Fermented Porcine Placenta and Its Dipeptides Modulate Cellular Senescence in Human Keratinocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Cell Culture
2.3. SA-β-Galactosidase Staining Assay
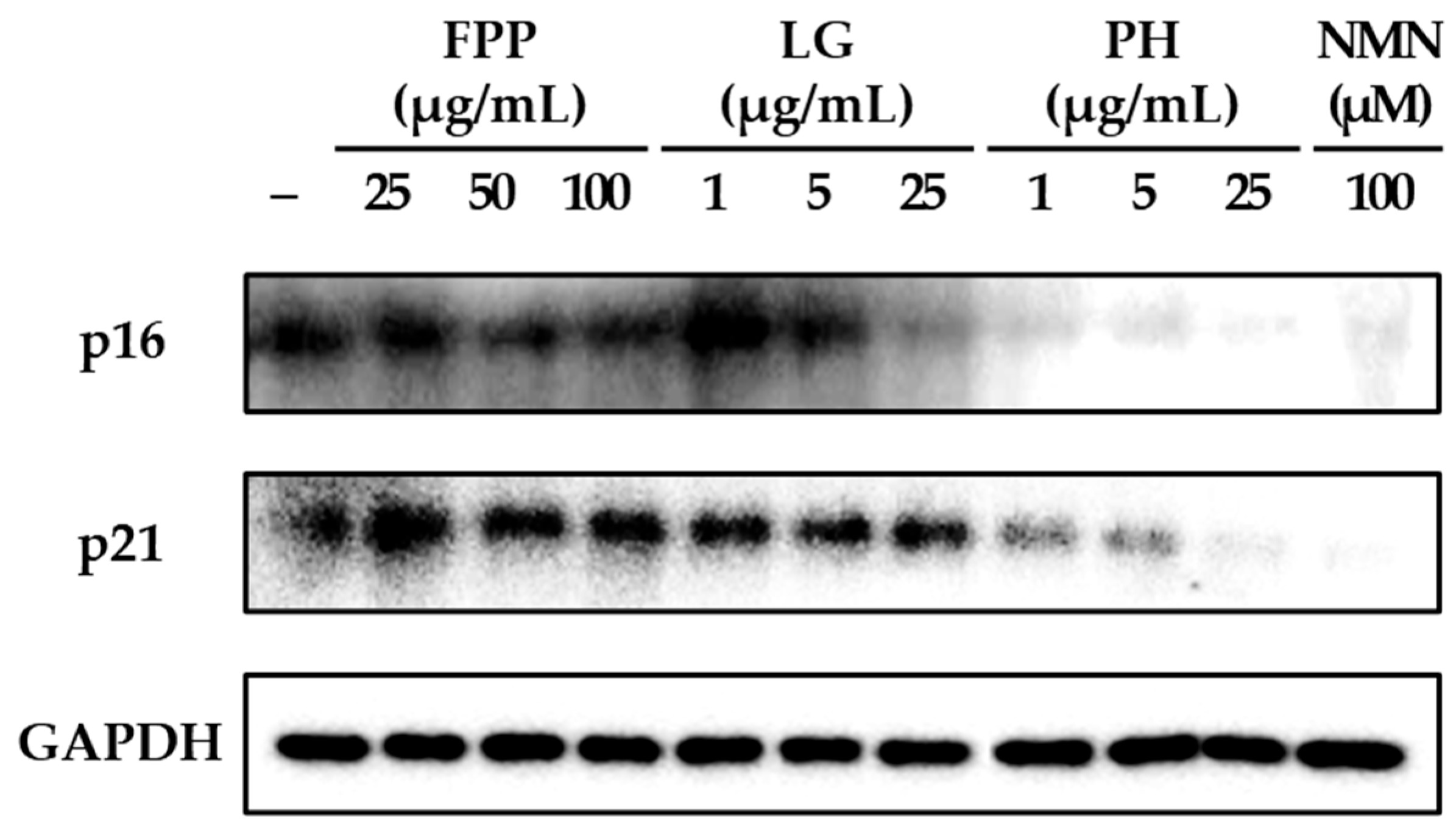
2.4. Western Blotting
2.5. ROS Assay
2.6. NAD+/NADH Assay
2.7. Measurement of NQO1 Activity
2.8. Statistical Analyses
3. Results
3.1. β-Galactosidase Activity as a Marker of Cellular Senescence
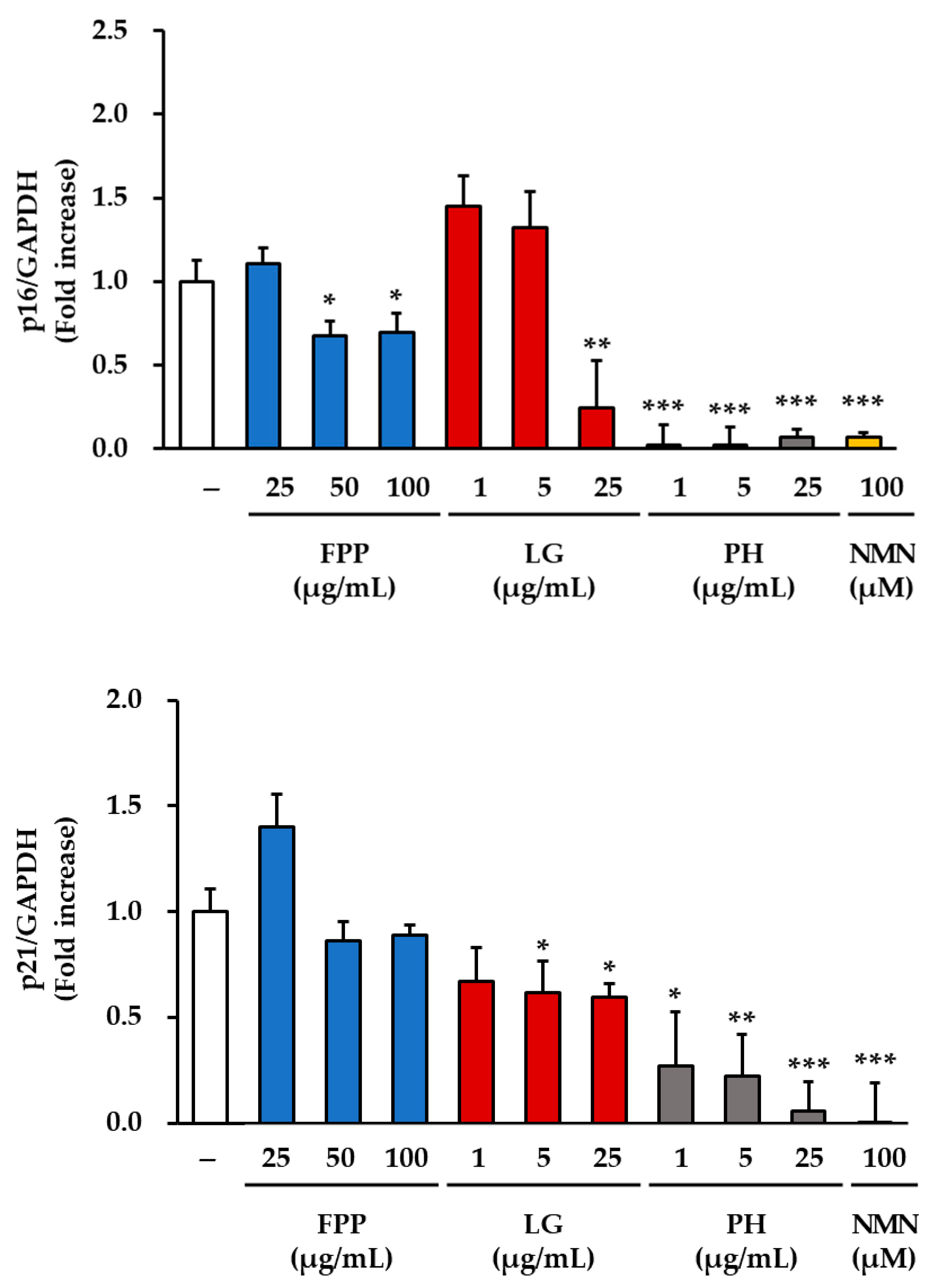
3.2. p16 and p21 Expression as Indicators of Cellular Senescence
3.3. ROS Levels as an Indicator of Oxidative Stress
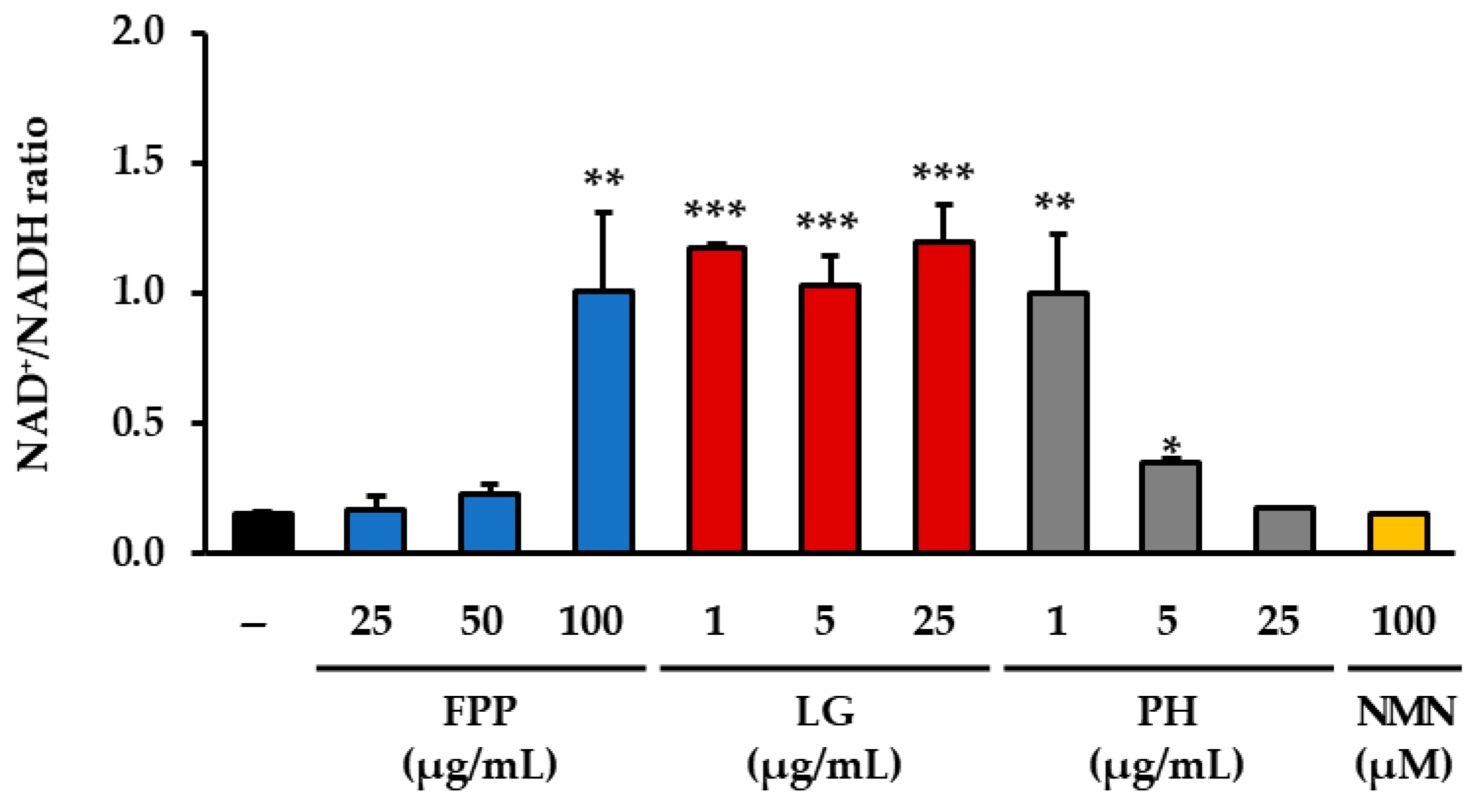
3.4. NAD+/NADH Ratio as an Indicator of Cellular Redox Balance
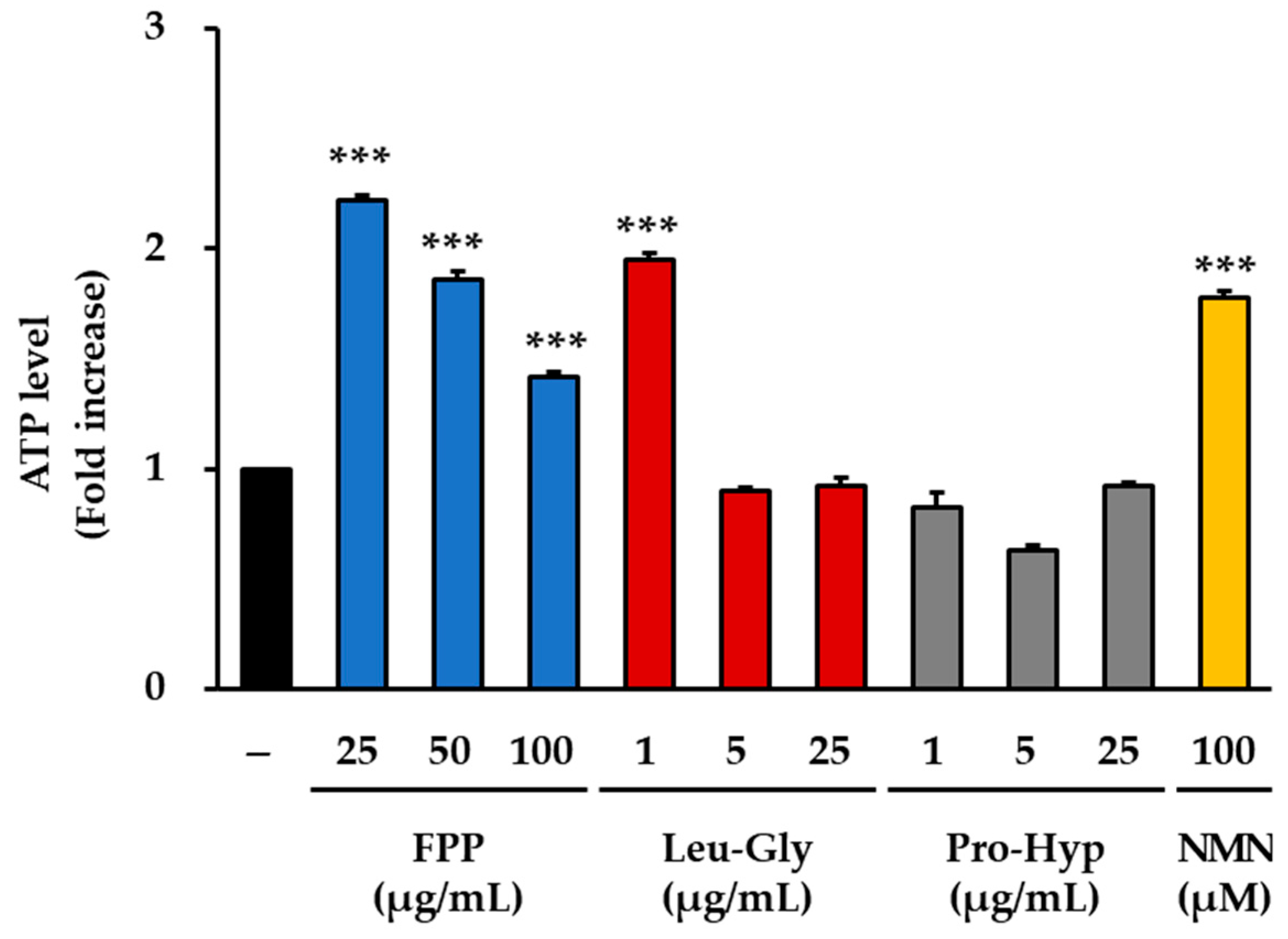
3.5. ATP Production as an Indicator of Cellular Energy Metabolism
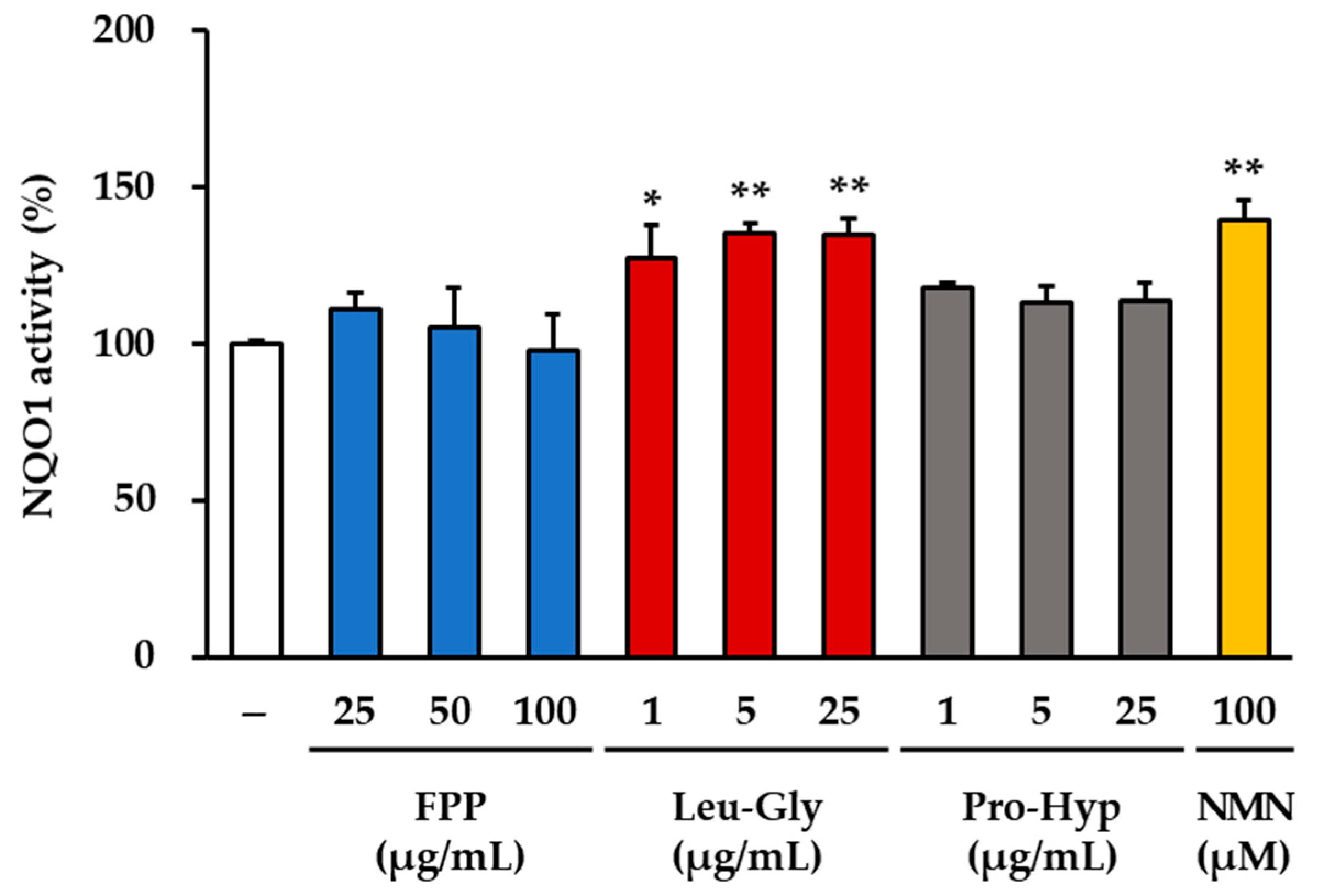
3.6. NQO1 Activity as an Indicator of Antioxidant Response
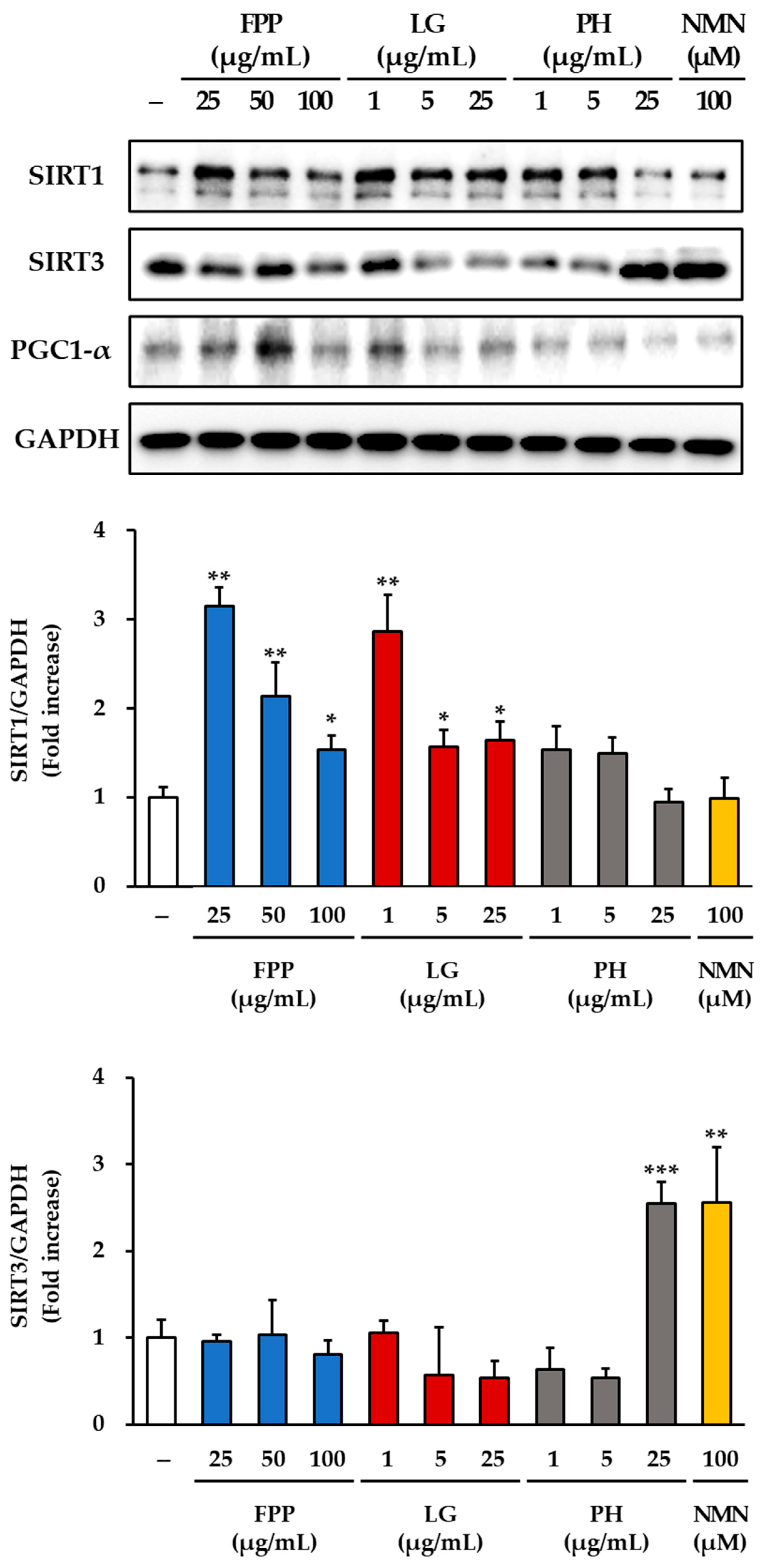
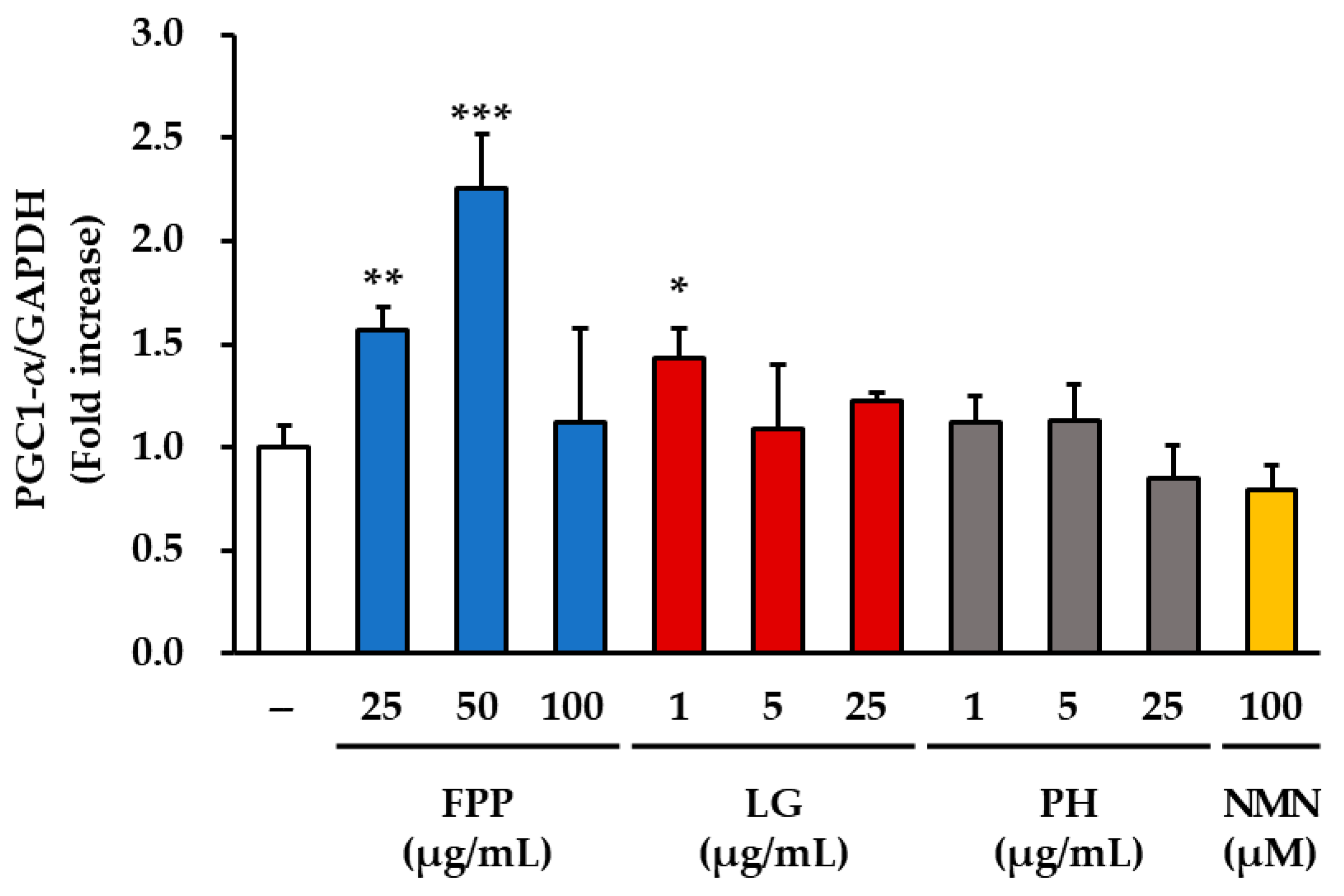
3.7. SIRT1, SIRT3 and PGC-1α Expression as Markers of Mitochondrial Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | Adenosine Triphosphate |
| AMPK | AMP-activated protein kinase |
| BCA | Bicinchoninic Acid Assay |
| DCFDA | 2′,7′-Dichlorofluorescin Diacetate |
| DNA | Deoxyribonucleic acid |
| FPP | fermented porcine placenta |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| HEKs | Human Epidermal Keratinocytes |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| LG | Leucine–Glycine |
| NAD | Nicotinamide Adenine Dinucleotide |
| NR | Nicotinamide riboside |
| NADH | Reduced Nicotinamide Adenine Dinucleotide |
| NMN | Nicotinamide Mononucleotide |
| NQO1 | NAD(P)H Quinone Dehydrogenase 1 |
| P21 | Cyclin-Dependent Kinase Inhibitor 1A (CDKN1A) |
| P53 | Tumor Protein P53 |
| P16 | Cyclin-dependent kinase inhibitor 2A (CDKN2A) |
| PBS | Phosphate-Buffered Saline |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha |
| PH | proline–hydroxyproline |
| Pla-Ext | Placental Extract |
| RIPA | Radioimmunoprecipitation Assay Buffer |
| ROS | Reactive Oxygen Species |
| SA-β-gal | Senescence-Associated β-Galactosidase |
| SIRT1 | Sirtuin 1 |
| SIRT3 | Sirtuin 3 |
| tBHP | tert-Butyl Hydroperoxide |
| TFAM | Transcription Factor A |
| UV | Ultraviolet Radiation |
References
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental stressors on skin aging. Mechanistic insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Hussein, R.S.; Bin Dayel, S.; Abahussein, O.; El-Sherbiny, A.A. Influences on skin and intrinsic aging: Biological, environmental, and therapeutic insights. J. Cosmet. Dermatol. 2025, 24, e16688. [Google Scholar] [CrossRef]
- Wickett, R.R.; Visscher, M.O. Structure and function of the epidermal barrier. Am. J. Infect. Control. 2006, 34, S98–S110. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The immune functions of keratinocytes in skin wound healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Al-Dhubaibi, M.S.; Mohammed, G.F.; Bahaj, S.S.; AbdElneam, A.I.; Al-Dhubaibi, A.M.; Atef, L.M. The Role of Keratinocytes in Skin Health and Disease. Dermatol. Rev. 2025, 6, e70028. [Google Scholar] [CrossRef]
- Papaccio, F.; Caputo, S.; Bellei, B. Focus on the contribution of oxidative stress in skin aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Bekou, V.; Zouboulis, C.C. Genetics and skin aging. Dermato-Endocrinol. 2012, 4, 280–284. [Google Scholar] [CrossRef]
- Brand, R.M.; Wipf, P.; Durham, A.; Epperly, M.W.; Greenberger, J.S.; Falo, L.D., Jr. Targeting mitochondrial oxidative stress to mitigate UV-induced skin damage. Front. Pharmacol. 2018, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Martic, I.; Papaccio, F.; Bellei, B.; Cavinato, M. Mitochondrial dynamics and metabolism across skin cells: Implications for skin homeostasis and aging. Front. Physiol. 2023, 14, 1284410. [Google Scholar] [CrossRef]
- Stojanovic, B.; Jovanovic, I.; Dimitrijevic Stojanovic, M.; Stojanovic, B.S.; Kovacevic, V.; Radosavljevic, I.; Jovanovic, D.; Miletic Kovacevic, M.; Zornic, N.; Arsic, A.A.; et al. Oxidative Stress-Driven Cellular Senescence: Mechanistic Crosstalk and Therapeutic Horizons. Antioxidants 2025, 14, 987. [Google Scholar] [CrossRef]
- Matsuoka, T.; Dan, K.; Takanashi, K.; Ogino, A. Early effects of porcine placental extracts and stem cell-derived exosomes on aging stress in skin cells. J. Funct. Biomater. 2024, 15, 306. [Google Scholar] [CrossRef]
- Matsunaga, K.; Komatsu, Y. Novel placenta-derived liquid product suitable for Cosmetic Application produced by fermentation and digestion of porcine or equine placenta using Lactic Acid Bacterium Enterococcus faecalis PR31. Fermentation 2024, 10, 89. [Google Scholar] [CrossRef]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet radiation--induced skin aging: The role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, H.-J.; Yoon, E.-J.; Lee, H.; Ji, Y.; Kim, Y.; Park, S.-J.; Kim, J.; Bae, S. Protective Effect of Iris germanica L. Rhizome-Derived Exosome against Oxidative-Stress-Induced Cellular Senescence in Human Epidermal Keratinocytes. Appl. Sci. 2023, 13, 11681. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, M.; Zhang, Y.; Liang, Y.; Ying, W. NAD+ administration profoundly decreases UVC-induced skin damage by attenuating oxidative stress, inflammation, DNA damage and apoptosis. Int. J. Physiol. Pathophysiol. Pharmacol. 2023, 15, 41. [Google Scholar]
- Katayoshi, T.; Nakajo, T.; Tsuji-Naito, K. Restoring NAD+ by NAMPT is essential for the SIRT1/p53-mediated survival of UVA-and UVB-irradiated epidermal keratinocytes. J. Photochem. Photobiol. B Biol. 2021, 221, 112238. [Google Scholar] [CrossRef]
- Katayoshi, T.; Yamaura, N.; Nakajo, T.; Kitajima, N.; Tsuji-Naito, K. Porcine placental extract increase the cellular NAD levels in human epidermal keratinocytes. Sci. Rep. 2022, 12, 19040. [Google Scholar] [CrossRef] [PubMed]
- Long, D.J.; Waikel, R.L.; Wang, X.-J.; Roop, D.R.; Jaiswal, A.K. NAD(P)H:quinone oxidoreductase 1 deficiency and increased susceptibility to 7,12-dimethylbenz [a]-anthracene-induced carcinogenesis in mouse skin. J. Natl. Cancer Inst. 2001, 93, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef] [PubMed]
- Oblong, J.E. The evolving role of the NAD+/nicotinamide metabolome in skin homeostasis, cellular bioenergetics, and aging. DNA Repair 2014, 23, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Bielach-Bazyluk, A.; Zbroch, E.; Mysliwiec, H.; Rydzewska-Rosolowska, A.; Kakareko, K.; Flisiak, I.; Hryszko, T. Sirtuin 1 and skin: Implications in intrinsic and extrinsic aging—A systematic review. Cells 2021, 10, 813. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Ndiaye, M.; Singh, C.K.; Ahmad, N. Mitochondrial sirtuins in skin and skin cancers. Photochem. Photobiol. 2020, 96, 973–980. [Google Scholar] [CrossRef]
- Quan, T.; Li, R.; Gao, T. Role of Mitochondrial Dynamics in Skin Homeostasis: An Update. Int. J. Mol. Sci. 2025, 26, 1803. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Han, N.-R.; Kim, N.-R.; Lee, M.; Kim, J.; Kim, C.-J.; Jeong, H.-J.; Kim, H.-M. Effect of fermented porcine placenta on physical fatigue in mice. Exp. Biol. Med. 2016, 241, 1985–1996. [Google Scholar] [CrossRef]
- Banerjee, J.; Bruckbauer, A.; Zemel, M.B. Activation of the AMPK/Sirt1 pathway by a leucine–metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans. Metabolism 2016, 65, 1679–1691. [Google Scholar] [CrossRef]
- Zemel, M.B. Modulation of energy sensing by leucine synergy with natural sirtuin activators: Effects on health span. J. Med. Food 2020, 23, 1129–1135. [Google Scholar] [CrossRef]
- Nomura, K.; Kimira, Y.; Kobayashi, R.; Shiobara, Y.; Osawa, Y.; Kataoka-Matsushita, A.; Shimizu, J.; Wada, M.; Mano, H. Collagen-derived dipeptide prolyl-hydroxyproline cooperates with Foxg1 to activate the PGC-1α promoter and induce brown adipocyte-like phenotype in rosiglitazone-treated C3H10T1/2 cells. Front. Nutr. 2024, 11, 1375532. [Google Scholar] [CrossRef]
- Guo, K.; Liu, R.; Jing, R.; Wang, L.; Li, X.; Zhang, K.; Fu, M.; Ye, J.; Hu, Z.; Zhao, W. Cryptotanshinone protects skin cells from ultraviolet radiation-induced photoaging via its antioxidant effect and by reducing mitochondrial dysfunction and inhibiting apoptosis. Front. Pharmacol. 2022, 13, 1036013. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, B.; Du, W.; Zeng, M.; He, K.; Yin, T.; Shang, S.; Su, T.; Han, D.; Gan, X. The role of nicotinamide mononucleotide supplementation in psoriasis treatment. Antioxidants 2024, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Pace, M.; Giorgi, C.; Lombardozzi, G.; Cimini, A.; Castelli, V.; d’Angelo, M. Exploring the Antioxidant Roles of Cysteine and Selenocysteine in Cellular Aging and Redox Regulation. Biomolecules 2025, 15, 1115. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.J.; Kang, M.; Jin, M.H.; Kim, J.; Lee, W.K.; Roh, S.-S.; Kang, K.S.; Hwang, G.S.; Park, S.; Lee, S. Fermented Porcine Placenta and Its Dipeptides Modulate Cellular Senescence in Human Keratinocytes. Curr. Issues Mol. Biol. 2025, 47, 941. https://doi.org/10.3390/cimb47110941
Choi YJ, Kang M, Jin MH, Kim J, Lee WK, Roh S-S, Kang KS, Hwang GS, Park S, Lee S. Fermented Porcine Placenta and Its Dipeptides Modulate Cellular Senescence in Human Keratinocytes. Current Issues in Molecular Biology. 2025; 47(11):941. https://doi.org/10.3390/cimb47110941
Chicago/Turabian StyleChoi, Yea Jung, Minseo Kang, Mu Hyun Jin, Jongbae Kim, Won Kyung Lee, Seok-Seon Roh, Ki Sung Kang, Gwi Seo Hwang, Sangki Park, and Sullim Lee. 2025. "Fermented Porcine Placenta and Its Dipeptides Modulate Cellular Senescence in Human Keratinocytes" Current Issues in Molecular Biology 47, no. 11: 941. https://doi.org/10.3390/cimb47110941
APA StyleChoi, Y. J., Kang, M., Jin, M. H., Kim, J., Lee, W. K., Roh, S.-S., Kang, K. S., Hwang, G. S., Park, S., & Lee, S. (2025). Fermented Porcine Placenta and Its Dipeptides Modulate Cellular Senescence in Human Keratinocytes. Current Issues in Molecular Biology, 47(11), 941. https://doi.org/10.3390/cimb47110941






