Regulation of TGF-β and BMP Signaling by Natural Triterpene Compounds in Pulmonary Arterial Hypertension (PAH)
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation Studies
2.2. Cell Culture, Plasmid DNA Isolation, and Transfection Procedure
2.3. Western Blotting
2.4. SMAD-Responsive Reporter Assay
2.5. RT-PCR Analysis
2.6. Q-PCR Analysis
2.7. Proliferation Assay
2.8. Calculations and Statistical Analysis
2.9. In Silico Analysis
3. Results
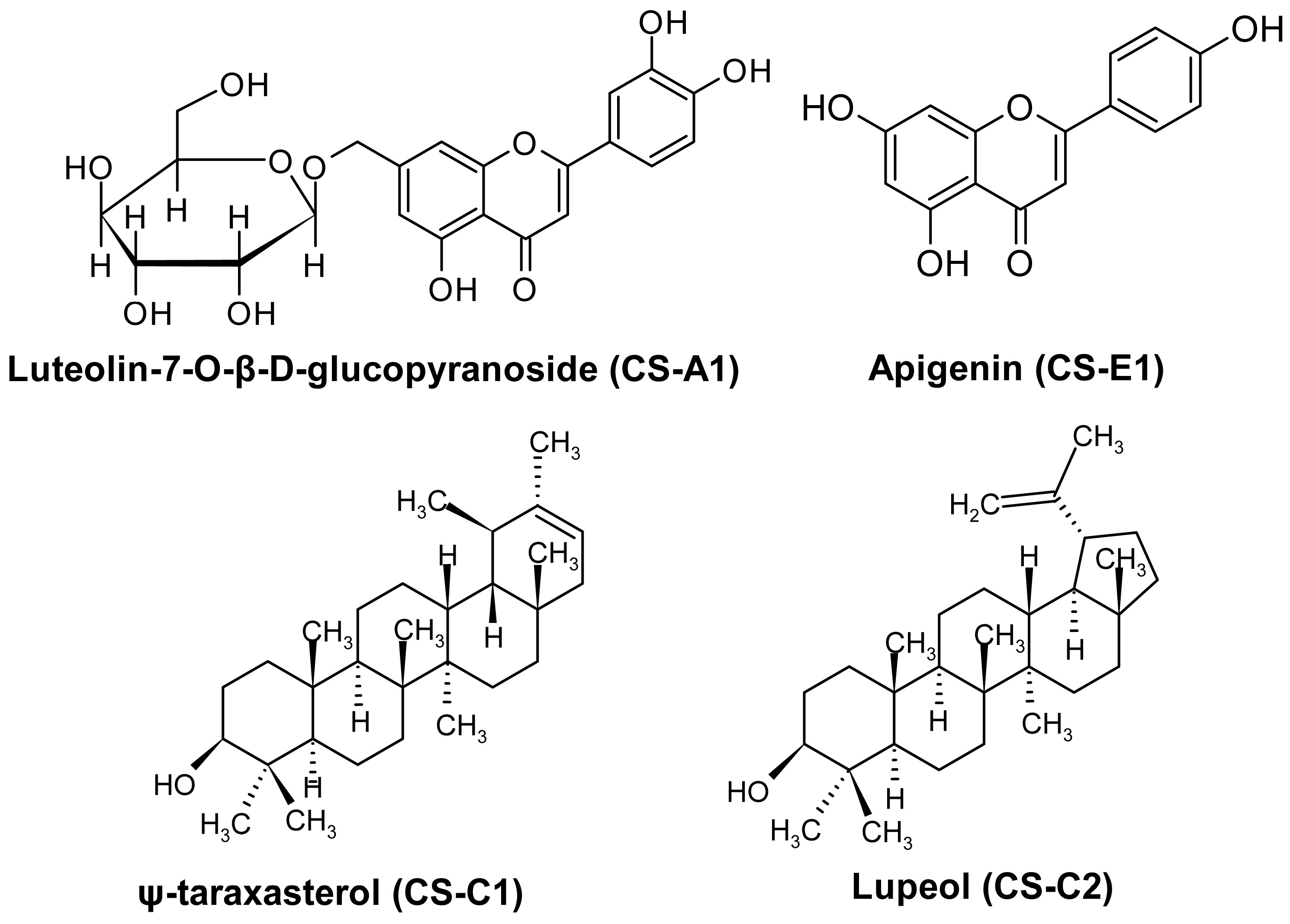
3.1. Isolation of Triterpene Compounds
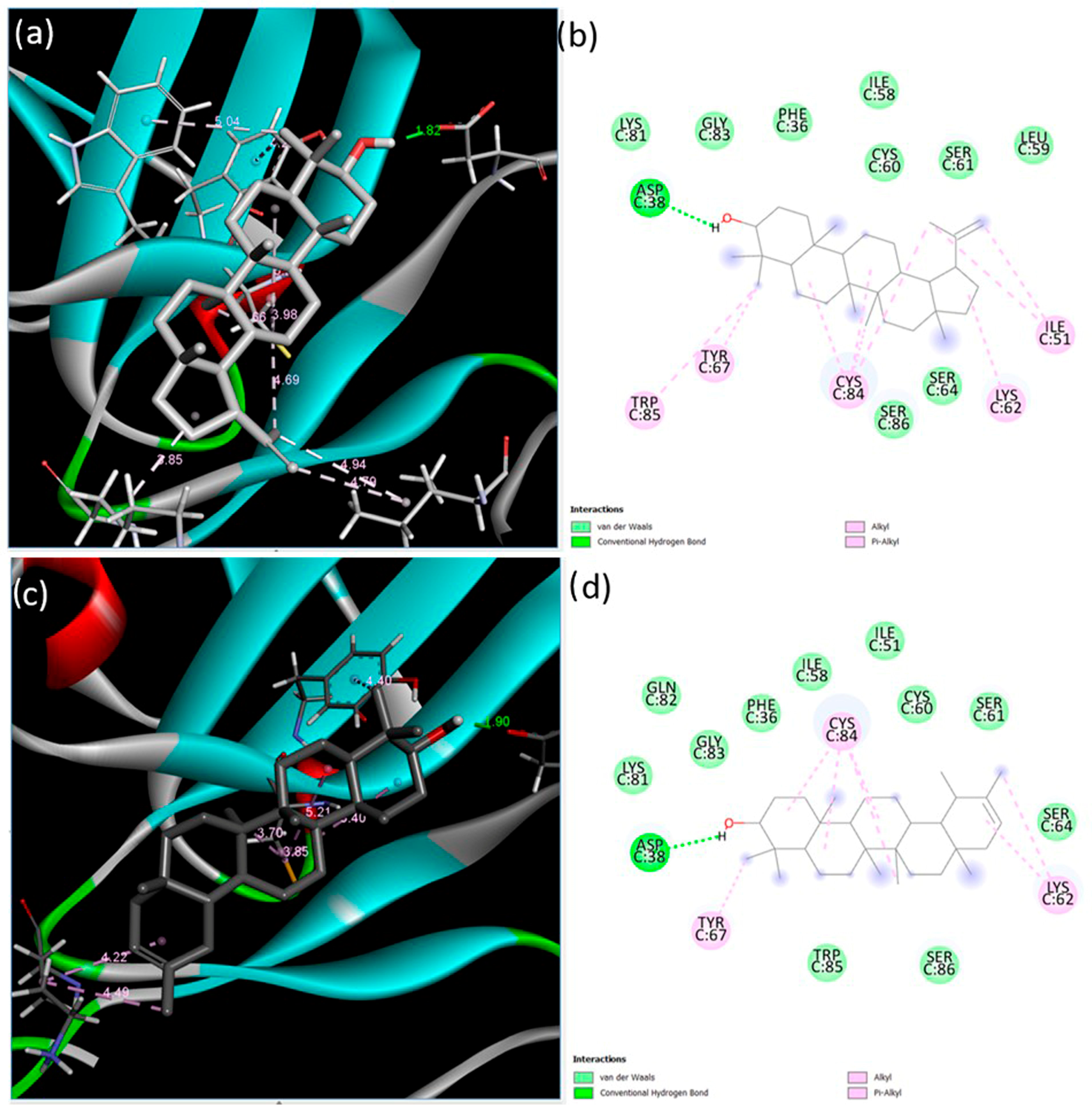
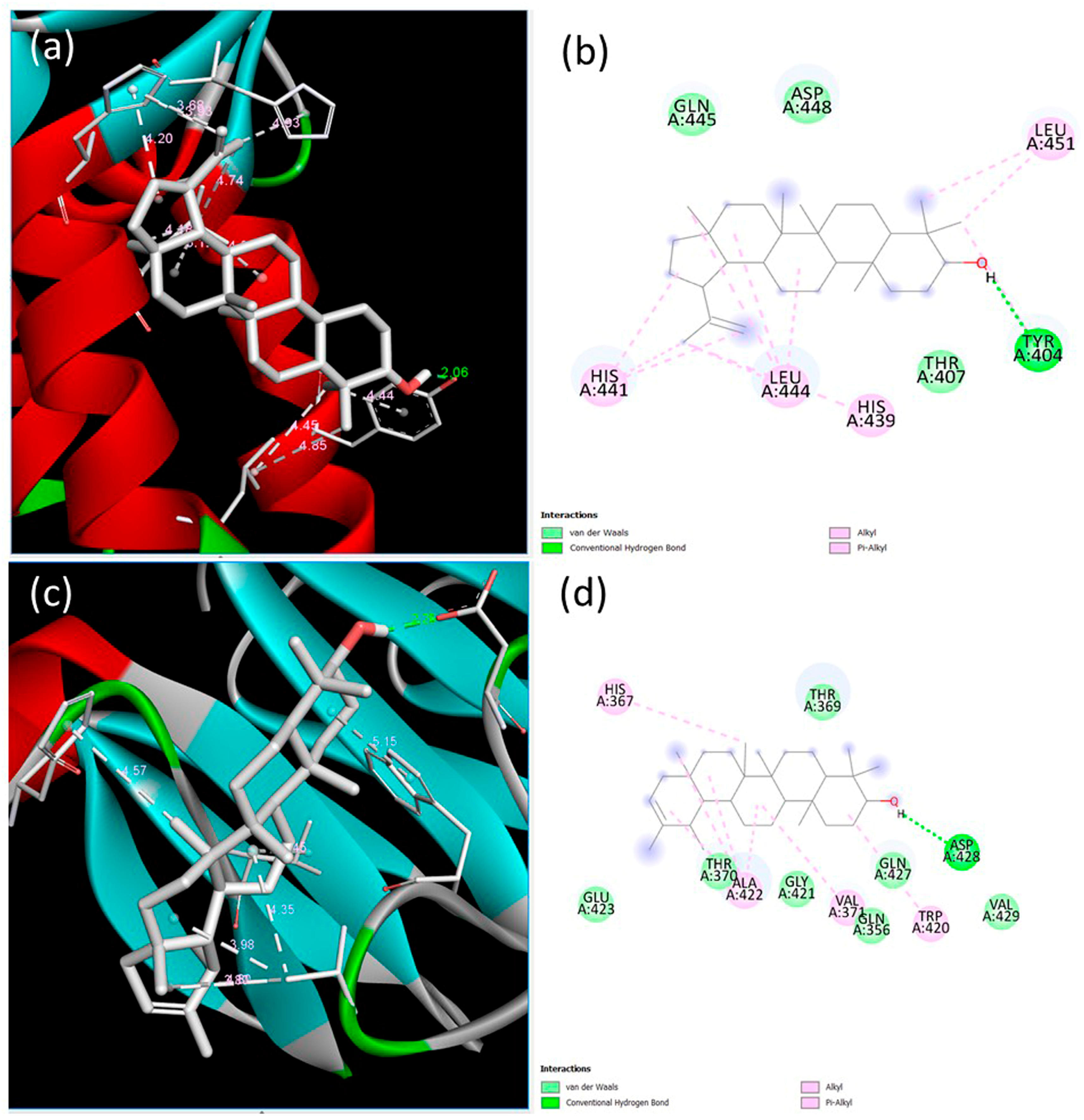
3.2. Determination of Lupeol and ψ-Taraxasterol Interactions with the Components of BMP and TGF-β Pathways Using In Silico Analysis
3.3. Lupeol and ψ-Taraxasterol Modulate SMAD-Responsive Reporter Activities
3.4. Lupeol and ψ-Taraxasterol Reduced the Phosphorylation of SMAD3 and Increased SMAD1/5 Proteins as Determined by Western Blotting
3.5. Lupeol and ψ-Taraxasterol Modulate the Expression of Target Genes Determined by RT-PCR and Q-PCR Analyses
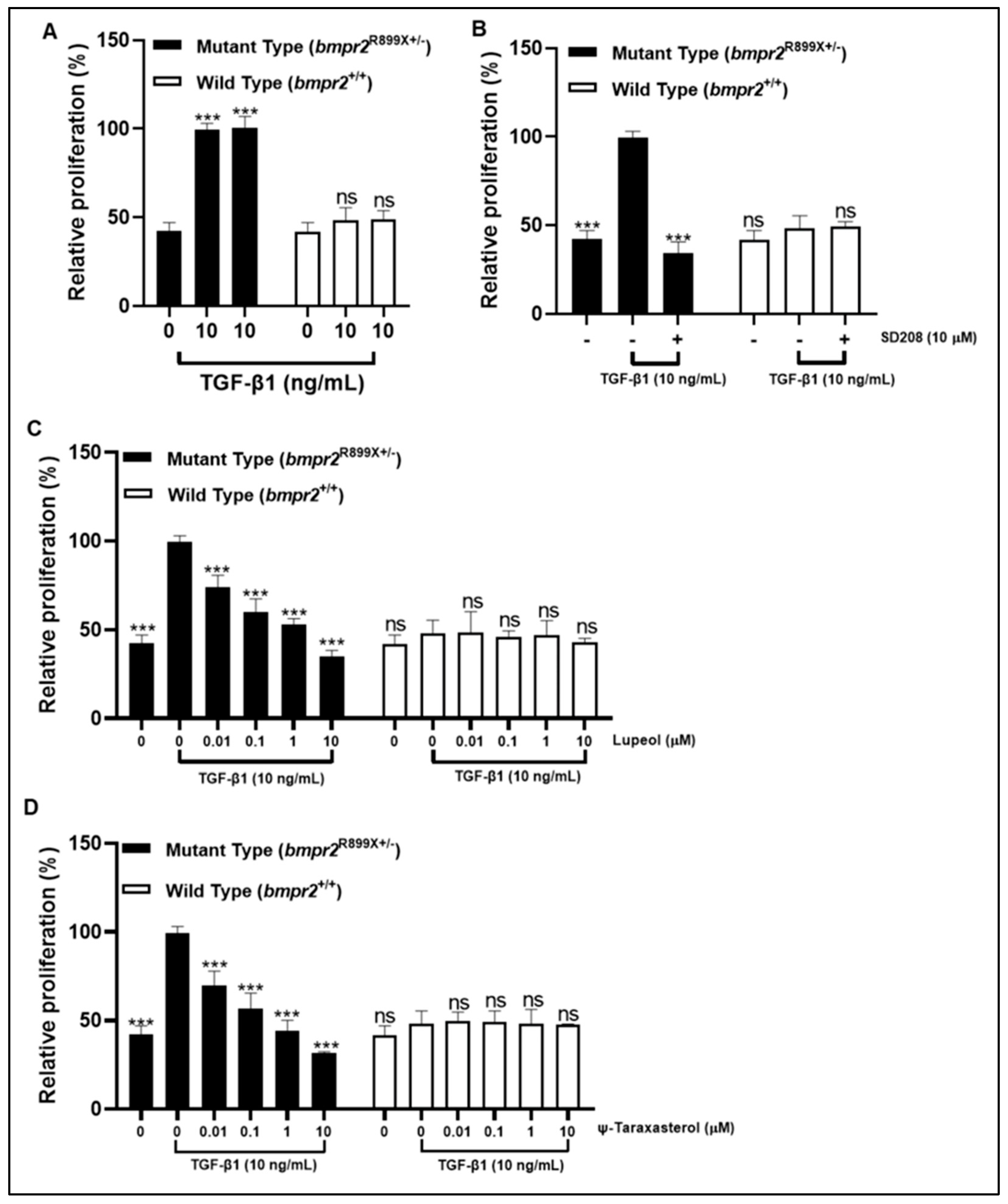
3.6. Lupeol and ψ-Taraxasterol Reduced Excessive Cell Proliferation of PASMCs Harboring a Pathogenic BMPR2 Mutation
4. Discussion
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| βGAL | Beta-galactosidase (reporter gene) |
| BMPR-II | Bone Morphogenetic Protein Receptor Type II |
| BMP | Bone Morphogenetic Protein |
| CD3OD | Deuterated Methanol (solvent for NMR spectroscopy) |
| CDCl3 | Deuterated Chloroform (solvent for NMR spectroscopy) |
| CS-A1 | Luteolin-7-O-β-D-glucopyranoside |
| CS-C1 | ψ-Taraxasterol |
| CS-C2 | Lupeol |
| CS-E1 | Apigenin |
| id-1 | Inhibitor of DNA Binding 1 (BMP target gene) |
| MS | Mass Spectrometry |
| NMR | Nuclear Magnetic Resonance Spectroscopy |
| NO | Nitric Oxide |
| PAH | Pulmonary Arterial Hypertension |
| pai-1 | Plasminogen Activator Inhibitor-1 (TGF-β target gene) |
| PAMSCs | Pulmonary Artery Smooth Muscle Cells |
| PH | Pulmonary Hypertension |
| qPCR | Quantitative Polymerase Chain Reaction |
| R-SMADs | Receptor-regulated SMADs (SMAD1/2/3/5 proteins) |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| SBE-LUC | SMAD Binding Element–Luciferase Reporter Assay |
| TGF-β | Transforming Growth Factor Beta |
| TGFBR2 | Transforming Growth Factor Beta Receptor 2 |
References
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur. Heart J. 2015, 46, 903–9759. [Google Scholar] [CrossRef]
- Tielemans, B.; Delcroix, M.; Belge, C.; Quarck, R. TGFβ and BMPRII Signalling Pathways in the Pathogenesis of Pulmonary Arterial Hypertension. Drug Discov. Today 2019, 24, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Dannewitz Prosseda, S.; Ali, M.K.; Spiekerkoetter, E. Novel Advances in Modifying BMPR2 Signaling in PAH. Genes 2021, 12, 8. [Google Scholar] [CrossRef]
- Osei-Wusuansa, M.; Mohammed, N.; Makanjuola, D.; Habas, K.; Sener, S.O.; Assi, K.H.; Nasim, R.; Nawaz, S.; Gopalan, R.J.; Wright, C.W.; et al. Therapeutic Resolution of Pulmonary Arterial Hypertension (PAH) Using Natural Products. Targets 2024, 2, 428–445. [Google Scholar] [CrossRef]
- Nasim, M.T.; Ogo, T.; Ahmed, M.; Randall, R.; Chowdhury, H.M.; Snape, K.M.; Bradshaw, T.Y.; Southgate, L.; Lee, G.J.; Jackson, I.; et al. Molecular Genetic Characterization of SMAD Signaling Molecules in Pulmonary Arterial Hypertension. Hum. Mutat. 2011, 32, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Nasim, M.T.; Ogo, T.; Chowdhury, H.M.; Zhao, L.; Chen, C.N.; Rhodes, C.; Trembath, R.C. BMPR-II Deficiency Elicits Pro-Proliferative and Anti-Apoptotic Responses through the Activation of TGFβ-TAK1-MAPK Pathways in PAH. Hum. Mol. Genet. 2012, 21, 2548–2558. [Google Scholar] [CrossRef]
- Sztrymf, B.; Coulet, F.; Girerd, B.; Yaici, A.; Jais, X.; Sitbon, O.; Montani, D.; Souza, R.; Simonneau, G.; Soubrier, F.; et al. Clinical Outcomes of Pulmonary Arterial Hypertension in Carriers of BMPR2 Mutation. Am. J. Respir. Crit. Care Med. 2008, 177, 1377–1383. [Google Scholar] [CrossRef]
- Tatius, B.; Wasityastuti, W.; Astarini, F.D.; Nugrahaningsih, D.A.A. Significance of BMPR2 Mutations in Pulmonary Arterial Hypertension. Respir. Investig. 2021, 59, 397–407. [Google Scholar] [CrossRef]
- Andruska, A.; Spiekerkoetter, E. Consequences of BMPR2 Deficiency in the Pulmonary Vasculature and Beyond: Contributions to Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2018, 19, 2499. [Google Scholar] [CrossRef]
- Ogo, T.; Chowdhury, H.M.; Yang, J.; Long, L.; Li, X.; Torres Cleuren, Y.N.; Morrell, N.W.; Schermuly, R.T.; Trembath, R.C.; Nasim, M.T. Inhibition of Overactive Transforming Growth Factor-β Signaling by Prostacyclin Analogs in Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 2013, 48, 733–741. [Google Scholar] [CrossRef]
- Long, L.; Crosby, A.; Yang, X.; Southwood, M.; Upton, P.D.; Kim, D.K.; Morrell, N.W. Altered Bone Morphogenetic Protein and Transforming Growth Factor-β Signaling in Rat Models of Pulmonary Hypertension: Potential for Activin Receptor-Like Kinase-5 Inhibition in Prevention and Progression of Disease. Circulation 2009, 119, 566–576. [Google Scholar] [CrossRef]
- Andre, P.; Joshi, S.R.; Briscoe, S.D.; Alexander, M.J.; Li, G.; Kumar, R. Therapeutic Approaches for Treating Pulmonary Arterial Hypertension by Correcting Imbalanced TGF-β Superfamily Signaling. Front. Med. 2022, 8, 814222. [Google Scholar] [CrossRef]
- Sharmin, N.; Nganwuchu, C.C.; Nasim, T. Targeting the TGF-β Signaling Pathway for Resolution of Pulmonary Arterial Hypertension. Trends Pharmacol. Sci. 2021, 42, 510–513. [Google Scholar] [CrossRef]
- Rol, N.; Kurakula, K.B.; Happe, C.; Bogaard, H.J.; Goumans, M.J. TGF-β and BMPR2 Signaling in PAH: Two Black Sheep in One Family. Int. J. Mol. Sci. 2018, 19, 2585. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.L.; Chen, Z.H.; Teng, Y.Y.; Liu, S.Y.; Jia, Y.; Zhang, K.W.; Sun, Z.L.; Wu, J.J.; Yuan, Z.D.; Feng, Y.; et al. The Smad-Dependent TGF-β and BMP Signaling Pathway in Bone Remodeling and Therapies. Front. Mol. Biosci. 2021, 8, 593310. [Google Scholar] [CrossRef] [PubMed]
- Kouri, F.M.; Queisser, M.A.; Königshoff, M.; Chrobak, I.; Preissner, K.T.; Seeger, W.; Eickelberg, O. Plasminogen Activator Inhibitor Type 1 Inhibits Smooth Muscle Cell Proliferation in Pulmonary Arterial Hypertension. Int. J. Biochem. Cell Biol. 2008, 40, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, M.; Wu, N.; Liu, B.; Liu, Q.; Fan, X. TGF-β/Smads Signaling Pathway, Hippo-YAP/TAZ Signaling Pathway, and VEGF: Their Mechanisms and Roles in Vascular Remodeling Related Diseases. Immun. Inflamm. Dis. 2023, 11, e1060. [Google Scholar] [CrossRef]
- Yang, J.; Davies, R.J.; Southwood, M.; Long, L.; Yang, X.; Sobolewski, A.; Upton, P.D.; Trembath, R.C.; Morrell, N.W. Mutations in Bone Morphogenetic Protein Type II Receptor Cause Dysregulation of Id Gene Expression in Pulmonary Artery Smooth Muscle Cells: Implications for Familial Pulmonary Arterial Hypertension. Circ. Res. 2008, 102, 1212–1221. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Morrell, N.W. Id proteins in the vasculature: From molecular biology to cardiopulmonary medicine. Cardiovasc. Res. 2014, 104, 388–398. [Google Scholar] [CrossRef]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Sener, S.O.; Nasim, R.; Nasim, T. The Prospect and Challenges of Repurposing Established Drugs in Pulmonary Arterial Hypertension. BioChem 2024, 4, 236–251. [Google Scholar] [CrossRef]
- Yu, Z.; Xiao, J.; Chen, X.; Ruan, Y.; Chen, Y.; Zheng, X.; Wang, Q. Bioactivities and Mechanisms of Natural Medicines in the Management of Pulmonary Arterial Hypertension. Chin. Med. 2022, 17, 13. [Google Scholar] [CrossRef]
- Sener, S.O.; Ozgen, U.; Kanbolat, S.; Korkmaz, N.; Badem, M.; Hanci, H.; Dirmenci, T.; Arabaci, T.; Aliyazicioglu, R.; Yenilmez, E.; et al. Investigation of Therapeutic Potential of Three Endemic Cirsium Species for Global Health Problem Obesity. S. Afr. J. Bot. 2021, 141, 243–254. [Google Scholar] [CrossRef]
- Kim, E.Y.; Jho, H.K.; Kim, D.I.; Rhyu, M.R. Cirsium japonicum elicits endothelium-dependent relaxation via histamine H(1)-receptor in rat thoracic aorta. J. Ethnopharmacol. 2008, 116, 223–227. [Google Scholar] [CrossRef]
- Yang, X.; Shao, H.; Chen, Y.; Ding, N.; Yang, A.; Tian, J.; Jiang, Y.; Li, G.; Jiang, Y. In renal hypertension, Cirsium japonicum strengthens cardiac function via the intermedin/nitric oxide pathway. Biomed. Pharmacother. 2018, 101, 787–791. [Google Scholar] [CrossRef]
- Saleem, M. Lupeol, a Novel Anti-Inflammatory and Anti-Cancer Dietary Triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Siddique, H.R.; Saleem, M. Beneficial Health Effects of Lupeol Triterpene: A Review of Preclinical Studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Parmar, S.K.; Sharma, T.P.; Airao, V.B.; Bhatt, R.; Aghara, R.; Chavda, S.; Rabadiya, S.O.; Gangwal, A.P. Neuropharmacological Effects of Triterpenoids. Phytopharmacology 2013, 4, 354–372. [Google Scholar]
- Reynolds, W.F.; McLean, S.; Poplawski, J.; Enriquez, R.G.; Escobar, L.I.; Leon, I. Total Assignment of 13C and 1H Spectra of Three Isomeric Triterpenol Derivatives by 2D NMR: An Investigation of the Potential Utility of 1H Chemical Shifts in Structural Investigations of Complex Natural Products. Tetrahedron 1986, 42, 3419–3428. [Google Scholar] [CrossRef]
- Stiti, N.; Hartmann, M.A. Nonsterol Triterpenoids as Major Constituents of Olea europaea. J. Lipids 2012, 2012, 476595. [Google Scholar] [CrossRef]
- da Silva, F.A.N.; de Farias Freire, S.M.; da Rocha Borges, M.O.; Barros, F.E.V.; da de Sousa, M.; de Sousa Ribeiro, M.N.; Pinheiro Guilhon, G.M.S.; Müller, A.H.; Romão Borges, A.C. Antinociceptive and Anti-Inflammatory Effects of Triterpenes from Pluchea quitoc DC. Aerial Parts. Pharmacogn. Res. 2017, 9, S1. [Google Scholar] [CrossRef] [PubMed]
- Nasim, M.T.; Eperon, I.C. A Double-Reporter Splicing Assay for Determining Splicing Efficiency in Mammalian Cells. Nat. Protoc. 2006, 1, 1022–1028. [Google Scholar] [CrossRef]
- Nasim, M.T.; Ghouri, A.; Patel, B.; James, V.; Rudarakanchana, N.; Morrell, N.W.; Trembath, R.C. Stoichiometric Imbalance in the Receptor Complex Contributes to Dysfunctional BMPR-II Mediated Signalling in Pulmonary Arterial Hypertension. Hum. Mol. Genet. 2008, 17, 1683–1694. [Google Scholar] [CrossRef]
- Lento, S.; Brioschi, M.; Barcella, S.; Nasim, M.T.; Ghilardi, S.; Barbieri, S.S.; Tremoli, E.; Banfi, C. Proteomics of Tissue Factor Silencing in Cardiomyocytic Cells Reveals a New Role for This Coagulation Factor in Splicing Machinery Control. J. Proteom. 2015, 119, 75–89. [Google Scholar] [CrossRef]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational Protein-Ligand Docking and Virtual Drug Screening with the AutoDock Suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Terstappen, G.C.; Reggiani, A. In Silico Research in Drug Discovery. Trends Pharmacol. Sci. 2001, 22, 23–26. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA. Discovery Studio Modeling Environment, Release 2017; Dassault Systèmes: San Diego, CA, USA, 2016. [Google Scholar]
- Sezen Karaoğlan, E.; Hancı, H.; Koca, M.; Kazaz, C. Some Bioactivities of Isolated Apigenin-7-O-Glucoside and Luteolin-7-O-Glucoside. Appl. Sci. 2023, 13, 1503. [Google Scholar] [CrossRef]
- Wawer, I.; Zielinska, A. 13C CP/MAS NMR Studies of Flavonoids. Magn. Reson. Chem. 2001, 39, 374–380. [Google Scholar] [CrossRef]
- Boyong, L.; Robinson, D.H.; Birt, F.D. Evaluation of Properties of Apigenin and [G-3H] Apigenin and Analytic Method Development. J. Pharm. Sci. 1997, 86, 721–725. [Google Scholar]
- Zitterl-Eglseer, K.; Sosa, S.; Jurenitsch, J.; Schubert-Zsilavecz, M.; Della Loggia, R.; Tubaro, A.; Bertoldi, M.; Franz, C. Anti-Oedematous Activities of the Main Triterpendiol Esters of Marigold (Calendula officinalis L.). J. Ethnopharmacol. 1997, 57, 139–144. [Google Scholar] [CrossRef]
- Silva, A.T.M.E.; Magalhães, C.G.; Duarte, L.P.; Mussel, W.D.N.; Ruiz, A.L.T.G.; Shiozawa, L.; Carvalho, J.E.D.; Trindade, I.C.; Vieira Filho, S.A. Lupeol and Its Esters: NMR, Powder XRD Data and In Vitro Evaluation of Cancer Cell Growth. Braz. J. Pharm. Sci. 2017, 53, e00251. [Google Scholar] [CrossRef]
- Viswanathan, G.; Rajagopal, S. De novo purine synthesis: A new target in pulmonary arterial hypertension? Eur. Heart J. 2023, 44, 1280–1282. [Google Scholar] [CrossRef]
- Zuo, W.; Liu, N.; Zeng, Y.; Xiao, Z.; Wu, K.; Yang, F.; Li, B.; Song, Q.; Xiao, Y.; Liu, Q. Luteolin Ameliorates Experimental Pulmonary Arterial Hypertension via Suppressing Hippo-YAP/PI3K/AKT Signaling Pathway. Front. Pharmacol. 2021, 12, 663551. [Google Scholar] [CrossRef]
- Ji, L.; Su, S.; Xin, M.; Zhang, Z.; Nan, X.; Li, Z.; Lu, D. Luteolin Ameliorates Hypoxia-Induced Pulmonary Hypertension via Regulating HIF-2α-Arg-NO Axis and PI3K-AKT-eNOS-NO Signaling Pathway. Phytomedicine 2022, 104, 154329. [Google Scholar] [CrossRef]
- He, Y.; Fang, X.; Shi, J.; Li, X.; Xie, M.; Liu, X. Apigenin Attenuates Pulmonary Hypertension by Inducing Mitochondria-Dependent Apoptosis of PASMCs via Inhibiting the Hypoxia Inducible Factor 1α–KV1.5 Channel Pathway. Chem. Biol. Interact. 2020, 317, 108942. [Google Scholar] [CrossRef]
- Gao, H.L.; Yu, X.J.; Hu, H.B.; Yang, Q.W.; Liu, K.L.; Chen, Y.M.; Zhang, Y.; Zhang, D.-D.; Tian, H.; Zhu, G.-Q.; et al. Apigenin Improves Hypertension and Cardiac Hypertrophy through Modulating NADPH Oxidase-Dependent ROS Generation and Cytokines in Hypothalamic Paraventricular Nucleus. Cardiovasc. Toxicol. 2021, 21, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Zafar, R. Occurrence of Taraxerol and Taraxasterol in Medicinal Plants. Pharmacogn. Rev. 2015, 9, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Banu, Z. Therapeutic Promise of Lupeol: A Comprehensive Review of Its Pharmacological Potential. Ann. Phytomed. 2024, 13, 63–74. [Google Scholar] [CrossRef]
- Jiao, F.; Tan, Z.; Yu, Z.; Zhou, B.; Meng, L.; Shi, X. The phytochemical and pharmacological profile of taraxasterol. Front. Pharmacol. 2022, 13, 927365. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xing, Y.; Zhang, J.; He, M.; Dong, J.; Chen, S.; Wu, H.; Huang, H.Y.; Chou, C.H.; Bai, L.; et al. MED1 Regulates BMP/TGF-β in Endothelium: Implication for Pulmonary Hypertension. Circ. Res. 2022, 131, 828–841. [Google Scholar] [CrossRef]
- Lakhan, S.E. In silico research is rewriting the rules of drug development: Is it the end of human trials? Cureus 2025, 17, e84007. [Google Scholar] [CrossRef]
- Al-Mohaya, M.; Mesut, B.; Kurt, A.; Çelik, Y.S. In silico approaches which are used in pharmacy. J. Appl. Pharm. Sci. 2024, 14, 239–253. [Google Scholar] [CrossRef]
- Prescott, M.J.; Langermans, J.A.; Ragan, I. Applying the 3Rs to non-human primate research: Barriers and solutions. Drug Discov. Today Dis. Models 2017, 23, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Becher, C.; Wits, M.; de Man, F.S.; Sanchez-Duffhues, G.; Goumans, M.J. Targeting Soluble TGF-β Factors: Advances in Precision Therapy for Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. Basic Trans. Sci. 2024, 9, 1360–1374. [Google Scholar] [CrossRef]
- Hodgson, J.; Swietlik, E.M.; Salmon, R.M.; Hadinnapola, C.; Nikolic, I.; Wharton, J.; Guo, J.; Liley, J.; Haimel, M.; Bleda, M.; et al. Characterization of GDF2 Mutations and Levels of BMP9 and BMP10 in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2020, 201, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.A.; Dunmore, B.J.; Long, L.; Crosby, A.; Al-Lamki, R.; Deighton, J.; Southwood, M.; Yang, X.; Skepper, J.; Howard, L.S.; et al. TNFα drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat. Commun. 2017, 8, 14079. [Google Scholar] [CrossRef]
- Cuthbertson, I.; Morrell, N.W.; Caruso, P. BMPR2 mutation and metabolic reprogramming in pulmonary arterial hypertension. Circ. Res. 2023, 132, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Liu, J.; Lu, J.; Zeng, C.; Chen, H.; Duan, Z.; Zhang, Y.; Li, X.; Wang, J.; Zhao, Y.; et al. HMGB2 release promotes pulmonary hypertension and predicts severity and mortality of patients with pulmonary arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 172–195. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Heng, H.H. Transient and stable vector transfection: Pitfalls, off-target effects, artifacts. Mutat. Res. Rev. Mutat. Res. 2017, 773, 91–103. [Google Scholar] [CrossRef]
- Thomas, P.; Smart, T.G. HEK293 cell line: A vehicle for the expression of recombinant proteins. J. Pharmacol. Toxicol. Methods 2005, 51, 187–200. [Google Scholar] [CrossRef]
- Chowdhury, H.M.; Sharmin, N.; Yuzbasioglu Baran, M.; Long, L.; Morrell, N.W.; Trembath, R.C.; Nasim, M.T. BMPRII deficiency impairs apoptosis via the BMPRII-ALK1-BclX-mediated pathway in pulmonary arterial hypertension. Hum. Mol. Genet. 2019, 28, 2161–2173. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, R.; He, X.; Ding, Y.; Chang, Y.; Yang, R.; Zhong, G.; Yang, H.; Li, J. Targeted delivery of BMPR2 mRNA attenuates pulmonary arterial hypertension by reversing pulmonary vascular remodeling. Acta Pharm. Sin. B 2025, 15, 5416–5430. [Google Scholar] [CrossRef]
- Shah, A.J.; Beckmann, T.; Vorla, M.; Kalra, D.K. New Drugs and Therapies in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2023, 24, 5850. [Google Scholar] [CrossRef]
- Upton, P.D.; Dunmore, B.J.; Li, W.; Morrell, N.W. An Emerging Class of New Therapeutics Targeting TGF, Activin, and BMP Ligands in Pulmonary Arterial Hypertension. Dev. Dyn. 2023, 252, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Sitbon, O.; Guignabert, C.; Savale, L.; Boucly, A.; Gallant-Dewavrin, M.; McLaughlin, V.; Hoeper, M.M.; Weatherald, J. Treatment of Pulmonary Arterial Hypertension: Recent Progress and a Look to the Future. Lancet Respir. Med. 2023, 11, 804–819. [Google Scholar] [CrossRef] [PubMed]
- Sanada, T.J.; Sun, X.Q.; Happé, C.; Guignabert, C.; Tu, L.; Schalij, I.; Bogaard, H.J.; Goumans, M.J.; Kurakula, K. Altered TGFβ/SMAD Signaling in Human and Rat Models of Pulmonary Hypertension: An Old Target Needs Attention. Cells 2021, 10, 84. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Sun, S.; Zhu, J.; Gao, S.; Pang, J.; Zhu, D.; Sun, Z. Transforming Growth Factor-Beta1 Upregulation Triggers Pulmonary Artery Smooth Muscle Cell Proliferation and Apoptosis Imbalance in Rats with Hypoxic Pulmonary Hypertension via the PTEN/AKT Pathways. Int. J. Biochem. Cell Biol. 2016, 77, 141–154. [Google Scholar] [CrossRef]
- Erewele, E.O.; Castellon, M.; Loya, O.; Marshboom, G.; Schwartz, A.; Yerlioglu, K.; Callahan, C.; Chen, J.; Minshall, R.D.; Oliveira, S.D. Hypoxia-Induced Pulmonary Hypertension Upregulates eNOS and TGF-β Contributing to Sex-Linked Differences in BMPR2+/R899X Mutant Mice. Pulm. Circ. 2022, 12, e12163. [Google Scholar] [CrossRef]
- Feng, F.; Harper, R.L.; Reynolds, P.N. BMPR2 Gene Delivery Reduces Mutation-Related PAH and Counteracts TGF-β-Mediated Pulmonary Cell Signalling. Respirology 2016, 21, 526–532. [Google Scholar] [CrossRef]
- Saleem, M.; Maddodi, N.; Abu Zaid, M.; Khan, N.; Bin Hafeez, B.; Asim, M.; Suh, Y.; Yun, J.M.; Setaluri, V.; Mukhtar, H. Lupeol inhibits growth of highly aggressive human metastatic melanoma cells in vitro and in vivo by inducing apoptosis. Clin. Cancer Res. 2008, 14, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Piao, T.; Ma, Z.; Li, X.; Liu, J. Taraxasterol inhibits IL-1β-induced inflammatory response in human osteoarthritic chondrocytes. Eur. J. Pharmacol. 2015, 756, 38–42. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sener, S.O.; Shaha, S.; İşcan, S.G.; Ozgen, U.; Baran, M.Y.; Nalcaoğlu, A.; Nasim, M.T. Regulation of TGF-β and BMP Signaling by Natural Triterpene Compounds in Pulmonary Arterial Hypertension (PAH). Curr. Issues Mol. Biol. 2025, 47, 939. https://doi.org/10.3390/cimb47110939
Sener SO, Shaha S, İşcan SG, Ozgen U, Baran MY, Nalcaoğlu A, Nasim MT. Regulation of TGF-β and BMP Signaling by Natural Triterpene Compounds in Pulmonary Arterial Hypertension (PAH). Current Issues in Molecular Biology. 2025; 47(11):939. https://doi.org/10.3390/cimb47110939
Chicago/Turabian StyleSener, Sila Ozlem, Sabita Shaha, Saltan Gülçin İşcan, Ufuk Ozgen, Merve Yuzbasioglu Baran, Aleyna Nalcaoğlu, and Md Talat Nasim. 2025. "Regulation of TGF-β and BMP Signaling by Natural Triterpene Compounds in Pulmonary Arterial Hypertension (PAH)" Current Issues in Molecular Biology 47, no. 11: 939. https://doi.org/10.3390/cimb47110939
APA StyleSener, S. O., Shaha, S., İşcan, S. G., Ozgen, U., Baran, M. Y., Nalcaoğlu, A., & Nasim, M. T. (2025). Regulation of TGF-β and BMP Signaling by Natural Triterpene Compounds in Pulmonary Arterial Hypertension (PAH). Current Issues in Molecular Biology, 47(11), 939. https://doi.org/10.3390/cimb47110939







