchelor, W.; Capers, Q.; Cavallari, L.H.; Leifer, D.; Luzum, J.A.; Roden, D.M.; Stellos, K.; et al. CYP2C19 Genetic Testing for Oral P2Y12 Inhibitor Therapy: A Scientific Statement From the American Heart Association. Circulation 2024, 150, e129–e150. [Google Scholar] [CrossRef]
- Mahady, S.E.; Margolis, K.L.; Chan, A.; Polekhina, G.; Woods, R.L.; Wolfe, R.; Nelson, M.R.; Lockery, J.E.; Wood, E.M.; Reid, C.; et al. Major GI Bleeding in Older Persons Using Aspirin: Incidence and Risk Factors in the ASPREE Randomised Controlled Trial. Gut 2021, 70, 717–724. [Google Scholar] [CrossRef]
- Wang, J.; Zou, J.; Shi, Y.; Zeng, N.; Guo, D.; Wang, H.; Zhao, C.; Luan, F.; Zhang, X.; Sun, J. Traditional Chinese Medicine and Mitophagy: A Novel Approach for Cardiovascular Disease Management. Phytomedicine 2024, 128, 155472. [Google Scholar] [CrossRef]
- Hou, Y.; Li, H.; Zhu, L.; Li, Y.; Zeng, Y.; Quan, T.; Xiang, Z.; Zhang, Y.; Bian, Y.; Wei, Y. A Review of Natural Compounds to Regulate Platelet Aggregation: Molecular Mechanism and Research Advance. Front. Pharmacol. 2025, 16, 1537776. [Google Scholar] [CrossRef]
- Xin, Q.-Q.; Chen, X.; Yuan, R.; Yuan, Y.-H.; Hui, J.-Q.; Miao, Y.; Cong, W.-H.; Chen, K.-J. Correlation of Platelet and Coagulation Function with Blood Stasis Syndrome in Coronary Heart Disease: A Systematic Review and Meta-Analysis. Chin. J. Integr. Med. 2021, 27, 858–866. [Google Scholar] [CrossRef]
- Lan, T.; Yu, D.; Zhao, Q.; Qu, C.; Wu, Q. Ethnomedicine, Phytochemistry, Pharmacology, Pharmacokinetics, and Clinical Application of Salvia miltiorrhiza Bunge (Lamiaceae): A Comprehensive Review. J. Ethnopharmacol. 2025, 350, 120032. [Google Scholar] [CrossRef]
- Chen, W.; Chen, G. Danshen (Salvia miltiorrhiza Bunge): A Prospective Healing Sage for Cardiovascular Diseases. Curr. Pharm. Des. 2017, 23, 5125–5135. [Google Scholar] [CrossRef]
- Li, H.; Gao, C.; Liu, C.; Liu, L.; Zhuang, J.; Yang, J.; Zhou, C.; Feng, F.; Sun, C.; Wu, J. A Review of the Biological Activity and Pharmacology of Cryptotanshinone, an Important Active Constituent in Danshen. Biomed. Pharmacother. 2021, 137, 111332. [Google Scholar] [CrossRef]
- Zheng, Z.; Ke, L.; Ye, S.; Shi, P.; Yao, H. Pharmacological Mechanisms of Cryptotanshinone: Recent Advances in Cardiovascular, Cancer, and Neurological Disease Applications. Drug Des. Devel Ther. 2024, 18, 6031–6060. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, Z.; Tu, C.; Zhang, H.; Gao, D.; Li, C.; He, Q.; Li, R.; Guo, Y.; Niu, M.; et al. An Activity-Calibrated Chemical Standardization Approach for Quality Evaluation of Salvia miltiorrhiza Bge. RSC Adv. 2017, 7, 5331–5339. [Google Scholar] [CrossRef]
- Nie, Z.-Y.; Zhang, J.-Q.; Shen, Y.-J.-Y.; Xi, J.-Q.; Cao, Y.-B.; Zhang, L.-C.; Li, L. Natural Active Herbal Monomers for the Treatment of Thromboembolic Diseases: A Review. Front. Pharmacol. 2025, 16, 1607415. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Yang, Z.; Yu, Q.; Zhao, L.; Wang, Y. Synergistic Effects of Cryptotanshinone and Senkyunolide I in Guanxinning Tablet Against Endogenous Thrombus Formation in Zebrafish. Front. Pharmacol. 2020, 11, 622787. [Google Scholar] [CrossRef]
- Lin, A.X.; Chan, G.; Hu, Y.; Ouyang, D.; Ung, C.O.L.; Shi, L.; Hu, H. Internationalization of Traditional Chinese Medicine: Current International Market, Internationalization Challenges and Prospective Suggestions. Chin. Med. 2018, 13, 9. [Google Scholar] [CrossRef]
- Singh, R.; Sahu, N.; Tyagi, R.; Alam, P.; Akhtar, A.; Walia, R.; Chandra, A.; Madan, S. Integrative Network Pharmacology, Molecular Docking, and Dynamics Simulations Reveal the Mechanisms of Cinnamomum tamala in Diabetic Nephropathy Treatment: An In Silico Study. Curr. Issues Mol. Biol. 2024, 46, 11868–11889. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Li, W.; Tang, Y.; Wang, S. Mechanism of Taxanes in the Treatment of Lung Cancer Based on Network Pharmacology and Molecular Docking. Curr. Issues Mol. Biol. 2023, 45, 6564–6582. [Google Scholar] [CrossRef]
- Cui, L.; Lu, H.; Lee, Y.H. Challenges and Emergent Solutions for LC-MS/MS Based Untargeted Metabolomics in Diseases. Mass. Spectrom. Rev. 2018, 37, 772–792. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Liu, X.; Wang, Q.; Zhao, D.; Su, M.; Jia, Z.; Shen, S. Integrating Metabolomics and Network Pharmacology to Decipher the Hepatoprotective Effect Mechanisms of Magnesium Isoglycyrrhizinate Injection. Curr. Issues Mol. Biol. 2024, 46, 279–298. [Google Scholar] [CrossRef]
- Huang, S.; Xu, F.; Wang, Y.-Y.; Shang, M.-Y.; Wang, C.-Q.; Wang, X.; Cai, S.-Q. Improvement and Application of Acute Blood Stasis Rat Model Aligned with the 3Rs (Reduction, Refinement and Replacement) of Humane Animal Experimentation. Chin. J. Integr. Med. 2020, 26, 292–298. [Google Scholar] [CrossRef]
- Le Blanc, J.; Mullier, F.; Vayne, C.; Lordkipanidzé, M. Advances in Platelet Function Testing-Light Transmission Aggregometry and Beyond. J. Clin. Med. 2020, 9, 2636. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, P.P.; Eichelberger, B.; Kopp, C.W.; Pultar, J.; Seidinger, D.; Koppensteiner, R.; Lang, I.M.; Panzer, S.; Gremmel, T. Disaggregation Following Agonist-Induced Platelet Activation in Patients on Dual Antiplatelet Therapy. J. Cardiovasc. Transl. Res. 2017, 10, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-X.; Feng, Y.; Zhang, Y.; Liu, Y.; Li, S.-D.; Yang, M.-H. Hemorheology Index Changes in a Rat Acute Blood Stasis Model: A Systematic Review and Meta-Analysis. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 96–107. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Y.; Li, S.; Liu, Y.; Zhang, Y.; Guo, Y.; Yang, M. Microvascular Pathological Features and Changes in Related Injury Factors in a Rat Acute Blood Stasis Model. J. Tradit. Chin. Med. 2017, 37, 108–115. [Google Scholar] [CrossRef]
- Undas, A.; Brummel-Ziedins, K.E.; Mann, K.G. Antithrombotic Properties of Aspirin and Resistance to Aspirin: Beyond Strictly Antiplatelet Actions. Blood 2007, 109, 2285–2292. [Google Scholar] [CrossRef]
- Weber, A.A.; Reimann, S.; Schrör, K. Specific Inhibition of ADP-Induced Platelet Aggregation by Clopidogrel in Vitro. Br. J. Pharmacol. 1999, 126, 415–420. [Google Scholar] [CrossRef]
- Maione, F.; Cantone, V.; Chini, M.G.; De Feo, V.; Mascolo, N.; Bifulco, G. Molecular Mechanism of Tanshinone IIA and Cryptotanshinone in Platelet Anti-Aggregating Effects: An Integrated Study of Pharmacology and Computational Analysis. Fitoterapia 2015, 100, 174–178. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, R.; Hua, C.; Wu, M.; Yuan, Y.; Zhang, L.; Guo, F.; Liu, J.; Yang, Z.; Liu, G. P2Y12 Receptor-Independent Antiplatelet Mechanism of Cryptotanshinone: Network Pharmacology and Experimental Validation of Multi-Target Signaling Pathways. J. Ethnopharmacol. 2025, 341, 119321. [Google Scholar] [CrossRef]
- Hildreth, K.; Kodani, S.D.; Hammock, B.D.; Zhao, L. Cytochrome P450-Derived Linoleic Acid Metabolites EpOMEs and DiHOMEs: A Review of Recent Studies. J. Nutr. Biochem. 2020, 86, 108484. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Moon, Y.S.; Cho, K.K. ω-6 and ω-3 Polyunsaturated Fatty Acids: Inflammation, Obesity and Foods of Animal Resources. Food Sci. Anim. Resour. 2024, 44, 988–1010. [Google Scholar] [CrossRef]
- Octave, M.; Pirotton, L.; de Cartier d’Yves, E.; Marino, A.; Ambroise, J.; Giera, M.; Guigas, B.; Ginion, A.; Robaux, V.; Kuijpers, M.; et al. Deleting ACC1 in Platelets Alters Phospholipidome and Reduces Platelet Activation and Thrombosis in Mice. Blood Adv. 2025, 9, 4553–4567. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Chen, C.-H.; Hsu, M.-C.; Chang, R.-W.; Wang, C.-H.; Lee, T.-S. Advances in the Molecular Mechanisms of Statins in Regulating Endothelial Nitric Oxide Bioavailability: Interlocking Biology between eNOS Activity and L-Arginine Metabolism. Biomed. Pharmacother. 2024, 171, 116192. [Google Scholar] [CrossRef] [PubMed]
- Gawrys, J.; Gajecki, D.; Szahidewicz-Krupska, E.; Doroszko, A. Intraplatelet L-Arginine-Nitric Oxide Metabolic Pathway: From Discovery to Clinical Implications in Prevention and Treatment of Cardiovascular Disorders. Oxid. Med. Cell Longev. 2020, 2020, 1015908. [Google Scholar] [CrossRef] [PubMed]
- Erens, C.; Van Broeckhoven, J.; Bronckaers, A.; Lemmens, S.; Hendrix, S. The Dark Side of an Essential Amino Acid: L-Arginine in Spinal Cord Injury. J. Neurotrauma 2023, 40, 820–832. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism Pathways of Arachidonic Acids: Mechanisms and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Cheng, Y.; Austin, S.C.; Rocca, B.; Koller, B.H.; Coffman, T.M.; Grosser, T.; Lawson, J.A.; FitzGerald, G.A. Role of Prostacyclin in the Cardiovascular Response to Thromboxane A2. Science 2002, 296, 539–541. [Google Scholar] [CrossRef]
- Li, L.; Sluter, M.N.; Yu, Y.; Jiang, J. Prostaglandin E Receptors as Targets for Ischemic Stroke: Novel Evidence and Molecular Mechanisms of Efficacy. Pharmacol. Res. 2021, 163, 105238. [Google Scholar] [CrossRef]
- Ershov, P.V.; Yablokov, E.O.; Mezentsev, Y.V.; Ivanov, A.S. Human Prostacyclin and Thromboxane Synthases: Molecular Interactions, Regulation, and Pharmacology. Biochimie 2025, 234, 76–88. [Google Scholar] [CrossRef]
- Li, W.; Busu, C.; Circu, M.L.; Aw, T.Y. Glutathione in Cerebral Microvascular Endothelial Biology and Pathobiology: Implications for Brain Homeostasis. Int. J. Cell Biol. 2012, 2012, 434971. [Google Scholar] [CrossRef]
- Arrivi, A.; Barillà, F.; Carnevale, R.; Sordi, M.; Pucci, G.; Tanzilli, G.; Prandi, F.R.; Mangieri, E. Protective Biomolecular Mechanisms of Glutathione Sodium Salt in Ischemia-Reperfusion Injury in Patients with Acute Coronary Syndrome-ST-Elevation Myocardial Infarction. Cells 2022, 11, 3964. [Google Scholar] [CrossRef]
- Kim, M.E.; Lee, J.S. Advances in the Regulation of Inflammatory Mediators in Nitric Oxide Synthase: Implications for Disease Modulation and Therapeutic Approaches. Int. J. Mol. Sci. 2025, 26, 1204. [Google Scholar] [CrossRef]
- Wei, C.; Wang, M.; Wang, X.-J. Evolutionary Conservation Analysis of Human Arachidonic Acid Metabolism Pathway Genes. Life Med. 2023, 2, lnad004. [Google Scholar] [CrossRef]
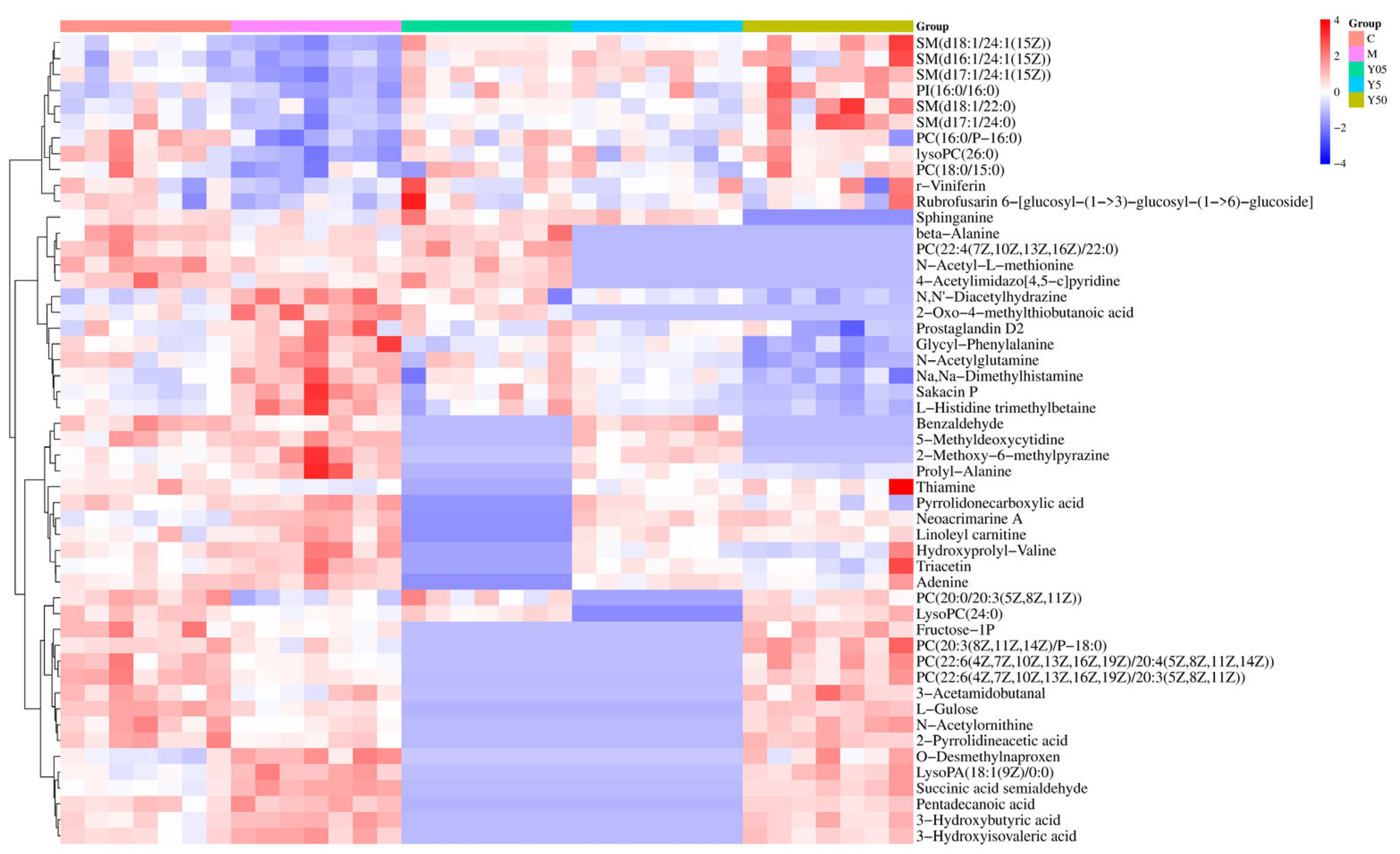

| NO. | RT (s) | m/z | HMDB ID | Metabolite | Classification | Lon Mode | Trend | |||
|---|---|---|---|---|---|---|---|---|---|---|
| C vs. M | M vs. Y05 | M vs. Y5 | M vs. Y50 | |||||||
| 1 | 217.5330 | 101.0241 | HMDB0001259 | Succinic acid semialdehyde | B | NRG | ↑ | - | - | ↓ |
| 2 | 431.1600 | 682.2992 | HMDB0040384 | Neoacrimarine A | B | POS | ↑ | - | ↓ | ↓ |
| 3 | 216.8970 | 435.2512 | HMDB0007855 | LysoPA(18:1(9Z)/0:0) | A | NRG | ↑ | - | - | ↓ |
| 4 | 78.9896 | 147.0117 | HMDB0001553 | 2-Oxo-4-methylthiobutanoic acid | B | NRG | ↑ | ↓ | - | - |
| 5 | 160.3015 | 840.6418 | HMDB0008277 | PC(20:0/20:3(5Z,8Z,11Z)) | A | POS | ↓ | ↑ | - | ↑ |
| 6 | 166.0875 | 718.5734 | HMDB0007994 | PC(16:0/P-16:0) | A | POS | ↓ | ↑ | ↑ | ↑ |
| 7 | 407.2230 | 217.1174 | HMDB0038239 | Sakacin P | B | POS | ↑ | ↓ | ↓ | ↓ |
| 8 | 286.5010 | 103.0398 | HMDB0000357 | 3-Hydroxybutyric acid | B | NRG | ↑ | - | - | ↓ |
| 9 | 197.6575 | 813.6816 | HMDB0012107 | SM(d18:1/24:1(15Z)) | A | POS | ↓ | ↑ | ↑ | ↑ |
| 10 | 347.4185 | 115.0512 | HMDB0060496 | N,N′-Diacetylhydrazine | B | NRG | ↑ | ↓ | ↓ | ↓ |
| 11 | 248.1410 | 215.0725 | HMDB0013989 | O-Desmethylnaproxen | B | NRG | ↑ | - | - | ↓ |
| 12 | 308.3890 | 203.0517 | HMDB0012326 | L-Gulose | B | POS | ↓ | - | - | ↑ |
| 13 | 291.6910 | 198.1228 | HMDB0029422 | L-Histidine trimethylbetaine | B | POS | ↑ | ↓ | ↓ | ↓ |
| 14 | 155.2270 | 856.5802 | HMDB0008737 | PC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/20:3(5Z,8Z,11Z)) | A | POS | ↓ | - | - | ↑ |
| 15 | 293.1655 | 140.1176 | HMDB0033438 | Na,Na-Dimethylhistamine | B | POS | ↑ | ↓ | ↓ | ↓ |
| 16 | 355.6875 | 265.1105 | HMDB0000235 | Thiamine | B | POS | ↓ | - | ↑ | ↑ |
| 17 | 206.5500 | 608.4628 | HMDB0010405 | LysoPC(24:0) | A | POS | ↓ | ↑ | - | ↑ |
| 18 | 198.0780 | 799.6664 | HMDB0011696 | SM(d17:1/24:1(15Z)) | A | POS | ↓ | ↑ | ↑ | ↑ |
| 19 | 205.1945 | 636.4930 | HMDB0029205 | lysoPC(26:0) | A | POS | ↓ | ↑ | ↑ | ↑ |
| 20 | 379.5950 | 219.0842 | HMDB0029592 | Triacetin | B | POS | ↑ | - | ↓ | ↓ |
| 21 | 214.6055 | 117.0554 | HMDB0000754 | 3-Hydroxyisovaleric acid | B | NRG | ↑ | - | - | ↓ |
| 22 | 153.9160 | 854.5663 | HMDB0008739 | PC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/20:4(5Z,8Z,11Z,14Z)) | A | POS | ↓ | - | - | ↑ |
| 23 | 168.0830 | 748.5792 | HMDB0008033 | PC(18:0/15:0) | A | POS | ↓ | ↑ | ↑ | ↑ |
| 24 | 221.2975 | 809.5157 | HMDB0009778 | PI(16:0/16:0) | A | NRG | ↓ | ↑ | ↑ | ↑ |
| 25 | 290.7780 | 223.1066 | HMDB0028848 | Glycyl-Phenylalanine | B | POS | ↑ | ↓ | ↓ | ↓ |
| 26 | 373.0340 | 175.1067 | HMDB0003357 | N-Acetylornithine | B | POS | ↓ | - | - | ↑ |
| 27 | 151.9330 | 125.0703 | HMDB0040143 | 2-Methoxy-6-methylpyrazine | B | POS | ↑ | - | ↓ | - |
| 28 | 198.3560 | 801.6802 | HMDB0011695 | SM(d17:1/24:0) | A | POS | ↓ | ↑ | ↑ | ↑ |
| 29 | 232.7680 | 107.0488 | HMDB0006115 | Benzaldehyde | B | POS | ↓ | - | ↑ | - |
| 30 | 380.6705 | 88.0403 | HMDB0000056 | beta-Alanine | B | NRG | ↓ | ↑ | - | - |
| 31 | 101.5005 | 130.0856 | HMDB0029444 | 2-Pyrrolidineacetic acid | B | POS | ↓ | - | - | ↑ |
| 32 | 313.2795 | 179.0555 | HMDB0062538 | Fructose-1P | B | NRG | ↓ | - | - | ↑ |
| 33 | 169.9790 | 136.0612 | HMDB0000034 | Adenine | B | POS | ↑ | - | ↓ | ↓ |
| 34 | 394.3850 | 231.1329 | HMDB0028876 | Hydroxyprolyl-Valine | B | POS | ↑ | - | ↓ | ↓ |
| 35 | 52.7136 | 130.0856 | HMDB0059649 | 3-Acetamidobutanal | B | POS | ↓ | - | - | ↑ |
| 36 | 404.3080 | 189.0861 | HMDB0006029 | N-Acetylglutamine | B | POS | ↑ | ↓ | ↓ | ↓ |
| 37 | 197.0020 | 424.3403 | HMDB0006469 | Linoleyl carnitine | A | POS | ↑ | - | ↓ | ↓ |
| 38 | 28.4327 | 907.2543 | HMDB0041291 | r-Viniferin | B | POS | ↓ | ↑ | ↑ | ↑ |
| 39 | 45.2212 | 351.2199 | HMDB0001403 | Prostaglandin D2 | A | NRG | ↑ | ↓ | ↓ | ↓ |
| 40 | 45.7435 | 241.2167 | HMDB0000826 | Pentadecanoic acid | B | NRG | ↑ | - | - | ↓ |
| 41 | 150.6600 | 162.0654 | HMDB0034888 | 4-Acetylimidazo[4,5-c]pyridine | B | POS | ↓ | ↑ | - | - |
| 42 | 211.7070 | 242.1124 | HMDB0002224 | 5-Methyldeoxycytidine | B | POS | ↑ | - | ↓ | - |
| 43 | 215.8790 | 190.0536 | HMDB0011745 | N-Acetyl-L-methionine | B | NRG | ↓ | ↑ | - | - |
| 44 | 42.3529 | 302.3035 | HMDB0000269 | Sphinganine | A | POS | ↓ | ↑ | ↑ | - |
| 45 | 316.9175 | 187.1067 | HMDB0029010 | Prolyl-Alanine | B | POS | ↑ | - | ↓ | ↓ |
| 46 | 272.4340 | 130.0492 | HMDB0000805 | Pyrrolidonecarboxylic acid | B | POS | ↑ | - | ↓ | ↓ |
| 47 | 28.5982 | 759.2177 | HMDB0034569 | Rubrofusarin 6-[glucosyl-(1->3)-glucosyl-(1->6)-glucoside] | B | POS | ↓ | ↑ | ↑ | ↑ |
| 48 | 156.3890 | 796.6173 | HMDB0008423 | PC(20:3(8Z,11Z,14Z)/P-18:0) | A | POS | ↓ | - | - | ↑ |
| 49 | 198.7930 | 785.6506 | HMDB0011694 | SM(d16:1/24:1(15Z)) | A | POS | ↓ | ↑ | ↑ | ↑ |
| 50 | 199.5415 | 787.6648 | HMDB0012103 | SM(d18:1/22:0) | A | POS | ↓ | ↑ | ↑ | ↑ |
| 51 | 153.3870 | 894.6874 | HMDB0008643 | PC(22:4(7Z,10Z,13Z,16Z)/22:0) | A | POS | ↓ | ↑ | - | - |
| Pathway Name | Total | Hits | p | FDR | Impact |
|---|---|---|---|---|---|
| Glycerophospholipid metabolism | 36 | 3 | 0.00434 | 0.34238 | 0.25096 |
| Butanoate metabolism | 15 | 2 | 0.00856 | 0.34238 | 0.03175 |
| Sphingolipid metabolism | 32 | 2 | 0.036766 | 0.88018 | 0.07838 |
| Linoleic acid metabolism | 5 | 1 | 0.04758 | 0.88018 | 0 |
| Arachidonic acid metabolism | 44 | 2 | 0.065589 | 0.88018 | 0.01528 |
| Thiamine metabolism | 7 | 1 | 0.066014 | 0.88018 | 0 |
| beta-Alanine metabolism | 21 | 1 | 0.18602 | 1 | 0.39925 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Liu, Z.; Wang, B.; Qiu, H.; Chen, Q.; Xian, J.; Liu, S.; Shi, X.; Xia, T.; Tan, X.; et al. Integrating Network Pharmacology and Metabolomics to Elucidate the Mechanism of Cryptotanshinone Against Platelet Aggregation. Curr. Issues Mol. Biol. 2025, 47, 953. https://doi.org/10.3390/cimb47110953
Huang J, Liu Z, Wang B, Qiu H, Chen Q, Xian J, Liu S, Shi X, Xia T, Tan X, et al. Integrating Network Pharmacology and Metabolomics to Elucidate the Mechanism of Cryptotanshinone Against Platelet Aggregation. Current Issues in Molecular Biology. 2025; 47(11):953. https://doi.org/10.3390/cimb47110953
Chicago/Turabian StyleHuang, Jielan, Zhenjie Liu, Baolin Wang, Haixin Qiu, Qiujie Chen, Jinyan Xian, Shen Liu, Xiaoxiu Shi, Ting Xia, Xiaoqing Tan, and et al. 2025. "Integrating Network Pharmacology and Metabolomics to Elucidate the Mechanism of Cryptotanshinone Against Platelet Aggregation" Current Issues in Molecular Biology 47, no. 11: 953. https://doi.org/10.3390/cimb47110953
APA StyleHuang, J., Liu, Z., Wang, B., Qiu, H., Chen, Q., Xian, J., Liu, S., Shi, X., Xia, T., Tan, X., Jiang, W., Shen, Y., Wang, L., & Feng, J. (2025). Integrating Network Pharmacology and Metabolomics to Elucidate the Mechanism of Cryptotanshinone Against Platelet Aggregation. Current Issues in Molecular Biology, 47(11), 953. https://doi.org/10.3390/cimb47110953






